Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review
Abstract
1. Introduction
2. Estimation of Removal Percentage and Adsorption Capacity
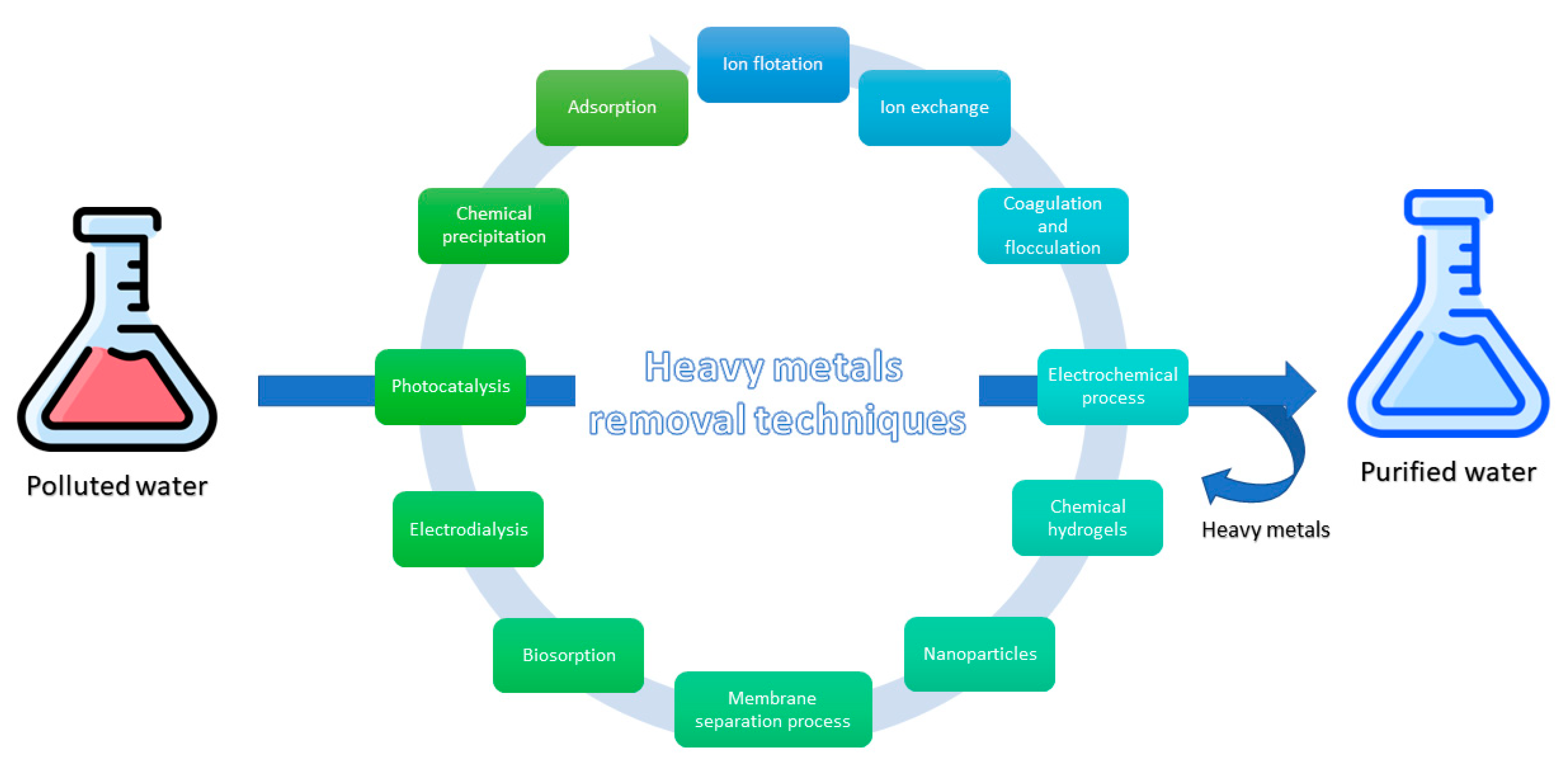
3. Removal Techniques of Potentially Toxic Metals
4. Key Factors That Affect the Adsorption Process
5. Surface Modification of Agricultural Wastes
6. Adsorption Capacity and Removal Performances of Different Types of Agricultural Waste
7. Recyclability and Regeneration of Agricultural Adsorbents
| Process | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Microbiological regeneration | Ecofriendly, efficient, time effective, cost effective | Very slow, blocking of the pore, slow regeneration, not applicable for surfactant-modified adsorbents, low efficiency | [217,220,221] |
| Thermal regeneration | Efficient, useful for adsorbents loaded with heterogeneous mixture of adsorbates | Expensive, time-consuming, modification of structure adsorption pore, high energy consumes, air pollution problems associated with gases released during the process, loss of carbon surface area | [217,220,221] |
| Oxidative regeneration | Efficient | Expensive, degradation of pores, time-consuming | [217,221] |
| Microwave regeneration | Ecofriendly, efficient, time effective, short regeneration time, suitable for multicomponent adsorbents, energy-saving process | Expensive, degradation of pores, further secondary treatments are needed | [217,221] |
| Ultrasound regeneration | Ecofriendly, efficient, time effective, low energy requirement, low carbon loss | Expensive, degradation of pores | [217,220,221] |
| Chemical regeneration | Cost effective, can be coupled easily with other techniques, fast regeneration, high regeneration efficiency, almost zero carbon loss, quick regeneration, possible adsorbate recovery | Modification of structure of the adsorbent, oxidant wastage, low solubility of the adsorbate, occasional sludge generation, toxicity issues, require of further treatment | [217,220,221] |
| Ozonation | Efficient | Surface modification of adsorbents, increasing costs due to calcination of adsorbents before use, acidification of adsorption surface which interfere with anionic pollutants | [219,220] |
| Photo-assisted oxidation | Ecofriendly, fast degradation of organic pollutants | Release of some harmful byproducts, long regeneration required, decreasing of efficiency with increasing of regeneration cycles | [220] |
| Supercritical fluids regeneration | Very fast regeneration times, efficient, less time consuming | Cost of pressure vessels, degradation of pores of adsorbent material | [219,220] |
| Ultrasonic regeneration | Higher desorption efficiency, low energy consumption, simple equipment required, efficient, eco-friendly, less time consuming | Highly expensive, degradation of pores of adsorbent | [219,220] |
8. Challenges and Future Perspectives on Agricultural Waste Adsorbents
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Role of Potentially Toxic Elements in Soils. In Soil Components and Human Health; Nieder, R., Benbi, D.K., Reichl, F.X., Eds.; Springer: Dordrecht, Germany, 2018; pp. 375–450. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2017, 119, 157–184. [Google Scholar]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. Environ. Anal. Chem. 2020, 102, 342–379. [Google Scholar]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Poll. 2018, 229, 225. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources and remediation techniques. Soil Sediment Contam Int. J. 2019, 28, 380–394. [Google Scholar]
- Rafique, M.; Hajra, S.; Tahir, M.B.; Gillani, S.S.A.; Irshad, M. A review on sources of heavy metals, their toxicity and removal technique using physico-chemical processes from wastewater. Environ. Sci. Pollut. Res. 2022, 29, 16772–16781. [Google Scholar]
- Wessling-Resnick, M. Excess iron: Considerations related to development and early growth. Am. J. Clin. Nutr. 2017, 106, 1600–1605. [Google Scholar]
- Angelova, I.; Ivanov, I.; Venelinov, T. Origin of Aluminum in the Raw Drinking Water of Sofia City, Bulgaria. Wat. Air Soil Poll. 2020, 231, 455. [Google Scholar]
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; Rehman, K.U.; Islam, R.U.; Wahab, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2021, 321, 114455. [Google Scholar]
- Kwikima, M.M.; Mateso, S.; Chebude, Y. Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review. Sci. Afr. 2021, 13, e00934. [Google Scholar]
- Lawinsider. Available online: https://www.lawinsider.com/dictionary/percent-removal (accessed on 1 December 2022).
- Jaber, L.; Ihsanullah, I.; Almanassra, I.W.; Backer, S.N.; Abushawish, A.; Khalil, A.K.A.; Alawadhi, H.; Shanableh, A.; Atieh, M.A. Adsorptive Removal of Lead and Chromate Ions from Water by Using Iron-Doped Granular Activated Carbon Obtained from Coconut Shells. Sustainability 2022, 14, 10877. [Google Scholar]
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Natural Gas Dehydration and Mercaptans Removal. In Handbook of Natural Gas Transmission and Processing, 4th ed.; Gulf Professional Publishing: Oxford, UK, 2019; pp. 307–348. [Google Scholar]
- Hussain, A.; Madan, S.; Madan, R. Removal of heavy metals from wastewater by adsorption. In Heavy metals—Their Environmental Impacts and Mitigation; Nazal, M.K., Zhao, H., Eds.; IntechOpen Publishing: London, UK, 2021; pp. 1–24. [Google Scholar]
- Abin-Bazaine, A.; Trujillo, A.C.; Olmos-Marquez, M. Adsorption Isotherms: Enlightenment of the Phenomenon of Adsorption. In Wastewater Treatment; Ince, M., Ince, O.K., Eds.; IntechOpen Publishing: London, UK, 2022; pp. 1–15. [Google Scholar]
- Tcheka, C.; Abia, D.; Iyedjolbo, B.; Akpomie, K.G.; Harouna, M.; Conradie, J. Biosorption of cadmium ions from aqueous solution onto alkaline-treated coconut shell powder: Kinetics, isotherm, and thermodynamics studies. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [PubMed]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.; Ul, H. Recent advances in application of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar]
- Shrestha, R.; Ban, S.; Devkkota, S.; Sharma, S.; Joshi, R.; Tiwari, P.; Kim, H.Y.; Joshi, K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar]
- Xiang, H.; Min, x.; Tang, C.J.; Sillanpaa, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. JWPE 2022, 49, 103023. [Google Scholar]
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6509–6518. [Google Scholar]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Technol. 2015, 1, 12–18. [Google Scholar]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Archour, S.; Menasra, H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arab. J. Sci. Eng. 2021, 47, 5587–5599. [Google Scholar]
- Ungureanu, N.; Vladut, V.; Cristea, M.; Cujbescu, D. Wastewater electrooxidation using stainless steel electrodes. E3S Web Conf. 2020, 180, 03015. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metals ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar]
- Xu, Z.; Zhang, Q.; Li, X.; Huang, X. A critical review on chemical analysis of heavy metal complexes in water/wastewater and the mechanism of treatment methods. Chem. Eng. J. 2022, 429, 131688. [Google Scholar]
- Zhang, X.; Yan, Y.; Li, N.; Yang, P.; Yang, Y.; Duan, G.; Wang, X. A robust and 3D-printed solar evaporator based on naturally occurring molecules. Sci. Bull. 2023, 68, 203–213. [Google Scholar]
- Fan, Z.; Ren, J.; Bai, H.; He, P.; Hao, L.; Liu, N.; Chen, B.; Niu, R.; Gong, J. Shape-controlled fabrication of MnO/C hybrid nanoparticle from waste polyester for solar evaporation and thermoelectricity generation. Chem. Eng. J. 2023, 451, 138534. [Google Scholar]
- Carbotecnia. Available online: https://www.carbotecnia.info/learning-center/filtration-methods/what-is-microfiltration/?lang=en (accessed on 11 January 2023).
