Synthesis, Stability and Microstructure of a One-Step Mixed Geopolymer Backfill Paste Derived from Diverse Waste Slags
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
- (1)
- Low-calcium silica-alumina precursors
- (2)
- High-calcium-based slags
- (3)
- Additives for backfill paste
- (4)
- Cement material comparison
- (5)
- Sodium hydroxide pellet and water
2.2. Mixing Design and Sample Preparation
2.2.1. Sample Preparation Method
2.2.2. Mixing Ratio Design
- (1)
- Twelve samples for self-hardening
- (2)
- Nine samples for mixing ratio optimization
- (3)
- Three samples for properties comparison and microstructural characterization
2.3. Testing Methods
2.3.1. Determination of Fresh and Hardened Properties
Measurement of pH and EC Values
Measurement of Fluidity
Measurement of Setting Time
Measurement of Water Absorption
Measurement of Bulk Density
Determination of Unconfined Compressive Strength (UCS)
Determination of Drying Shrinkage
Determination of Permeability Coefficient
Characterizations of Na2SO4 and NaCl Solution Attacks
2.3.2. Microstructures, Products, and Thermal Stabilities of Hardened Pastes
3. Results and Discussion
3.1. Self-Hardening and Alkali Seepage of Pastes
3.2. Effects of Raw Materials on Fresh and Hardened Properties
3.3. Performance Assessment of Shrinkage, Permeability, and Chemical Attack
3.3.1. Determination of the Optimal Mixing Proportion of Backfill Paste
3.3.2. Drying Shrinkage Analysis
3.3.3. Permeability Coefficient Analysis
3.3.4. Changes in Mass and UCS under Sulfate and Chloride Attack
