Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities
Abstract
1. Introduction
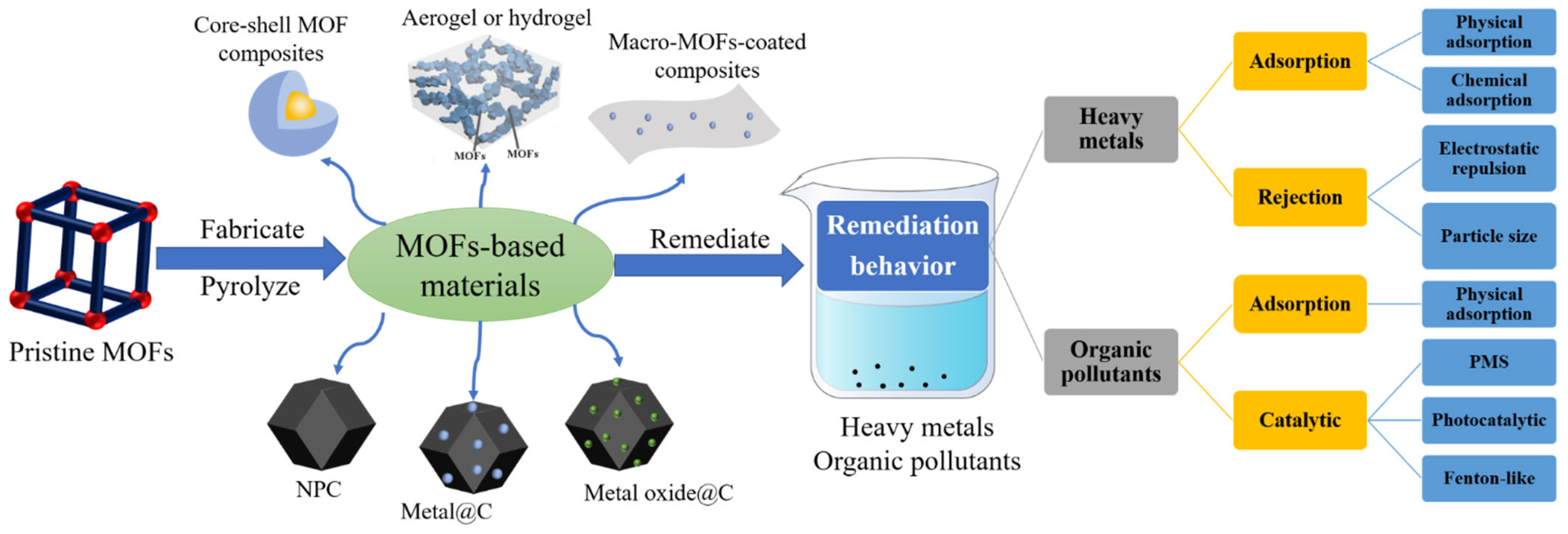
2. What Are MOF-Based Materials?
2.1. MOF Composites
2.2. MOF Derivatives
3. Remediation of HMs Using MOF-Based Materials
3.1. Typical Cationic HMs
3.1.1. Adsorption Behavior of MOF Composites
3.1.2. Adsorption Behavior of MOF Derivatives
3.2. Typical Anionic HMs
3.2.1. Capture of As(III) and As(V)
3.2.2. Capture of Cr (Cr(III) and Cr(VI))
3.3. Possible Mechanisms of HM Removal
4. Remediation of OPs using MOF-Based Materials
4.1. Conventional Industrial Organics
4.2. Organic Dyes
4.2.1. Adsorption Behavior of MOF-Based Materials
4.2.2. Catalytic Behavior of MOF-Based Materials
4.3. Pesticides
4.3.1. Adsorption Behavior of MOF-Based Materials
4.3.2. Catalytic Behavior of MOF-Based Materials
4.4. Pharmaceuticals and Personal Care Products
4.4.1. Adsorption Behavior of MOF-Based Materials
4.4.2. Catalytic Behavior of MOF-Based Materials
4.5. Possible Mechanisms of OP Removal
5. Conclusions and Prospects
- Production cost
- 2.
- Material stability
- 3.
- Material safety
- 4.
- Material recyclability
- 5.
- Extension from water to other fields
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dong, H.; Zhang, L.; Shao, P.H.; Hu, Z.C.; Yao, Z.W.; Xiao, Q.Y.; Li, D.W.; Li, M.; Yang, L.M.; Luo, S.L.; et al. A metal-organic framework surrounded with conjugate acid-base pairs for the efficient capture of Cr(VI) via hydrogen bonding over a wide pH range. J. Hazard. Mater. 2023, 441, 129945. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Dehghani, M.H.; Nabizadeh, R.; Mesdaghinia, A.; Alimohammadi, M.; Najafpoor, A.A. Adsorption of phosphorus from aqueous solution by cubic zeolitic imidazolate framework-8: Modeling, mechanical agitation versus sonication. J. Mol. Liq. 2016, 224, 151–157. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- USEPA. Drinking Water Standards and Health Advisories (EPA 820-R-11-002); Office of Water, USEPA: Washington, DC, USA, 2011. [Google Scholar]
- Fu, K.X.; Liu, X.C.; Lv, Y.; Luo, J.M.; Sun, M.X.; Luo, S.L.; Crittenden, J.C. Superselective Hg(II) Removal from Water Using a Thiol-Laced MOF-Based Sponge Monolith: Performance and Mechanism. Environ. Sci. Technol. 2022, 56, 2677–2688. [Google Scholar] [CrossRef]
- Wen, J.; Hu, X.H. Metal selectivity and effects of co-existing ions on the removal of Cd, Cu, Ni, and Cr by ZIF-8-EGCG nanoparticles. J. Colloid. Interf. Sci. 2021, 589, 578–586. [Google Scholar] [CrossRef]
- Ma, S.; Hou, Y.; Xiao, Y.; Chu, F.; Cai, T.; Hu, W.; Hu, Y. Metal-organic framework@polyaniline nanoarchitecture for improved fire safety and mechanical performance of epoxy resin. Mater. Chem. Phys. 2020, 247, 122875. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, C.; Kaneti, Y.V.; Eguchi, M.; Lin, J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231–4249. [Google Scholar] [CrossRef]
- Wen, J.; Fang, Y.; Zeng, G.M. Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal-organic frameworks: A review of studies from the last decade. Chemosphere 2018, 201, 627–643. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Trens, P.; Belarbi, H.; Shepherd, C.; Gonzalez, P.; Ramsahye, N.A.; Lee, U.H.; Seo, Y.K.; Chang, J.S. Adsorption and separation of xylene isomers vapors onto the chromium terephthalate-based porous material MIL-101(Cr): An experimental and computational study. Micropor. Mesopor. Mater. 2014, 183, 17–22. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Ghaderpoori, M.; Shahsavani, A.; Amini, M.M. Adsorption of fluoride over a metal organic framework Uio-66 functionalized with amine groups and optimization with response surface methodology. J. Mol. Liq. 2016, 221, 279–286. [Google Scholar] [CrossRef]
- Kayal, S.; Sun, B.; Chakraborty, A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks). Energy 2015, 91, 772–781. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Edit. 2020, 59, 20561–20567. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Pan, Y.; Liu, G.; Jin, W. Metal-organic framework adsorbents and membranes for separation applications. Curr. Opin. Chem. Eng. 2018, 20, 122–131. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, B.X.; Liu, W.L. An adjustable dual-emission fluorescent metal-organic framework: Effective detection of multiple metal ions, nitro-based molecules and DMA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117283. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Polyukhov, D.M.; Kudriavykh, N.A.; Gromilov, S.A.; Kiryutin, A.S.; Poryvaev, A.S.; Fedin, M.V. Efficient MOF-Catalyzed Ortho–Para Hydrogen Conversion for Practical Liquefaction and Energy Storage. ACS Energy Lett. 2022, 7, 4336–4341. [Google Scholar] [CrossRef]
- Zhong, F.; Li, C.; Xie, Y.; Xu, H.; Gao, J. Titanium metal-organic framework nanorods for highly sensitive nitroaromatic explosives detection and nanomolar sensing of Fe3+. J. Solid State Chem. 2019, 278, 120892. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazavenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal–Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Malgras, V.; Na, J.; Lin, J.; You, J.; Zhang, M.; Li, J.; Yamauchi, Y. Metal-Organic Frameworks and Their Derived Materials: Emerging Catalysts for a Sulfate Radicals-Based Advanced Oxidation Process in Water Purification. Small 2019, 15, e1900744. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Megha, R.; Lakhani, V.; Vala, S.; Dharaskar, S.; Reddy Paluvai, N.; Kumar Sinha, M.; Sasikumar Jampa, S. Removal of heavy metals and dyes from its aqueous solution utilizing metal organic Frameworks (MOFs): Review. Mater. Today Proc. 2023, 77, 188–200. [Google Scholar]
- Visa, A.; Maranescu, B.; Lupa, L.; Crisan, L.; Borota, A. New Efficient Adsorbent Materials for the Removal of Cd(II) from Aqueous Solutions. Nanomaterials 2020, 10, 899. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Nguyen, D.T.C.; Le, H.T.N.; Tu, T.T.K.; Le, N.D.; Lim, K.T.; Bach, L.G.; Nguyen, T.D. MIL-53 (Fe)-directed synthesis of hierarchically mesoporous carbon and its utilization for ciprofloxacin antibiotic remediation. J. Environ. Chem. Eng. 2019, 7, 102881. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, Z.; Zeng, G.; Yang, Z.H.; Xiao, R.; Li, X.; Cao, J.; Zhou, C.; Chen, H.; Jia, M.; et al. Metal–organic frameworks derived magnetic carbon-αFe/Fe3C composites as a highly effective adsorbent for tetracycline removal from aqueous solution. Chem. Eng. J. 2019, 374, 91–99. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Wang, Z.; Wang, Z.L.; Bush, R. Highly efficient and rapid removal of arsenic(III) from aqueous solutions by nanoscale zero-valent iron supported on a zirconium 1,4-dicarboxybenzene metal-organic framework (UiO-66 MOF). RSC Adv. 2019, 9, 39475–39487. [Google Scholar] [CrossRef]
- Mao, J.; Ge, M.; Huang, J.; Lai, Y.; Lin, C.; Zhang, K.; Meng, K.; Tang, Y. Constructing multifunctional MOF@rGO hydro-/aerogels by the self-assembly process for customized water remediation. J. Mater. Chem. A 2017, 5, 11873–11881. [Google Scholar] [CrossRef]
- Abdollahi, N.; Akbar Razavi, S.A.; Morsali, A.; Hu, M.L. High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef]
