From Hazardous Chrysotile and Polyamide Wastes into Sustainable Serpentine/Polyamide Nanocomposite Membrane: Fabrication, Characterization, and Environmental Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Collected Raw Materials
2.2.1. Preparation of Natural Mining Waste
2.2.2. Preparation of Industrial Wastes
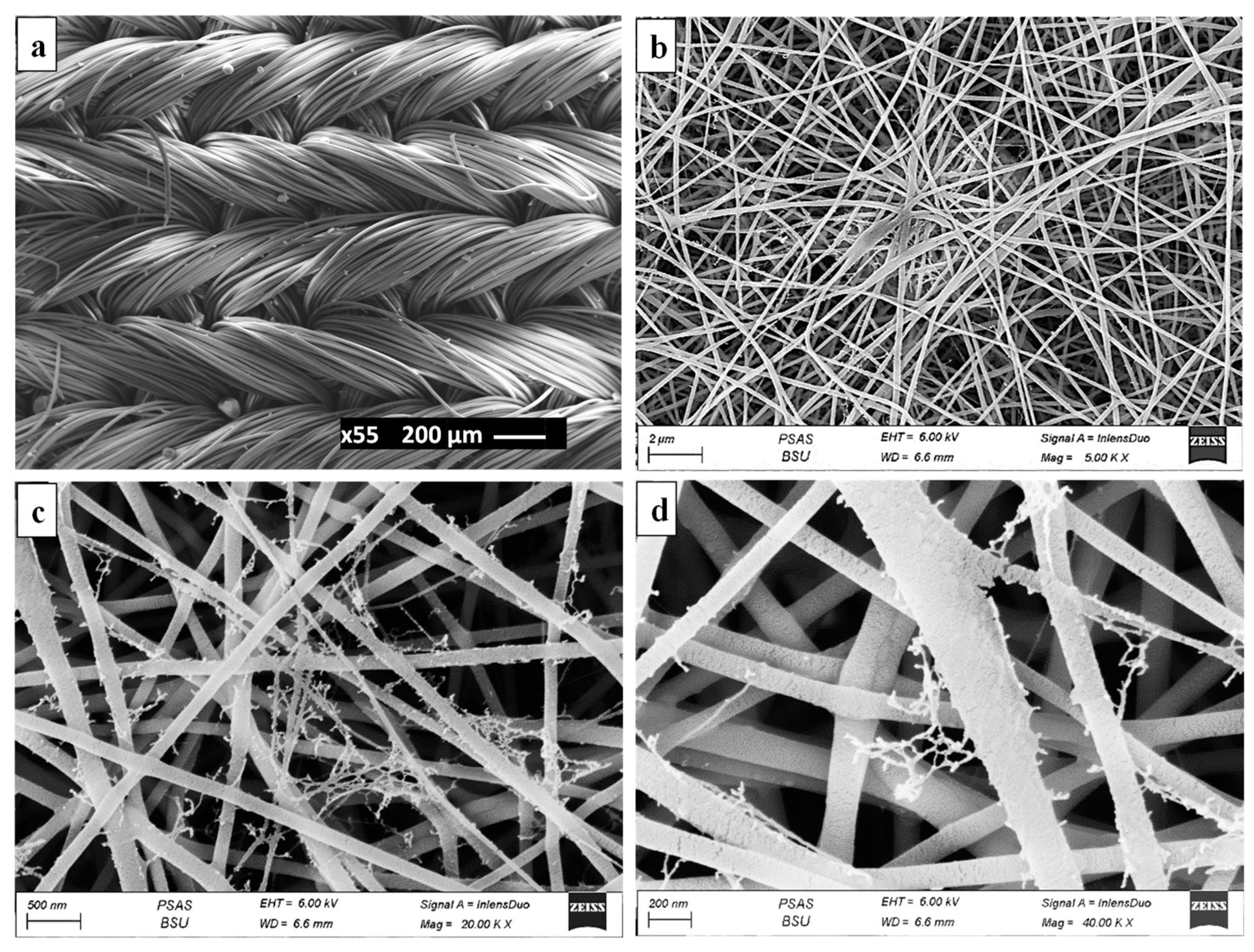
2.3. Fabrication of Serpentine/Polyamide Nanofiber Membrane (SP/PAM)
2.4. Characterizations of Raw Materials and the Fabricated SP/PAM
2.5. Batch System Studies Using Aqueous Solutions
2.5.1. Effect of pH, SP/PAM Dose, Initial Concentration, and Contact Time
2.5.2. Isotherm and Kinetic Studies
3. Results and Discussion
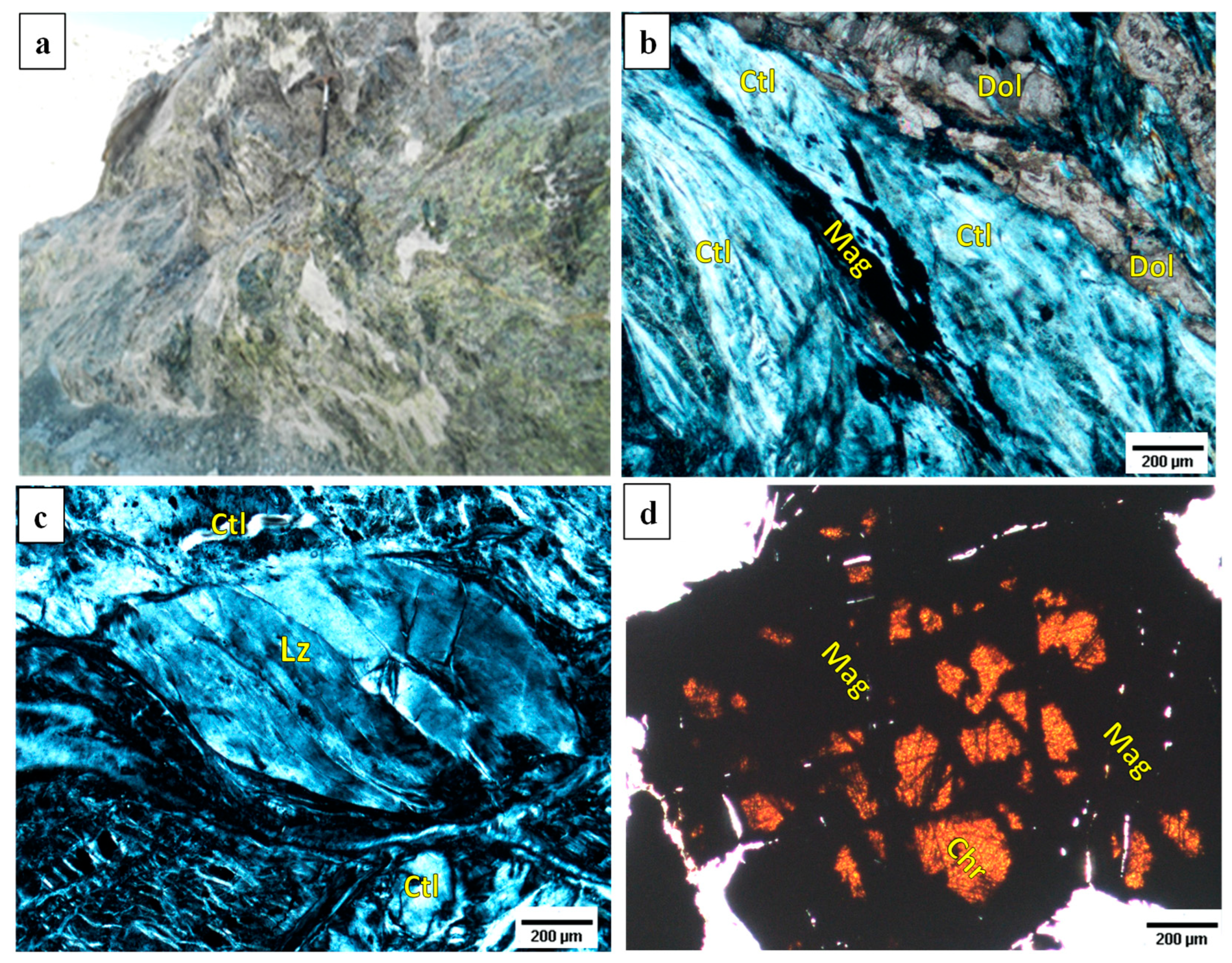
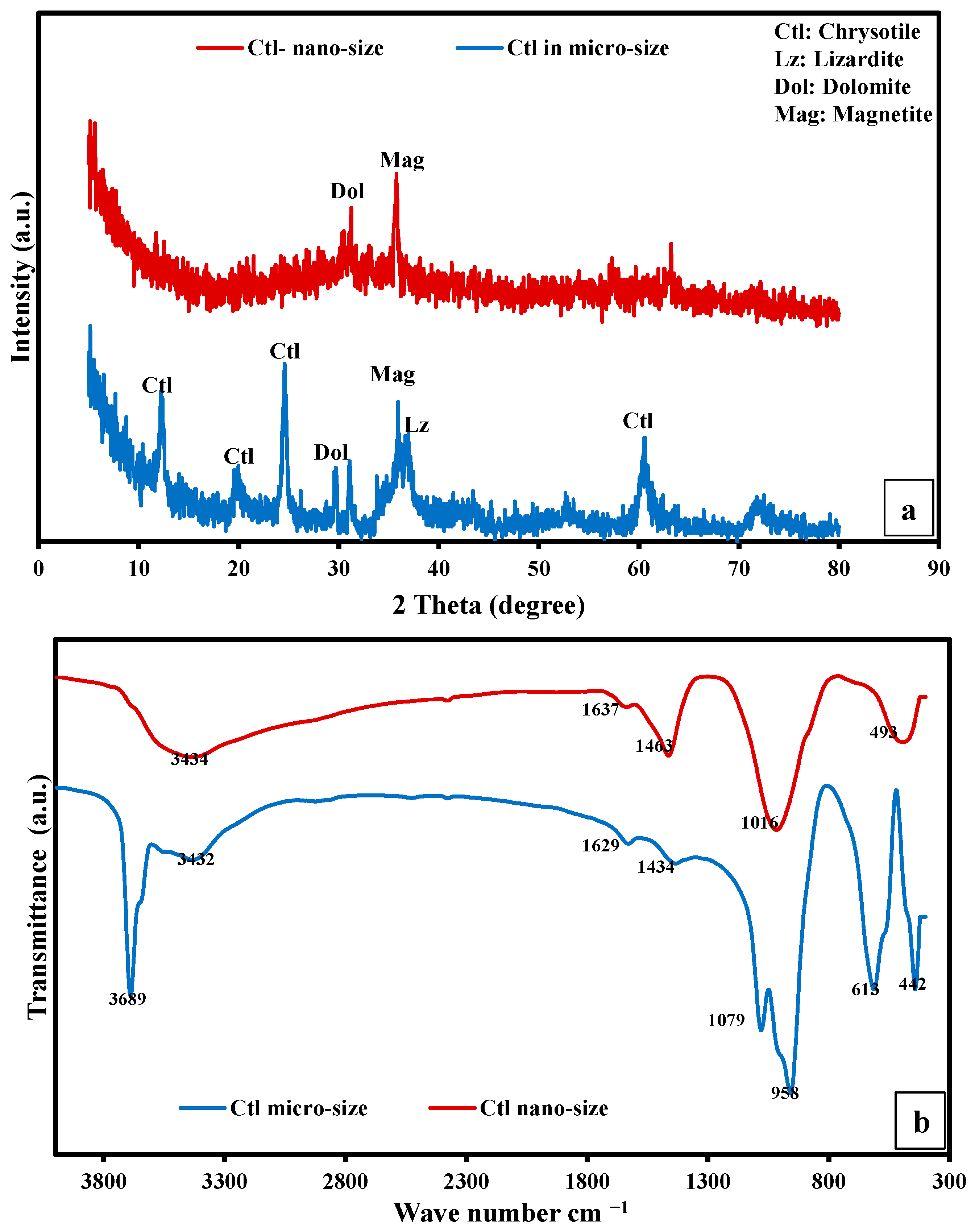
3.1. Characterization of Raw Materials
Characterization of Natural Mining Waste
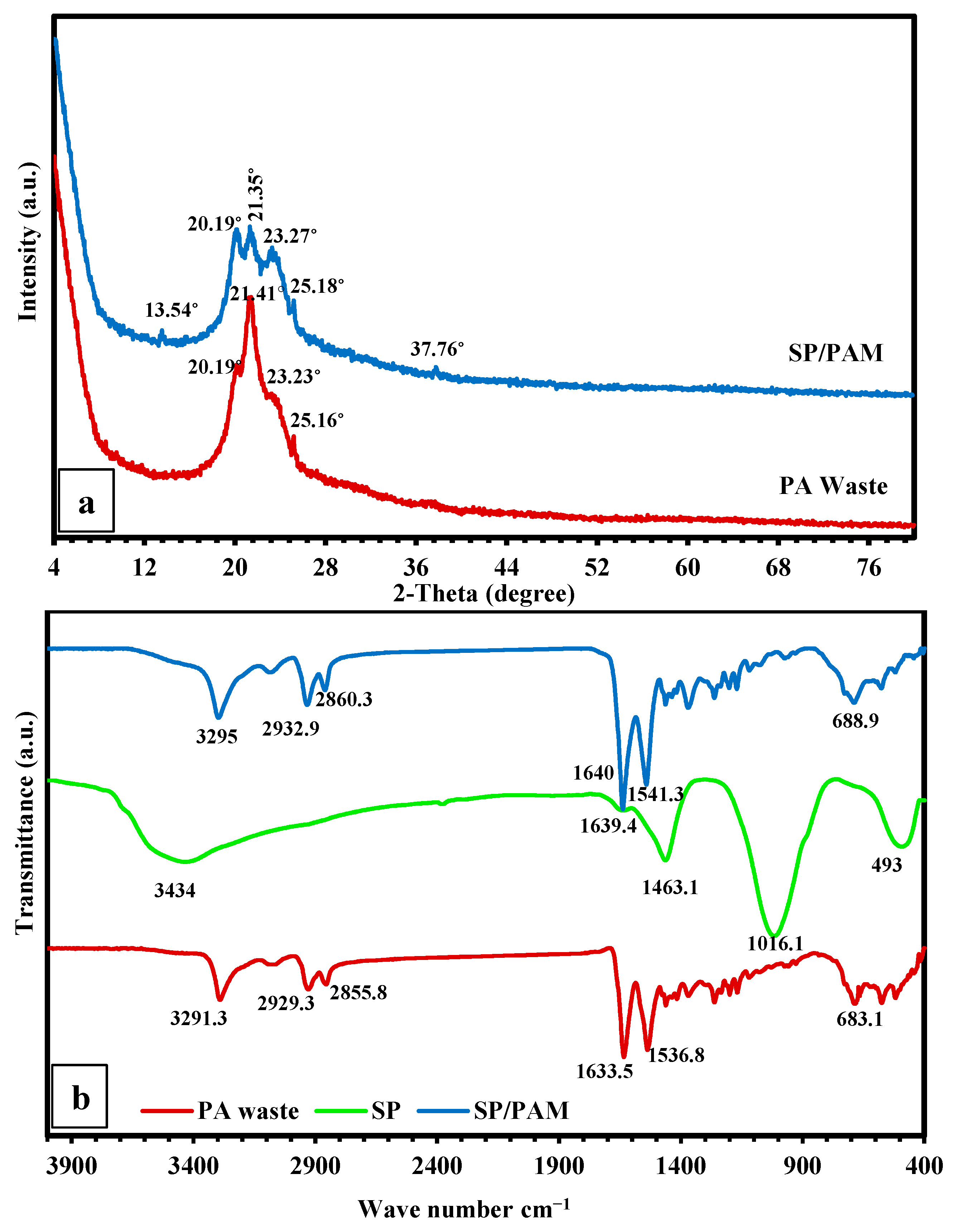
3.2. Characterization of the Fabricated SP/PAM and the Precursor PA6
3.3. Batch System Experiments Using Aqueous Solutions
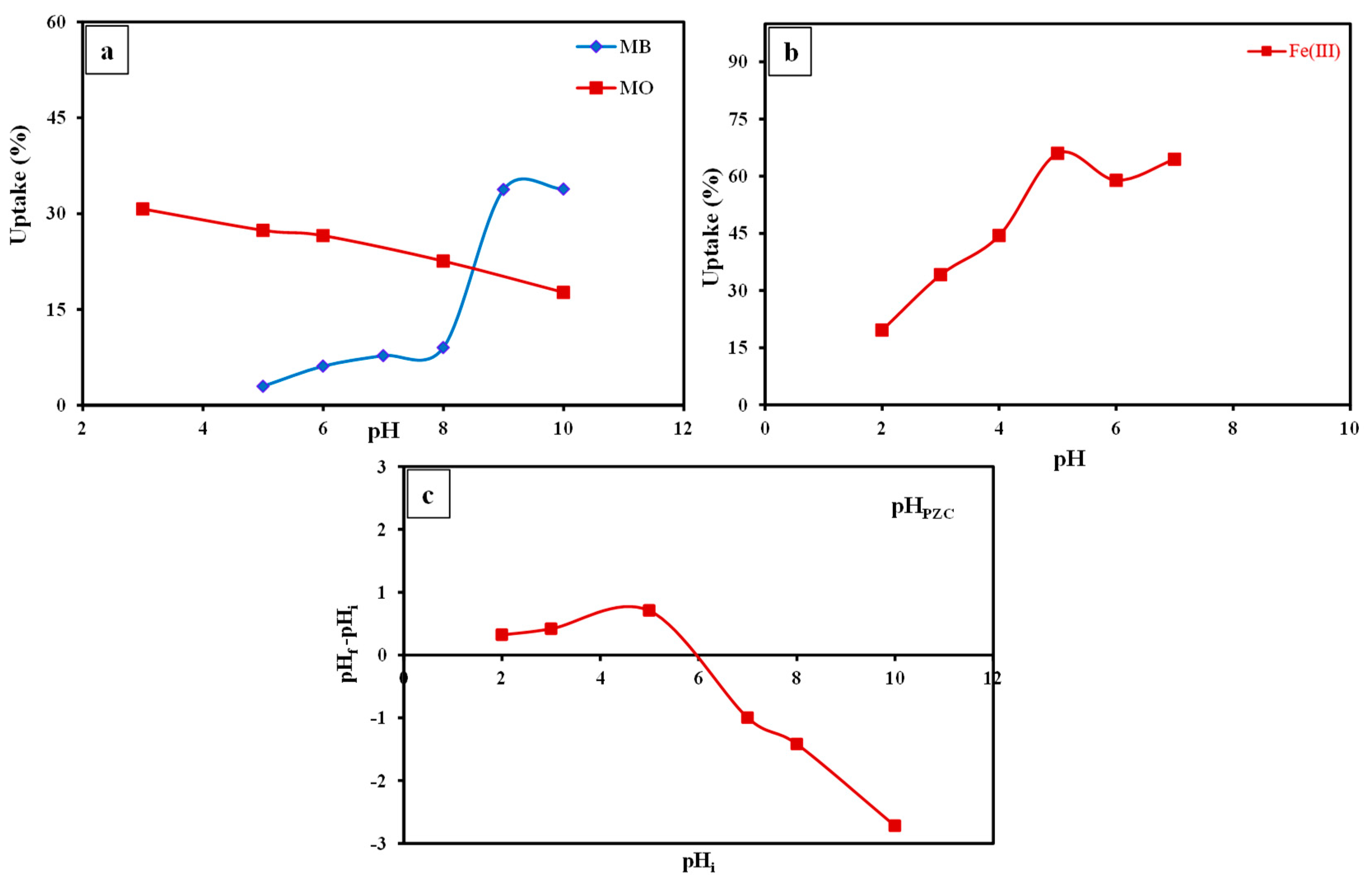
3.3.1. Effect of pH
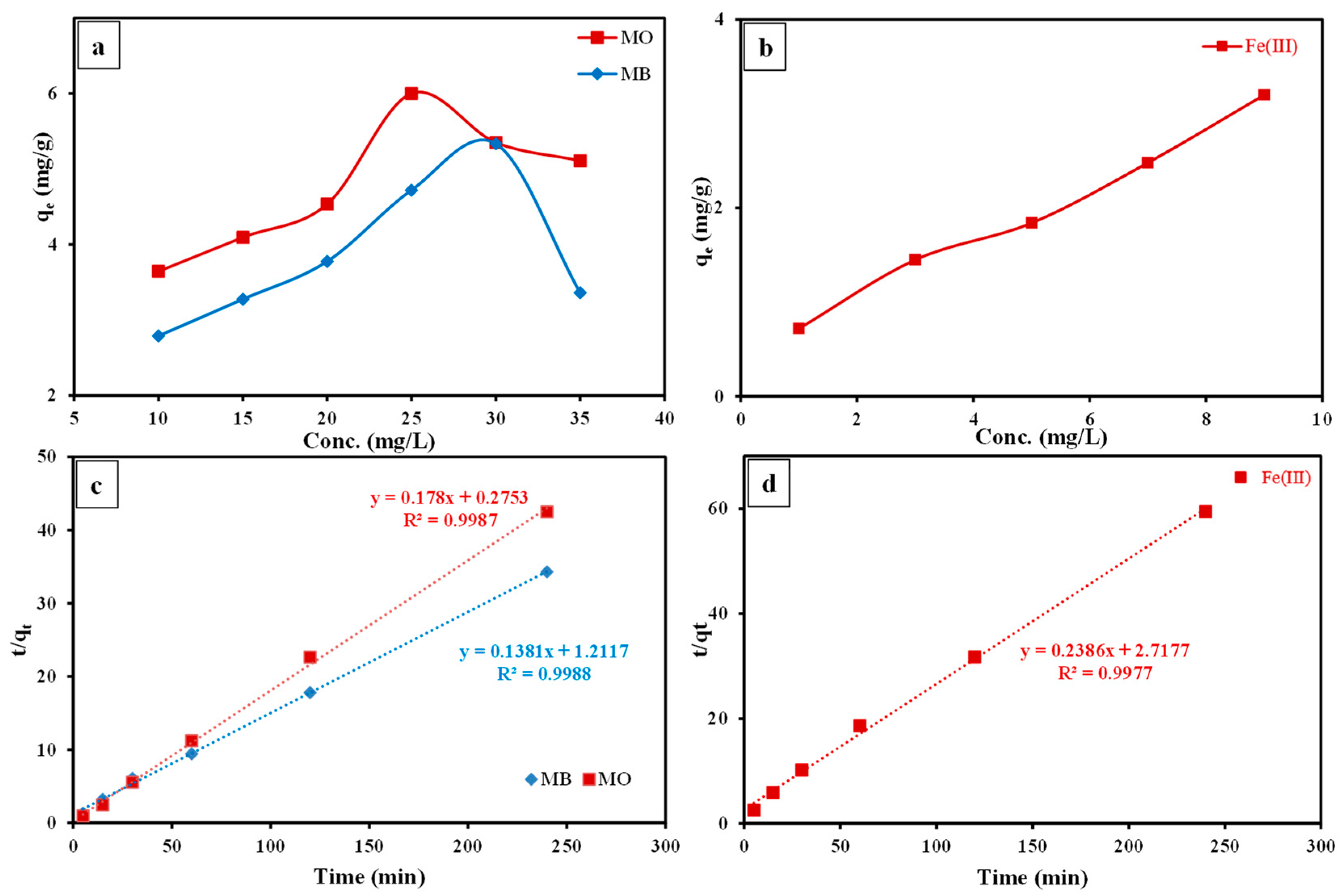
3.3.2. Effect of Contact Time
3.3.3. Effect of Adsorbent Dose
3.3.4. Effect of Initial Concentration
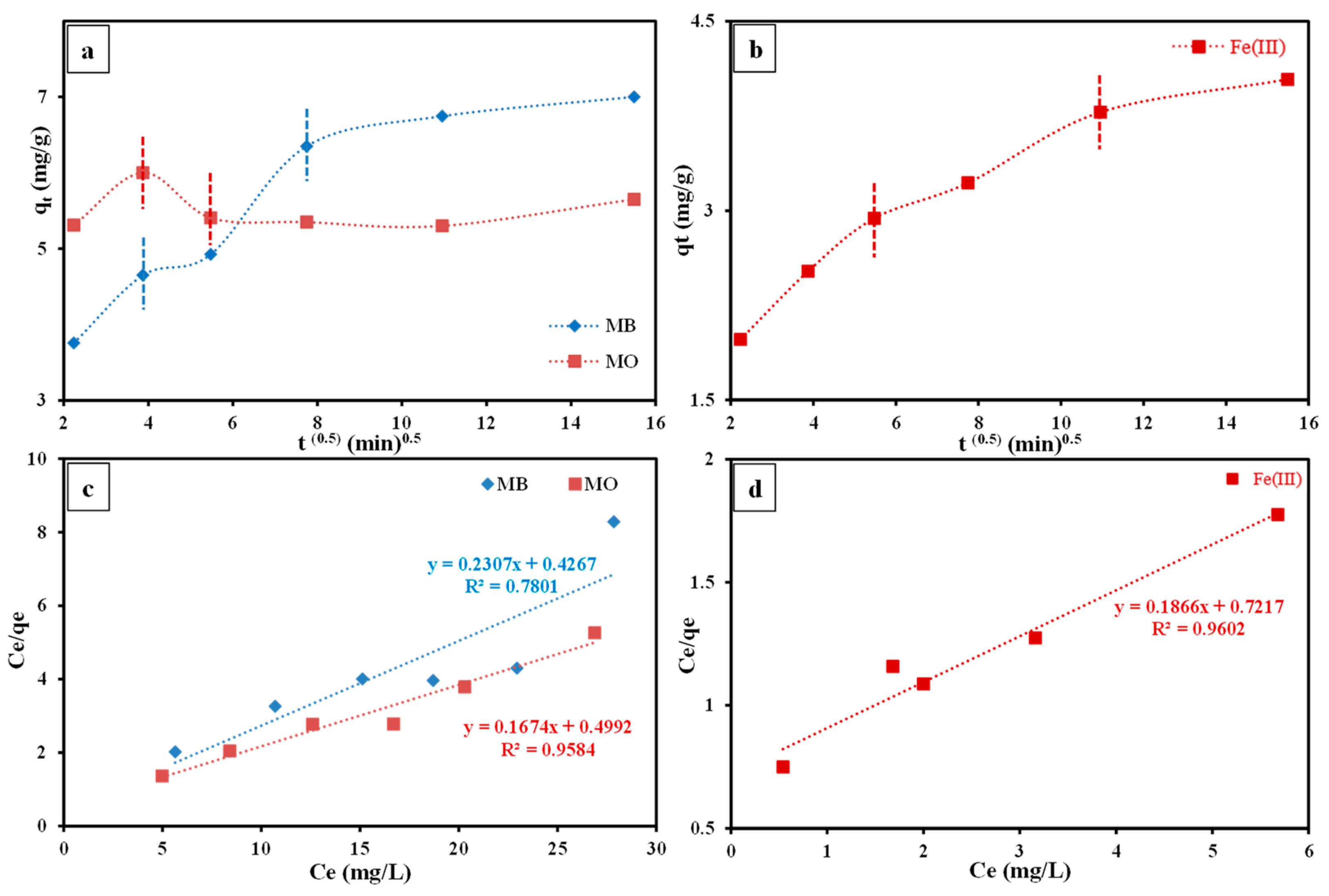
3.3.5. Kinetic Studies
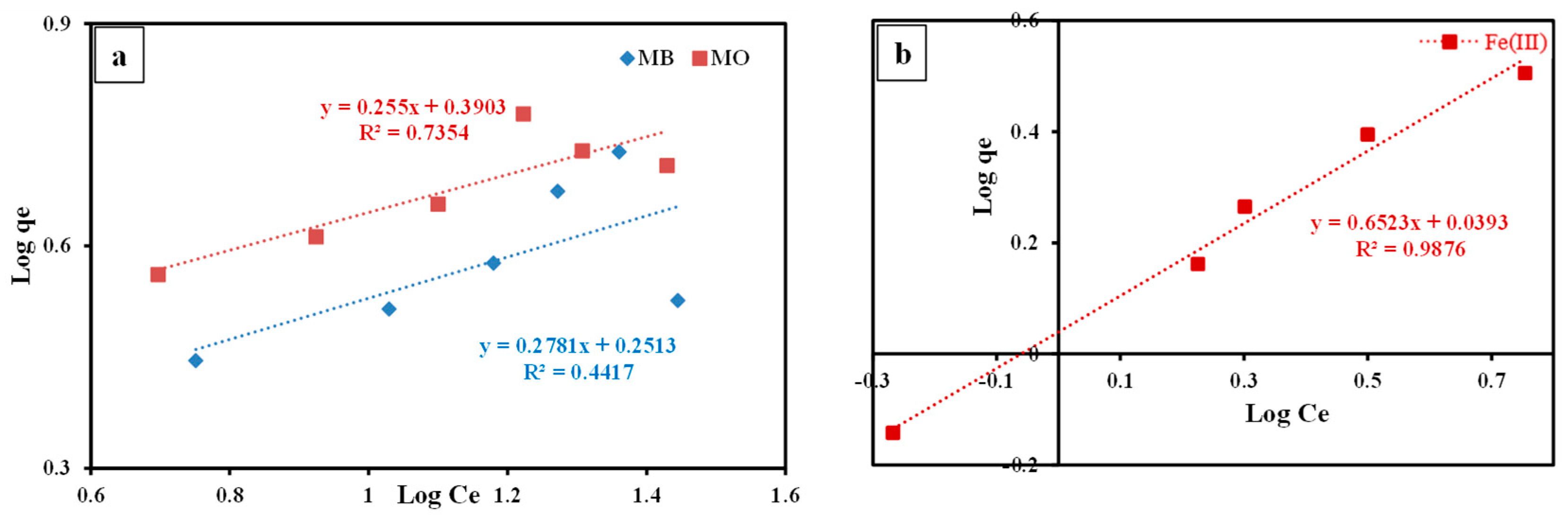
3.3.6. Isotherm Studies
4. Conclusions
- (a)
- The successful impregnation of SP Ctl within PA6 via the electro-spinning technique created an internal network structure within the polymer fibers by molecular self-assembly, producing intermitted charge upon the surface and hence multiplying the sorption capacity of the produced hybrid nanocomposite.
- (b)
- The hybrid, hydrophilic, and hydrophobic nature that was gained from the precursor SP Ctl and PA6 wastes, respectively, nominated the fabricated nanofibrous membrane composite as a promising adsorbent for various sorts of water contaminants with adequate removal efficiency.
- (c)
- The remediation of MO and MB and Fe(III) by the fabricated SP/PAM nanocomposite was a pH- and time-reliant procedure and their R% was attained at pH 3.0, 9.0, and 5.0, as well as 15, 60, and 120 min as retention time, respectively.
- (d)
- The PSO equation illustrated the kinetic of the sorption procedure of the regarded contaminants by the prepared nanocomposite in an adequate manner as was assured by the high R2 (≈0.999) and the close matching among the values of both experimental and theoretical qe.
- (e)
- The IPD multi-linearity specified that intra-particle diffusion is one of the driving stages in the sorption of the regarded contaminants by the investigated nanocomposite in collaboration with several styles of diffusion.
- (f)
- In accordance with the R2 results, the sorption data of the investigated dyes and Fe(Ⅲ) by the studied nanocomposite was explained well by the Langmuir equation compared to the Freundlich equation, indicating mono-layer sorption of these contaminants by the O2- and N2-holding sites on the composite surface.
