Intergrading Water Quality Parameters, Benthic Fauna and Acute Toxicity Test for Risk Assessment on an Urban-Rural River
Abstract
1. Introduction
2. Materials and Methods
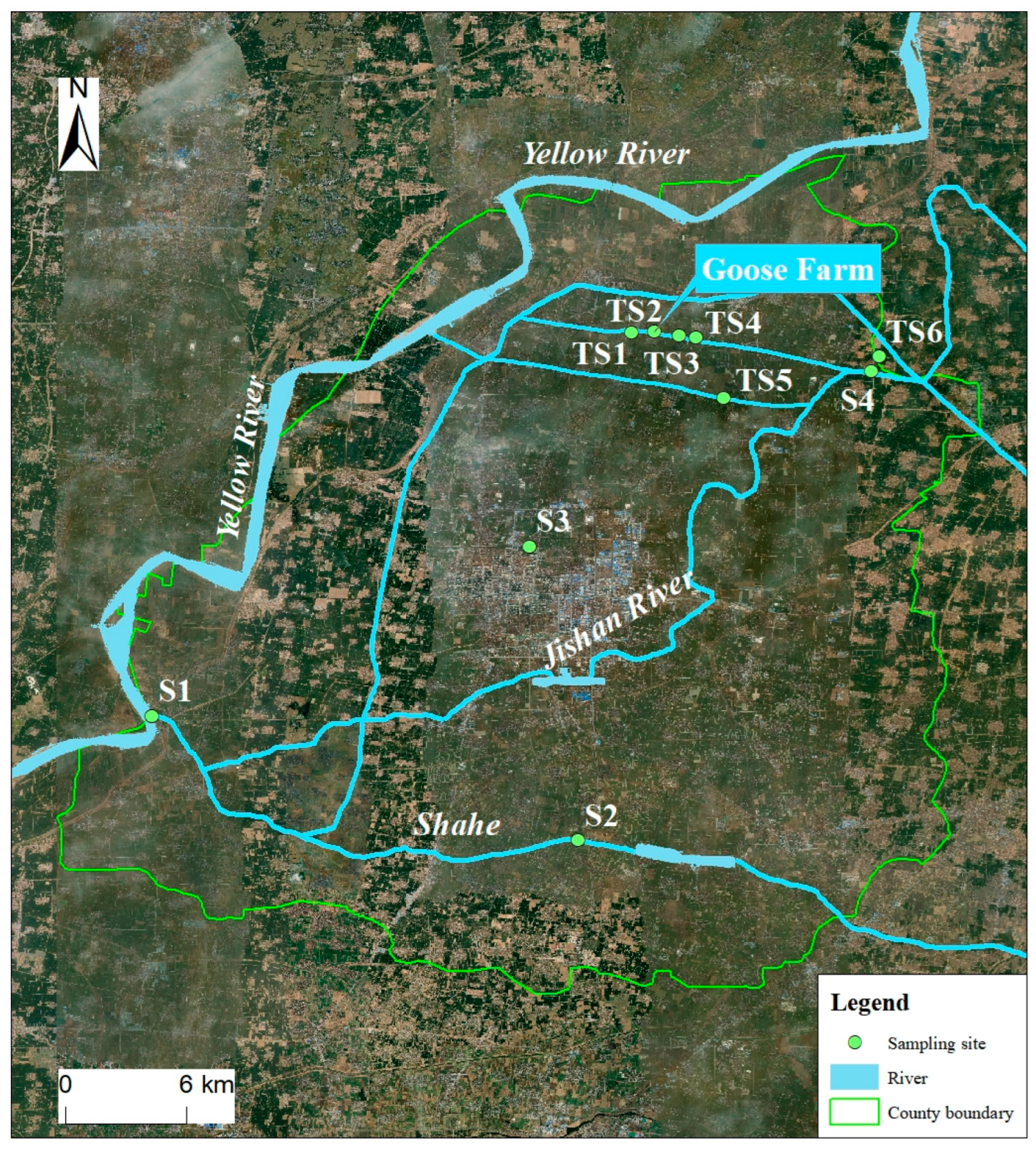
2.1. Study Area
2.2. Sampling and Sample Treatments
2.3. Zebrafish Embryotoxicity Test (ZET)
2.4. Biodiversity Index
2.5. Benthic Index of Biological Integrity (B-IBI)
2.6. Physico-Chemical Properties Analysis
2.7. Data Analysis
3. Results
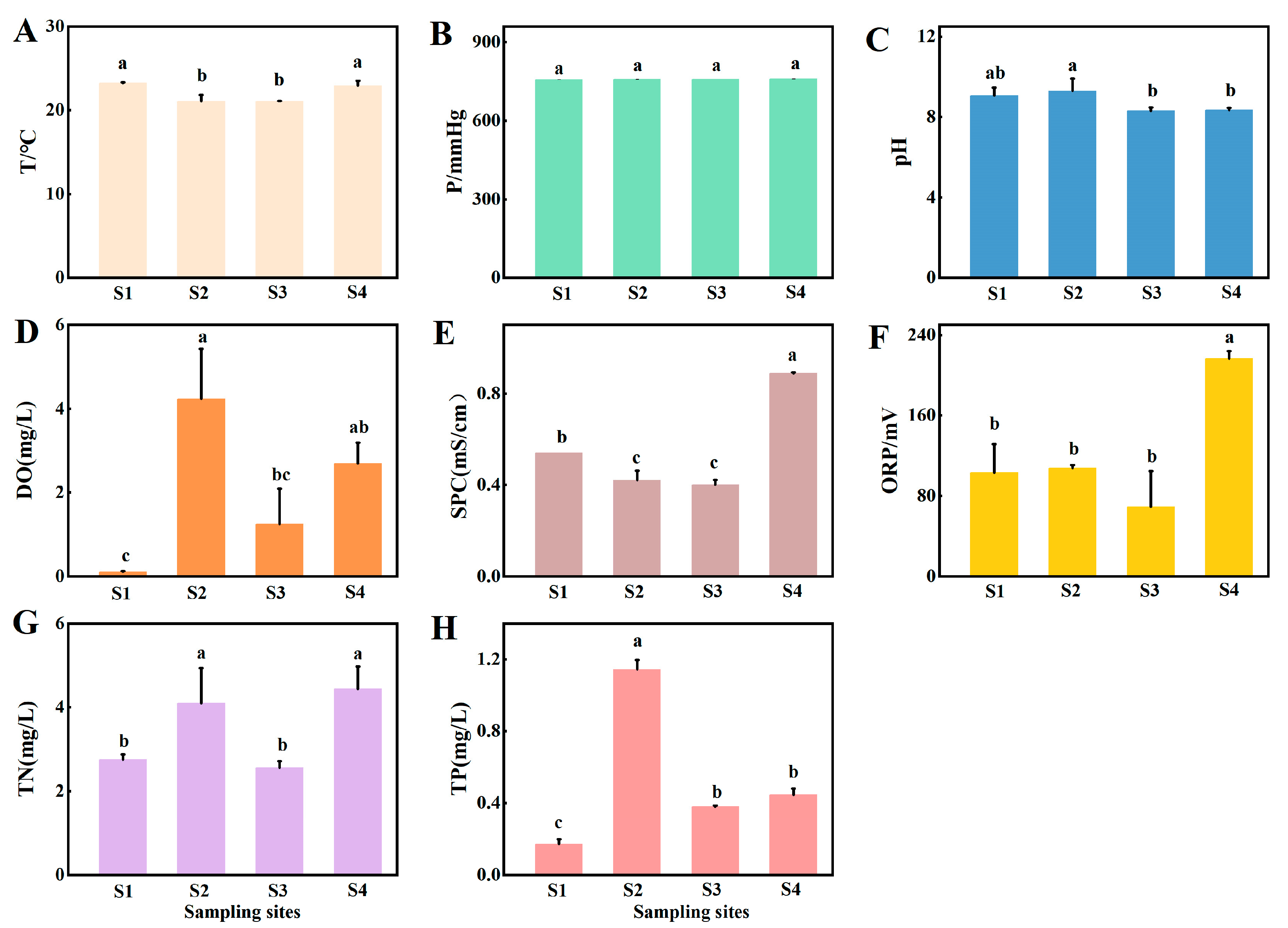
3.1. Physicochemical Parameters
3.2. Benthic Diversity
3.2.1. Species Composition, Distribution and Diversity
3.2.2. Ecological Health Evaluation Using B-IBI
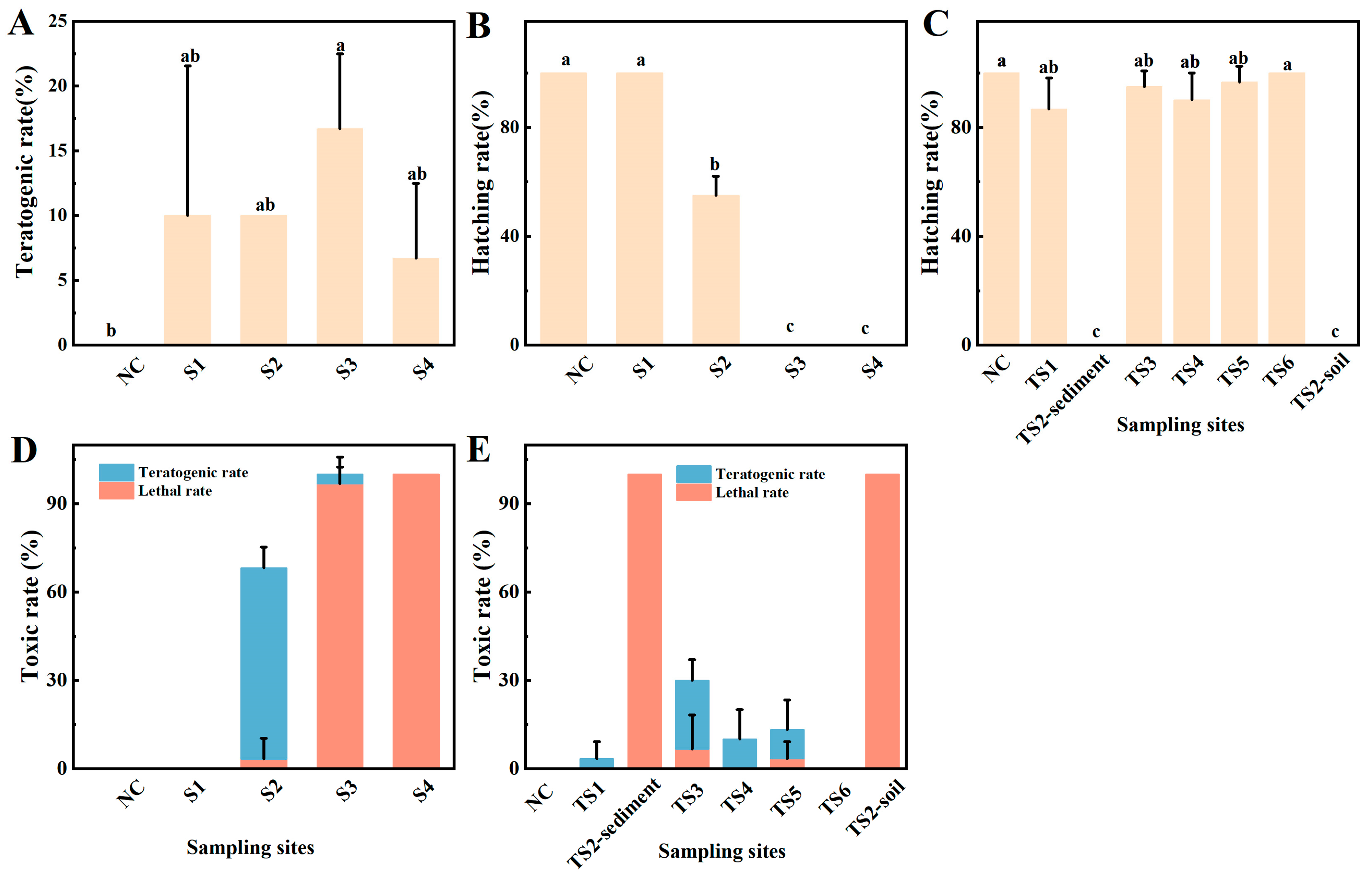
3.3. Effects of ZET
3.3.1. Effects of Raw Water and Sediments
3.3.2. Dose–Response Curve and LC50
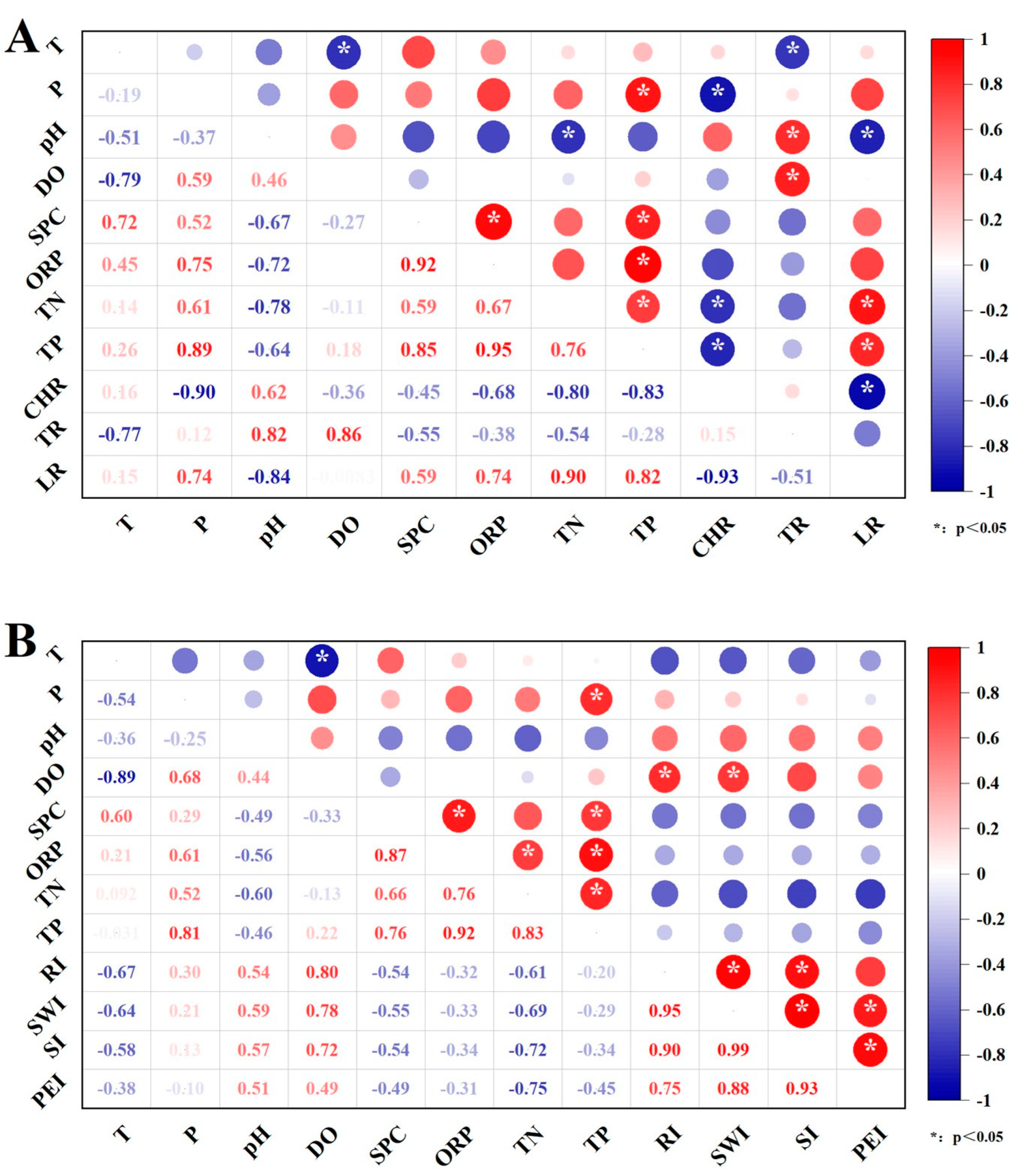
3.4. Correlation Analysis
4. Discussions
4.1. Water Quality and Benthic Fauna
4.2. Toxic Effects to Zebrafish Embryos and Ecosystem Risk
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Y.; Schoups, G.; van de Giesen, N. Organic Pollution of Rivers: Combined Threats of Urbanization, Livestock Farming and Global Climate Change. Sci. Rep. 2017, 7, 43289. [Google Scholar] [CrossRef]
- Ding, X.; Liu, L. Long-Term Effects of Anthropogenic Factors on Nonpoint Source Pollution in the Upper Reaches of the Yangtze River. Sustainability 2019, 11, 2246. [Google Scholar] [CrossRef]
- Fernández-Luqueño, F.; López-Valdez, F.; Gamero-Melo, P.; Luna, S.; Aguilera-González, E.N.; Martínez, A.I.; Hernández-Martínez, G.; Herrera-Mendoza, R.; Álvarez, M.A.; Pérez-Velázquez, I.R. Heavy Metal Pollution in Drinking Water—A Global Risk for Human Health: A Review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- Li, X.-D.; Masuda, H.; Kusakabe, M.; Yanagisawa, F.; Zeng, H.-A. Degradation of Groundwater Quality due to Anthropogenic Sulfur and Nitrogen Contamination in the Sichuan Basin, China. Geochem. J. 2006, 40, 309–332. [Google Scholar] [CrossRef]