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar]
- The, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar]
- Chen, L.; Ren, J.; Gong, J.; Qu, J.; Niu, R. Cost-effective, scalable fabrication of self-floating xerogel foam for simultaneous photothermal water evaporation and thermoelectric power generation. Chem. Eng. J. 2023, 454, 140383. [Google Scholar]
- Esmaeili, H.; Foroutan, R. Investigation into ion exchange and adsorption methods for removing heavy metals from aqueous solutions. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 620–629. [Google Scholar]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organicand inorganic pollutants from industrial wastewater. IJEST 2020, 18, 3273–3294. [Google Scholar]
- Rudi, N.N.; Muhamad, M.S.; Chuan, L.T.; Alipal, J.; Omar, S.; Hamidon, N.; Hamid, N.H.A.; Sunar, N.M.; Ali, R.; Harun, H. Evolution of adsorption process for manganese removal in water via agricultural waste adsorbents. Heliyon 2020, 6, e05049. [Google Scholar]
- Celebi, H.; Gok, G.; Gok, O. Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions. Sci. Rep. 2020, 10, 17570. [Google Scholar]
- Wan, S.; Ma, Z.; Xue, Y.; Ma, M.; Xu, S.; Qian, L.; Zhang, Q. Sorption of Lead(II), Cadmium(II), and Copper(II) Ions from Aqueous Solutions Using Tea Waste. Ind. Eng. Chem. Res. 2014, 53, 3629–3635. [Google Scholar]
- Celebi, H.; Gok, O. Evaluation of Lead Adsorption Kinetics and Isotherms from Aqueous Solution Using Natural Walnut Shell. Int. J. Environ. Res. 2017, 11, 83–90. [Google Scholar]
- Ghasemi, S.; Gholami, R.M.; Yazdanian, M. Biosorption of heavy metal from cadmium rich aqueous solutions by tea waste as a low-cost bio-adsorbent. Jundishapur J. Health Sci. 2017, 9, e37301. [Google Scholar] [CrossRef]
- Malakahmad, A.; Tan, S.; Yavari, S. Valorization of wasted black tea as a low-cost adsorbent for nickel and zinc removal from aqueous solution. J. Chem. 2016, 2016, 5680983. [Google Scholar]
- Futalan, C.M.; Kim, J.; Yee, J.J. Adsorptive treatment via simultaneous removal of copper, lead and zinc from soil washing wastewater using spent cofee grounds. Water Sci. Technol. 2019, 79, 1029–1041. [Google Scholar] [PubMed]
- Yang, S.; Wu, Y.; Aierken, A.; Zhang, M.; Fang, P.; Fan, Y.; Ming, Z. Mono/competitive adsorption of Arsenic(III) and Nickel(II) using modified green tea waste. J. Taiwan Inst. Chem. Eng. 2016, 60, 213–221. [Google Scholar]
- Mutongo, F.; Kuipa, O.; Kuipa, P.K. Removal of Cr(VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg. Chem. Appl. 2014, 2014, 973153. [Google Scholar]
- Sabri, M.U.; Qayyum, A.A.; Akhtar, M.; Munawar, Z. Adsorption kinetics of iron(II) from waste/aqueous solution by using potato peel as carbonaceous material. Int. J. Biosci. 2018, 13, 212–220. [Google Scholar]
- Witek-Krowiak, A.; Szafran, R.G.; Modelski, S. Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 2011, 265, 126–134. [Google Scholar]
- Abdelfattah, I.; Ismail, A.A.; Al Sayed, F.; Almedolab, A.; Aboelghait, K. Biosorption of heavy metals ions in real industrial wastewater using peanuthusk as efficient and cost-effective adsorbent. Environ. Nanotechnol. Monit. Manag. 2016, 6, 176–183. [Google Scholar]
- Liu, Y.; Sun, X.; Li, B. Adsorption of Hg2+ and Cd2+ by ethylenediamine modified peanut shells. Carbohydr. Polym. 2010, 81, 335–339. [Google Scholar]
- Wasewar, K.L.; Atif, M.; Prasad, B.; Mishra, I.M. Batch adsorption of zinc on tea factory waste. Desalination 2009, 244, 66–71. [Google Scholar]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar]
- Liu, G.; Liao, L.; Dai, Z.; Qi, Q.; Wu, J.; Ma, L.Q.; Tang, C.; Xu, J. Organic adsorbents modified with citric acid and Fe3O4 enhance the removal of Cd and Pb in contaminated solutions. Chem. Eng. J. 2020, 395, 125108. [Google Scholar]
- Huang, B.; Liu, G.; Wang, P.; Zhao, X.; Xu, H. Effect of nitric acid modification on characteristics and adsorption properties of lignite. Processes 2019, 7, 167. [Google Scholar] [CrossRef]
- Lesoana, M.; Mlaba, R.P.V.; Mtunzi, F.M.; Klink, M.J.; Ejidike, P.; Pakade, V.E. Influence of inorganic acid modification on Cr (VI) adsorption performance and the physicochemical properties of activated carbon. S. Afr. J. Chem. Eng. 2019, 28, 8–18. [Google Scholar] [CrossRef]
- Boeykens, S.P.; Redondo, N.; Obeso, R.A.; Caracciolo, N.; Vázquez, C. Chromium and Lead adsorption by avocado seed biomass study through the use of Total Reflection X-Ray Fluorescence analysis. Appl. Radiat. Isot. 2019, 153, 108809. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ko, R.-A.; Lee, S.; Chon, K. Removal Efficiencies of Manganese and Iron Using Pristine and Phosphoric Acid Pre-Treated Biochars Made from Banana Peels. Water 2020, 12, 1173. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Naidoo, E.B. Adsorption studies of toxic cadmium (II) and chromium (VI) ions from aqueous solution by activated black cumin (Nigella sativa) seeds. J. Environ. Chem. Eng. 2020, 8, 104045. [Google Scholar] [CrossRef]
- Wanja, N.E.; Murungi, J.; Hassanali, A. Application of chemically modified avocado seed for removal of Copper (II), Lead(II), and Cadmium(II) ions from aqueous solutions. Int. J. Appl. Sci. Eng. 2016, 6, 1–15. [Google Scholar]
- Abid, M.; Niazi, N.K.; Bibi, I.; Farroqi, A.; Ok, Y.S.; Kunhikrishnan, A.; Ali, F.; Ali, S.; Igalavithana, A.D.; Arshad, M. Arsenic (V) biosorption by charred orange peel in aqueous environments. Int. J. Phytoremediation 2016, 18, 442–449. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Ruiz Paternina, E.; Gallo Mercado, J.; Moscote Bohorquez, J. Evaluation of the biosorption with african palm bagasse for the removal of Pb (II) in solution. Prospect 2015, 13, 59–67. [Google Scholar]
- Ding, Z.; Yu, R.; Hu, X.; Chen, Y. Adsorptive Removal of Hg(II) Ions from Aqueous Solutions Using Chemical-Modified Peanut Hull Powder. Pol. J. Environ. Stud. 2014, 23, 1115–1121. [Google Scholar]
- Shooto, N.D.; Thabede, P.M.; Naidoo, E.B. Simultaneous adsorptive study of toxic metal ions in quaternary system from aqueous solution using low-cost black cumin seeds (Nigella sativa) adsorbents. S. Afr. J. Chem. Eng. 2019, 30, 15–27. [Google Scholar] [CrossRef]
- Zheng, C.; Ling, Z.; Xiaobai, Z.; Zhimin, F.; An, L. Treatment technologies for organic wastewater. Water Treat. 2013, 11, 250–286. [Google Scholar]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Comparisons of porous, surface chemistry and adsorption properties of carbon derived from Enteromorpha prolifera activated by H4P2O7 and KOH. Chem. Eng. J. 2013, 232, 582–590. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment—Conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb (II), Cu(II), and Cd (II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Shakoor, A.; Mahmood, M.; Chen, D.Y. Enhanced adsorption of aqueous Pb(II) by modified biochar produced through pyrolysis of watermelon seeds. Sci. Total Environ. 2021, 784, 147136. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Herrera-Barros, A.; Villabona-Ortiz, A.; Gonzalez-Delgado, A.; Nunez-Zarur, J. Hexavalent chromium adsorption from aqueous solution using orange peel modified with calcium chloride: Equilibrium and kinetics study. Indian J. Sci. Technol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, Y.; Yue, Q.; Kong, W.; Gao, B.; Wang, W.; Jiang, W. Degradation of chlortetracycline with simultaneous removal of copper (II) from aqueous solution using wheat straw-supported nanoscale zero-valent iron. Chem. Eng. J. 2020, 379, 122384. [Google Scholar] [CrossRef]
- Soldatkina, L.; Zavrichko, M. Equilibrium, kinetic, and thermodynamic studies of anionic dyes adsorption on corn stalks modified by cetylpyridinium bromide. Colloids Interfaces 2019, 3, 4. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, J.; Si, H.; Wang, B. Environmental effects and carbon sequestration potential of returning agricultural waste to field by carbonization. WJASS 2022, 8, 1–7. [Google Scholar]
- Goswami, M.; Phukan, P. Enhanced adsorption of cationic dyes using sulfonic acid modified activated carbon. J. Environ. Chem. Eng. 2017, 5, 3508–3517. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Wu, M. Simultaneous removal of dye and heavy metal by banana peels derived hierarchically porous carbons. J. Taiwan Inst. Chem. 2018, 93, 543–553. [Google Scholar] [CrossRef]