3.4. Morphology and Microstructure Analysis of Hardened Paste by SEM-EDS
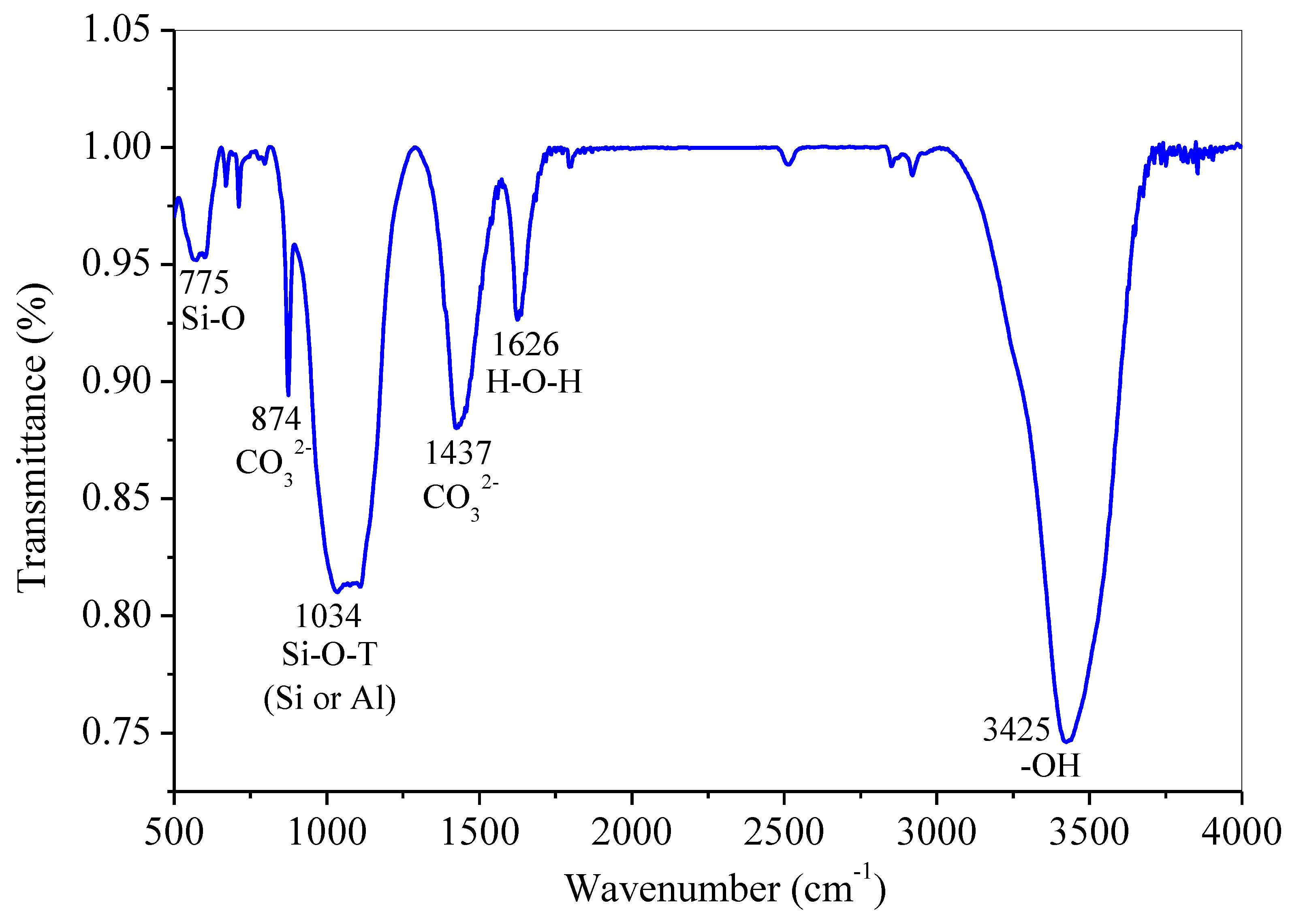
3.5. Mineral Components and Chemical Products Analysis of Hardened Paste by XRD and FTIR
→(Na+,K+,Ca2+) + m(OH)3-Si-O-Al−(OH)2-O-Si-(OH)3
→4mH2O + (Na+,K+,Ca2+)-[Si(OH)2-O-Al−(OH)2-O-Si-(OH)3-O-]m [(N,C)-A-S-H]
3.6. Thermal Decomposition by TG-DSC Analysis
3.7. Discussion
4. Conclusions
- (1)
- Direct synthesis of geopolymer paste for goaf backfill from a variety of solid wastes (FFAI, FFAII, SR, CSG, GBFS, RM, and BRS) and additives (LP, and GP) is possible with the addition of an adequate amount of water. The paste used as backfill hardens well and has no environmental impact on surrounding soils and water due to the seepage of less alkalinity. Even though the fresh and hardened characteristics of the new backfill paste are affected by the amounts of FFAI, RM, GBFS, and LP used, the paste can be adjusted to meet the requirements of goaf backfill because of its physical and chemical characteristics.
- (2)
- With a W/B of 0.70, the optimal mass mixing ratio of new backfill paste P1 with different solid wastes is 1:4:1:2:1:1:1:1:2 (FFAI:FFAII:RM:CSG:SR:GBFS:BRS:GP:LP). Solid wastes (SR, CSG, GBFS, FFAI, FFAII, RM, and BRS) account for 46.0% by mass. Paste P1 has a 28-day UCS of 2.1 MPa and fluidity of 201 mm. The initial setting time was 920 min and the final setting time was 1220 min. In addition, after 90 days in terms of drying shrinkage, permeability, and Na2SO4 and NaCl solution attack, paste P1 was superior to the OPC paste.