- Burdon, F.J.; Munz, N.A.; Reyes, M.; Focks, A.; Joss, A.; Räsänen, K.; Altermatt, F.; Eggen, R.I.L.; Stamm, C. Agriculture versus Wastewater Pollution as Drivers of Macroinvertebrate Community Structure in Streams. Sci. Total Environ. 2019, 659, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Kenworthy, J.M.; Rolland, G.; Samadi, S.; Lejeusne, C. Local Variation within Marinas: Effects of Pollutants and Implications for Invasive Species. Mar. Pollut. Bull. 2018, 133, 96–106. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Yuan, Z.; Liu, H.; Lam, P.K.S. Presence of Arsenic, Mercury and Vanadium in Aquatic Organisms of Laizhou Bay and Their Potential Health Risk. Mar. Pollut. Bull. 2017, 125, 334–340. [Google Scholar] [CrossRef]
- Ogbeide, O.; Uhunamure, G.; Okundaye, F.; Ejeomo, C. First Report on Probabilistic Risk Assessment of Pesticide Residues in a Riverine Ecosystem in South-South Nigeria. Chemophere 2019, 231, 546–561. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X. Risk Assessment of Metals in Road-Deposited Sediment along an Urban–Rural Gradient. Environ. Pollut. 2013, 174, 297–304. [Google Scholar] [CrossRef]
- Chetty, S.; Pillay, L. Assessing the Influence of Human Activities on River Health: A Case for two South African Rivers with Differing Pollutant Sources. Environ. Monit. Assess. 2019, 191, 168. [Google Scholar] [CrossRef]
- Liu, J.-S.; Guo, L.-C.; Luo, X.-L.; Chen, F.-R.; Zeng, E.Y. Impact of Anthropogenic Activities on Urban Stream Water Quality: A Case Study in Guangzhou, China. Environ. Sci. Pollut. Res. 2014, 21, 13412–13419. [Google Scholar] [CrossRef] [PubMed]