- Ali, A.; Saeed, K. Decontamination of Cr (VI) and Mn (II) from aqueous media by untreated and chemically treated banana peel: A comparative study. Desalin. Water Treat. 2015, 53, 3586–3591. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, W.; Li, G.; Wang, X.; Zhu, L. Effect of Alkali Treatment of Wheat Straw on Adsorption of Cu(II) under Acidic Condition. J. Chem. 2016, 2016, 6326372. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Zimmerman, A.R.; Gao, B. Sorption and cosorption of lead (II) and methylene blue on chemically modified biomass. Bioresour. Technol. 2014, 167, 569–573. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Parlayici, S. Modified barley straw as a potential biosorbent for removal of copper ions from aqueous solutions. Food chem. 2012, 135, 2229–2234. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K. Synthesis and characterization of cellulose based adsorbents for removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions. React. Funct. Polym. 2019, 140, 82–92. [Google Scholar] [CrossRef]
- Wang, F.; Pan, Y.; Cai, P.; Guo, T.; Xiao, H. Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent. Bioresour. Technol. 2017, 241, 482–490. [Google Scholar] [CrossRef]
- Zheng, L.; Dang, Z.; Yi, X.; Zhang, H. Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk. J. Hazard. Mater. 2010, 176, 650–656. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, Y.; Zhang, M.; Ming, Z.; Yang, S.; Arkin, A.; Fang, P. Functionalized agricultural biomass as a low-cost adsorbent: Utilization of rice straw incorporated with amine groups for the adsorption of Cr(VI) and Ni(II) from single and binary systems. Biochem. Eng. J. 2016, 105, 27–35. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X. Removal of Cr(VI) from aqueous solution using modified corn stalks: Characteristic, equilibrium, kinetic and thermodynamic study. Chem. Eng. J. 2011, 168, 909–917. [Google Scholar] [CrossRef]
- Khandanlou, R.; Ahmad, M.B.; Fard Masoumi, H.R.; Shameli, K.; Basri, M.; Kalantari, K. Rapid adsorption of copper(II) and Lead(II) by rice straw/Fe3O4 nanocomposite: Optimization, equilibriumisotherms, and adsorption kinetics study. PLoS ONE 2015, 10, e120264. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Tran, H.T.; Ouédraogo, W.K.I.; Schmidt, C.; Zachmann, D.; Bahadir, M. Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As(V) from aqueous solutions. Food Chem. 2013, 138, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Zhang, M.; Liu, B.; Xu, X.; Li, X.; Yue, Q.; Ma, C. Characteristics of amine surfactant modified peanut shell and its sorption property for Cr(VI). Chin. J. Chem. Eng. 2013, 21, 1260–1268. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sureshkumar, M.V. Removal of chromium(VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour. Technol. 2008, 99, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Esteki, M.; Mousavi, S.F.; Mostafazadeh-Fard, B. Removal of Ni(II), Cd(II) and Cr(III) from industrial wastewater by raw and modified cotton stalk. J. Biodivers. Environ. Sci. 2014, 5, 579–582. [Google Scholar]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Comparing the adsorption mechanism of cd by rice straw pristine and KOH-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 11875–11883. [Google Scholar] [CrossRef]
- Fouladi Tajar, A.; Kaghazchi, T.; Soleimani, M. Adsorption of cadmium from aqueous solutions on sulfurized activated carbon prepared from nut shells. J. Hazard. Mater. 2009, 165, 1159–1164. [Google Scholar] [CrossRef]
- Karic, N.; Maia, A.S.; Teodorovic, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.L.; Dolic, M. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Radenkovic, M.; Momcilovic, M.; Petrovic, J.; Mrakovic, A.; Relic, D.; Popovic, A. Removal of heavy metals from aqueous media by sunflower husk: A comparative study of biosorption efficiency by using ICP-OES and LIBS. J. Serb. Chem. Soc. 2022, 87, 939–952. [Google Scholar] [CrossRef]
- Ismail, M.S.; Yahya, M.D.; Anuta, M.; Obayomi, K.S. Facile preparation of amine -functionalized corn husk derived activated carbon for effective removal of selected heavy metals from battery recycling wastewater. Heliyon 2022, 8, e09516. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Bernal, D.F.; Ortiz, M.Y.C.; Gutierrez Cifuentes, J.A.; Bastos-Arrieta, J.; Palet, C.; Candela, A.M. Coffee Husk and Lignin Revalorization: Modification with Ag Nanoparticles for Heavy Metals Removal and Antifungal Assays. Water 2022, 14, 1796. [Google Scholar] [CrossRef]
- Dalali, N.; Hagghi, A. Removal of cadmium from aqueous solutions by walnut green husk as a low-cost biosorbent. Desalin. Water Treat. 2015, 57, 13782–13794. [Google Scholar] [CrossRef]
- Olguin, M.T.; Lopez-Gonzalez, H.; Serrano-Gomez, J. Hexavalent chromium removal from aqueous solutions by Fe-modified peanut husk. Water Air Soil Pollut. 2013, 224, 1654. [Google Scholar] [CrossRef]
- Sugashini, S.; Begum, K.M.M.S. Preparation of activated carbon from carbonized rice husk by ozone activation for Cr(VI) removal. Xinxing Tan Cailiao/New Carbon Mater. 2015, 30, 252–261. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+ & Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar]
- Sobhanardakani, S.; Parvizimosaed, H.; Olyaie, E. Heavy metals removal from wastewaters using organic solid waste—Rice husk. Environ. Sci. Pollut. Res. 2013, 20, 5265–5271. [Google Scholar] [CrossRef]
- Lawal, O.S.; Ayanda, O.S.; Rabiu, O.O.; Adebowale, K.O. Application of black walnut (Juglan nigra) husk for the removal of lead (II) ion from aqueous solution. Water Sci. Technol. 2017, 75, 2454–2464. [Google Scholar] [CrossRef]
- Dang, V.B.H.; Doan, H.D.; Dang-Vu, T.; Lohi, A. Equilibrium and kinetics of biosorption of cadmium (II) and copper (II) ions by wheat straw. Bioresour. Technol. 2009, 100, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Dhir, B.; Kumar, R. Adsorption of heavy metals by salvinia biomass and agricultural residues. Int. J. Environ. Res. 2010, 4, 427–432. [Google Scholar]
- Farooq, U.; Khan, M.A.; Atharc, M.; Kozinskia, J.A. Effect of modification of environmentally friendly bioadsorbents wheat (Triticum aestivum) on the biosorptive removal of cadmium(II) ions from aqueous solution. Chem. Eng. J. 2011, 171, 400–441. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Parlayici, S. Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J. Hazard. Mater. 2009, 164, 982–986. [Google Scholar] [CrossRef]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Vijay, V.K.; Kaushal, P. Efficacy of rice straw derived biochar for removal of Pb+2 and Zn+2 from aqueous: Adsorption, thermodynamic and cost analysis. Bioresour. Technol. Rep. 2022, 17, 100920. [Google Scholar] [CrossRef]
- Jia, D.; Li, C. Adsorption of Pb(II) from aqueous solutions using corn straw. Desalin. Water Treat. 2015, 56, 223–231. [Google Scholar] [CrossRef]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L. Kinetic and Thermodynamic Studies of the Biosorption of Ni(II) by Modified Rape Straw. Procedia Environ. Sci. 2016, 31, 75–80. [Google Scholar] [CrossRef]
- El-Razik, E.M.A.; Abdel-Karim, A.M. Uses of Some Nano-Sized Organic Wastes to Treat Industrial Wastewater. Egypt. J. Chem. 2021, 64, 3413–3421. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Mahmood-ul-Hassan, M.; Surthor, V.; Rafique, E.; Yasin, M. Removal of Cd, Cr, and Pb from aqueous solution by unmodified and modified agricultural wastes. Environ. Monit. Assess. 2015, 187, 19. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Mukherjee, K.; Saha, I.; Ghosh, A.; Ghosh, S.K.; Saha, B. Removal of hexavalent chromium from water by adsorption on mosambi (Citrus limetta) peel. Res. Chem. Intermed. 2013, 39, 2245–2257. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Yirga, A.; Yadav, O.P.; Dey, T. Waste Orange Peel Adsorbent for Heavy Metal Removal from Water. Pollution 2022, 8, 553–566. [Google Scholar]
- Guechi, E.K.; Hamdaowio, O. Evaluation of potato peel as a novel adsorbent for the removal of Cu(II) from aqueous solutions: Equilibrium, kinetics and thermodynamic studies. Desalin. Water Treat. 2015, 57, 10677–10688. [Google Scholar] [CrossRef]
- Abdic, S.; Memic, M.; Sabanovic, E.; Sulejmanovic, J.; Begic, S. Adsorptive removal of eight heavy metals from aqueous solution by unmodifed and modifed agricultural waste: Tangerine peel. Int. J. Environ. Sci. Technol. 2017, 15, 2511–2518. [Google Scholar] [CrossRef]
- Sabanovic, E.; Memic, M.; Sulejmanovic, J.; Selovic, A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Pol. J. Chem. Technol. 2020, 22, 46–53. [Google Scholar] [CrossRef]