- Boix, G.; Troyano, J.; Garzon-Tovar, L.; Camur, C.; Bermejo, N.; Yazdi, A.; Piella, J.; Bastus, N.G.; Puntes, V.F.; Imaz, I.; et al. MOF-Beads Containing Inorganic Nanoparticles for the Simultaneous Removal of Multiple Heavy Metals from Water. ACS Appl. Mater. Interfaces 2020, 12, 10554–10562. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Surface Decoration of Amino-Functionalized Metal–Organic Framework/Graphene Oxide Composite onto Polydopamine-Coated Membrane Substrate for Highly Efficient Heavy Metal Removal. ACS Appl. Mater. Interfaces 2017, 9, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zheng, X.; Ou, H.; Yu, P.; Li, Q.; Feng, S. Fabrication of an amine-modified ZIF-8@GO membrane for high-efficiency adsorption of copper ions. New J. Chem. 2019, 43, 5603–5610. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.L.; Zeng, G.M.; Song, B.; Liu, H.Y.; Huan, S.Y.; Li, J. Ultrathin reduced graphene oxide/MOF nanofiltration membrane with improved purification performance at low pressure. Chemosphere 2018, 204, 378–389. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight Studies on Metal–Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari Rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Z.; Li, Y.; Xiao, J. Formation of willow leaf-like structures composed of NH2-MIL68(In) on a multifunctional multiwalled carbon nanotube backbone for enhanced photocatalytic reduction of Cr(VI). Nano Res. 2017, 10, 3543–3556. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N.; Yang, L.Y.; Wang, Y.G.; Yu, D.; Ouyang, X.K. Facile Fabrication of ZIF-8/Calcium Alginate Microparticles for Highly Efficient Adsorption of Pb(II) from Aqueous Solutions. Ind. Eng. Chem. Res. 2019, 58, 6394–6401. [Google Scholar] [CrossRef]
- Bo, S.; Ren, W.; Lei, C.; Xie, Y.; Cai, Y.; Wang, S.; Gao, J.; Ni, Q.; Yao, J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr(IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Yin, N.; Wang, K.; Xia, Y.A.; Li, Z. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb(II). Desalination 2018, 430, 120–127. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Ren, X.; Zhang, W.; Zhang, T.; Liu, X.; Du, T.; Li, T.; Wang, J. Internally extended growth of core–shell NH2-MIL-101(Al)@ZIF-8 nanoflowers for the simultaneous detection and removal of Cu(II). J. Mater. Chem. A 2018, 6, 21029–21038. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Zhong, D.; Zhong, N. CTAB-surface-functionalized magnetic MOF@MOF composite adsorbent for Cr(VI) efficient removal from aqueous solution. Colloids Surf. A 2020, 586, 124255. [Google Scholar] [CrossRef]
- Jian, M.; Wang, H.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Self-assembled one-dimensional MnO2@zeolitic imidazolate framework-8 nanostructures for highly efficient arsenite removal. Environ. Sci. Nano 2016, 3, 1186–1194. [Google Scholar] [CrossRef]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin-alginic acid composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Chen, C.; Xu, H.; Gao, Y.; Tan, X.; Alsaedi, A.; Hayat, T. Cr(VI) Reduction and Immobilization by Core-Double-Shell Structured Magnetic Polydopamine@Zeolitic Idazolate Frameworks-8 Microspheres. ACS Sustain. Chem. Eng. 2017, 5, 6795–6802. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal-Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef]
- Wang, N.; Ouyang, X.K.; Yang, L.Y.; Omer, A.M. Fabrication of a Magnetic Cellulose Nanocrystal/Metal–Organic Framework Composite for Removal of Pb(II) from Water. ACS Sustain. Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Ricco, R.; Konstas, K.; Styles, M.J.; Richardson, J.J.; Babarao, R.; Suzuki, K.; Scopece, P.; Falcaro, P. Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. A 2015, 3, 19822–19831. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Leong, S.; Lin, X.; Wei, J.; Kong, B.; Xu, Y.; Low, Z.X.; Yao, J.; Wang, H. Rapid Construction of ZnO@ZIF-8 Heterostructures with Size-Selective Photocatalysis Properties. ACS Appl. Mater. Interfaces 2016, 8, 9080–9087. [Google Scholar] [CrossRef]
- Ma, X.; Lou, Y.; Chen, X.-B.; Shi, Z.; Xu, Y. Multifunctional flexible composite aerogels constructed through in-situ growth of metal-organic framework nanoparticles on bacterial cellulose. Chem. Eng. J. 2019, 356, 227–235. [Google Scholar] [CrossRef]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Shooto, N.D.; Dikio, C.W.; Wankasi, D.; Sikhwivhilu, L.M.; Mtunzi, F.M.; Dikio, E.D. Novel PVA/MOF Nanofibres: Fabrication, Evaluation and Adsorption of Lead Ions from Aqueous Solution. Nanoscale Res. Lett. 2016, 11, 414. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal-organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Bahmani, E.; Koushkbaghi, S.; Darabi, M.; ZabihiSahebi, A.; Askari, A.; Irani, M. Fabrication of novel chitosan-g-PNVCL/ZIF-8 composite nanofibers for adsorption of Cr(VI), As(V) and phenol in a single and ternary systems. Carbohydr. Polym. 2019, 224, 115148. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Zhai, S.; Gao, G.; Ding, J.; Zhang, W.; Liu, Y.; Zhao, X.; Pan, B.; Lv, L. Efficient removal of nickel(II) from high salinity wastewater by a novel PAA/ZIF-8/PVDF hybrid ultrafiltration membrane. Water Res. 2018, 143, 87–98. [Google Scholar] [CrossRef]
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-Reduced Graphene Oxide Nanocomposites by a Simple Self-Assembly Strategy for Increasing Interfacial Contact: Efficient Visible-Light Photocatalysts. ACS Appl. Mater. Interf. 2015, 7, 9507–9515. [Google Scholar] [CrossRef]
- Tang, J.; Yamauchi, Y. Carbon materials: MOF morphologies in control. Nat. Chem. 2016, 8, 638–639. [Google Scholar] [CrossRef]
- Hu, M.; Reboul, J.; Furukawa, S.; Torad, N.L.; Ji, Q.; Srinivasu, P.; Ariga, K.; Kitagawa, S.; Yamauchi, Y. Direct carbonization of Al-based porous coordination polymer for synthesis of nanoporous carbon. J. Am. Chem. Soc. 2012, 134, 2864–2867. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, C.; Wen, T.; Zhuang, L.; Wang, X.; Song, G.; Chen, D.; Ai, Y.; Hayat, T.; et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem. Eng. J. 2018, 336, 241–252. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, F.; Zhang, Y.; Ou, P.; Wu, P.; Fang, Q.; Chen, Z.; Li, S. UiO-66 derived N-doped carbon nanoparticles coated by PANI for simultaneous adsorption and reduction of hexavalent chromium from waste water. Chem. Eng. J. 2019, 378, 122069. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Catalytic chromium reduction using formic acid and metal nanoparticles immobilized in a metal-organic framework. Chem. Commun. 2013, 49, 3327–3329. [Google Scholar] [CrossRef] [PubMed]
- Gadipelli, S.; Travis, W.; Zhou, W.; Guo, Z. A thermally derived and optimized structure from ZIF-8 with giant enhancement in CO2 uptake. Energy Environ. Sci. 2014, 7, 2232–2238. [Google Scholar] [CrossRef]
- Chen, D.; Shen, W.; Wu, S.; Chen, C.; Luo, X.; Guo, L. Ion exchange induced removal of Pb(II) by MOF-derived magnetic inorganic sorbents. Nanoscale 2016, 8, 7172–7179. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bhattacharyya, S.; Hazra, A.; Ghosh, A.C.; Maji, T.K. Post-synthetic metalation in an anionic MOF for efficient catalytic activity and removal of heavy metal ions from aqueous solution. Chem Commun. 2016, 52, 2831–2834. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Li, C.; Wu, C. Exploring a thiol-functionalized MOF for elimination of lead and cadmium from aqueous solution. J. Mol. Liq. 2016, 221, 43–50. [Google Scholar] [CrossRef]