- (g)
- Electrostatic attraction was the driving mechanism for sorption of both dyes by the iso-energetic sites of the SP/PAM, whereas the coupling between chelation and electrostatic attraction was the controlling mechanisms for Fe(Ⅲ) sorption.
- (h)
- Finally, the fabricated SP/PAM nanocomposite can be classified as sustainable adsorbent with adequate efficiency for dyes and heavy metals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed]
- Metwally, B.S.; El-Sayed, A.A.; Radwan, E.K.; Hamouda, A.S.; El-Sheikh, M.; Salama, M. Fabrication, characterization, and dye adsorption capability of recycled modified polyamide nanofibers. Egypt. J. Chem. 2018, 61, 867–882. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M. Impact of Textile Dyes on Human Health and Environment. In Impact of Textile Dyes on Public Health and the Environment; Wani, K.A., Jangid, N.K., Bhat, A.R., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 162–169. [Google Scholar] [CrossRef]
- Bai, L.; Wang, S.; Wang, Z.; Hong, E.; Wang, Y.; Xia, C.; Wang, B. Kinetics and mechanism of photocatalytic degradation of methyl orange in water by mesoporous Nd-TiO2-SBA-15 nanocatalyst. Environ. Pollut. 2019, 248, 516–525. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, M.N.; Metwally, B.S.; Mubarak, M.F.; Ahmed, H.A.; Abdel Moghny, T.; Zayed, A.M. Fabrication of electrospun polyamide–weathered basalt nano-composite as a non-conventional membrane for basic and acid dye removal. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Zayed, A.M.; Fathy, M.; Sillanpää, M.; Abdel Wahed, M.S.M. Talc-graphite schist as a natural organo-mineral complex for methylene blue remediation: Kinetic and isotherm study. SN Appl. Sci. 2020, 2, 740. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Silver, J. Chemistry of Iron; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Tagyan, A.I.; Yasser, M.M.; Mousa, A.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Marzouk, M.A. A Feasibility Study on the Recall of Metallophilic Fungi from Fe(III)-Contaminated Soil and Evaluating Their Mycoremediation Capacity: Experimental and Theoretical Study. J. Fungi 2023, 9, 382. [Google Scholar] [CrossRef]
- Saud, P.S.; Pant, B.; Ghouri, Z.K.; Panthi, G.; Park, S.-J.; Han, W.; Park, M.; Kim, H.-Y. Synthesis and characterization of photocatalytic and antibacterial PAN/Ag2CO3 composite nanofibers by ion exchange method. Fibers Polym. 2015, 16, 1336–1342. [Google Scholar] [CrossRef]
- Khatri, N.; Tyagi, S.; Rawtani, D. Recent strategies for the removal of iron from water: A review. J. Water Process Eng. 2017, 19, 291–304. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Dong, M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes. J. Colloid Interface Sci. 2018, 509, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Ghorai, U.K.; Chattopadhyay, K.K.; Banerjee, D. Removal of textile dyes by carbon nanotubes: A comparison between adsorption and UV assisted photocatalysis. Phys. E Low Dimens. Syst. Nanostruct. 2018, 99, 6–15. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Mohammad, A.-T.; Jawad, A.H. Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. J. Clean. Prod. 2019, 232, 43–56. [Google Scholar] [CrossRef]