- Ahmadi, H.; Hafiz, S.S.; Sharifi, H.; Rene, N.N.; Habibi, S.S.; Hussain, S. Low cost biosorbent (Melon Peel) for effective removal of Cu (II), Cd (II), and Pb (II) ions from aqueous solution. CSCEE 2022, 6, 100242. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Murugesa, A.; Sivanesan, S. Removal of cadmium(II) from aqueous solution by agricultural waste cashew nut shell. Korean J. Chem. Eng. 2012, 29, 756–768. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T.; Cetin, S.; Bhanger, M.I. Lead sorption by waste biomass of hazelnut and almond shell. J. Hazard. Mater. 2009, 167, 1203–2128. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhong, M.; Zhou, S.; Li, W.; Wang, T.; Li, J. Study on adsorption of Cu2+, Pb2+, Cd2+, and Zn2+ by the KMnO4 modified biochar derived from walnut shell. Int. J. Environ. Sci. Technol. 2022, 20, 1551–1568. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Abhinaya, R.V.; Kirupha, S.D.; Murugesan, A.; Sivanesan, S. Adsorption of Metal Ions onto the Chemically Modified Agricultural Waste. Clean—Soil Air Water 2012, 40, 188–197. [Google Scholar] [CrossRef]
- Anandkumar, J.; Mandal, B. Removal of Cr(VI) from aqueous solution using Bael fruit (Aegle marmelos correa) shell as an adsorbent. J. Hazard. Mater 2009, 168, 633–640. [Google Scholar] [CrossRef]
- Okoye, A.I.; Ejikeme, P.M.; Onukwuli, O.D. Lead removal from wastewater using fluted pumpkin seed hull activated carbon: Adsorption modelling and kinetics. Int. J. Environ. Sci. Technol. 2010, 7, 793–800. [Google Scholar] [CrossRef]
- Ercag, E.; Kanmaz, N.; Bugdayci, M.; Hizal, J. Cr(VI) adsorption on binary and ternary composites of raw cocoa shell with magnetic nanoparticle and Prussian blue. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100625. [Google Scholar]
- Jalali, M.; Aboulghazi, F. Sunflower stalk, an agricultural waste, as an adsorbent for the removal of lead and cadmium from aqueous solutions. J. Mater. Cycles Waste Manag. 2013, 15, 548–555. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, C.; Dang, Z.; Zhang, H.; Yi, X.; Liu, C. Preparation of cellulose derived from corn stalk and its application for cadmium ion adsorption from aqueous solution. Carbohydr. Polym. 2012, 90, 1008–1015. [Google Scholar] [CrossRef]
- Yang, W.; Lei, G.; Quan, S.; Zhang, L.; Wang, B.; Hu, H.; Li, L.; Ma, H.; Yin, C.; Feng, F.; et al. The Removal of Cr(VI) from Aqueous Solutions with Corn Stalk Biochar. Int. J. Environ. Res. Public Health 2022, 19, 14188. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, W.; Qiu, Z. The study of copper (II) removal from aqueous solutions by adsorption using corn stalk material. Adv. Mater. Res. 2012, 610–613, 1950–1953. [Google Scholar] [CrossRef]
- Deng, H.; Li, Q.; Huang, M.; Li, A.; Zang, J.; Li, Y.; Li, S.; Kang, C.; Mo, W. Removal of Zn(II), Mn(II) and Cu(II) by adsorption onto banana stalk biochar: Adsorption process and mechanisms. Water Sci. Technol. 2020, 82, 2962–2974. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z.; Yi, W.; Li, Y.; Zhang, P.; Zhang, A.; Wang, L. Impacts of pyrolysis temperature on lead adsorption by cotton stalk-derived biochar and related mechanisms. J. Environ. Chem. Eng. 2021, 9, 105602. [Google Scholar] [CrossRef]
- Erdem, M.; Duran, H.; Sahin, M.; Ozdemir, I. Kinetics, thermodynamics, and isotherms studies of Cd(II) adsorption onto grape stalk. Desalin. Water Treat. 2015, 54, 3348–3357. [Google Scholar] [CrossRef]
- Dong, J.; Hu, J.; Wang, J. Radiation-induced grafting of sweet sorghum stalk for copper(II) removal from aqueous solution. J. Hazard. Mater. 2013, 262, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kirbiyik, C.; Kilic, M.; Cepeliogullar, O.; Putun, A.E. Use of sesame stalk biomass for the removal of Ni(II) and Zn(II) from aqueous solutions. Water Sci. Technol. 2012, 66, 231–238. [Google Scholar] [CrossRef]
- Kang, C.; Li, Q.; Deng, H.; Mo, W.; Meng, M.; Huang, S. EDTAD-modified cassava stalks loaded with Fe3O4: Highly efficient removal of Pb2+ and Zn2+ from aqueous solution. Environ. Sci. Pollut. Res. 2021, 28, 6733–6745. [Google Scholar] [CrossRef] [PubMed]
- Mingzhen, Z.; Guijan, L.; Ruijia, L.; Arif, M.; Jinzhao, X. Competitive adsorption of copper and zinc on tea stalk biochar prepared at 2 different pyrolysis temperatures: Mechanism and characteristics. SSRN 2022. [Google Scholar] [CrossRef]
- Prokopov, T.; Nikolova, M.; Ivanova, T.; Popova, V.; Dimov, M.; Taneva, D. Equilibrium study of Cr (VI) removal from aqueous solution by stalks from three tobacco species (Nicotiana) grown in Bulgaria. J. Environ. Res. Eng. Manag. 2019, 75, 46–54. [Google Scholar] [CrossRef]
- Abbas, M.; Kaddour, S.; Trari, M. Kinetic and equilibrium studies of cobalt adsorption on apricot stone activated carbon. J. Ind. Eng. Eng. Chem. 2014, 20, 745–751. [Google Scholar] [CrossRef]
- El-Saharty, A.; Mahmoud, S.N.; Manjood, A.H.; Nassar, A.A.H.; Ahmed, A.M. Effect of Apricot Stone Activated Carbon Adsorbent on the Removal of Toxic Heavy Metals Ions from Aqueous Solutions. IJEE 2018, 3, 51–62. [Google Scholar] [CrossRef]
- Amar, M.B.; Walha, K.; Salvado, V. Evaluation of Olive Stones for Cd(II), Cu(II), Pb(II) and Cr(VI) Biosorption from Aqueous Solution: Equilibrium and Kinetics. Int. J. Environ. Res. 2020, 14, 193–204. [Google Scholar] [CrossRef]
- Yan, J.; Lan, G.; Qiu, H.; Chen, C.; Liu, Y.; Du, G.; Zhang, J. Adsorption of heavy metals and methylene blue from aqueous solution with citric acid modified peach stone. Sep. Sci. Technol. 2018, 53, 1678–1688. [Google Scholar] [CrossRef]
- Khemmari, F.; Benrachedi, K. Peach stones valorized to high efficient biosorbent for hexavalent chromium removal from aqueous solution: Adsorption kinetics, equilibrium and thermodynamic studies. Rev. Roum. Chim. 2019, 64, 603–613. [Google Scholar] [CrossRef]
- Ku, K.; Seong-Jik, P.; Woo-Seok, S.; Byoung-Hwan, U.; Young-Kee, K. Removal of Synthetic Heavy Metal (Cr6+, Cu2+, As3+, Pb2+) from Water Using Red Mud and Lime Stone. J. Korean Soc. Environ. Eng. 2012, 34, 566–573. [Google Scholar]
- Parlayici, S.; Pehlivan, E. Removal of metals by Fe3O4 loaded activated carbon prepared from plum stone (Prunus nigra): Kinetics and modelling study. Powder Technol. 2017, 317, 23–30. [Google Scholar] [CrossRef]
- Pap, S.; Knudsen, T.S.; Radonic, J.; Maletic, S.; Igic, S.M.; Sekulic, M.T. Utilization of fruit processing industry waste as green activated carbon for the treatment of heavy metals and chlorophenols contaminated water. J. Clean. Prod. 2017, 162, 958–972. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Opotu, O.U.; Adebowale, K.O.; Ogunsolu, O.; Alujimi, O.O. 2012. Biosorption of Cd2+ and Pb2+ ions onto mango stone and cocoa pod waste: Kinetic and equilibrium studies. Sci. Res. Essays. 2012, 7, 1614–1629. [Google Scholar] [CrossRef]
- Kahraman, H.T.; Pehlivan, E. Cr6+ removal using oleaster (Elaeagnus) seed and cherry (Prunus avium) stone biochar. Powder Technol. 2017, 306, 61–67. [Google Scholar] [CrossRef]
- Paneru, K.R.; Jha, V.K. Adsorptive removal of Pb(II) ions from aqueous solution by activated carbon prepared from cabbage waste. Nepal J. Environ. Sci. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nguyan, T.V.; Vigneswaran, S. Performance of cabbage and cauliflower wastes for heavy metal removal. Desalin. Water Treat. 2014, 52, 844–860. [Google Scholar] [CrossRef]
- Vafakhah, S.; Bahrololoom, M.E.; Saeedikhani, M. Adsorption Kinetics of Cupric Ions on Mixture of Modified Corn Stalk and Modified Tomato Waste. J. Water Resour. Prot. 2016, 8, 1238–1250. [Google Scholar] [CrossRef]
- Yargıç, A.Ş.; Yarbay Şahin, R.Z.; Özbay, N.; Önal, E. Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J. Clean. Prod. 2015, 88, 152–159. [Google Scholar] [CrossRef]
- Heraldy, E.; Lestati, W.W.; Permatasari, D.; Arimurti, D.D. Biosorbent from tomato waste and apple juice residue for lead removal. J. Environ. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Nasir, A.W.; Hanif, M.A. Efficacy of Daucus carota L. waste biomass for the removal of chromium from aqueous solutions. Desalination 2010, 253, 78–87. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Owolabi, J.O.; Igbomezie, C.O. Optimization of process parameters for adsorption of heavy metals from aqueous solutions by alumina-onion skin composite. Chem. Eng. Commun. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Negi, R.; Satpathy, G.; Tyagi, Y.K.; Gupta, R.K. Biosorption of heavy metals by utilizing onion and garlic wastes. Int. J. Environ. Pollut. 2012, 49, 179. [Google Scholar] [CrossRef]
- Hegazy, I.; Ali, M.E.A.; Zaghlool, E.H.; Elsheikh, R. Heavy metals adsorption from contaminated water using moringa seeds/ olive pomace byproducts. Appl. Water Sci. 2021, 11, 95. [Google Scholar] [CrossRef]
- Muthuraman, R.M.; Murugappan, A.; Soundharajan, B. A sustainable material for removal of heavy metals from water: Adsorption of Cd(II), Pb(II), and Cu(II) using kinetic mechanism. Desalin. Water Treat. 2021, 220, 192–198. [Google Scholar] [CrossRef]