- (3)
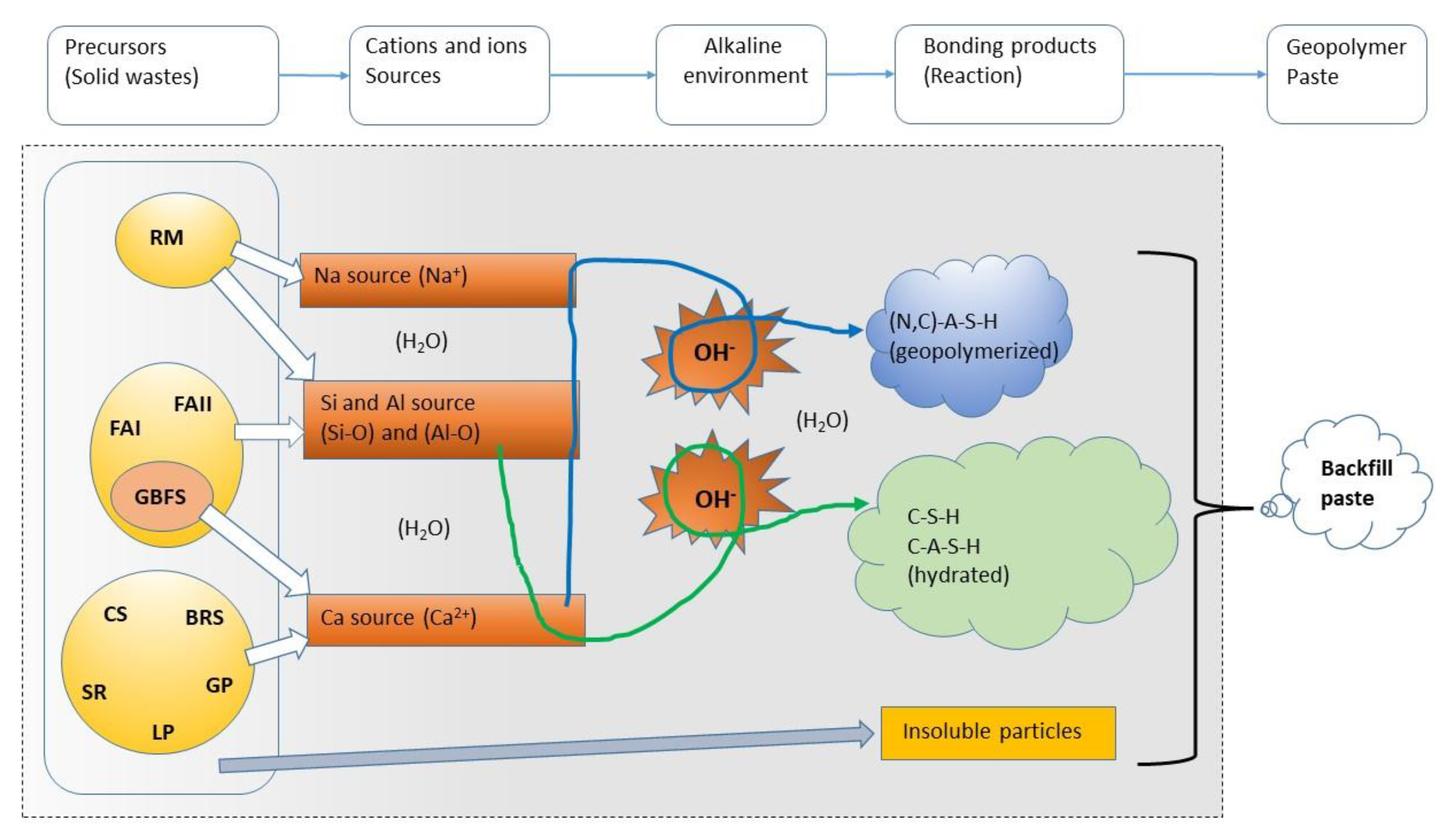
- In alkaline circumstances, FFAI, FFAII, GBFS, and RM provide SiO2 and Al2O3 precursors, while CSG, SR, GBFS, BRS, LP, and GP supply calcium sources such as CaO, Ca(OH)2, and CaCl2, among others. Furthermore, LP, RM, and GBFS raise the pH. The presence of (N,C)-A-S-H, C-S-H, and C-A-S-H gels from geopolymerization and hydration reactions in paste P1 is confirmed to play a crucial role in chemical cementation to support mechanical strength at a high pH of 12.37, while other unreacted particles (CaCO3 and unreactive FA particles, etc.) act as the physical filling aggregate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.-Y.; Han, R.; Yu, B.; Wei, Y.-M. Accounting process-related CO2 emissions from global cement production under Shared Socioeconomic Pathways. J. Clean. Prod. 2018, 184, 451–465. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y. Use of carbide slag from acetylene industry for activation of ground granulated blast-furnace slag. Constr. Build. Mater. 2020, 238, 117713. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. Earth Syst. Sci. Data 2019, 10, 2213–2239. [Google Scholar] [CrossRef]
- Cai, B.; Wang, J.; He, J.; Geng, Y. Evaluating CO2 emission performance in China’s cement industry: An enterprise perspective. Appl. Energy 2016, 166, 191–200. [Google Scholar] [CrossRef]
- Guo, S.; Ma, C.; Long, G.; Xie, Y. Cleaner one-part geopolymer prepared by introducing fly ash sinking spherical beads: Properties and geopolymerization mechanism. J. Clean. Prod. 2019, 219, 686–697. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Mann, D.; Lumsden, K.; Tao, M. Durability of red mud-fly ash based geopolymer and leaching behavior of heavy metals in sulfuric acid solutions and deionized water. Constr. Build. Mater. 2016, 124, 373–382. [Google Scholar] [CrossRef]
- Qi, J.M.; Zhou, P.X.; Yang, D.; Wang, Z.X.; Li, B. Desulphurization mechanism and engineering practice of carbide slag. Environ. Sci. Pollut. R. 2022, 29, (59), 88519–88530. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Zhou, B.; Gao, H.; Lin, Y. Resistance of Soda Residue-Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments. Materials 2021, 14, 785. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Liu, Y.; Zhou, B. Synthesis and characterization of fly ash geopolymer paste for goaf backfill: Reuse of soda residue. J. Clean. Prod. 2020, 260, 121045. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Xu, Z.; Zhang, Z.; Zhang, C.; Bai, Y.; Zhao, Q. Mechanical performance and microstructure improvement of soda residue–carbide slag–ground granulated blast furnace slag binder by optimizing its preparation process and curing method. Constr. Build. Mater. 2021, 302, 124403. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, B.; Guo, S.; Long, G.; Xie, Y. Properties and characterization of green one-part geopolymer activated by composite activators. J. Clean. Prod. 2019, 220, 188–199. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Thumrongvut, J.; Seangatith, S.; Phetchuay, C.; Suksiripattanapong, C. Comparative Experimental Study of Sustainable Reinforced Portland Cement Concrete and Geopolymer Concrete Beams Using Rice Husk Ash. Sustainability 2022, 14, 9856. [Google Scholar] [CrossRef]
- Suksiripattanapong, C.; Krosoongnern, K.; Thumrongvut, J.; Sukontasukkul, P.; Horpibulsuk, S.; Chindaprasirt, P. Properties of cellular lightweight high calcium bottom ash-portland cement geopolymer mortar. Case Stud. Constr. Mater. 2020, 12, e00337. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T. Shear Bond Strength of Fa-Pc Geopoylmer under Different Sand to Binder Ratios and Sodium Hydroxide Concentrations. Int. J. GEOMATE 2018, 14, 52–57. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.; Huang, B.; Shu, X.; He, Q. Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, A.G.; Lyu, B.C.; Liu, K.W.; Chu, Y.J.; Ma, R.; Xu, H.Y.; Jing, Y.; Sun, D.S. Proportioning Factors of Alkali-Activated Materials and Interaction Relationship Revealed by Response Surface Modeling. Materials 2023, 16, 2042. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Singh, N.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Guo, W.C.; Zhao, Q.X.; Sun, Y.J.; Xue, C.H.; Bai, Y.Y.; Shi, Y.X. Effects of various curing methods on the compressive strength and microstructure of blast furnace slag-fly ash-based cementitious material activated by alkaline solid wastes. Constr. Build. Mater. 2022, 357, 129397. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rahman, M.K.; Najamuddin, S.K.; Alhelal, Z.S.; Acero, C.E. A review on utilization of industrial by-products in the production of controlled low strength materials and factors influencing the properties. Constr. Build. Mater. 2022, 325, 126704. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Wang, T.; Su, L.; Zhou, B.; Lin, Y.; Mohan, S. Determination of Gel Products in Alkali-Activated Fly Ash-Based Composites Incorporating Inorganic Calcium Additives. Adv. Mater. Sci. Eng. 2022, 2022, 7476671. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Xiao, B.; Huang, Q. Synthesis and strength optimization of one-part geopolymer based on red mud. Constr. Build. Mater. 2016, 111, 317–325. [Google Scholar] [CrossRef]
- National Development and Reform Commission of China. Implementing Scheme of Mainly Solid Waste Utilization; Beijing, China. 2011. Available online: https://www.docin.com/p-1851492371.html (accessed on 20 December 2022).