- Hosseini Ravandi, S.A.; Sadrjahani, M.; Valipouri, A.; Dabirian, F.; Ko, F.K. Recently developed electrospinning methods: A review. Text. Res. J. 2022, 92, 5130–5145. [Google Scholar] [CrossRef]
- Masoud, M.A.; Rashad, A.M.; Sakr, K.; Shahien, M.G.; Zayed, A.M. Possibility of using different types of Egyptian serpentine as fine and coarse aggregates for concrete production. Mater. Struct. 2020, 53, 87. [Google Scholar] [CrossRef]
- Deschamps, F.; Godard, M.; Guillot, S.; Hattori, K. Geochemistry of subduction zone serpentinites: A review. Lithos 2013, 178, 96–127. [Google Scholar] [CrossRef]
- Evans, B.W. Control of the Products of Serpentinization by the Fe2+Mg−1 Exchange Potential of Olivine and Orthopyroxene. J. Petrol. 2008, 49, 1873–1887. [Google Scholar] [CrossRef]
- Momčilović, M.Z.; Ranđelović, M.S.; Purenović, M.M.; Đorđević, J.S.; Onjia, A.; Matović, B. Morpho-structural, adsorption and electrochemical characteristics of serpentinite. Sep. Purif. Technol. 2016, 163, 72–78. [Google Scholar] [CrossRef]
- Belluso, E.; Cavallo, A.; Halterman, D. Crystal habit of mineral fibres. Miner. Fibres Cryst. Chem. Chem. Phys. Prop. Biol. Interact. Toxic. 2017, 18, 65–110. [Google Scholar] [CrossRef]
- World Health Organization. Reference methods for measuring airborne man-made mineral fibres (MMMF). In Environmental Health (WHO-EURO); World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 1985. [Google Scholar]
- Herzog, B.; Kohan, M.I.; Mestemacher, S.A.; Pagilagan, R.U.; Redmond, K. Polyamides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar] [CrossRef]
- Saleh, M.; Demir, D.; Ozay, Y.; Yalvac, M.; Bolgen, N.; Dizge, N. Fabrication of basalt embedded composite fiber membrane using electrospinning method and response surface methodology. J. Appl. Polym. Sci. 2021, 138, 50599. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Masoud, M.A.; El-Khayatt, A.M.; Kansouh, W.A.; Sakr, K.; Shahien, M.G.; Zayed, A.M. Insights into the effect of the mineralogical composition of serpentine aggregates on the radiation attenuation properties of their concretes. Constr. Build. Mater. 2020, 263, 120141. [Google Scholar] [CrossRef]
- Zayed, A.M.; Masoud, M.A.; Shahien, M.G.; Gökçe, H.S.; Sakr, K.; Kansouh, W.A.; El-Khayatt, A.M. Physical, mechanical, and radiation attenuation properties of serpentine concrete containing boric acid. Constr. Build. Mater. 2021, 272, 121641. [Google Scholar] [CrossRef]