- Sinche, F.; Cabrera, M.; Vaca, L.; Segura, E.; Carrera, P. Determination of the Ecological Water Quality in the Orienco Stream Using Benthic Macroinvertebrates in the Northern Ecuadorian Amazon. Integr. Environ. Assess. Manag. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gerber, R.; Smit, N.J.; van Vuren, J.H.J.; Ikenaka, Y.; Wepener, V. Biomarkers in Tigerfish (Hydrocynus vittatus) as Indicators of Metal and Organic Pollution in Ecologically Sensitive Subtropical Rivers. Ecotoxicol. Environ. Saf. 2018, 157, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Dalzochio, T.; Ressel Simões, L.A.; Santos de Souza, M.; Prado Rodrigues, G.Z.; Petry, I.E.; Andriguetti, N.B.; Herbert Silva, G.J.; Gehlen, G.; Basso da Silva, L. Water Quality Parameters, Biomarkers and Metal Bioaccumulation in Native Fish Captured in the Ilha River, Southern Brazil. Chemosphere 2017, 189, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Journey, C.A.; Berninger, J.P.; Button, D.T.; Clark, J.M.; Corsi, S.R.; DeCicco, L.A.; Hopkins, K.G.; Huffman, B.J.; Nakagaki, N.; et al. Mixed-Chemical Exposure and Predicted Effects Potential in Wadeable Southeastern USA Streams. Sci. Total Environ. 2019, 655, 70–83. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Kaboré, I.; Ouéda, A.; Moog, O.; Meulenbroek, P.; Tampo, L.; Bancé, V.; Melcher, A.H. A Benthic Invertebrates-Based Biotic Index to Assess the Ecological Status of West African Sahel Rivers, Burkina Faso. J. Environ. Manag. 2022, 307, 114503. [Google Scholar] [CrossRef]
- Wu, J.; Mao, R.; Li, M.; Xia, J.; Song, J.; Cheng, D.; Sun, H. Assessment of Aquatic Ecological Health Based on Determination of Biological Community Variability of Fish and Macroinvertebrates in the Weihe River Basin, China. J. Environ. Manag. 2020, 267, 110651. [Google Scholar] [CrossRef]
- Brack, W.; Aissa, S.A.; Backhaus, T.; Dulio, V.; Escher, B.I.; Faust, M.; Hilscherova, K.; Hollender, J.; Hollert, H.; Müller, C.; et al. Effect-Based Methods Are Key. The European Collaborative Project Solutions Recommends Integrating Effect-Based Methods for Diagnosis and Monitoring of Water Quality. Environ. Sci. Eur. 2019, 31, 10. [Google Scholar] [CrossRef]