- Ke, F.; Jiang, J.; Li, Y.; Liang, J.; Wan, X.; Ko, S. Highly selective removal of Hg2+ and Pb2+ by thiol-functionalized Fe3O4 @metal-organic framework core-shell magnetic microspheres. Appl. Surf. Sci. 2017, 413, 266–274. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Panda, A.; Kim, E.; Yoon, M. Biopolymer-Coated Magnetite Nanoparticles and Metal-Organic Framework Ternary Composites for Cooperative Pb(II) Adsorption. ACS Appl. Nano Mater. 2018, 1, 4198–4210. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef]
- Song, Y.; Qiang, T.; Ye, M.; Ma, Q.; Fang, Z. Metal organic framework derived magnetically separable 3-dimensional hierarchical Ni@C nanocomposites: Synthesis and adsorption properties. Appl. Surf. Sci. 2015, 359, 834–840. [Google Scholar] [CrossRef]
- Afshariazar, F.; Morsali, A.; Wang, J.; Junk, P.C. Highest and Fastest Removal Rate of Pb(II) Ions through Rational Functionalized Decoration of a Metal-Organic Framework Cavity. Chemistry 2020, 26, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.B.; Xu, L.; Yang, J.C.E.; Cui, H.J.; Yuan, B.; Fu, M.L. Magnetic responsive Fe3O4-ZIF-8 core-shell composites for efficient removal of As(III) from water. Colloids Surf. A 2018, 539, 59–68. [Google Scholar] [CrossRef]
- Huo, J.-B.; Xu, L.; Chen, X.; Zhang, Y.; Yang, J.-C.E.; Yuan, B.; Fu, M.-L. Direct epitaxial synthesis of magnetic Fe3O4@UiO-66 composite for efficient removal of arsenate from water. Micropor. Mesopor. Mater. 2019, 276, 68–75. [Google Scholar] [CrossRef]
- Folens, K.; Leus, K.; Nicomel, N.R.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Laing, G.D.; Van Der Voort, P. Fe3O4@MIL-101—A Selective and Regenerable Adsorbent for the Removal of As Species from Water. Eur. J. Inorg. Chem. 2016, 27, 4395–4401. [Google Scholar] [CrossRef]
- Yang, J.C.; Yin, X.B. CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity. Sci. Rep. 2017, 7, 40955. [Google Scholar] [CrossRef]
- Wang, D.; Gilliland, S.E.; Yi, X.; Logan, K.; Heitger, D.R.; Lucas, H.R.; Wang, W.N. Iron Mesh-Based Metal Organic Framework Filter for Efficient Arsenic Removal. Environ. Sci. Technol. 2018, 52, 4275–4284. [Google Scholar] [CrossRef]
- Lv, Z.; Fan, Q.; Xie, Y.; Chen, Z.; Alsaedi, A.; Hayat, T.; Wang, X.; Chen, C. MOFs-derived magnetic chestnut shell-like hollow sphere NiO/Ni@C composites and their removal performance for arsenic(V). Chem. Eng. J. 2019, 362, 413–421. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Fu, H.; Yi, X.H.; Wang, P.; Zhao, C.; Wang, C.C.; Zheng, W. Simultaneous Cr(VI) reduction and Cr(III) removal of bifunctional MOF/Titanate nanotube composites. Environ. Pollut. 2019, 249, 502–511. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. M@MIL-100(Fe) (M = Au, Pd, Pt) nanocomposites fabricated by a facile photodeposition process: Efficient visible-light photocatalysts for redox reactions in water. Nano Res. 2015, 8, 3237–3249. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, C.; Lu, S.; Zhang, X.; Alsaedi, A.; Hayat, T. MOFs-induced encapsulation of ultrafine Ni nanoparticles into 3D N-doped graphene-CNT frameworks as a recyclable catalyst for Cr(VI) reduction with formic acid. Carbon 2019, 148, 52–63. [Google Scholar] [CrossRef]
- Gao, G.; Nie, L.; Yang, S.; Jin, P.; Chen, R.; Ding, D.; Wang, X.C.; Wang, W.; Wu, K.; Zhang, Q. Well-defined strategy for development of adsorbent using metal organic frameworks (MOF) template for high performance removal of hexavalent chromium. Appl. Surf. Sci. 2018, 457, 1208–1217. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wu, S.H.; Jia, S.Y.; Qin, F.-X.; Zhou, S.M.; Ren, H.T.; Na, P.; Liu, Y. Novel hematite nanorods and magnetite nanoparticles prepared from MIL-100(Fe) template for the removal of As(V). Mater. Lett. 2014, 132, 8–10. [Google Scholar] [CrossRef]
- Shen, L.; Huang, L.; Liang, S.; Liang, R.; Qin, N.; Wu, L. Electrostatically derived self-assembly of NH2-mediated zirconium MOFs with graphene for photocatalytic reduction of Cr(VI). RSC Adv. 2014, 4, 2546–2549. [Google Scholar] [CrossRef]
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Q.; Ren, S.; Chen, Z.; Zheng, H. Assembly of Zr-MOF crystals onto magnetic beads as a highly adsorbent for recycling nitrophenol. Chem. Eng. J. 2017, 323, 74–83. [Google Scholar] [CrossRef]
- Niu, H.; Zheng, Y.; Wang, S.; Zhao, L.; Yang, S.; Cai, Y. Continuous generation of hydroxyl radicals for highly efficient elimination of chlorophenols and phenols catalyzed by heterogeneous Fenton-like catalysts yolk/shell Pd@Fe3O4@metal organic frameworks. J. Hazard. Mater. 2018, 346, 174–183. [Google Scholar] [CrossRef]
- Teng, W.; Bai, N.; Chen, Z.; Shi, J.; Fan, J.; Zhang, W.X. Hierarchically porous carbon derived from metal-organic frameworks for separation of aromatic pollutants. Chem. Eng. J. 2018, 346, 388–396. [Google Scholar] [CrossRef]
- He, D.; Niu, H.; He, S.; Mao, L.; Cai, Y.; Liang, Y. Strengthened Fenton degradation of phenol catalyzed by core/shell Fe-Pd@C nanocomposites derived from mechanochemically synthesized Fe-Metal organic frameworks. Water Res. 2019, 162, 151–160. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Fernandez-Delgado, O.; Deemer, E.; Wang, H.; El-Gendy, A.A.; Curry, M.L.; Noveron, J.C. Carbonization of Co-BDC MOF results in magnetic C@Co nanoparticles that catalyze the reduction of methyl orange and 4-nitrophenol in water. J. Mol. Liq. 2019, 290, 111059. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Liang, J.; Ning, X.A.; Sun, J.; Song, J.; Lu, J.; Cai, H.; Hong, Y. Toxicity evaluation of textile dyeing effluent and its possible relationship with chemical oxygen demand. Ecotoxicol. Environ. Saf. 2018, 166, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, X.; Bai, Y.; Liu, H. Applications of nanomaterials in enantioseparation and related techniques. Trends Anal. Chem. 2012, 39, 195–206. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Ding, J. Adsorption behavior of magnetic amino-functionalized metal–organic framework for cationic and anionic dyes from aqueous solution. RSC Adv. 2016, 6, 48884–48895. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, C.; Guan, H.; Li, L.; Fan, L.; Wang, Y.; Liu, L.; Meng, Q.; Zhang, R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surf. A 2018, 539, 382–390. [Google Scholar] [CrossRef]
- Xu, S.; Lv, Y.; Zeng, X.; Cao, D. ZIF-derived nitrogen-doped porous carbons as highly efficient adsorbents for removal of organic compounds from wastewater. Chem. Eng. J. 2017, 323, 502–511. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Synthesis of graphene oxide/metal–organic frameworks hybrid materials for enhanced removal of Methylene blue in acidic and alkaline solutions. J. Chem. Technol. Biotechnol. 2017, 93, 698–709. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Removal of Congo red dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason. Sonochem. 2017, 39, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, J.; Chen, X.; Yang, Y.; Yang, S. Selective adsorption performance of H6P2Mo15W3O62-based Cu3(BTC)2 composite in treatment of simulated cationic dye wastewater. Chem. Res. Chin. Univ. 2017, 33, 268–273. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Bao, C.; Ma, J.; Liu, M.; Wang, F. Magnetic responsive metal–organic frameworks nanosphere with core–shell structure for highly efficient removal of methylene blue. Chem. Eng. J. 2016, 283, 1127–1136. [Google Scholar] [CrossRef]