- Zayed, A.M.; Masoud, M.A.; Rashad, A.M.; El-Khayatt, A.M.; Sakr, K.; Kansouh, W.A.; Shahien, M.G. Influence of heavyweight aggregates on the physico-mechanical and radiation attenuation properties of serpentine-based concrete. Constr. Build. Mater. 2020, 260, 120473. [Google Scholar] [CrossRef]
- Masoud, M.A.; El-Khayatt, A.M.; Shahien, M.G.; Bakhit, B.R.; Suliman, I.I.; Zayed, A.M. Radiation Attenuation Assessment of Serpentinite Rocks from a Geological Perspective. Toxics 2022, 10, 697. [Google Scholar] [CrossRef]
- Gahlan, H.A.; Arai, S.; Almadani, S.A. Petrogenesis of carbonated meta-ultramafic lenses from the Neoproterozoic Heiani ophiolite, South Eastern Desert, Egypt: A natural analogue to CO2 sequestration. J. Afr. Earth Sci. 2015, 102, 102–115. [Google Scholar] [CrossRef]
- ASTM E1621; Standard Guide for Elemental Analysis by Wavelength Dispersive X-Ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D7348; Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. ASTM International: West Conshohocken, PA, USA, 2008.
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of Coal and Shale: Insights from Gas Adsorption and SANS/USANS Techniques. Energy Fuels 2012, 26, 5109–5120. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Vasanthan, N.; Salem, D.R. FTIR spectroscopic characterization of structural changes in polyamide-6 fibers during annealing and drawing. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 536–547. [Google Scholar] [CrossRef]
- Xu, J.-R.; Ren, X.-K.; Yang, T.; Jiang, X.-Q.; Chang, W.-Y.; Yang, S.; Stroeks, A.; Chen, E.-Q. Revisiting the Thermal Transition of β-Form Polyamide-6: Evolution of Structure and Morphology in Uniaxially Stretched Films. Macromolecules 2018, 51, 137–150. [Google Scholar] [CrossRef]
- Tsou, S.-Y.; Lin, H.-S.; Wang, C. Studies on the electrospun Nylon 6 nanofibers from polyelectrolyte solutions: 1. Effects of solution concentration and temperature. Polymer 2011, 52, 3127–3136. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Tao, L.; Xiao, R. Preparation and characterization of polyamide 6 fibre based on a phosphorus-containing flame retardant. RSC Adv. 2018, 8, 9261–9271. [Google Scholar] [CrossRef] [PubMed]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Sustainable biocomposites from biobased polyamide 6,10 and biocarbon from pyrolyzed miscanthus fibers. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Wan, J.; Bu, Z.-Y.; Li, C.; Fan, H.; Li, B.-G. Preparation, melting, glass relaxation and nonisothermal crystallization kinetics of a novel dendritic nylon-11. Thermochim. Acta 2011, 524, 117–127. [Google Scholar] [CrossRef]
- Hanno, I.; Anselmi, C.; Bouchemal, K. Polyamide Nanocapsules and Nano-emulsions Containing Parsol® MCX and Parsol® 1789: In Vitro Release, Ex Vivo Skin Penetration and Photo-Stability Studies. Pharm. Res. 2012, 29, 559–573. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Chou, C.C.; Yu, P.H.; Lin, K.-L. Effects of applied voltage on the morphology and phases of electrospun poly(vinylidene difluoride) nanofibers. Polym. Int. 2022, 71, 1176–1183. [Google Scholar] [CrossRef]