- Shang, J.; Dai, J.-G.; Zhao, T.-J.; Guo, S.-Y.; Zhang, P.; Mu, B. Alternation of traditional cement mortars using fly ash-based geopolymer mortars modified by slag. J. Clean. Prod. 2018, 203, 746–756. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- Haruna, S.; Fall, M. Time- and temperature-dependent rheological properties of cemented paste backfill that contains superplasticizer. Powder Technol. 2020, 360, 731–740. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T. The setting time of a clay-slag geopolymer matrix: The influence of blast-furnace-slag addition and the mixing method. J. Clean. Prod. 2016, 112, 1150–1155. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Huang, B.; Shu, X.; He, Q. Synergistic utilization of red mud for flue-gas desulfurization and fly ash-based geopolymer preparation. J. Hazard. Mater. 2019, 369, 503–511. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Zhou, B.; Lin, Y.; Gao, H. Performance Optimization and Characterization of Soda Residue-Fly Ash Geopolymer Paste for Goaf Backfill: Beta-Hemihydrate Gypsum Alternative to Sodium Silicate. Materials 2020, 13, 5604. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Zhu, Q.; Ma, W.; Liu, Y. Preparation and characterization of press-formed fly ash cement incorporating soda residue. Mater. Lett. 2020, 259, 126852. [Google Scholar] [CrossRef]
- He, K.; Shen, Z.; Zhang, B.; Sun, J.; Zou, H.; Zhou, M.; Zhang, Z.; Xu, H.; Ho, S.S.H.; Cao, J. Emission profiles of volatile organic compounds from various geological maturity coal and its clean coal briquetting in China. Atmos. Res. 2022, 274, 106200. [Google Scholar] [CrossRef]
- Silva, D.; Filleti, R.; Musule, R.; Matheus, T.; Freire, F. A systematic review and life cycle assessment of biomass pellets and briquettes production in Latin America. Renew. Sustain. Energy Rev. 2022, 157, 112042. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Injorhor, B.; Phoo-Ngernkham, T.; Damrongwiriyanupap, N.; Li, L.-Y.; Sukontasukkul, P.; Chindaprasirt, P. Drying shrinkage, strength and microstructure of alkali-activated high-calcium fly ash using FGD-gypsum and dolomite as expansive additive. Cem. Concr. Compos. 2020, 114, 103760. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Compos. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-Ngernkham, T.; Li, L.-Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Niroshan, N.; Sivakugan, N.; Veenstra, R.L. Laboratory Study on Strength Development in Cemented Paste Backfills. J. Mater. Civil Eng. 2017, 29, 4017027. [Google Scholar] [CrossRef]
- Li, H.; Wu, A.; Wang, H. Evaluation of short-term strength development of cemented backfill with varying sulphide contents and the use of additives. J. Environ. Manag. 2019, 239, 279–286. [Google Scholar] [CrossRef] [PubMed]
- JC/T 603-2004; Standard Test Method for Drying Shrinkage of Mortar. 2004. Available online: http://www.cssn.net.cn/app/home/productDetail/a8105c02d10d9a7a92a9fef608f6879d (accessed on 14 February 2023). (In Chinese)