- Zhang, C.F.; Qiu, L.G.; Ke, F.; Zhu, Y.J.; Yuan, Y.P.; Xu, G.S.; Jiang, X. A novel magnetic recyclable photocatalyst based on a core–shell metal–organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J. Mater. Chem. A 2013, 1, 14329. [Google Scholar] [CrossRef]
- Yue, X.; Guo, W.; Li, X.; Zhou, H.; Wang, R. Core-shell Fe3O4@MIL-101(Fe) composites as heterogeneous catalysts of persulfate activation for the removal of Acid Orange 7. Environ. Sci. Pollut. Res. 2016, 23, 15218–15226. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Feng, Q.; Chen, X.; Hu, Z. Visible light photocatalytic degradation of MB using UiO-66/g-C3N4 heterojunction nanocatalyst. Chemosphere 2018, 212, 523–532. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Luo, R.; Liu, C.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Metal-organic framework one-dimensional fibers as efficient catalysts for activating peroxymonosulfate. Chem. Eng. J. 2017, 330, 262–271. [Google Scholar] [CrossRef]
- Wu, C.H.; Yun, W.C.; Wi-Afedzi, T.; Lin, K.A. ZIF-67 supported on marcoscale resin as an efficient and convenient heterogeneous catalyst for Oxone activation. J. Colloid Interf. Sci. 2018, 514, 262–271. [Google Scholar] [CrossRef]
- Ren, W.; Gao, J.; Lei, C.; Xie, Y.; Cai, Y.; Ni, Q.; Yao, J. Recyclable metal-organic framework/cellulose aerogels for activating peroxymonosulfate to degrade organic pollutants. Chem. Eng. J. 2018, 349, 766–774. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid Interf. Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef]
- Lin, H.Y.; Zhao, J.; Song, G.; Luan, J.; Liu, X.X.; Liu, G.C. High quality and high performance adsorption of Congo red using as-grown MWCNTs synthesized over a Co-MOF as a catalyst precursor via the CVD method. Dalton Trans. 2017, 46, 17067–17073. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants. Carbon 2017, 115, 730–739. [Google Scholar] [CrossRef]
- Ma, W.; Du, Y.; Wang, N.; Miao, P. ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation. Environ. Sci. Pollut. Res. Int. 2017, 24, 16276–16288. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, W.; Ren, Z.; Du, Y.; Xu, P.; Han, X. Prussian blue analogues derived porous nitrogen-doped carbon microspheres as high-performance metal-free peroxymonosulfate activators for non-radical-dominated degradation of organic pollutants. J. Mater. Chem. A 2018, 6, 884–895. [Google Scholar] [CrossRef]
- Lin, K.A.; Chen, B.J. Prussian blue analogue derived magnetic carbon/cobalt/iron nanocomposite as an efficient and recyclable catalyst for activation of peroxymonosulfate. Chemosphere 2017, 166, 146–156. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qiu, L.G.; Yuan, Y.P.; Zhu, Y.J.; Jiang, X.; Xiao, J.D. Magnetic Fe3O4@C/Cu and Fe3O4@CuO core–shell composites constructed from MOF-based materials and their photocatalytic properties under visible light. Appl. Catal. B 2014, 144, 863–869. [Google Scholar] [CrossRef]
- Andrew Lin, K.Y.; Hsu, F.K.; Lee, W.D. Magnetic cobalt–graphene nanocomposite derived from self-assembly of MOFs with graphene oxide as an activator for peroxymonosulfate. J. Mater. Chem. A 2015, 3, 9480–9490. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Li, L.; Jiang, J. Iron terephthalate metal–organic framework: Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation. Appl. Catal. B 2014, 148–149, 191–200. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Z.; Ke, X.; Jaatinen, E.; Xie, T.; Wang, D.; Guo, C.; Zhao, J.; Zhu, H. Supported silver nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Green Chem. 2010, 12, 414. [Google Scholar] [CrossRef]
- Li, M.; Meng, J.; Li, Q.; Huang, M.; Liu, X.; Owusu, K.A.; Liu, Z.; Mai, L. Finely Crafted 3D Electrodes for Dendrite-Free and High-Performance Flexible Fiber-Shaped Zn-Co Batteries. Adv. Funct. Mater. 2018, 28, 1802016. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, D.; Guo, Z.; Jia, P.; Xing, H. Eosin Y-Embedded Zirconium-Based Metal-Organic Framework as a Dual-Emitting Built-In Self-Calibrating Platform for Pesticide Detection. Inorg. Chem. 2020, 59, 5386–5393. [Google Scholar] [CrossRef]
- Li, Z. Spatiotemporal pattern models for bioaccumulation of pesticides in common herbaceous and woody plants. J. Environ. Manag. 2020, 276, 111334. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; He, M.; Kong, F. Risk attitude, risk perception, and farmers’ pesticide application behavior in China: A moderation and mediation model. J. Clean. Prod. 2020, 276, 124241. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Elshahat, M.; Emam, H.E. Cu–BTC@cotton composite: Design and removal of ethion insecticide from water. RSC Adv. 2016, 6, 42324–42333. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Huang, P.; Wang, J.; Du, T.; Du, X.; Lu, X. Magnetic Cu-MOFs embedded within graphene oxide nanocomposites for enhanced preconcentration of benzenoid-containing insecticides. Talanta 2018, 181, 112–117. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Xu, D.; Huang, X.; Xu, X.; Zheng, S.; Zhang, Y.; Lin, H. Metal-organic framework preparation using magnetic graphene oxide-beta-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr. Polym. 2017, 175, 584–591. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Vaziri, R.; Abureesh, M.A. Highly robust AgIO3 /MIL-53 (Fe) nanohybrid composites for degradation of organophosphorus pesticides in single and binary systems: Application of artificial neural networks modelling. J. Taiwan Inst. Chem. Eng. 2018, 83, 133–142. [Google Scholar] [CrossRef]
- Naimi Joubani, M.; Zanjanchi, M.A.; Sohrabnezhad, S. The carboxylate magnetic—Zinc based metal-organic framework heterojunction: Fe3O4-COOH@ZIF-8/Ag/Ag3PO4 for plasmon enhanced visible light Z-scheme photocatalysis. Adv. Powder Technol. 2020, 31, 29–39. [Google Scholar] [CrossRef]
- Shen, K.; Chen, L.; Long, J.; Zhong, W.; Li, Y. MOFs-Templated Co@Pd Core–Shell NPs Embedded in N-Doped Carbon Matrix with Superior Hydrogenation Activities. ACS Catal. 2015, 5, 5264–5271. [Google Scholar] [CrossRef]
- Long, J.; Shen, K.; Chen, L.; Li, Y. Multimetal-MOF-derived transition metal alloy NPs embedded in an N-doped carbon matrix: Highly active catalysts for hydrogenation reactions. J. Mater. Chem. A 2016, 4, 10254–10262. [Google Scholar] [CrossRef]
- Ye, G.; Luo, P.; Zhao, Y.; Qiu, G.; Hu, Y.; Preis, S.; Wei, C. Three-dimensional Co/Ni bimetallic organic frameworks for high-efficient catalytic ozonation of atrazine: Mechanism, effect parameters, and degradation pathways analysis. Chemosphere 2020, 253, 126767. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Correa, E.M.; Dominguez-Vargas, J.R.; Olivares-Marin, F.J.; de Heredia, J.B. On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazard. Mater. 2010, 177, 1046–1053. [Google Scholar] [CrossRef]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209–210, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jiménez, S.M.; Hernández-Maldonado, A.J. Nickel(II) grafted MCM-41: A novel sorbent for the removal of Naproxen from water. Micropor. Mesopor. Mater. 2008, 116, 246–252. [Google Scholar] [CrossRef]