- Abubakar, M.; Mohd Noor, S.F.B.; Ahmad, N. Effect of milling time on the performance of ceramic membrane from ball clay for the treatment of nickel plating wastewater. J. Aust. Ceram. Soc. 2019, 55, 667–679. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Atta, M.M.; Taha, E.O. Optical spectroscopy, thermal analysis, and dynamic mechanical properties of graphene nano-platelets reinforced polyvinylchloride. J. Mater. Sci. Mater. Electron. 2021, 32, 22699–22717. [Google Scholar] [CrossRef]
- Selim, H.; Elshypany, R.; El-Bahy, S.M.; Mubarak, M.F.; Taha, E.O. Fabrication of electro spun nylon6.12/chitosan @PES nanofibrous UF membrane towards dyes rejection from synthetic wastewater. Polym. Bull. 2022, 80, 977–999. [Google Scholar] [CrossRef]
- Arigbabowo, O.K.; Tate, J.S. Additive manufacturing of polyamide nanocomposites for electrostatic charge dissipation applications. Mater. Sci. Eng. B 2021, 271, 115251. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Kim, D.-S. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Verma, S.; Gautam, R.K.; Krishna, V. Copper adsorption onto synthesized nitrilotriacetic acid functionalized Fe3O4 nanoparticles: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2015, 3, 2161–2171. [Google Scholar] [CrossRef]
- Asuha, S.; Zhou, X.G.; Zhao, S. Adsorption of methyl orange and Cr(VI) on mesoporous TiO2 prepared by hydrothermal method. J. Hazard. Mater. 2010, 181, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Al-Saadi, A.A.; Gupta, V.K. Carbonaceous adsorbent prepared from waste tires: Experimental and computational evaluations of organic dye methyl orange. J. Mol. Liq. 2014, 191, 85–91. [Google Scholar] [CrossRef]
- Zayed, A.M.; Abdel Wahed, M.S.M.; Mohamed, E.A.; Sillanpää, M. Insights on the role of organic matters of some Egyptian clays in methyl orange adsorption: Isotherm and kinetic studies. Appl. Clay Sci. 2018, 166, 49–60. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, R.; Baskar, C.; Chung, W.-J. Removal of methylene blue from aqueous waste using rice husk and rice husk ash. Desalination 2010, 259, 249–257. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Ma, L.; Zhang, Y.; Cui, B. Direct regeneration of hydrogels based on lemon peel and its isolated microcrystalline cellulose: Characterization and application for methylene blue adsorption. Int. J. Biol. Macromol. 2021, 191, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J. Colloid Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef]
- Kasim, N.; Mahmoudi, E.; Mohammad, A.W.; Abdullah, S.R.S.T. Influence of feed concentration and pH on iron and manganese rejection via nanohybrid polysulfone/Ag-GO ultrafiltration membrane. Desalination Water Treat. 2017, 61, 29–41. [Google Scholar] [CrossRef]