- Sheng, J.; Tang, W.; Webber, M. Can Interbasin Water Transfer Affect Water Consumption and Pollution? Lessons from China’s South–North Water Transfer Project. Environ. Policy Gov. 2020, 30, 345–358. [Google Scholar] [CrossRef]
- Kong, X.; Wang, S.; Liu, B.; Sun, H.; Sheng, Z. Impact of Water Transfer on Interaction between Surface Water and Groundwater in the Lowland Area of North China Plain. Hydrol. Process. 2018, 32, 2044–2057. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; de VRIEND, H.J. Impact of Water Diversion on the Morphological Development of the Lower Yellow River. Int. J. Sediment Res. 2008, 23, 13–27. [Google Scholar] [CrossRef]
- Cui, W.-Y.; Guo, S.-Y.; Meng, X.-Z.; Kong, F.-Q. Application of Adapted Benthic Index of Biotic Integrity (B-IBI) for River Ecosystem Health Assessment in Zhanghe River Watershed, China. Pol. J. Ecol. 2019, 66, 407. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, X. Coupling Driving Factors of Eco-Environmental Protection and High-Quality Development in the Yellow River Basin. Front. Environ. Sci. 2022, 10, 951218. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, S.; Zhang, Z.; Aihemaiti, Y.; Yang, L.; Shao, Y.; Chen, Z.; Jiang, Y.; Jin, C.; Zheng, G. Dilution or Enrichment: The Effects of Flood on Pollutants in Urban Rivers. Environ. Sci. Eur. 2022, 34, 61. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; Wang, K. Biodiversity and Temporal Patterns of Macrozoobenthos in a Coal Mining Subsidence Area in North China. PeerJ 2019, 7, e6456. [Google Scholar] [CrossRef]
- Lammer, E.; Kamp, H.G.; Hisgen, V.; Koch, M.; Reinhard, D.; Salinas, E.R.; Wendler, K.; Zok, S.; Braunbeck, T. Development of a flow-through system for the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Toxicol. Vitr. 2009, 23, 1436–1442. [Google Scholar] [CrossRef]
- Beekhuijzen, M.; de Koning, C.; Flores-Guillén, M.-E.; de Vries-Buitenweg, S.; Tobor-Kaplon, M.; van de Waart, B.; Emmen, H. From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef]