- Park, S.; Lee, W. Removal of selected pharmaceuticals and personal care products in reclaimed water during simulated managed aquifer recharge. Sci. Total Environ. 2018, 640–641, 671–677. [Google Scholar] [CrossRef]
- Jamil, K. Health effects of pharmaceuticals and personal care products. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology, 1st ed.; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Butterworth-Heinemann: Kidlington, UK, 2019; pp. 115–128. [Google Scholar]
- Mendez-Arriaga, F.; Esplugas, S.; Gimenez, J. Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585–594. [Google Scholar] [CrossRef]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.E.; Haji Shabani, A.M.; Dadfarnia, S.; Emami, S. Effective removal of ciprofloxacin from aqueous solutions using magnetic metal–organic framework sorbents: Mechanisms, isotherms and kinetics. J. Iran. Chem. Soc. 2016, 13, 1617–1627. [Google Scholar] [CrossRef]
- Bayazit, S.S.; Danalioglu, S.T.; Abdel Salam, M.; Kerkez Kuyumcu, O. Preparation of magnetic MIL-101 (Cr) for efficient removal of ciprofloxacin. Environ. Sci. Pollut. Res. Int. 2017, 24, 25452–25461. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Liu, H.; Zhu, Y.; Wang, D.; Yan, M. Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial Uio-66-(OH)2/GO. Appl. Surf. Sci. 2020, 525, 146614. [Google Scholar] [CrossRef]
- Sun, X.; Hu, D.; Yang, L.Y.; Wang, N.; Wang, Y.G.; Ouyang, X.K. Efficient adsorption of Levofloxacin from aqueous solution using calcium alginate/metal organic frameworks composite beads. J. Sol-Gel Sci. Technol. 2019, 91, 353–363. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, H.; Wu, S.; Yang, C.; Cheng, J. Enhanced removal of bisphenol A from aqueous solution by aluminum-based MOF/sodium alginate-chitosan composite beads. Chemosphere 2019, 237, 124493. [Google Scholar] [CrossRef]
- Mohamed, A.K.; Mahmoud, M.E. Encapsulation of starch hydrogel and doping nanomagnetite onto metal-organic frameworks for efficient removal of fluvastatin antibiotic from water. Carbohydr. Polym. 2020, 245, 116438. [Google Scholar] [CrossRef]
- Yang, W.; Han, Y.; Li, C.; Zhu, L.; Shi, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Shapeable three-dimensional CMC aerogels decorated with Ni/Co-MOF for rapid and highly efficient tetracycline hydrochloride removal. Chem. Eng. J. 2019, 375, 122076. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Dai, W.; Wei, Y.; Wang, Y.; Zhang, Y.; Zhi, L.; Huang, H.; Gao, Z. A metal-organic framework with large 1-D channels and rich OH sites for high-efficiency chloramphenicol removal from water. J. Colloid Interf. Sci. 2018, 526, 28–34. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, J.; Liu, D.; Kang, R.; Yu, G.; Deng, S. Synthesis of mixed-linker Zr-MOFs for emerging contaminant adsorption and photodegradation under visible light. Chem. Eng. J. 2019, 378, 122118. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Adsorptive removal of wide range of pharmaceuticals and personal care products from water using bio-MOF-1 derived porous carbon. Micropor. Mesopor. Mater. 2018, 270, 102–108. [Google Scholar] [CrossRef]
- Sarker, M.; An, H.J.; Yoo, D.K.; Jhung, S.H. Nitrogen-doped porous carbon from ionic liquid@Al-metal-organic framework: A prominent adsorbent for purification of both aqueous and non-aqueous solutions. Chem. Eng. J. 2018, 338, 107–116. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]
- Peng, H.; Cao, J.; Xiong, W.; Yang, Z.; Jia, M.; Sun, S.; Xu, Z.; Zhang, Y.; Cai, H. Two-dimension N-doped nanoporous carbon from KCl thermal exfoliation of Zn-ZIF-L: Efficient adsorption for tetracycline and optimizing of response surface model. J. Hazard. Mater. 2020, 402, 123498. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Bhadra, B.N.; Lee, H.J.; Jhung, S.H. Metal-organic framework-derived carbons: Preparation from ZIF-8 and application in the adsorptive removal of sulfamethoxazole from water. Catal. Today 2018, 301, 90–97. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Huang, Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Shi, S.; Fan, Y.; Huang, Y. Facile Low Temperature Hydrothermal Synthesis of Magnetic Mesoporous Carbon Nanocomposite for Adsorption Removal of Ciprofloxacin Antibiotics. Ind. Eng. Chem. Res. 2013, 52, 2604–2612. [Google Scholar] [CrossRef]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Carabineiro, S.A.; Thavorn-Amornsri, T.; Pereira, M.F.; Figueiredo, J.L. Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res. 2011, 45, 4583–4591. [Google Scholar] [CrossRef]
- Ke, Q.; Shi, Y.; Liu, Y.; Chen, F.; Wang, H.; Wu, X.L.; Lin, H.; Chen, J. Enhanced catalytic degradation of bisphenol A by hemin-MOFs supported on boron nitride via the photo-assisted heterogeneous activation of persulfate. Sep. Purif. Technol. 2019, 229, 115822. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, X.; Qu, K.; Huang, Z.; Liu, S.; Dong, M.; Guo, Z. Bimetallic metal-organic frameworks anchored corncob-derived porous carbon photocatalysts for synergistic degradation of organic pollutants. Chemosphere 2020, 259, 127389. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Luo, S.; Jing, F.; Shen, L.; Qin, N.; Wu, L. A simple strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl. Catal. B 2015, 176–177, 240–248. [Google Scholar] [CrossRef]
- He, L.; Dong, Y.; Zheng, Y.; Jia, Q.; Shan, S.; Zhang, Y. A novel magnetic MIL-101(Fe)/TiO2 composite for photo degradation of tetracycline under solar light. J. Hazard. Mater. 2019, 361, 85–94. [Google Scholar] [CrossRef]
- Liu, F.; Cao, J.; Yang, Z.; Xiong, W.; Xu, Z.; Song, P.; Jia, M.; Sun, S.; Zhang, Y.; Zhong, X. Heterogeneous activation of peroxymonosulfate by cobalt-doped MIL-53(Al) for efficient tetracycline degradation in water: Coexistence of radical and non-radical reactions. J. Colloid Interf. Sci. 2020, 581, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Ma, L.; Zhao, P.; Cao, Y.; Gao, H.; Wang, C.; Li, Q.; Dong, S.; Sun, J. Facile green synthetic graphene-based Co-Fe Prussian blue analogues as an activator of peroxymonosulfate for the degradation of levofloxacin hydrochloride. J. Colloid Interf. Sci. 2018, 526, 18–27. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Liu, C.; Luo, R.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Metal–organic framework derived Co3O4/C@SiO2 yolk–shell nanoreactors with enhanced catalytic performance. J. Mater. Chem. A 2018, 6, 11226–11235. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Zhang, H.; Yang, B.; Xiao, K.; Guo, T.; Zhang, J.; Shao, H.; Wang, Y.; Yu, G. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation. Appl. Catal. B 2018, 233, 35–45. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Wu, Y.; Jia, M.; Sun, S.; Zhou, C.; Zhang, Y.; Zhong, R. Peroxymonosulfate activation of magnetic Co nanoparticles relative to an N-doped porous carbon under confinement: Boosting stability and performance. Sep. Purif. Technol. 2020, 250, 117237. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Yang, C.; Li, X.; Lin, Y.; Yin, K.; Sun, J.; Teng, Q.; Du, C.; Zhong, Y. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation. Chem. Eng. J. 2020, 392, 123683. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation. Chem. Eng. J. 2019, 375, 122007. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Chu, L.; Wang, J. Enhancement of ionizing radiation-induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe-based MOFs. J. Hazard. Mater. 2020, 389, 122148. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, X.; Jin, P.; Dzakpasu, M.; Wang, X.C.; Zhang, Q.; Zhang, L.; Yang, L.; Ding, D.; Wang, W.; et al. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Song, W.; Ge, P.; Ke, Q.; Sun, Y.; Chen, F.; Wang, H.; Shi, Y.; Wu, X.L.; Lin, H.; Chen, J.; et al. Insight into the mechanisms for hexavalent chromium reduction and sulfisoxazole degradation catalyzed by graphitic carbon nitride: The Yin and Yang in the photo-assisted processes. Chemosphere 2019, 221, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Lei, Y.; Wei, L.; Zhai, S.; Wang, Y.; Karahan, H.E.; Chen, X.; Zhou, Z.; Wang, C.; Sui, X.; Chen, Y. Metal-free bifunctional carbon electrocatalysts derived from zeolitic imidazolate frameworks for efficient water splitting. Mater. Chem. Front. 2018, 2, 102–111. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, J.; He, Y.B.; Wang, Z.; Tanaka, S.; Hossain, M.S.A.; Pan, Z.Z.; Xiang, B.; Yang, Q.H.; Yamauchi, Y. Fabrication of an MOF-derived heteroatom-doped Co/CoO/carbon hybrid with superior sodium storage performance for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 15356–15366. [Google Scholar] [CrossRef]
- Sarker, M.; Shin, S.; Jeong, J.H.; Jhung, S.H. Mesoporous metal-organic framework PCN-222(Fe): Promising adsorbent for removal of big anionic and cationic dyes from water. Chem. Eng. J. 2019, 371, 252–259. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chem. Eng. J. 2017, 310, 197–215. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Fenton-like degradation of sulfamethoxazole using Fe-based magnetic nanoparticles embedded into mesoporous carbon hybrid as an efficient catalyst. Chem. Eng. J. 2018, 351, 1085–1094. [Google Scholar] [CrossRef]
- Hu, B.; Yuan, J.Y.; Tian, J.Y.; Wang, M.; Wang, X.; He, L.; Zhang, Z.; Wang, Z.W.; Liu, C.S. Co/Fe-bimetallic organic framework-derived carbon-incorporated cobalt-ferric mixed metal phosphide as a highly efficient photocatalyst under visible light. J. Colloid Interf. Sci. 2018, 531, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Wen, J.; Xue, Z.Z.; Yin, X.Y.; Li, Y.F.; Yuan, L. The accumulation and toxicity of ZIF-8 nanoparticles in Corbicula fluminea. J. Environ. Sci. 2023, 127, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ruyra, A.; Yazdi, A.; Espin, J.A.; Carne-Sanchez, N.; Roher, J.; Lorenzo, I.; Imaz, D. Maspoch, Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal-organic framework nanoparticles. Chemistry 2015, 21, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Hong, L.; Zheng, X.; Zhou, J.; Zhan, J.; Chen, Z.; Liu, S. Growth inhibition of Microcystic aeruginosa by metal–organic frameworks: Effect of variety, metal ion and organic ligand. RSC Adv. 2018, 8, 35314–35326. [Google Scholar] [CrossRef]










| HMs | MCLG 1 (mg/L) | MCL 2(mg/L) |
| Mercury (inorganic) | 0.002 | 0.002 |
| Lead | zero | 0.015 |
| Cadmium | 0.005 | 0.005 |
| Copper | 1.3 | 1.3 |
| Arsenic | zero | 0.05 |
| Antimony | 0.006 | 0.006 |
| Chromium (total) | 0.1 | 0.1 |
| OPs | MCLG (mg/L) | MCL (mg/L) |
| Chlorobenzene | 0.1 | 0.1 |
| Dichloromethane | zero | 0.005 |
| Ethylbenzene | 0.7 | 0.7 |
| Four Vinyl Chloride | zero | 0.005 |
| Atrazine | 0.003 | 0.003 |
| Diquat | 0.02 | 0.02 |
| Lindane | 0.0002 | 0.0002 |
| Gebutox | 0.007 | 0.007 |
| Glyphosate | 0.7 | 0.7 |
| Polychlorinated biphenyls | zero | 0.0005 |
| Xylenes | 10 | 10 |
| MOF-Based Materials | HMs | Qe (mg/g) or Removal Rate (%) | Selectivity | Reusability | Mechanism | Ref. | |
|---|---|---|---|---|---|---|---|
| Core–shell composites | Fe-BTC/PDA | Hg(II) Pb(II) | 1643 394 | Ca2+, Mg2+, Na+, K+, Sr2+, B2+ | Reusable | Electrostatic interaction Coordination, Diffusion | [47] |
| Fe3O4@Cu3(btc)2-SH | Hg(II) Pb(II) | 348.43 215.05 | Ni2+, Na+, Mg2+, Ca2+, Zn2+, Cd2+ | Reusable | Electrostatic interaction Coordination | [69] | |
| Fe3O4@TMU-32 | Hg(II) Pb(II) | 909 1607 | Cr3+, Cu2+ | Reusable | Electrostatic interaction Coordination | [31] | |
| PFe3O4@NH2-MIL-125 (Ti) | Pb(II) | 561.7 | Ag+, Be2+, Cd2+, Zn2+, Ni2+, Mn2+, Mg2+, As3+, Cr3+ | Reusable | Chemical bonding (C-N-Pb(II)-O-Fe) Coordination | [70] | |
| ZIF-8@CA | Pb(II) | 1321.21 | NO3−, SO42−, Cl− Cd2+, Cu2+, Zn2+ | Reusable | Electrostatic interaction Ion exchange | [39] | |
| melamine-MOFs | Pb(II) | 205 | Not mentioned | Reusable | Coordination | [41] | |
| MCNC@Zn-BTC | Pb(II) | 558.66 | Cu2+, Cd2+, Zn2+ | Reusable | Coordination | [48] | |
| Fe3O4@SiO2@UiO-66 Fe3O4@SiO2@UiO-66-NH2 Fe3O4@SiO2@UiO-66-Urea | Pb(II) MB MO | 102 128 219 | Not mentioned | Reusable | Electrostatic interaction Coordination | [71] | |
| NH2-MIL-101(Al)@ZIF-8 | Cu(II) | 526.74 | Li+, Na+, K+, Ca2+, Mg2+, Mn2+, Co2+, Ni2+, Hg2+, Pb2+, Cd2+ | Not mentioned | Coordination Diffusion | [42] | |
| Macro-MOF composites | PA 300 nanofibers PA 808 nanofibers | Hg(II) Pb(II) Hg(II) Pb(II) | 265.45 150.95 254.4 119.9 | Not mentioned | Reusable | Electrostatic interaction Ion exchange | [55] |
| PVA/Sb-TBC nanofibers PVA/Sr-TBC nanofibers PVA/La-TBC nanofibers | Pb(II) | 91 124 194 | Ca2+, Mg2+ | Not mentioned | Electrostatic interaction Ion exchange | [54] | |
| UiO-66-NH2@CA aerogels UiO-66@CA aerogels | Pb(II) Cu(II) Pb(II) Cu(II) | 89.40 39.33 81.30 31.23 | Not mentioned | Reusable | Electrostatic interaction Coordination | [52] | |
| ZIF-8/rGA aerogels | Pb(II) Cd(II) | 281.5 101.1 | Not mentioned | Reusable | Ion exchange Electrostatic interaction | [30] | |
| BC@ZIF-8 aerogels | Pb(II) Cd(II) | 390 220 | Pb2+, Co2+ | Reusable | Coordination Diffusion | [51] | |
| ZIF-67/BC/CH aerogels | Cu(II) Cr(VI) | 200.6 152.1 | Not mentioned | Not mentioned | Coordination Ion exchange Electrostatic interaction | [53] | |
| PDA/MOF-TFN membrane | Pb(II) Cd(II) Ni(II) | 94–99.2% | Not mentioned | Not mentioned | Electrostatic interaction Ion exchange Coordination Size exclusion | [57] | |
| IRMOF-3/GO-1 PSF@PDA@IRMOF-3/GO-1 membrane | Cu(II) | 254.14 89.3% | Na+, K+, Ca2+, Mg2+ Pb2+, Ni2+, Co2+, Fe3+ | Not mentioned | Coordination Electrostatic interaction Size exclusion | [33] | |
| f-ZIF-8@GO membrane | Cu(II) | 1872.24 | Pb2+, Co2+ | Reusable | Electrostatic interaction Coordination | [34] | |
| PAN/MOF-808 membrane | Cd(II) Zn(II) | 225.05 287.06 | Not mentioned | Reusable | Electrostatic interaction Ion exchange Size exclusion | [36] | |
| UiO-66-(COOH)2/prGO membrane | Cu(II) Cd(II) | 96.5–83.1% 92.6–80.4% | Not mentioned | Not mentioned | Electrostatic interaction Size exclusion | [35] | |
| PAA/ZIF-8/PVDF membrane | Ni(II) | 219.09 | Na+ | Reusable | Hydrogen bonding Ion exchange Coordination | [58] | |
| MOF derivatives | ZnO/ZnFe2O4/C | Pb(II) | 344.83 | Not mentioned | Reusable | Ion exchange | [66] |
| Ni@C | Pb(II) Cu(II) Cd(II) | 92.5 63.4 41.4 | Not mentioned | Reusable | van der Waals forces Diffusion | [72] | |
| MOF-Based Materials | Target HMs | Qe (mg/g) or Removal Rate (%) | Reusability | Mechanism | Ref. | |
|---|---|---|---|---|---|---|