- Sun, W.; Wang, H.J.; Hou, K.P. Control of waste rock-tailings paste backfill for active mining subsidence areas. J. Clean. Prod. 2018, 171, 567–579. [Google Scholar] [CrossRef]
- Chi, M. Effects of dosage of alkali-activated solution and curing conditions on the properties and durability of alkali-activated slag concrete. Constr. Build. Mater. 2012, 35, 240–245. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, S.; Sun, Q.; Li, B.; Cai, C.; Xia, Y.; Wei, X.; Mu, Q. Preparation and microstructure of fly ash geopolymer. J. Clean. Prod. 2019, 225, 376–390. [Google Scholar] [CrossRef]
- Kirkelund, G.M.; Magro, C.; Guedes, P.; Jensen, P.E.; Ribeiro, A.B.; Ottosen, L.M. Electrodialytic removal of heavy metals and chloride from municipal solid waste incineration fly ash and air pollution control residue in suspension—Test of a new two compartment experimental cell. Electrochim. Acta 2015, 181, 73–81. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Elmoaty, A.E.M.A.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Ai, T.; Huang, B.; Shu, X.; He, Q. Strength properties of geopolymers derived from original and desulfurized red mud cured at ambient temperature. Constr. Build. Mater. 2016, 125, 905–911. [Google Scholar] [CrossRef]
- Lee, N.; Jang, J.; Lee, H. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Early strength development and hydration of alkali-activated blast furnace slag/fly ash blends. Adv. Cem. Res. 1999, 11, 189–196. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. Drying Shrinkage of Slag Blended Fly Ash Geopolymer Concrete Cured at Room Temperature. Procedia Eng. 2015, 125, 594–600. [Google Scholar] [CrossRef]
- Sadeghian, G.; Behfarnia, K.; Teymouri, M. Drying shrinkage of one-part alkali-activated slag concrete. J. Build. Eng. 2022, 51, 104263. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.; Cheng, Y.-B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Tong, K.T. Mechanical and durability properties of alkali-activated fly ash concrete with increasing slag content. Constr. Build. Mater. 2021, 301, 124330. [Google Scholar] [CrossRef]
- Wang, W.-C.; Wang, H.-Y.; Lo, M.-H. The fresh and engineering properties of alkali activated slag as a function of fly ash replacement and alkali concentration. Constr. Build. Mater. 2015, 84, 224–229. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, K.; Wang, X. A binder prepared by low-reactivity blast furnace slags for cemented paste backfill: Influence of super-fine fly ash and chemical additives. Constr. Build. Mater. 2022, 327, 126988. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Zhai, Q.; Kurumisawa, K. Effect of accelerators on Ca(OH)2 activated ground granulated blast-furnace slag at low curing temperature. Cem. Concr. Compo. 2021, 124, 104272. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr. Build. Mater. 2016, 106, 500–511. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Wang, J.; He, L.; Zhang, A.; Gao, H.; Yang, J.; Liang, L. Properties and Cementation Mechanism of Geopolymer Backfill Paste Incorporating Diverse Industrial Solid Wastes. Materials 2023, 16, 480. [Google Scholar] [CrossRef] [PubMed]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Haha, M.B.; LE Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Ming, F.; Yang, C.; Chen, L.; Liu, Y. Influence of low-temperature curing on the mechanical strength, hydration process, and microstructure of alkali-activated fly ash and ground granulated blast furnace slag mortar. Constr. Build. Mater. 2021, 269, 121811. [Google Scholar] [CrossRef]
- Shah, S.F.A.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Improvement of early strength of fly ash-slag based one-part alkali activated mortar. Constr. Build. Mater. 2020, 246, 118533. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 2010, 82, 742–748. [Google Scholar] [CrossRef]
- Fall, M.; Célestin, J.C.; Pokharel, M.; Touré, M. A contribution to understanding the effects of curing temperature on the mechanical properties of mine cemented tailings backfill. Eng. Geol. 2010, 114, 397–413. [Google Scholar] [CrossRef]
- Petlovanyi, M.; Mamaikin, O. Assessment of an expediency of binder material mechanical activation in cemented rockfill. ARPN J. Eng. Appl. Sci. 2019, 14, 3492–3503. [Google Scholar]
- Petlovanyi, M.V.; Zubko, S.A.; Popovych, V.V.; Sai, K.S. Physicochemical mechanism of structure formation and strengthening in the backfill massif when filling underground cavities. Vopr. Khimii I Khimicheskoi Tekhnologii 2020, 6, 142–150. [Google Scholar]
- Fernandez-Jimenez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]











| Materials | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | SO3 | Na2O | P2O5 | TiO2 | Cl− | Other | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFAI | 53.6 | 33.5 | 2.8 | 0 | 1.6 | 1.5 | 0.9 | 0 | 0.4 | 1.3 | 0 | 1.5 | 3 |
| FFAII | 46.1 | 23.2 | 5.4 | 8 | 2.6 | 1.8 | 0.8 | 0 | 0.6 | 0.3 | 0 | 3.5 | 7.6 |
| RM | 27.5 | 28.4 | 2.5 | 25.8 | 0.2 | 0.1 | 0.8 | 14.7 | 0 | 0 | 0 | 0 | 0 |
| CSG | 8.7 | 0.5 | 62.6 | 1 | 1.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25.4 |
| BRS | 14.7 | 1.2 | 72.6 | 1.1 | 0 | 0 | 1.4 | 0 | 0 | 0 | 0 | 4.7 | 4.3 |
| SR | 9.2 | 8.7 | 36.4 | 3.4 | 6.8 | 0.3 | 5.5 | 3.9 | 0 | 0.1 | 23.1 | 0 | 2.8 |
| GBFS | 35.1 | 16.2 | 33.6 | 0 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 4.1 | 0 |
| GP | 3 | 3.6 | 30.5 | 0 | 0.3 | 0 | 40.6 | 0.3 | 0 | 0 | 2.3 | 0 | 19.5 |
| LP | 0.1 | 0 | 71.2 | 0 | 1.8 | 0 | 1.6 | 0 | 0 | 0 | 0 | 0 | 25.3 |
| OPC | 21.8 | 5.7 | 65.3 | 3.5 | 0 | 0 | 2.8 | 0 | 0 | 0 | 0 | 0 | 0.9 |
| Materials | pH (−) | EC (µS/cm) | SSA (m2/kg) | SG (−) |
|---|---|---|---|---|
| FFAI | 10.24 | 1075.0 | 640 | 2.48 |
| FFAII | 8.30 | 2750.0 | 390 | 2.25 |
| RM | 10.52 | 2180.0 | 360 | 2.56 |
| CSG | 8.55 | 2700.0 | 420 | 1.80 |
| BRS | 10.07 | 1632.0 | 345 | 1.10 |
| SR | 9.32 | 3710.0 | 307 | 2.35 |
| GBFS | 11.45 | 440.0 | 660 | 2.67 |
| GP | 8.25 | 4.6 | 300 | 2.98 |
| LP | 12.86 | 7580.0 | 320 | 1.10 |
| OPC | 12.87 | 8970.0 | 370 | 2.89 |