- Kurniawati, D.; Bahrizal; Sari, T.K.; Adella, F.; Sy, S. Effect of Contact Time Adsorption of Rhodamine B, Methyl Orange and Methylene Blue Colours on Langsat Shell with Batch Methods. J. Phys. Conf. Ser. 2021, 1788, 012008. [Google Scholar] [CrossRef]
- Mzinyane, N.N.; Ofomaja, A.E.; Naidoo, E.B. Synthesis of poly (hydroxamic acid) ligand for removal of Cu (II) and Fe (II) ions in a single component aqueous solution. S. Afr. J. Chem. Eng. 2021, 35, 137–152. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, J.O.; Abubakre, O.K.; Roos, W.D. Selected Heavy Metals Removal From Electroplating Wastewater by Purified and Polyhydroxylbutyrate Functionalized Carbon Nanotubes Adsorbents. Sci. Rep. 2019, 9, 4475. [Google Scholar] [CrossRef]
- Mohamed, F.M.; Li, Z.; Zayed, A.M. Carbon nanotube impregnated anthracite (An/CNT) as a superior sorbent for azo dye removal. RSC Adv. 2020, 10, 25586–25601. [Google Scholar] [CrossRef]
- Petzold, G.; Schwarz, S.; Dutschk, V. Polyelectrolyte-surfactant complexes and their influence on the wettability of different polymer surfaces. Colloid. Polym. Sci. 2014, 292, 2197–2205. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Feng, Q.; Zhang, M.; Gu, Y. Talc–serpentine interactions and implications for talc depression. Miner. Eng. 2012, 32, 68–73. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Advances in water pollution research. In Proceedings of the First International Conference on Water Pollution Research, London, UK, 3–7 September 1962; p. 231. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem 1906, 57, 1100–1107. [Google Scholar]
- Jawad, A.H.; Ismail, K.; Ishak, M.A.M.; Wilson, L.D. Conversion of Malaysian low-rank coal to mesoporous activated carbon: Structure characterization and adsorption properties. Chin. J. Chem. Eng. 2019, 27, 1716–1727. [Google Scholar] [CrossRef]
- Selim, A.Q.; Mohamed, E.A.; Seliem, M.K.; Zayed, A.M. Synthesis of sole cancrinite phase from raw muscovite: Characterization and optimization. J. Alloys Compd. 2018, 762, 653–667. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mamat, N.F.H.; Hameed, B.H.; Ismail, K. Biofilm of cross-linked Chitosan-Ethylene Glycol Diglycidyl Ether for removal of Reactive Red 120 and Methyl Orange: Adsorption and mechanism studies. J. Environ. Chem. Eng. 2019, 7, 102965. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Lima, D.R.; da Silva, R.S.; Thue, P.S.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Larsson, S.H. Removal of captopril pharmaceutical from synthetic pharmaceutical-industry wastewaters: Use of activated carbon derived from Butia catarinensis. J. Environ. Chem. Eng. 2020, 8, 104506. [Google Scholar] [CrossRef]




| Parameter | Conditions | Other Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| pH | MO | - | 3 | 5 | 6 | 8 | 10 | 25 mg/L initial concentration for MO and MB and 3 mg/L for Fe(Ⅲ), 25 mg dose, 200 rpm/60 min (speed/contact time), 25 and 50 mL solution for dyes and Fe(Ⅲ), respectively. |