| Core–shell composites | Fe3O4@ZIF-8 | As(III) | 100 | Reusable | Ligand exchange Chemical bonding (Zn-O-As) | [74] |
| β-MnO2@ZIF-8 | As(III) | 140.27 | Reusable | Electrostatic attraction Oxidation-adsorption Chemical bonding (As-O) | [44] | |
| MOF-NZVI | As(III) | 360.6 | Reusable | Oxidation-adsorption Chemical bonding (As-O) | [29] | |
| Fe3O4@UiO-66 | As(V) | 73.2 | Reusable | Ion exchange Chemical bonding (As-O) | [75] | |
| Fe3O4@MIL-101 | As(III) As(V) | 121.5 80.0 | Reusable | Oxidation/reduction-adsorption Chemical bonding (As-O) | [76] | |
| CoFe2O4@MIL-100(Fe) | As(III) As(V) | 143.6 114.8 | Not mentioned | Ion exchange Chemical bonding (As-O) Hydrogen bonding | [77] | |
| MIL-100(Fe) based filters | As(III) As(V) | 90% 100% | Reusable | Oxidation-adsorption Fenton-like reaction | [78] | |
| MOF derivatives | NiOx/Ni@C 300 400 500 600 Ni-MOF | As(V) | 210.40 454.94 290.89 342.77 133.93 | Reusable | Electrostatic interaction Chemical bonding (As-O) | [79] |
| Core–shell composites | MOR-1-HA | Cr(VI) | 259 | Reusable | Electrostatic interaction Ion exchange Coordination | [45] |
| MP@ZIF-8 | Cr(VI) | 136.56 | Reusable | Electrostatic interaction Adsorption-reduction Coordination | [46] | |
| CeO2@UiO-66-(SH)2 CeO2/Fe3O4@UiO-66-(SH)2 | As Cd(II) Cr Cu(II) Pb(II) Hg(II) | 56% 87% 93% 99% 99% 98% | Reusable | Electrostatic interaction Coordination Chemical bonding (Zr-O-As) | [32] | |
| BUC-21/TNTs | Cr(VI) | 100% (20 min) | Reusable | Ion exchange Electrostatic interaction Photocatalysis-reduction Adsorption-reduction | [80] | |
| ZnO@ZIF-8 | Cr(VI) MB | 88% (240 min) | Not mentioned | Electrostatic interaction Photocatalysis-reduction Diffusion Adsorption-reduction | [50] | |
| MWCNT/NH2-MIL-68 | Cr(VI) | 100% (120 min) | Reusable | Photocatalysis-reduction Diffusion Adsorption-reduction | [38] | |
| M@MIL-100(Fe) | Cr(VI) | 100% (8 min) | Reusable | Photocatalysis-reduction Adsorption-reduction | [81] | |
| Fe3O4@UiO-66@UiO-67/CTAB | Cr(VI) | 932.1 | Reusable | Electrostatic interaction Hydrogen bonding van der Waals forces | [43] | |
| MOF derivatives | PANI@NC | Cr(VI) | 198.04 | Reusable | Adsorption Coordination-reduction | [63] |
| Ni@N-CNTs/NG | Cr(VI) | 72.325 | Reusable | Electrostatic interaction Diffusion Adsorption-reduction (HCOOH) | [82] | |
| P-Fe2O3 | Cr(VI) Pb(II) Cu(II) Co(II) | 175.5 97.8 66.2 60.4 | Not mentioned | Electrostatic interaction Ion exchange Coordination | [62] | |
| Fe0.72(0) Fe2.28(II)C | Cr(VI) | 354.6 | Reusable | Adsorption-reduction | [83] | |
| NZVI@ZD | Cr(VI) | 226.5 | Not mentioned | Electrostatic interaction Diffusion Adsorption-reduction | [84] |
| MOF-Based Materials | MOF Precursors | Removal Type | Organic Dyes | Performance | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| MOF composites | |||||||
| GO/MOFs | Ni-MOF | Adsorption | MB | 274 mg/g for MB | It could be used at least five times after washing with ethanol. | Electrostatic interaction and acid–base interaction | [101] |
| Ni-MOF/GO | Ni-MOF | Adsorption | CR | 2489 mg/g for CR | Not mentioned | Electrostatic interaction, acid–base interaction | [102] |
| MFC-N | UiO-66 | Adsorption | MB | 128 mg/g for MB | There was no obvious loss of MFC adsorption capacity after 6 cycles. | Electrostatic attraction interaction | [71] |
| MFC-O | MO | 219 mg/g for MO | |||||
| Magnetic NH2-MIL-101(Al) | MIL-101(Al) | Adsorption | MG IC | 274.4 mg/g for MG 135 mg/g for IC | The removal rate of MG and IC decreased slightly after 5 cycles. | Electrostatic interaction, π–π stacking interaction and hydrogen bonding | [97] |
| POM@MOF | Cu3(BTC)2 | Adsorption | MB | 77.22 mg/g for MB | Not mentioned | Electrostatic attraction | [103] |
| Cu-MOFs/Fe3O4 | Cu-MOFs | Adsorption | MG | 113.67 mg/g for MG | MG removal rate could still reach 90% after 5 cycles. | Physical adsorption | [99] |
| MZIF-67 | ZIF-67 | Adsorption | MO | 738 mg/g for MO | Not mentioned | Electrostatic interaction | [98] |
| Fe3O4@MIL-100(Fe) | MIL-100(Fe) | Adsorption | MB | 73.8 mg/g for MO | Adsorption did not decrease significantly after 5 cycles. | Not mentioned | [104] |
| Fe3O4@MIL-100(Fe) | MIL-100(Fe) | Photocatalytic degradation | MB | 99.77% photodegradation for MB within 200 min | The photocatalytic activity had no obvious loss after repeated use. | Photogenerated holes (h+), photoelectron transfer, ·OH | [105] |
| Fe3O4@MIL-101(Fe) | MIL-101(Fe) | PMS degradation | AO7 | Degraded completely within 60 min | The removal rate of AO7 ranged from 98.1% to 95.0% in 3 cycles. | , ·OH, ·O2− | [106] |
| UiO-66/g-C3N4 | UiO-66 | Photocatalytic degradation | MB | 100% photodegradation for MB within 240 min | Not mentioned | ·O2− | [107] |
| ZIF-67/PAN | ZIF-67 | PMS degradation | AY | 95.1% degradation rate within 10 min | The catalytic effect remained stable (above 98%) after 5 cycles. | [108] | |
| ZIF@R | ZIF-67 | PMS degradation | RhB | Removed completely within 20 min | No loss of catalytic activity of ZIF@R after 5 cycles. | and ·OH | [109] |
| ZIF-9@GEL | ZIF-9 | PMS degradation | RhB | 99% degradation rate within 10 min | The degradation performance did not decrease significantly (about 90%) after 3 cycles. | and ·OH | [110] |
| ZIF-12@GEL | ZIF-12 | ||||||
| MOF derivatives | |||||||
| Ni@C | Ni-MOF | Adsorption | RhB | Almost 99% adsorption for RhB within 10 min | Not mentioned | van der Waals forces, hydrogen bonding | [72] |
| Ni/PC-CNT | Ni/Zn-MOF | Adsorption | MG CR | 898 mg/g for MG 818 mg/g for CR | The adsorption rate of Ni/PC-CNT for MG and CR remained above 85%. | π–π interaction and electrostatic interaction | [111] |
| MWCNTs | Co-MOF | Adsorption | CR | 1639 mg/g for CR | Not mentioned | Hydrogen-bonding interactions, π–π stacking interactions and the effect of mesopores | [112] |
| Carbon-ZD | ZIF-8 | Adsorption | MB | 1148.2 mg/g for MB | The adsorption efficiency of the Carbon-ZD for MB was still very high after 5 cycles. | Nitrogen doping and electrostatic interaction | [100] |
| Carbon-ZS | 791.3 mg/g for MB | ||||||
| Carbon-Z | 505.3 mg/g for MB | ||||||
| Co-BiFeO3 | PABs | Photocatalytic degradation | MO | Nearly 89.8% degradation rate in 120 min | The degradation rates in 4 cycles were 89.8%, 86.3%, 83.5% and 81.4%, respectively. | A larger range of light response and more oxygen vacancies | [80] |
| NPCs | ZIF-8 | PMS degradation | MO RhB | 100% MO and 90% RhB were removed by NPC/PMS within 60 min. | Not mentioned | Excellent electron transfer ability of graphite nitrogen | [113] |
| NH2-MIL-53 | |||||||
| IRMOF-3 | |||||||
| NPC-800 | ZIF-8 | PMS degradation | RhB | The degradation rate of RhB was 85.0%. | The removal rate of RhB decreased slightly from 85.0% to 68.8% after 3 cycles. | and ·OH | [114] |
| PNC-800 | Zn-Co PBAS | PMS degradation | MB RhB OII | The degradation rate was 100%, 92.8 and 93.2%, respectively. | PNC-800 has good catalytic stability after 3 cycles. | Non-radical process | [115] |
| MCCI | Co/Fe-MOF | PMS degradation | RhB | 80% degradation rate within 30 min | The catalytic effect remained good after 6 cycles. | [116] | |
| Fe3O4@C/Cu | HKUST-1 | Photocatalytic degradation | MB | Completely removed within 150 min | The photocatalytic activity had no obvious loss after 5 cycles | Photoelectron transfer and ·OH | [117] |