- Giri, D.D.; Jha, J.M.; Srivastava, N.; Shah, M.; Almalki, A.H.; Alkhanani, M.F.; Pal, D.B. Waste seeds of Mangifera indica, Artocarpus heterophyllus and Schizizium commune as biochar for heavy metal removal from simulated wastewater. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Fristak, V.; Moreno-Jimenez, E.; Fresno, T.; Diaz, E. Effect of Physical and Chemical Activation on Arsenic Sorption Separation by Grape Seeds-Derived Biochar. Separations 2018, 5, 59. [Google Scholar] [CrossRef]
- Sheikh, Z.; Amin, M.; Khan, N.; Khan, M.N.; Sami, S.K.; Khan, S.B.; Hafeez, I.; Khan, S.A.; Bakhsh, E.; Cheng, C.K. Potential application of Allium Cepa seeds as a novel biosorbent for efficient biosorption of heavy metals ions from aqueous solution. Chemosphere 2021, 279, 130545. [Google Scholar] [CrossRef] [PubMed]
- Garba, Z.N.; Bello, I.; Galadima, A.; Lawal, A.Y. Optimization of adsorption conditions using central composite design for the removal of copper (II) and lead (II) by defatted papaya seed. Karbala Int. J. Mod. Sci. 2016, 2, 20–28. [Google Scholar] [CrossRef]
- Nagashanmugam, K.B.; Srinivasan, K. Evaluation of carbon derived from Gingelly oil cake for the removal of lead(II) from aqueous solutions. J. Environ. Sci. Eng. 2010, 52, 349–360. [Google Scholar]
- Fernandez-Gonzalez, R.; Martin-Lara, M.A.; Ianez-Rodriguez, I.; Calero, M. Removal of heavy metals from acid mining effluents by hydrolyzed olive cake. Bioresour. Technol. 2018, 268, 169–175. [Google Scholar] [CrossRef]
- Meneghel, A.P.; Goncalves, A.C., Jr.; Strey, L.; Rubio, T.; Schwantes, D.; Casarin, J. Biosorption and removal of chromium from water by using moringa seed cake (Moringa oleifera Lam.). Quim. Nova 2013, 36, 104–110. [Google Scholar] [CrossRef]
- Yusoff, M.E.M.; Idris, J.; Zainal, N.H.; Ibrahim, M.F.; Abd-Aziz, S. Adsorption of Heavy Metal Ions by Oil Palm Decanter Cake Activated Carbon. Makara J. Sci. 2019, 23, 59–64. [Google Scholar] [CrossRef]
- Sireesha, S.; Sreedhar, I. Modified low cost engineered biochar prepared from neem de-oiled cake for heavy metal sorption. Mater. Today Proc. 2022, 72, 34–40. [Google Scholar] [CrossRef]
- Vishnuvardhan Reddy, T.; Chauhan, S.; Chakraborty, S. Adsorption isotherm and kinetics analysis of hexavalent chromium and mercury on mustard oil cake. Environ. Eng. Res. 2017, 22, 95–107. [Google Scholar] [CrossRef]
- Ucar, S.; Erdem, M.; Tay, T.; Karagoz, S. Removal of lead (II) and nickel (II) ions from aqueous solution using activated carbon prepared from rapeseed oil cake by Na2CO3 activation. Clean Technol. Environ. Policy 2015, 14, 747–756. [Google Scholar] [CrossRef]
- Mazurek, K.; Druzynski, S.; Kielkowska, U.; Szlyk, E. New Separation Material Obtained from Waste Rapeseed Cake for Copper(II) and Zinc(II) Removal from the Industrial Wastewater. Materials 2021, 14, 2566. [Google Scholar] [CrossRef] [PubMed]
- Coskun, Y.I. Biosorption of Copper by a Natural Byproduct Material: Pressed Black Cumin Cakes. Anal. Lett. 2019, 53, 1247–1265. [Google Scholar] [CrossRef]
- Mondal, D.K.; Nandi, B.K.; Purkait, M.K. Removal of mercury (II) from aqueous solution using bamboo leaf powder: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2013, 1, 891–898. [Google Scholar] [CrossRef]
- Al Rmalli, S.W.; Dahmani, A.A.; Abuein, M.M.; Gleza, A.A. Biosorption of mercury from aqueous solutions by powdered leaves of castor tree (Ricinus communis L.). J. Hazard. Mater. 2008, 152, 955–959. [Google Scholar] [CrossRef]
- Shafique, U.; Ijaz, A.; Salman, M.; Zaman, W.; Jamil, N.; Rehman, R.; Javaid, A. Removal of arsenic from water using pine leaves. J. Taiwan Inst. Chem. Eng. 2012, 43, 256–263. [Google Scholar] [CrossRef]
- Ge, Q.; Tian, Q.; Moeen, M.; Wang, S. Facile Synthesis of Cauliflower Leaves Biochar at Low Temperature in the Air Atmosphere for Cu(II) and Pb(II) Removal from Water. Materials 2020, 13, 3163. [Google Scholar] [CrossRef]
- Kamar, F.H.; Nechifor, A.C.; Ridha, M.J.M.; Mohammed Altaieemi, M.B.; Nechifor, G. Study on Adsorption of Lead Ions from Industrial Wastewater by Dry Cabbage Leaves. Rev. Chim. 2015, 66, 921–925. [Google Scholar]
- Malik, R.; Lata, S.; Singhal, S. Removal of heavy metal from waste water by the use of modified aloe vera leaf powder. Int. J. Basic Appl. Sci. 2015, 5, 6–17. [Google Scholar]
- Nur-E-Alam, M.; Mia, A.S.M.; Ahmad, F.; Rahman, M.M. Adsorption of chromium (Cr) from tannery wastewater using low-cost spent tea leaves adsorbent. Appl. Water Sci. 2018, 8, 129. [Google Scholar] [CrossRef]
- Al Prol, A.E.; El Azeem, M.A.; Amer, A.; El-Metwally, M.E.A.; El-Hamid, H.T.A.; El-Moselhy, K.M. Adsorption of Cadmium (II) Ions from Aqueous Solution onto Mango Leaves. Asian J. Chem. Sci. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Saeedi, Z.; Akbarpour, B.; Rasoulzadeh, H.; Yetilmezsoy, K.; Al-Ghouti, M.A.; Khraisheh, M.; McKay, G. Adsorptive Removal of Arsenic and Mercury from Aqueous Solutions by Eucalyptus Leaves. Water Air Soil Pollut. 2017, 228, 429. [Google Scholar] [CrossRef]
- Sathish, T.; Vinithkumar, N.V.; Dharani, G.; Kirubagarn, R. Efficacy of mangrove leaf powder for bioremediation of chromium (VI) from aqueous solutions: Kinetic and thermodynamic evaluation. Appl. Water Sci. 2014, 5, 153–160. [Google Scholar] [CrossRef]
- Aloma, I.; Martın-Lara, M.A.; Rodrıguez, I.L.; Blazquez, G.; Calero, M. Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2012, 43, 275–281. [Google Scholar] [CrossRef]
- Demiral, H.; Gungor, C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Cholico-Gonzalez, D.; Lara, N.O.; Macedo, A.M.F.; Salas, J.C. Adsorption Behavior of Pb(II), Cd(II), and Zn(II) onto Agave Bagasse, Characterization, and Mechanism. ACS Omega 2020, 5, 3302–3314. [Google Scholar] [CrossRef]
- Velazquez-Jimenez, L.H.; Pavlick, A.; Rangel-Mendez, J.R. Chemical characterization of raw and treated agave bagasse and its potential as adsorbent of metal cations from water. Ind. Crop. Prod. 2013, 43, 200–206. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S.; Zamani, S.; Akbari, S.; Salehi, M. Optimization and modeling of aqueous Cr(VI) adsorption onto activated carbon prepared from sugar beet bagasse agricultural waste by application of response surface methodology. Surf. Interfaces 2020, 18, 100444. [Google Scholar] [CrossRef]
- Mohan, D.; Markandeya; Dey, S.; Dwivedi, S.B.; Shukla, S.P. Adsorption of arsenic using low-cost adsorbents: Guava leaf biomass, mango bark and bagasse. Curr. Sci. 2019, 117, 649–661. [Google Scholar] [CrossRef]
- Villabona-Ortiz, A.; Tejada-Tovar, C.; Gonzalez-Delgado, A.D. Elimination of Chromium (VI) and Nickel (II) Ions in a Packed Column Using Oil Palm Bagasse and Yam Peels. Water 2020, 14, 1240. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Osman, H.E.; Badwy, R.K.; Ahmad, H.F. Usage of some agricultural by-products in the removal of some heavy metals from industrial wastewater. J. Phytol. 2010, 2, 51–62. [Google Scholar]
- Owalude, S.O.; Tella, A.C. Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 377–388. [Google Scholar] [CrossRef]
- Ghasemi, S.M.; Mohseni-Bandpei, A.; Ghaderpoori, M.; Fakri, Y.; Keramati, H.; Taghavi, M.; Moradi, B.; Karimyan, K. Application of modified maize hull for removal of Cu(II) ions from aqueous solutions. Environ. Prot. Eng. 2017, 43, 93–103. [Google Scholar] [CrossRef]
- Beidokhti, M.Z.; Naeeni, S.T.O.; Abdi Ghahroudi, M.S. Biosorption of Nickel (II) from Aqueous Solutions onto Pistachio Hull Waste as a Low-Cost Biosorbent. Civ. Eng. J. 2019, 5, 447–457. [Google Scholar] [CrossRef]
- Sheng-Quan, Y.; Si-Yuan, G.; Yi-Gang, Y.; Hui, W.; Han-Rui. Removal of the heavy metal ion Cr(VI) by soybean hulls in dyehouse wastewater treatment. Desalination Water Treat. 2012, 42, 197–201. [Google Scholar] [CrossRef]
- Sheibani, A.; Shishehbor, M.R.; Alaei, H. Removal of Fe(III) ions from aqueous solution by hazelnut hull as an adsorbent. Int. J. Ind. Chem. 2012, 3, 4. [Google Scholar] [CrossRef]
- Yahya, M.D.; Yohanna, I.; Auta, M.; Obayomi, K.S. Remediation of Pb (II) ions from Kagara gold mining effluent using cotton hull adsorbent. Sci. Afr. 2020, 8, e00399. [Google Scholar] [CrossRef]
- Sahranavard, M.; Ahmadpour, A.; Doosti, M.R. Biosorption of Hexavalent Chromium Ions from Aqueous Solutions using Almond Green Hull as a Low-Cost Biosorbent. Eur. J. Res. 2011, 58, 392–400. [Google Scholar]