- Adam, A.H.B.; de Haan, L.H.J.; Louisse, J.; Rietjens, I.M.C.M.; Kamelia, L. Assessment of the in vitro developmental toxicity of diethylstilbestrol and estradiol in the zebrafish embryotoxicity test. Toxicol. Vitr. 2021, 72, 105088. [Google Scholar] [CrossRef]
- Guan, B.; Chen, M.; Elsey-Quirk, T.; Yang, S.; Shang, W.; Li, Y.; Tian, X.; Han, G. Soil seed bank and vegetation differences following channel diversion in the Yellow River Delta. Sci. Total Environ. 2019, 693, 133600. [Google Scholar] [CrossRef]
- Li, T.; Zhu, Z.; Shao, Y.; Chen, Z.; Roß-Nickoll, M. Soil seedbank: Importance for revegetation in the water level fluctuation zone of the reservoir area. Sci. Total Environ. 2022, 829, 154686. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, J.; Liu, B.; Guan, X.; Li, J. Assessment of Aquatic Ecosystem Health with Indices of Biotic Integrity (IBIs) in the Ganjiang River System, China. Water 2022, 14, 278. [Google Scholar] [CrossRef]
- Wu, J.; He, Y.; Zhao, Y.; Chen, K.; Cui, Y.; Wang, H. A Simple Index of Lake Ecosystem Health Based on Species-Area Models of Macrobenthos. Int. J. Environ. Res. Public Health 2022, 19, 9678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-G. Development of a New Biotic Index to Assess Freshwater Pollution. Environ. Pollut. 2006, 139, 306–317. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Gippel, C.J.; Wang, H.-Z.; Leigh, C.; Jiang, X.-H. Assessment of the ecological health of heavily utilized, large lowland rivers: Example of the lower Yellow River, China. Limnology 2017, 18, 17–29. [Google Scholar] [CrossRef]
- Rodrigues, P.E.S.; Costa-Schmidt, L.E.; Ott, R.; Rodrigues, E.N.L. Influence of Forest Structure upon the Diversity and Composition of Edaphic Diplopods. J. Insect. Conserv. 2017, 21, 297–306. [Google Scholar] [CrossRef]