| MB | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Fe(Ⅲ) | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Dose (mg) | MO and MB | 10 | 15 | 20 | 25 | 30 | 35 | pH (3.0, 9.0, and 5.0) for MO, MB, and Fe(Ⅲ), respectively, 25 mg/L initial concentration of MO and MB and 3 mg/L for Fe(Ⅲ), 200 rpm/15, 60 and 120 min (speed/contact time) for MO, MB, and Fe(Ⅲ), respectively, 25 and 50 mL solution for dyes and Fe, respectively. |
| Fe(Ⅲ) | 20 | 30 | 40 | 50 | 60 | 70 | ||
| Contact time (min) | MO, MB, and Fe(Ⅲ) | 5 | 15 | 30 | 60 | 120 | 240 | pH (3.0, 9.0, and 5.0) for MO, MB, and Fe(Ⅲ), respectively, 25 mg/L initial concentration of MO and MB and 3.0 mg/L for Fe(Ⅲ), 25 mg dose, 200 rpm as a shaking speed, 25 and 50 mL solution for dyes and Fe(Ⅲ), respectively. |
| Initial dye concentration (mg/L) | MO and MB | 10 | 15 | 20 | 25 | 30 | 35 | pH (3.0, 9.0, and 5.0) for MO, MB, and Fe(Ⅲ), respectively, 25 mg dose, 200 rpm/15, 60 and 120 min (speed/contact time) for MO, MB, and Fe(Ⅲ), respectively, 25 and 50 mL solution for dyes and Fe(Ⅲ), respectively. |
| Equation No. | Linear Form | Parameters |
|---|---|---|
| (1) | qe (mg/g): adsorbed amount of MO, MB, and Fe(Ⅲ) at equilibrium. Ci: the initial MO, MB, and Fe(Ⅲ) concentration in solutions (mg/L). Cf: the concentration of MO, MB, and Fe(IⅡ) at equilibrium (mg/L). V: the volume of MO, MB, and Fe(Ⅲ) solutions (mL). m: the mass of adsorbents (mg). | |
| (2) | R%: removal efficiency of MO, MB, and Fe(Ⅲ) by addressed adsorbent. Ci: the initial MO, MB, and Fe(Ⅲ) concentration in solutions (mg/L). Ct: the concentration of MO, MB, and Fe(Ⅲ) (mg/L) at time t. | |
| (3) | qt (mg/g): adsorbed amount of MO, MB, and Fe(Ⅲ) at time t. Ci: the initial MO, MB, and Fe(Ⅲ) concentration in solutions (mg/L). Ct: the concentration of MO, MB, and Fe(Ⅲ) (mg/L) at time t. V: the volume of MO, MB, and Fe(Ⅲ) solutions (mL). m: the mass of adsorbents (mg). |
| Composition (wt. %) | Chrysotile (Average Ratios) |
|---|---|
| SiO2 | 35.61 |
| MgO | 37.72 |
| Fe2O3 | 6.12 |
| CaO | 2.65 |
| SO3 | 0.14 |
| K2O | 0.47 |
| Na2O | 0.22 |
| TiO2 | 0.01 |
| P2O5 | 0.12 |
| Cl | 0.05 |
| Cr2O3 | 0.12 |
| NiO | 0.24 |
| Co3O4 | 0.013 |
| SrO | 0.005 |
| V2O5 | 0.002 |
| ZnO | 0.003 |
| L.O.I | 16.50 |
| Total | 99.99 |
| Parameter | Surface Area (SBET, m2/g) | Total Pore Volume (Vt, cm3/g) | Average Pore Diameter (Dp, nm) |
|---|---|---|---|
| PA waste | 2875.26 | 5.06 | 1.93 |
| SP/PAM | 209.79 | 0.38 | 1.93 |
| Storage Modulus (MPa) | Glass Transition Temperature (Tg, °C) | ||
|---|---|---|---|
| (Glassy Region) at 25 °C | (Rubbery Region) at 100 °C | ||
| PA waste | 100.24 | 35.58 | 70.52 |
| SP/PAM | 117.27 | 30.82 | 75.13 |
| Kinetic/Isotherm Model | Linear Form | Parameters | Ref. |
|---|---|---|---|
| Pseudo-second-order | qt (mg/g): removed amount of MO, MB, and Fe(ⅡI) at time t. qe (mg/g): equilibrium adsorption uptake. k2 (g/mg min): rate constant of the second-order adsorption. qe(calc.) = 1/slope k2 = (slope)2/intercept | [66] | |