- Wang, Z.; Yin, P.; Qu, R.; Chen, H.; Wang, C.; Ren, S. Adsorption kinetics, thermodynamics and isotherm of Hg(II) from aqueous solutions using buckwheat hulls from Jiaodong of China. Food Chem. 2013, 136, 1508–1514. [Google Scholar] [CrossRef]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, M.N.; Wijaya, L. Adsorption-reduction performance of tea waste and rice husk biochars for Cr(VI) elimination from wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Tofan, L.; Teodosiu, C.; Paduraru, C.; Wenkert, R. Cobalt (II) removal from aqueous solutions by natural hemp fibers: Batch and fixed-bed column studies. Appl. Surf. Sci. 2013, 285, 33–39. [Google Scholar] [CrossRef]
- Hu, C.H.; Kuo, C.Y.; Guan, S.S. Adsorption of heavy metals from aqueous solutions by waste coffee residues: Kinetics, equilibrium, and thermodynamics. Desalin. Water Treat. 2014, 57, 5056–5064. [Google Scholar]
- Ghasemi, M.; Naushad, M.; Ghasemi, N.; Khosravi-fard, Y. A novel agricultural waste based adsorbent for the removal of Pb(II) from aqueous solution: Kinetics, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2014, 20, 454–461. [Google Scholar] [CrossRef]
- Asim, N.; Amin, M.H.; Samsudin, N.A.; Badiei, M.; Razali, H.; Akhtaruzzaman, M.D.; Amin, N.; Sopian, K. Development of effective and sustainable adsorbent biomaterial from an agricultural waste material: Cu(II) removal. Mater. Chem. Phys. 2020, 249, 123128. [Google Scholar] [CrossRef]
- Rahaman, M.; Das, A.; Bose, S. Development of copper—Iron bimetallic nanoparticle impregnated activated carbon derived from coconut husk and its efficacy as a novel adsorbent toward the removal of chromium (VI) from aqueous solution. Water 2021, 93, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Obayomi, K.S.; Bello, J.O.; Nnoruka, J.S.; Adediran, A.A.; Olajide, P.O. Development of low-cost bio-adsorbent from agricultural waste composite for Pb(II) and As(III) sorption from aqueous solution. Cogent Eng. 2019, 6, 1687274. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Chuong, C.S.; Mohd-Setapar, S.H.; Asli, U.A.; Pa’ee, K.F.; Len, K.Y.T. Trends in Adsorption Mechanisms of Fruit Peel Adsorbents to Remove Wastewater Pollutants (Cu (II), Cd (II) and Pb (II)). J. Water Environ. Technol. 2020, 18, 290–313. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. An eco-friendly approach for copper (II) ion adsorption onto cotton seed cake and its characterization: Simulation and validation. J. Taiwan Inst. Chem. Eng. 2015, 50, 198–204. [Google Scholar] [CrossRef]
- Ahsanul Haque, A.N.; Sultana, N.; Muhammad Sayem, A.S.; Smriti, S.A. Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability 2022, 14, 17. [Google Scholar]
- El-Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.N.; Mulla, S.I.; Kumar, V.; Americo-Pinheiro, J.H.P. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Convers. Biorefinery 2022, 5, 29. [Google Scholar]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A comprehensive review on the chemical regeneration of biochar adsorbent for sustainable wastewater treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar]
- Patel, H. Review on solvent desorption study from exhausted adsorbent. J. Saudi Chem. Soc. 2021, 25, 101302. [Google Scholar]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: A critical review. Desalin. Water Treat. 2016, 57, 518–544. [Google Scholar] [CrossRef]
- Othmani, A.; Magdouli, S.; Kumar, P.S.; Kapoor, A.; Chellam, P.V.; Gokkus, O. Agricultural waste materials for adsorptive removal of phenols, chromium (VI) and cadmium (II) from wastewater: A review. Environ. Res. 2022, 204, 111916. [Google Scholar] [CrossRef] [PubMed]
- Menia, S.; Abbaci, A.; Azzouz, N. Regeneration of Peel of Peas (Pisum sativum) After Zinc Adsorption. Green Energy Technol. 2018, 133, 136. [Google Scholar]
- Kalavathy, H.M.; Miranda, L.R. Moringa oleifera—A solid phase extractant for the removal of copper, nickel and zinc from aqueous solutions. Chem. Eng. J. 2010, 158, 188–199. [Google Scholar] [CrossRef]
- Kyzas, G.Z. Commercial coffee wastes as materials for adsorption of heavy metals from aqueous solutions. Materials 2012, 5, 1826–1840. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef]
- Wen, X.; Sun, N.; Yan, C.; Zhou, S.; Pang, T. Rapid removal of Cr(VI) ions by densely grafted corn stalk fibers: High adsorption capacity and excellent recyclable property. J. Taiwan Inst. Chem. Eng. 2018, 89, 95–104. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, A.K.; Verma, P.; Singh, S.; Mondal, M.K. Adsorption-Desorption Surface Bindings, Kinetics, and Mass Transfer Behavior of Thermally and Chemically Treated Great Millet Husk towards Cr(VI) Removal from Synthetic Wastewater. Adsorp. Sci. Technol. 2022, 2022, 3956977. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Edyvean, R.G.J. Bioremoval of antimony(III) from contaminated water using several plant wastes: Optimization of batch and dynamic flow conditions for sorption by green bean husk (Vigna radiata). Chem. Eng. J. 2013, 225, 192–201. [Google Scholar] [CrossRef]
- Poonam; Bhart, S.K.; Kumar, N. Kinetic study of lead (Pb2+) removal from battery manufacturing wastewater using bagasse biochar as biosorbent. Appl. Water Sci. 2018, 8, 119. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P.D. Batch and continuous (fixed-bed column) biosorption of Cu(II) by Tamarindus indica fruit shell. Korean, J. Chem. Eng. 2012, 30, 369–378. [Google Scholar]
- Shanmugaprakash, M.; Sivakumar, V. Batch and fixed-bed column studies for biosorption of Zn(II) ions onto pongamia oil cake (Pongamia pinnata) from biodiesel oil extraction. J. Environ. Manag. 2015, 164, 161–170. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar]
| Toxic Metal | Health Hazard | Permissible Limit | Reference |
|---|---|---|---|
| Lead (Pb) | Hypertension, anorexia, renal impairment, senile dementia, reduced fertility, cognitive impairment, abdominal pain | WHO—0.05 mg/L USEPA—0.005 mg/L | [2,5,6,7,8] |
| Cadmium (Cd) | Bone disease, lung and prostate cancer, pulmonary fibrosis, Itai–Itai disease, hypertension, renal toxicity, testicular atrophy | WHO—0.003 mg/L USEPA—0.005 mg/L | [2,5,6,7,8] |
| Mercury (Hg) | Ataxia, dermatitis, kidney damage, Minamata disease, miscarriages, DNA damage, pulmonary edema, dementia, gingivitis | WHO—0.001 mg/L USEPA—0.002 mg/L UE—0.001 mg/L | [2,5,7,8] |
| Arsenic (As) | Brain damage, liver tumor, melanosis, lung irritation, infertility and miscarriage, hemolysis, hepatomegaly, conjunctivitis | WHO—0.01 mg/L USEPA—0.05 mg/L | [2,5,6,7,8] |
| Chromium (Cr) | Chronic bronchitis, vomiting, lung cancer, pneumonia, liver damage, nausea and vomiting, renal failure, reproductive toxicity | WHO—0.05 mg/L USEPA—0.1 mg/L | [2,5,6,7,8] |
| Cobalt (Co) | Skin problems, congestion, edema, allergic dermatitis, respiratory problems, pneumonia and fibrosis, cardiac problems, asthma, nausea and vomiting, liver disorders | WHO—0.1 mg/L | [2,5] |
| Copper (Cu) | Anemia, metabolic disorders, metal fiver, Wilson disease, hepatic and kidney disease, reproductive and developmental toxicity, diabetes, dizziness | WHO—2.5 mg/L USEPA—1.3 mg/L | [2,5,6,7,8] |
| Manganese (Mn) | Hypertension, pneumonia, neurological problems (dullness, lethargy, weakness), mimicry of Parkinson’s disease, infertility problems, pneumonia | WHO—0.5 mg/L | [2,5] |
| Nickel (Ni) | Lung and nasal cancer, dermatitis, nausea and vomiting, kidney diseases, nausea and vomiting, dizziness, heart disorders, asthma | WHO—2.0 mg/L | [2,5,7,8] |
| Iron (Fe) | Neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease), effects on cell proliferation and differentiation, affects cognition and memory | WHO—0.3 mg/L | [5,9] |
| Zinc (Zn) | Ataxia, depression, gastrointestinal problems, respiratory disorders, anemia, impaired immune function, metal fume fever, prostate cancer | WHO—5.0 mg/L | [2,5,7,8] |
| Aluminum (Al) | Dementia, lung damage, pulmonary fibrosis, colitis, neurological diseases (Alzheimer’s, sclerosis, Parkinson’s disease), kidney issues | WHO—0.2 mg/L USEPA—0.2 mg/L | [2,10] |
| Isotherm/Kinetic Model | Equation | Reference |
|---|---|---|
| Langmuir isotherm | [18] | |
| Freundlich isotherm | ||
| Redlich–Peterson isotherm | ||
| Sips isotherm | ||
| PFO model | ) | |
| PSO model | ||
| Henry’s linear model | [19] | |
| Temkin isotherm | ||
| Toth isotherm |
| Removal Technique | Advantages | Drawbacks | Reference |
|---|---|---|---|
| Ultrafiltration | Cost effective, low-pressure demand, great performance | Secondary pollutants, requirement of posttreatments | [23] |
| Nanofiltration | Easy to operate, high removal performances, reliability, suitable for industrial practice | High energy consumption, lower water permeability | [24,25] |
| Microfiltration | Low operation pressure, cost effective, easy to operate, low energy demand | Low removal performances | [29,33] |