- Shanbhag, R.R.; Sundararaj, R. Assemblages and Species Diversity of Wood Destroying Termites in Different Land Use Systems in Western Ghat, India. J. For. Res. 2013, 24, 361–364. [Google Scholar] [CrossRef]
- Ohgushi, T. Eco-Evolutionary Dynamics of Plant–Herbivore Communities: Incorporating Plant Phenotypic Plasticity. Curr. Opin. Insect Sci. 2016, 14, 40–45. [Google Scholar] [CrossRef]
- Huh, M.K.; Joo, W.H.; Seo, J.-Y.; Jang, S.-H.; Choi, B.-K. Community Structure and Diversity across Spatial Scales of Macro-benthos in the Hwang River. J. Environ. Sci. 2013, 22, 1241–1251. [Google Scholar] [CrossRef]
- Nestlerode, J.A.; Murrell, M.C.; Hagy, J.D.; Harwell, L.; Lisa, J.A. Bioassessment of a Northwest Florida Estuary Using Benthic Macroinvertebrates. Integr. Environ. Assess. Manag. 2020, 16, 245–256. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine Flood Plains: Present State and Future Trends. Envir. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Graf, W.; Leitner, P.; Hanetseder, I.; Ittner, L.D.; Dossi, F.; Hauer, C. Ecological Degradation of a Meandering River by Local Channelization Effects: A Case Study in an Austrian Lowland River. Hydrobiologia 2016, 772, 145–160. [Google Scholar] [CrossRef]
- Siviglia, A.; Repetto, R.; Zolezzi, G.; Tubino, M. River Bed Evolution Due to Channel Expansion: General Behaviour and Application to a Case Study (Kugart River, Kyrgyz Republic). River Res. Appl. 2008, 24, 1271–1287. [Google Scholar] [CrossRef]
- Wesner, J.S.; Swanson, D.L.; Dixon, M.D.; Soluk, D.A.; Quist, D.J.; Yager, L.A.; Warmbold, J.W.; Oddy, E.; Seidel, T.C. Loss of Potential Aquatic-Terrestrial Subsidies Along the Missouri River Floodplain. Ecosystems 2020, 23, 111–123. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Echevarria, D.J.; Homechaudhuri, S.; Stewart, A.M.; Collier, A.D.; Kaluyeva, A.A.; Li, S.; Liu, Y.; Chen, P.; Wang, J.; et al. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat. Toxicol. 2016, 170, 297–309. [Google Scholar] [CrossRef]