| Intra-particle diffusion | qt (mg/g): removed amount of MO, MB, and Fe(ⅡI) at time t. Kp (mg/g min0.5): intra-particle diffusion rate constant. C (mg/g): intercept of the line which reflects the thickness of the boundary layer. kp = slope C = intercept | [67] | |
| Langmuir | (mg/L): equilibrium concentration of the resting MO, MB, and Fe(IⅡ) in the solution. (mg/g): removed amount of MO, MB, and Fe(IⅡ) at equilibrium. (mg/g): maximum adsorption capacity. (L/mg): Langmuir constant. qmax = 1/slope b = slope/intercept | [68] | |
| Freundlich | (mg/L): equilibrium concentration of the resting MO, MB, and Fe(ⅡI) in the solution. (mg/g): removed amount of MO, MB, and Fe(IⅡ) at equilibrium. (mg/g)*L/mg)(1/n): MO, MB, and Fe(ⅡI) adsorption capacity. n: heterogeneity factor. kF = 10intercept 1/n = slope | [69] |
| Adsorbent | Contaminant | Initial Concentration (mg/L) | qe(exp.) (mg/g) | Kinetic Model | |||||
| Pseudo-Second-Order | Intra-Particle Diffusion | ||||||||
| Parameters | Parameters | ||||||||
| SP/PAM | k2 (g/mg min) | qe (Calc.) (mg/g) | R2 | Kp (mg/g min0.5) | C (mg/g) | R2 | |||
| MO | 25 | 6 | 0.115 | 5.62 | 0.999 | - | - | - | |
| MB | 25 | 6.35 | 0.016 | 7.24 | 0.999 | 0.452 | 2.74 | 0.927 | |
| Fe(Ⅲ) | 3 | 3.78 | 0.021 | 4.2 | 0.998 | 0.177 | 1.89 | 0.955 | |
| Adsorbent | Contaminant | Initial Concentration (mg/L) | Isotherm Model | |||||
| Langmuir | Freundlich | |||||||
| Parameters | Parameters | |||||||
| SP/PAM | qmax (mg/g) | b (L/mg) | R2 | 1/n | KF (mg/g)*L/mg)(1/n) | R2 | ||
| MO | 10–35 | 5.97 | 0.335 | 0.958 | 0.255 | 2.46 | 0.735 | |
| MB | 4.33 | 0.541 | 0.780 | 0.278 | 1.78 | 0.442 | ||
| Fe(Ⅲ) | 1–9 | 5.36 | 0.259 | 0.960 | 0.652 | 1.09 | 0.988 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Maghrabi, A.H.; El-Rabiee, M.M.; Metwally, B.S.; Masoud, M.A.; Abdelaziz, M.H.; Petrounias, P.; Koukouzas, N.; Zayed, A.M. From Hazardous Chrysotile and Polyamide Wastes into Sustainable Serpentine/Polyamide Nanocomposite Membrane: Fabrication, Characterization, and Environmental Application. Sustainability 2023, 15, 7060. https://doi.org/10.3390/su15097060
El Maghrabi AH, El-Rabiee MM, Metwally BS, Masoud MA, Abdelaziz MH, Petrounias P, Koukouzas N, Zayed AM. From Hazardous Chrysotile and Polyamide Wastes into Sustainable Serpentine/Polyamide Nanocomposite Membrane: Fabrication, Characterization, and Environmental Application. Sustainability. 2023; 15(9):7060. https://doi.org/10.3390/su15097060
Chicago/Turabian StyleEl Maghrabi, Amal H., Mohmmed M. El-Rabiee, Bahaa S. Metwally, Mostafa A. Masoud, Mohamed H. Abdelaziz, Petros Petrounias, Nikolaos Koukouzas, and Ahmed M. Zayed. 2023. "From Hazardous Chrysotile and Polyamide Wastes into Sustainable Serpentine/Polyamide Nanocomposite Membrane: Fabrication, Characterization, and Environmental Application" Sustainability 15, no. 9: 7060. https://doi.org/10.3390/su15097060