| Reverse osmosis | High removal performances | Membrane fouling, membrane degradation, high power consumption | [24,29] |
| Forward osmosis | Energy-saving because it does not need hydraulic pressure, environmentally friendly, easy cleaning, low fouling | Internal and external concentration polarization, draw solution reconcentration, | [29] |
| Electrodialysis | High water recovery, can operate in a wide range of pH, no phase change, no reaction products | Membrane fouling, high operation cost, high cost of membranes, need of electric potential, periodic maintenance | [24,29] |
| Chemical precipitation | Simple operation, low cost of precipitant, ease of automatic control, inexpensive and simple, high removal performances, less dissolve solids | Requires a large quantity of precipitates, sludge generation, can produce hydrogen sulfide gas, extra cost for sludge disposal | [25,29,34] |
| Coagulation–flocculation | Suitable for broader pH range, simplicity in operation, low energy consumption, high versatility | Production of sludge, transfer of toxic compound into solid phase, toxicity, hazardousness of inorganic coagulants, results in a high quantity of sludge, selective technique for metals, inefficient for some emerging chemical contaminants, the flocculants are nonbiodegradable | [25,29,35] |
| Flotation | Low-energy demand, rapid and compact process, reduced volumes of sludge, moderate cost | Demand of selective treatments, requirement of efficient and nontoxic surfactants | [29] |
| Electro-deposition | Selective method, cost-effective, high capacity to recovery of valuable materials | High sensitivity, side reactions affect the removal capacity | [29] |
| Electrocoagulation | Sludge resulted is stable, easily removable and nontoxic, easy to operate | High energy consumption, resulting of harmful byproducts | [29] |
| Electroflotation | Short process time, resulting of stable sludge | Difficulty in controlling system pH | [29] |
| Electrooxidation | Oxidizes high toxic compounds, does not result in secondary pollutants | Corrosion of electrodes | [29] |
| Ion exchange | High removal efficiency, low cost, large applicability in a wide range of treatments, high selectivity, rapid kinetics, high treatment capacity, recovery of heavy metals, fast kinetics | Low longevity, high cost of reduction phase, resins pollution, residual sludge production, demand of resins | [25,33] |
| Photocatalysis | High efficiency, rapid destruction, less harmful byproducts | High cost, long duration time, limited applications | [24,34] |
| Solar steam generation | High efficiency, low cost, no consumption of additional energy, low heat loss, improves energy utilization of elaborated infrastructures | Difficulty of precise customization of conical arrays, extended processing time | [31,32,36] |
| Adsorption | Easy to operate and use, simplicity, low cost, high effect on contaminants removal, lack of sludge, recycling of adsorbed materials, usage of particles with different sizes, availability of adsorbent materials | Low selectivity | [34,37] |
| Adsorbent | Metal Ion | Optimum Parameters | R.E. * (%) | qe* (mg g−1) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| M (g) | pH | C0 (mg/L) | t (min) | T (°C) | |||||
| Brewed tea waste | Pb2+, Cd2+, Ni2+, Zn2+ | 1.0; 1.0; 2.0; 2.0 | 4.0; 4.0; 5.0; 5.0 | 100 | 2; 5; 30; 10 | 20 °C | 97.97; 84.74; 82; 76 | 1.197; 1.457; 1.163; 2.468 | [42] |
| Tea waste | Pb2+, Cd2+, Cu2+ | 0.1 | 5.0 | 100; 20 | 60 | 25 °C | - | 33.49; 16.87; 21.02 | [43] |
| Walnut shell | Pb2+ | 1.0 | 4.0 | 100 | 2 | 20 °C | 90 | 9.912 | [44] |
| Tea waste | Cd2+ | 10 | 5.0 | 5 | 90 | 20 °C | 99.5 | 1.76 | [45] |
| Waste black tea | Zn2+ | 20 | 5.0 | 100 | 250 | 20 °C | 80 | 166.7 | [46] |
| Coffee grounds | Zn2+, Cu2+, Pb2+ | 2.5 | 5.0 | 100 | 300 | 55 °C | 84.55; 68.73; 57.23 | 5.25; 24.28; 48.13 | [47] |
| Modified green tea waste | Ni2+, As3+ | 0.3 | 3.0 | 5 | 10 | 33 °C | - | 0.3116; 0.4212 | [48] |
| Potato peel | Cr6+ | 4.0 | 2.5 | 20 | 48 | - | 100 | 3.28 | [49] |
| Potato peels charcoal | Fe2+ | 0.8 | - | 50 | 20 | - | 90.4 | 121.95 | [50] |
| Peanut shells | Cr3+, Cu2+ | 10 | 5.0 | - | 60 | 20 °C | - | 27.86; 25.39 | [51] |
| Peanut husk | Pb2+, Cd2+, Co2+, Mn2+, Ni2+ | 5.0 | 6.0 | 100 | 180; 240 (Mn) | - | 99; 62; 30; 45; 38 | 27.03; 11.36; 6.10; 14.29; 56.82 | [52] |
| Peanut shells | Cd2+, Hg2+ | 0.2 | 3 | 10 | 30 | - | 37 (Hg) | 6.0; 1.90 | [53] |
| Peanut shells | 100 (Hg) | 14.17; 30.72 | |||||||
| Tea waste | Zn2+ | 0.2 | 4.2 | 25 | 30 | - | - | 8.90 | [54] |
| Adsorbent | Metal Ions | Modifying Agent | Adsorption Capacity (mg g−1) | Reference | |
|---|---|---|---|---|---|
| Unmodified Form | Modified Form | ||||
| Acidification | |||||
| Avocado seeds | Pb2+, Cr6+ | H3PO4 | 18.9; 3.39 | 26.6; 5.10 | [59] |
| Banana peel biochar | Mn2+, Fe2+ | H3PO4 | 0.796; 27.355 | 2.319; 29.550 | [60] |
| Black cumin seeds | Cd2+ | H3PO4 | 18.16 | 19.48 | [61] |
| Avocado seeds | Pb2+, Cd2+, Cu2+ | H2SO4 | 21.48; 11.78; 46.02 | 271.02; 237.14; 102.57 | [62] |
| Orange peel | As6+ | H2SO4 | 86.95 | 98.03 | [63] |
| Peanut hull | Hg2+ | Mercaptoacetic acid | 30.3 | 83.3 | [65] |
| Black cumin seeds | Co2+ | HCl | 28.05 | 78.58 | [66] |
| Banana peel | Mn2+ | HCl | 2.864 | 4.736 | [78] |
| Alkalization | |||||
| Black cumin seeds | Co2+ | NaOH | 28.05 | 87.54 | [66] |
| Banana peel | Mn2+ | NaOH | 2.864 | 3.601 | [78] |
| Wheat straw | Cu2+ | NaOH | 10.11 | 10.59 | [79] |
| Peanut hulls | Pb2+ | NaOH | 19.7 | 49.6 | [80] |
| Oxidizing agents | |||||
| Watermelon seeds biochar | Pb2+ | H2O2 | 44.32 | 60.87 | [71] |
| Orange peel | Cr6+ | CaCl2 | 12.29 | 4.950 | [72] |
| Black cumin seeds | Cd2+ | KMnO4 | 18.16 | 23.87 | [61] |
| Esterification | |||||
| Raw palm bagasse | Pb2+ | Citric acid | 162 | 451 | [64] |
| Barley straw | Cu2+ | Citric acid | 4.64 | 31.71 | [81] |
| Rice husk | Cu2+, Pb2+, Ni2+ | N-isopropylacrylamide and citric acid | 86.81; 79.75; 69.98 | 84.67; 118.3; 79.87 | [82] |
| Etherification | |||||
| Sugarcane bagasse | Pb2+, Cu2+, Zn2+ | Ethylenediamine, CS2 | - | 558.9; 446.2; 363.3 | [83] |
| Corn stalk | Cd2+ | Acrylonitrile | 3.39 | 12.73 | [84] |
| Rice straw | Cr6+, Ni2+ | Triethylamine | - | 15.82; 3.95 | [85] |
| Corn stalk | Cr6+ | Diethylenetriamine | - | 200.0 | [86] |
| Magnetization | |||||
| Sugarcane bagasse | Pb2+, Cd2+ | Citric acid and Fe2O3 | - | 33; 117 | [56] |
| Rice straw | Cu2+, Pb2+ | Fe3O4 | - | 16.31; 19.45 | [87] |
| Sugarcane bagasse | As5+ | Fe oxides | - | 22.1 | [88] |
| Wheat straw | Cu2+ | Nanoscale zero-valent | 376.4 | [73] | |
| Surfactant modification | |||||
| Peanut husk | Cr6+ | Epichlorohydrin | - | 138.34 | [89] |
| Coconut coir pith | Cr6+ | Hexadecyltrimethylammonium bromide | - | 76.3 | [90] |
| Cotton stalk | Ni2+, Cd2+, Cr3+ | Sodium dodecyl sulphate | 31.91; 19.58; 38.29 | 38.23; 24.93; 43.33 | [91] |
| Carbonization | |||||
| Peanut hull biochar | Pb2+ | H2O2 | - | 22.82 | [92] |
| Rice straw biochar | Cd2+ | H3PO4 | 12.17 | 41.9 | [93] |
| Nut shell biochar | Cd2+ | SO2 | 104.17 | 142.86 | [94] |
| Agricultural Waste | Metal Ions | Removal Efficiency (%) | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|
| Husks | ||||
| Peanut husk | Cr6+ | - | 33.1 | [102] |
| Rice husk | Cr3+, Cu2+ | - | 22.5; 30.0 | [105] |
| Rice husk | Cr6+ | - | 52.1 | [103] |
| Rice husk ash | Cr6+, Pb2+, Zn2+ | 87.12; 88.63; 99.28 | - | [104] |
| Sunflower husk | Pb2+, Cu2+, Cd2+, Ni2+ | 98.7; 90.3; 80.0; 94.0 | - | [98] |
| Corn husk | Pb2+, Cu2+, Ni2+ | 99.66; 96.01; 92.36 | - | [99] |
| Coffee husk | Pb2+, Cd2+, Cr3+, Cu2+ | - | 1.470; 0.174; 0.188; 0.259 | [100] |
| Walnut husk | Cd2+ | 96.11 | - | [101] |
| Walnut seed husk | Pb2+ | - | 4.0 | [106] |
| Straw | ||||
| Wheat straw | Cd2+, Cu2+ | - | 14.56; 11.43 | [107] |
| Wheat straw | Cr6+, Ni2+ | - | 47.16; 41.84 | [108] |
| Wheat straw | Cd2+ | - | 39.22 | [109] |
| Barley straw | Cu2+, Pb2+ | - | 4.64; 23.2 | [110] |
| Barley straw | Cu2+, Ni2+, Co2+, Cd2+ | - | 17.8; 8.25; 6.58; 1.42 | [111] |
| Rice straw | Pb2+, Zn2+ | - | 17.93; 25.73 | [112] |
| Corn straw | Pb2+ | - | 15.0236 | [113] |
| Corn straw | Cd2+, Pb2+ | 99.24; 98.62 | - | [114] |
| Rape straw | Cd2+ | - | 32.737 | [115] |
| Rape straw | Ni2+ | 99.7 | - | [116] |
| Peel | ||||
| Pomegranate peel | Cr6+ | 98.95 | - | [117] |
| Grapefruit peel | Cd2+ | - | 42.09 | [118] |
| Banana peel | Cd2+, Cr6+, Pb2+ | - | 3.66; 6.85; 20.90 | [119] |
| Sweet lime peel | Cr6+ | - | 250 | [120] |
| Orange peel | Cu2+, Pb2+ | - | 15.27; 73.53 | [121] |
| Orange peel | Cu2+, Cd2+ | 96.9; 98.1 | 2.78; 2.57 | [122] |