- Sebastiaan van Maren, D.; Yang, M.; Wang, Z.B. Predicting the Morphodynamic Response of Silt-Laden Rivers to Water and Sediment Release from Reservoirs: Lower Yellow River, China. J. Hydraul. Eng. 2011, 137, 90–99. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Bi, N.; Li, S.; Yuan, P.; Wang, A.; Syvitski, J.P.M.; Saito, Y.; Yang, Z.; Liu, S.; et al. Impacts of the dam-orientated water-sediment regulation scheme on the lower reaches and delta of the Yellow River (Huanghe): A review. Glob. Planet Chang. 2017, 157, 93–113. [Google Scholar] [CrossRef]
- Ostra, M.; Beklova, M.; Stoupalova, M.; Ostry, M. Ecotoxicity Evaluation In Municipal And Food Industry Wastewaters. Fresen. Environ. Bull. 2009, 18, 1674–1680. [Google Scholar]
- Stamenković, M.; Steinwall, E.; Wulff, A. Cultivation and Photophysiological Characteristics of Desmids in Moderately Saline Aquaculture Wastewater. J. Phycol. 2021, 57, 926–941. [Google Scholar] [CrossRef]
- Cao, S.T.; Tran, H.P.; Le, H.T.T.; Bui, H.P.K.; Nguyen, G.T.H.; Nguyen, L.T.; Nguyen, B.T.; Luong, A.D. Impacts of Effluent from Different Livestock Farm Types (Pig, Cow, and Poultry) on Surrounding Water Quality: A Comprehensive Assessment Using Individual Parameter Evaluation Method and Water Quality Indices. Environ. Sci. Pollut. Res. 2021, 28, 50302–50315. [Google Scholar] [CrossRef]
- Tan, J.; Liu, L.; Li, F.; Chen, Z.; Chen, G.Y.; Fang, F.; Guo, J.; He, M.; Zhou, X. Screening of Endocrine Disrupting Potential of Surface Waters via an Affinity-Based Biosensor in a Rural Community in the Yellow River Basin, China. Environ. Sci. Technol. 2022, 56, 14350–14360. [Google Scholar] [CrossRef] [PubMed]



| Classification | Latin Name | S1 | S2 | S3 | S4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | B | N | B | N | B | N | B | ||
| Annelid | Limnodrilus hoffmeisteri | 1 | 0.0001 | 20 | 0.0208 | 1 | 0.0001 | ||
| Branchiura sowerbyi | 1 | 0.0001 | |||||||