| MCG | ZIF-67 | PMS degradation | AY | Completely decolorized within 30 min | The removal rate remained 97.6% after 50 cycles. | and ·OH | [118] |
| MOF-Based Materials | MOF Precursors | Qe (mg/g) | Adsorption Thermodynamics | Adsorption Kinetics | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| MOF composites | |||||||
| Fe3O4@MIL-100(Fe) | MIL-100(Fe) | CIP 278.39 | Langmuir model | Elovich model and pseudo-second-order model | Not mentioned | Chemisorption and physical adsorption | [144] |
| Fe3O4@MOF-235(Fe) | MOF-235(Fe) | CIP 187.48 | Langmuir model | Elovich model | Not mentioned | Physical adsorption | [144] |
| MIL-101/Fe3O4 | MIL-101(Cr) | CIP 63.28 | Langmuir and Freundlich models | Pseudo-second-order model | Not mentioned | Film diffusion and intraparticle diffusion | [145] |
| MWCNT/MIL- 53(Fe) | MIL-53(Fe) | TCN 363.37 OTC 325.59 CTC 180.68 | Langmuir model | Pseudo-second-order model | The adsorption of TCs did not change obviously after 4 cycles. | π–π interaction, pore/size-selective adsorption and influence of metal ions | [146] |
| UIO-66-(OH)2/GO | UIO-66 | TC 37.96 | Freundlich model | Pseudo-second-order model | Not mentioned | Electrostatic interaction, π–π interaction, hydrogen bonding and acid–base interaction | [147] |
| UIO-66/CA | UIO-66 | LOFX 86.43 | Langmuir model | Pseudo-second-order model | The adsorption efficiency of LOFX was still above 70% even after 5 cycles. | Not mentioned | [148] |
| Al-MOF/SA-CS | Al-MOF | BPA 136.9 | Freundlich model | Pseudo-second-order model | The adsorption efficiency of BPA remained above 96% after 5 cycles. | π–π stacking, hydrogen bonding and cation–π interaction | [149] |
| NFe3O4@Zn(GA)/Starch-Hydrogel | Zn-MOF | FLV 782.05 | Langmuir model | Pseudo-second-order model | The adsorption capacity decreased to 700.09 mg/g after 5 cycles. | Not mentioned | [150] |
| Ni/Co-MOF@CMC aerogel | Ni/Co-MOF | TC 624.87 | Langmuir model | Pseudo-second-order model | Not mentioned | Surface hydroxyl interaction, complexation of metal ions and oxygen | [151] |
| MOF derivatives | |||||||
| PCN-222 | Zr-MOFs | CAP 379 | Not mentioned | pseudo-second-order model | Not mentioned | H-bond interaction, electrostatic interaction and the special pore structure of PCN-222 | [152] |
| PCN-134 | Zr-MOFs | DF 604.1 | Langmuir model | Pseudo-second-order model. | The removal rate was above 95% after 7 cycles. | Not mentioned | [153] |
| CDMs | MAF-6 | IBP 408 DCF 503 | Langmuir model | Pseudo-second-order model. | The adsorption decreased slightly after 1 cycle, but basically unchanged from 2 to 5 cycles. | van der Waals and hydrophobic interactions | [78] |
| BMDCs | bio-MOF-1 | ATLN 522 CLFA 540 | Langmuir model | Pseudo-second-order model | The adsorption of ATLN did not decrease appreciably after 4 cycles. | ATLN: Electrostatic interactions CLFA: H-bonding and electrostatic interactions | [154] |
| CDIL@AlPCP | Al-MOF | PCMX 338 TCS 326 | Langmuir model | Pseudo-second-order model | The adsorption efficiency did not decrease seriously with the increase in the number of cycles. | H-bonding | [155] |
| Co3S4 | ZIF-67 | CIP 471.7 | Langmuir model | Pseudo-second-order model and liquid-film diffusion model | There was no obvious loss in CIP removal after recycling five times. | Electrostatic interaction | [156] |
| NC-800 | Zn-ZIF-L | TC 347.06 | Langmuir model | Pseudo-second-order model | The adsorption of TC could maintain a high level after 4 cycles. | Electrostatic interaction and hydrogen bond interaction | [157] |
| MDC-1000 | ZIF-8 | SMX 435 | Langmuir model | Pseudo-second-order model | The adsorption capacity decreased slightly after 1 cycle, but was unchanged from 2 to 4 cycles. | H-bonding | [158] |
| NPC | ZIF-8 | CIP 416.7 | Freundlich model | Pseudo-second-order model | The adsorption of CIP had no obvious loss after 7 cycles. | Electrostatic interactions, hydrophobic interactions | [159] |
| MOF-Based Materials | MOF Precursors | Catalytic Type | PPCPs | Degradation Rate | Reusability | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| MOF composites | |||||||
| Cu-hemin MOFs/BN | Cu-MOF | Photo-PS degradation | BPA | 99% within 30 min | The degradation rate of BPA dropped to 78% in the third cycle. | [164] | |
| MOF/CCAC | Fe/Ni-MOF | Photocatalytic degradation | TC | 98% within 75 min | Photocatalysts were well-reused in ten on-off cycles. | ·O2− and h+ | [165] |
| ZIF-9@GEL | ZIF-9 | PMS degradation | TC | 90% within 1 h | The degradation performance did not decrease significantly (about 90%) after 3 cycles. | and ·OH | [110] |
| ZIF-12@GEL | ZIF-12 | ||||||
| Pd@MIL- 100(Fe) | MIL-100(Fe) | Photocatalytic degradation | TEH IBP BPA | 99.5% within 150 min 100% within 150 min 70% within 240 min | The photocatalytic activity did not obviously decrease after 4 cycles. | Photogenerated electron and ·OH | [166] |
| MIL-100(Fe)/TiO2 | MIL-100(Fe) | Photocatalytic degradation | TC | 92.76% within 10 min | Degradation rate of TC was similar after 5 cycles. | ·O2− and ·OH | [167] |
| Co-MIL- 53(Al) | MIL-53(Al) | PMS degradation | TC | 94.0% within 120 min | Co-MIL-53(Al) showed good activity after 4 cycles. | and 1O2 | [168] |
| Co-Fe PBAs@rGO | Co-MOF | PMS degradation | LVF | 97.6% after 60 min | Degradation rate exhibited no significant decrease after 5 cycles. | [169] | |
| MOF derivatives | |||||||
| YSCCSs | ZIF-67 | PMS degradation | BPA | 99.1% within 23 min | The degradation rate of BPA exceeded 90% after 7 cycles. | and ·OH | [170] |
| ZIF-CN/ g-C3N4 | ZIF-67 | Photo-PMS degradation | BPA | 97% after 60 min | The degradation rate only decreased by 8% after 7 cycles. | and ·OH | [171] |
| Co@NC-800 | ZIF-67 | PMS degradation | TC | 91.2% within 5 min | The degradation rate hardly decreased after 4 cycles. | , ·O2− and 1O2 | [172] |
| Fe-N/C | ZIF-8 | PMS degradation | BPF | 97.1% within 90 min | The removal rate decreased to 94.9%, 61.3%, and 42.1% in 3 cycles. | 1O2 | [173] |
| FeCu@C | [Fe, Cu] -BDC | Fenton-like degradation | SMT | 100% within 90 min | Not mentioned | π−π interaction, ·OH and surface hydroxyl groups | [174] |
| DMOFs | MIL-100(Fe) | Ionizing radiation degradation | CEP-C SM | 100% removal for CEP-C 95% removal for CEP-C | The removal rate of CEP-C and SMT decreased to 94% and 76% after 3 cycles, respectively. | ·OH | [175] |
| CoFe2O4 NC | Co/Fe bi-MOFs | PMS degradation | BPA | Over 97% within 90 min | Catalytic capacity dropped significantly after the first cycle and could be restored after 400 °C calcination for 15 min. | and ·OH | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xue, Z.; Wen, J. Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities. Sustainability 2023, 15, 6686. https://doi.org/10.3390/su15086686
Yang C, Xue Z, Wen J. Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities. Sustainability. 2023; 15(8):6686. https://doi.org/10.3390/su15086686
Chicago/Turabian StyleYang, Cuilian, Zhuangzhuang Xue, and Jia Wen. 2023. "Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities" Sustainability 15, no. 8: 6686. https://doi.org/10.3390/su15086686
APA StyleYang, C., Xue, Z., & Wen, J. (2023). Recent Advances in MOF-Based Materials for Remediation of Heavy Metals and Organic Pollutants: Insights into Performance, Mechanisms, and Future Opportunities. Sustainability, 15(8), 6686. https://doi.org/10.3390/su15086686