| Potato peel | Cu2+ | - | 84.74 | [123] |
| Tangerine peel | Cr3+, Cu2+, Mn2+, Co2+, Ni2+, Pb2+, Cd2+, Zn2+ | 88.92; 97.04; 92.48; 94.70; 93.50; 93.0; 97.90; 96.80 | - | [124] |
| Lemon peel | Cd2+, Co2+, Cr6+, Cu2+, Mn2+, Ni2+, Pb2+ | - | 7.34; 5.63; 7.56; 7.17; 5.17; 5.73; 8.17 | [125] |
| Melon peel | Cu2+, Cd2+, Pb2+ | - | 77.76; 76.16; 191.93 | [126] |
| Shells | ||||
| Cashew nut shell | Cd2+ | - | 22.11 | [127] |
| Almond shell | Pb2+ | - | 8.08; | [128] |
| Hazelnut shell | 28.18 | |||
| Walnut shell | Cu2+, Pb2+, Cd2+, Zn2+ | - | 30.18; 70.37; 44.94; 58.96 | [129] |
| Walnut shell | Cr6+ | - | 200 | [130] |
| Cashew nut shell | Cu2+, Cd2+, Zn2+, Ni2+ | - | 406.6; 436.7; 455.7; 456.3 | |
| Bael fruit shell | Cr6+ | - | 17.27 | [131] |
| Fluted pumpkin seed shell | Pb2+ | - | 14.286 | [132] |
| Coconut shell | Cd2+ | - | 14.22 | [18] |
| Cocoa shell | Cr6+ | - | 23.36 | [133] |
| Stalk | ||||
| Sunflower stalk | Pb2+, Cd2+ | 97; 87 | 182.90; 69.80 | [134] |
| Corn stalk | Cd2+ | - | 12.73 | [84] |
| Corn stalk | Cd2+ | - | 21.37 | [135] |
| Corn stalk | Cr6+ | 28.67 | - | [136] |
| Corn stalk | Cu2+ | - | 54.05 | [137] |
| Banana stalk | Cu2+, Mn2+, Zn2+ | - | 134.88; 109.10; 108.10 | [138] |
| Cotton stalk | Pb2+ | - | 146.78 | [139] |
| Cotton stalk | Cr3+, Ni2+, Cd2+ | 100; >90; >90 | 31.91; 19.58; 38.29 | [91] |
| Grape stalk | Cd2+ | - | 21.5 | [140] |
| Sweet sorghum stalk | Cu2+ | - | 13.32 | [141] |
| Sesame stalk | Ni2+, Zn2+ | - | 47.62; 100 | [142] |
| Cassava stalks | Pb2+, Zn2+ | - | 163.93; 84.74 | [143] |
| Tea stalk | Cu2+, Zn2+ | - | 50.34; 37.87 | [144] |
| Stalks of tobacco species | Cr6+ | 99.13; 98.33; 95.0 | - | [145] |
| Stone | ||||
| Apricot stone | Pb2+ | - | 111.11 | [146] |
| Apricot stone | Al3+, Zn2+ | - | 333.3; 500 | [147] |
| Olive stone | Cu2+, Cd2+, Pb2+, Cr6+ | 77.4; 80.5; 94.5; 46 | 0.557; 0.3; 0.581; 2.345 | [148] |
| Peach stone | Pb2+, Cd2+, Cu2+ | - | 118.76; 37.48; 32.22 | [149] |
| Peach stone | Cr6+ | 97 | - | [150] |
| Lime stone | Pb2+, Cu2+, Cr6+, As3+ | 30.8; 16.5; 11.5; 8.9 | - | [151] |
| Activated plum stone | Cu2+, Pb2+ | - | 48.31; 80.65 | [152] |
| Raw plum stone | Pb2+, Cd2+, Ni2+ | - | 9.93; 12.45; 5.63 | [153] |
| Mango stone | Cd2+, Pb2+ | - | 21.05; 1.90 | [154] |
| Cherry stones | Cr6+ | 81.3 | - | [155] |
| Vegetable waste | ||||
| Cabbage waste | Pb2+ | - | 54.945 | [156] |
| Cabbage waste | Pb2+, Cd2+ | - | 60.57; 20.57 | [157] |
| Cauliflower waste | Pb2+, Cd2+ | - | 47.63; 21.32 | |
| Tomato waste | Cu2+ | - | 25 | [158] |
| Tomato waste | Cu2+ | 92.08 | 34.48 | [159] |
| Tomato waste | Pb2+ | - | 152 | [160] |
| Carrot waste | Cr3+, Cr6+ | - | 86.65; 88.27 | [161] |
| Onion waste | Pb2+, Cd2+ | 92.05; 94.89 | 9.74; 14.17 | [162] |
| Onion waste | As3+, Fe2+, Pb2+, Sn2+, Cd2+, Hg2+ | - | 2.56; 8.012; 9.957; 7.812; 1.36; 4.95 | [163] |
| Garlic waste | As3+, Fe2+, Pb2+, Sn2+, Cd2+, Hg2+ | - | 2.304; 8.459; 10.496; 7.132; 1.47; 5.12 | |
| Seeds | ||||
| Moringa seeds | Fe2+, Mn2+ | 80.5; 93 | 10.28; 11.641 | [164] |
| Avocado seeds | Cd2+, Cu2+, Pb2+ | 98.23; 99.12; 99.29 | - | [165] |
| Avocado seeds | Pb2+, Cr6+ | - | 18.9; 3.39 | [59] |
| Mangifera indica seeds | As3+ | 94 | 0.365 | [166] |
| Artocarpus heterophyllus seeds | As3+ | 93 | 29.25 | |
| Schizizium commune seeds | As3+ | 92 | 0.360 | |
| Grape seeds biochar | As3+ | - | 5.082 | [167] |
| Allium Cepa seeds | Cr6+, Cd2+, Zn2+, Cu2+, Pb2+ | - | 1.78; 1.52; 1.40; 1.62; 1.68 | [168] |
| Watermelon seeds biochar | Pb2+ | - | 44.32 | [71] |
| Papaya seeds | Cu2+, Pb2+ | - | 97.55; 99.96 | [169] |
| Cakes | ||||
| Gingelly oil cake | Pb2+ | - | 105.26 | [170] |
| Neem cake | Cu2+, Cr6+, Ni2+ | 93.3; 85.4; 96.6 | - | [171] |
| Moringa seed cake | Cr6+ | - | 3.191 | [172] |
| Oil palm cake | Cu2+, Pb2+, Zn2+ | - | 45.01; 125.51; 39.21 | [173] |
| Cottom seed cake | Cu2+ | 88 | - | [174] |
| Mustard oil cake | Cr6+, Hg2+ | - | 29; 48 | [175] |
| Rapeseed oil cake | Pb2+, Ni2+ | - | 129.87; 133.33 | [176] |
| Rapeseed cake | Cu2+, Zn2+ | - | 52.196; 29.043 | [177] |
| Olive cake | Cu2+, Mn2+, Zn2+,Ni2+, Pb2+, Cr3+ | - | 30.031; 3.571; 12.693; 5.851; 41.539; 22.193 | [141] |
| Black cumin cake | Cu2+ | - | 106.38 | [178] |
| Leaves | ||||
| Bamboo leaf powder | Hg2+ | - | 27.11 | [179] |
| Castor leaf powder | Hg2+ | - | 37.20 | [180] |
| Pine leaf powder | As5+ | - | 3.27 | [181] |
| Cauliflower leaf biochar | Cu2+, Pb2+ | - | 81.43; 224.60 | [182] |
| Cabbage leaves | Pb2+ | 95.67 | 6.307 | [183] |
| Aloe vera leaf powder | Pb2+ | 96.2 | - | [184] |
| Tea leaves | Cr6+ | 95.42 | 10.64 | [185] |
| Mango leaves | Cd2+ | - | 4.08 | [186] |
| Eucalyptus leaves | As3+, Hg2+ | > 94% | 84.03; 129.87 | [187] |
| Mangrove leaf powder | Cr6+ | - | 60.24 | [188] |
| Bagasse | ||||
| Sugarcane bagasse | Ni2+ | - | 2.0 | [189] |
| Grape bagasse | Cu2+ | - | 43.47 | [190] |
| Agave bagasse | Pb2+, Cd2+, Zn2+ | - | 93.14; 28.50; 24.66 | [191] |
| Agave bagasse | Zn2+, Cd2+, Pb2+ | - | 8; 14; 36 | [192] |
| Sugar beet bagasse | Cr6+ | - | 52.87 | [193] |
| Mango bagasse | As3+ | - | 1.35 | [194] |
| Oil palm bagasse | Cr6+ | - | 111.45 | [195] |
| Palm bagasse | Pb2+ | - | 162 | [64] |
| Hulls | ||||
| Peanut hull | Cu2+ | - | 14.13 | [196] |
| Rice hull | Zn2+, Cd2+, Fe3+ | 91.017; 84.848; 94.667 | 1.3367; 0.137; 25.403 | [197] |
| Groundnut hull | Cr6+ | - | 90 | [198] |
| Maize hull | Cu2+ | 50 | - | [199] |
| Pistachio hull | Ni2+ | > 75 | 14 | [200] |
| Soybean hulls | Cr6+ | 91.991 | - | [201] |
| Hazelnut hull | Fe3+ | 83.5 | 13.59 | [202] |
| Cotton hull | Pb2+ | - | 27.65 | [203] |
| Almond hull | Cr6+ | > 94.14 | - | [204] |
| Buckwheat hulls | Hg2+ | - | 243.90 | [205] |
| Other agricultural wastes | ||||
| Green tea | Cr6+ | 99.98 | - | [117] |
| Tea waste biochar | Cr6+ | - | 198.0 | [206] |
| Brewed tea waste | Pb2+, Cd2+, Ni2+, Zn2+ | - | 1.1947; 1.457; 1.163; 2.468 | [42] |
| Hemp fiber | Co2+ | - | 13.58 | [207] |
| Corn cob | Cd2+, Cr3+, Pb2+ | - | 13.577; 18.782; 29.168 | [119] |
| Sunflower achene head | 11.404; 12.206; 22.644 | |||
| Cocoa pod | Cd2+, Pb2+ | - | 12.15; 5.31 | [154] |
| Apple juice residue | Pb2+ | - | 108 | [160] |
| Coffee waste | Cu2+, Pb2+, Zn2+ | - | 8.2; 27.6; 8.0 | [208] |
| Mango bark | As3+ | - | 1.25 | [194] |
| Fig sawdust | Pb2+ | 95.8 | 80.645 | [209] |
| Coconut coir | Cu2+ | 13.43–41.56 | 0.5–1.15 | [210] |
| Coconut husk-activated carbon | Cr6+ | 95.28 | 173.9 | [211] |
| Agricultural waste-activated carbon | As3+, Pb2+ | - | 200; 250 | [212] |
| Adsorbent | Metal Ions | Amount Desorbed | No. of Cycles | Desorbing Agent | Reference |
|---|---|---|---|---|---|
| Corn stalk fibers | Cr6+ | - | 20 | NaOH 0.1 M | [226] |
| Pea peel | Zn2+ | 10 mg g−1 | 5 | HCl 0.5 M | [222] |
| 5 mg g−1 | 5 | NaOH 0.5 M | |||
| Moringa oleifera biochar | Cu2+, Zn2+, Ni2+ | ↘ with 9.15%, ↘ with 10.3%, ↘ with 11.78% | 6; 7; 7 | H2SO4 0.1 M | [223] |
| Coffee waste | Cu2+, Cr6+ | 70–75%, 55–60% | 10 | - | [224] |
| Modified orange peel | Pb2+, Cd2+, Ni2+ | 43.5 mg L−1, 43.1 mg L−1, 41.3 mg L−1 | 3 | 0.05 mol/L HCl | [227] |
| Millet husk | Cr6+ | 74.48% | 6 | 0.1 M NaOH, followed by 0.1 M HCl | [228] |
| Green bean husk | Sb3+ | 83.7% | 7 | 0.1 M HCl | [229] |
| Sugarcane bagasse | Pb2+, Ni2+ | 89.9%, 96.11% | 1 | 0.1 M HNO3 | [225] |
| 75.28%, 79.6% | 1 | HCl | |||
| 44.7%, 55.3% | 1 | NaOH | |||
| Sugarcane bagasse | Pb2+ | 90.05%; 83.97%; 77.92 %; 70.01% | 1 | 0.1 M HNO3, 0.1 M HCl, 0.1 M H2SO4, 0.1 M NaOH | [230] |
| Tamarind fruit seed powder | Cu2+ | 90% | - | 0.5 N HCl | [231] |
| Pongamia oil cake | Zn2+ | 90.14% | 6 | 0.05–0.1 mM HCl; 0.05–0.1 mM H2SO4; 0.01–0.1 mM EDTA | [232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Panciu, C.M.; Berca, L.; Sionel, R.M.; Mustatea, G. Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability 2023, 15, 5999. https://doi.org/10.3390/su15075999
Ungureanu EL, Mocanu AL, Stroe CA, Panciu CM, Berca L, Sionel RM, Mustatea G. Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability. 2023; 15(7):5999. https://doi.org/10.3390/su15075999
Chicago/Turabian StyleUngureanu, Elena L., Andreea L. Mocanu, Corina A. Stroe, Corina M. Panciu, Laurentiu Berca, Robert M. Sionel, and Gabriel Mustatea. 2023. "Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review" Sustainability 15, no. 7: 5999. https://doi.org/10.3390/su15075999
APA StyleUngureanu, E. L., Mocanu, A. L., Stroe, C. A., Panciu, C. M., Berca, L., Sionel, R. M., & Mustatea, G. (2023). Agricultural Byproducts Used as Low-Cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability, 15(7), 5999. https://doi.org/10.3390/su15075999