| No. | Binders (g) | Water (g) | W/B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSG | SR | FFAII | FFAI | GBFS | LP | RM | BRS | GP | |||
| D01 | 100 | 50 | 200 | 50 | 50 | 100 | 50 | 100 | 50 | 200 | 0.27 |
| D02 | 100 | 100 | 200 | 100 | 100 | 100 | 100 | 100 | 100 | 200 | 0.20 |
| D03 | 200 | 100 | 200 | 100 | 100 | 100 | 100 | 100 | 100 | 200 | 0.18 |
| D04 | 200 | 100 | 200 | 200 | 100 | 100 | 100 | 100 | 100 | 200 | 0.17 |
| D05 | 200 | 100 | 200 | 100 | 200 | 100 | 100 | 100 | 100 | 200 | 0.17 |
| D06 | 200 | 100 | 200 | 100 | 200 | 100 | 100 | 100 | 200 | 200 | 0.15 |
| D07 | 50 | 50 | 200 | 50 | 50 | 100 | 50 | 100 | 50 | 200 | 0.29 |
| D08 | 50 | 50 | 300 | 50 | 50 | 100 | 50 | 200 | 50 | 200 | 0.22 |
| D09 | 50 | 50 | 300 | 50 | 50 | 100 | 50 | 300 | 50 | 200 | 0.20 |
| D10 | 50 | 50 | 400 | 50 | 50 | 50 | 50 | 300 | 50 | 200 | 0.19 |
| D11 | 50 | 50 | 400 | 50 | 50 | 50 | 50 | 400 | 50 | 200 | 0.17 |
| D12 | 50 | 50 | 500 | 50 | 50 | 50 | 50 | 400 | 50 | 200 | 0.16 |
| No. | Binders (g) | Water (g) | W/B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSG | SR | FFAII | BRS | GP | FFAI | LP | GBFS | RM | OPC | NaOH | |||
| F1 | 50 | 25 | 100 | 25 | 25 | 25 | 50 | 25 | 25 | — | — | 250 | 0.71 |
| F2-1 | 50 | 25 | 100 | 25 | 25 | 0 | 50 | 25 | 25 | — | — | 250 | 0.77 |
| F2-2 | 50 | 25 | 100 | 25 | 25 | 50 | 50 | 25 | 25 | — | — | 250 | 0.67 |
| F3-1 | 50 | 25 | 100 | 25 | 25 | 25 | 50 | 0 | 25 | — | — | 250 | 0.77 |
| F3-2 | 50 | 25 | 100 | 25 | 25 | 25 | 50 | 50 | 25 | — | — | 250 | 0.67 |
| F4-1 | 50 | 25 | 100 | 25 | 25 | 25 | 25 | 25 | 25 | — | — | 250 | 0.77 |
| F4-2 | 50 | 25 | 100 | 25 | 25 | 25 | 75 | 25 | 25 | — | — | 250 | 0.67 |
| F5-1 | 50 | 25 | 100 | 25 | 25 | 25 | 50 | 25 | 0 | — | — | 250 | 0.77 |
| F5-2 | 50 | 25 | 100 | 25 | 25 | 25 | 50 | 25 | 50 | — | — | 250 | 0.67 |
| P1 | 200 | 100 | 400 | 100 | 100 | 100 | 200 | 100 | 100 | — | — | 980 | 0.70 |
| P2 | — | — | — | — | — | — | — | — | — | 1400 | — | 900 | 0.64 |
| P3 | 200 | 100 | 400 | 100 | 100 | 100 | 200 | 100 | 100 | — | 200 | 980 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, H.; Gao, H.; Liang, L.; Yang, J. Synthesis, Stability and Microstructure of a One-Step Mixed Geopolymer Backfill Paste Derived from Diverse Waste Slags. Sustainability 2023, 15, 6708. https://doi.org/10.3390/su15086708
Zhao X, Wang H, Gao H, Liang L, Yang J. Synthesis, Stability and Microstructure of a One-Step Mixed Geopolymer Backfill Paste Derived from Diverse Waste Slags. Sustainability. 2023; 15(8):6708. https://doi.org/10.3390/su15086708
Chicago/Turabian StyleZhao, Xianhui, Haoyu Wang, Han Gao, Luhui Liang, and Jing Yang. 2023. "Synthesis, Stability and Microstructure of a One-Step Mixed Geopolymer Backfill Paste Derived from Diverse Waste Slags" Sustainability 15, no. 8: 6708. https://doi.org/10.3390/su15086708
APA StyleZhao, X., Wang, H., Gao, H., Liang, L., & Yang, J. (2023). Synthesis, Stability and Microstructure of a One-Step Mixed Geopolymer Backfill Paste Derived from Diverse Waste Slags. Sustainability, 15(8), 6708. https://doi.org/10.3390/su15086708