| Tubifex sinicus | 2 | 0.0812 | |||||||
| Mollusc | Plectotropis | 10 | 27.936 | ||||||
| Gyraulus compressus | 7 | 0.0377 | |||||||
| Physa acuta | 1 | 0.0208 | |||||||
| Parafossarulus striatulus | 2 | 0.4716 | |||||||
| Arthodpod | Macrobrachium nipponense | 1 | 0.0317 | ||||||
| Exopalaemon modestus | 1 | 0.2185 | |||||||
| Chironomus sp. | 1 | 0.0001 | 95 | 0.7597 | 149 | 0.7058 | |||
| Polypedilum sp. | 6 | 0.0003 | 1 | 0.0058 | |||||
| Dicrotendipes sp. | 1 | 0.0001 | |||||||
| Tanytarsus sp. | 1 | 0.0001 | |||||||
| Cryptotendipes sp. | 25 | 0.0006 | |||||||
| Cricotopus sp. | 1 | 0.0001 | 1 | 0.0001 | |||||
| Propsilocerus sp. | 3 | 0.0006 | 26 | 0.0595 | 1 | 0.0002 | |||
| Orthocladius sp. | |||||||||
| Cryptochironomus sp. | 1 | 0.0001 | |||||||
| Clinotanypus sp. | 5 | 0.0034 | |||||||
| Tanypus sp. | 12 | 0.0249 | |||||||
| Procladius sp. | 2 | 0.0041 | |||||||
| Total | 6 | 0.2193 | 229 | 29.3942 | 154 | 0.7328 | 8 | 0.0378 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, W.; Chen, Z.; Shao, Y. Intergrading Water Quality Parameters, Benthic Fauna and Acute Toxicity Test for Risk Assessment on an Urban-Rural River. Sustainability 2023, 15, 6423. https://doi.org/10.3390/su15086423
Shao W, Chen Z, Shao Y. Intergrading Water Quality Parameters, Benthic Fauna and Acute Toxicity Test for Risk Assessment on an Urban-Rural River. Sustainability. 2023; 15(8):6423. https://doi.org/10.3390/su15086423
Chicago/Turabian StyleShao, Wenhua, Zhongli Chen, and Ying Shao. 2023. "Intergrading Water Quality Parameters, Benthic Fauna and Acute Toxicity Test for Risk Assessment on an Urban-Rural River" Sustainability 15, no. 8: 6423. https://doi.org/10.3390/su15086423
APA StyleShao, W., Chen, Z., & Shao, Y. (2023). Intergrading Water Quality Parameters, Benthic Fauna and Acute Toxicity Test for Risk Assessment on an Urban-Rural River. Sustainability, 15(8), 6423. https://doi.org/10.3390/su15086423






