Adding Value to Reclaimed Water from Wastewater Treatment Plants: The Environmental Feasibility of a Minimal Liquid Discharge System for the Case Study of Larnaca
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Development of the MLD System for Larnaca WWTP
2.2. Life Cycle Assessment of the Proposed System
2.2.1. Goal and Scope
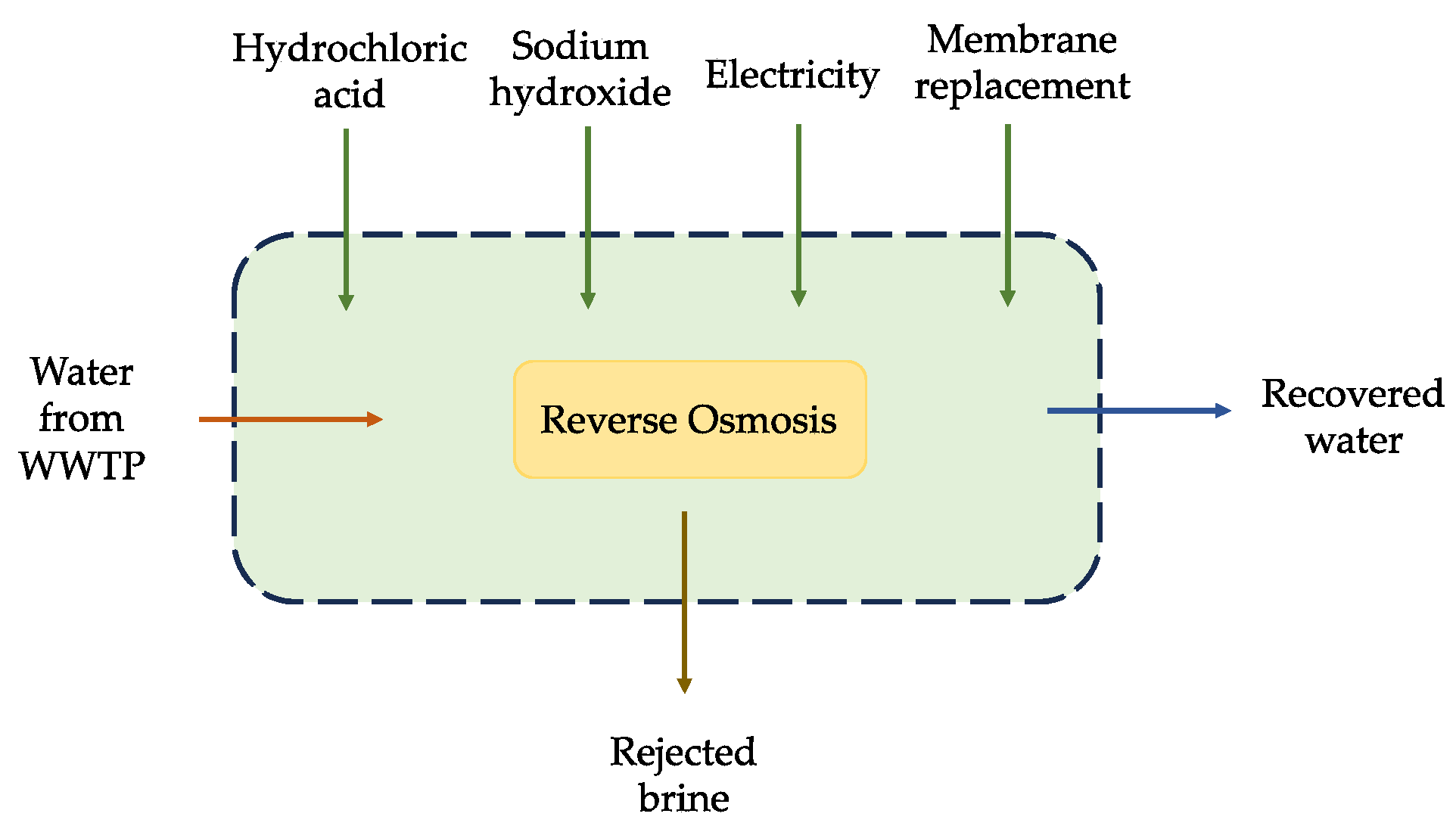
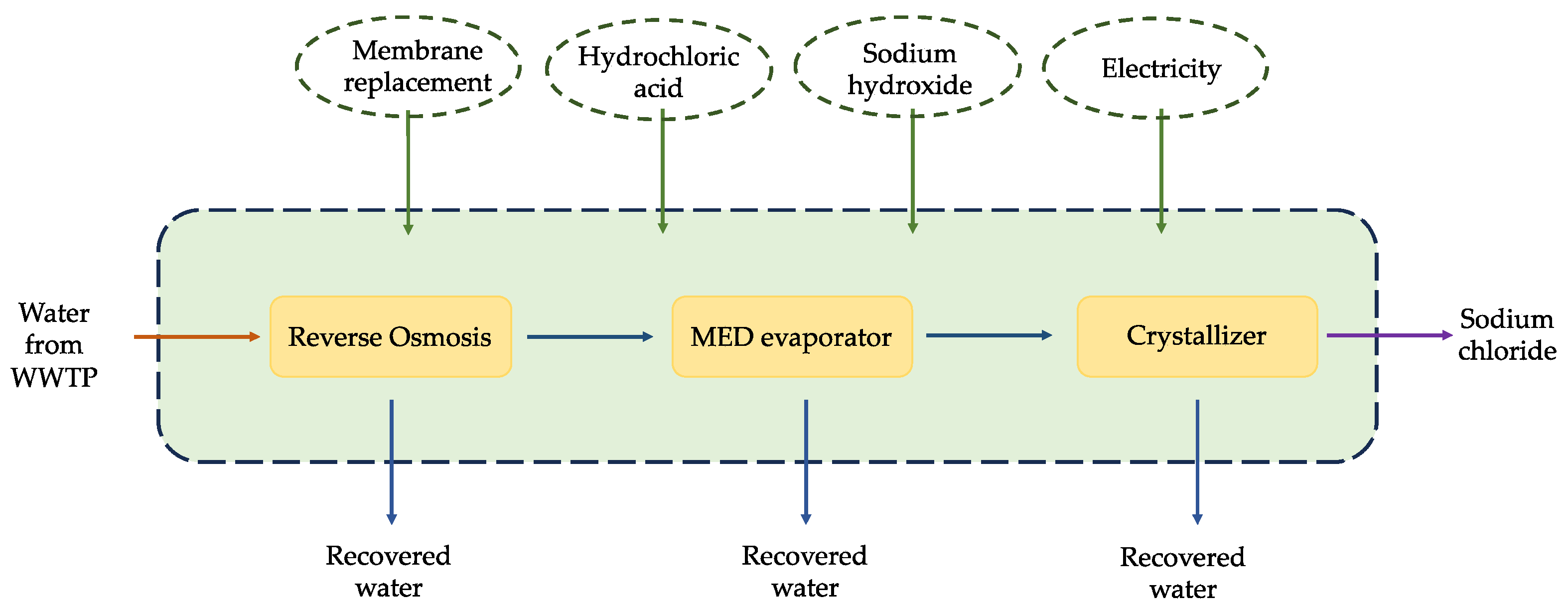
2.2.2. System Boundaries
2.2.3. Inventory Analysis
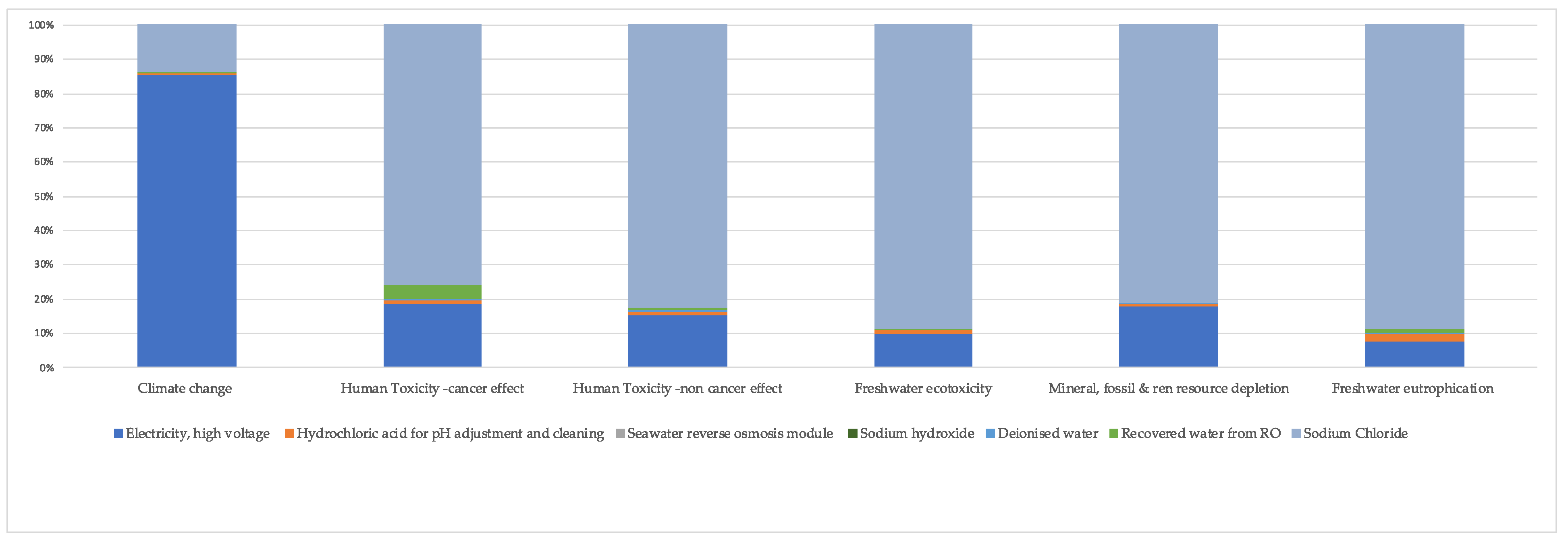
3. Results and Discussion
3.1. The Life Cycle Impact Assessment (LCIA)
3.1.1. Climate Change
3.1.2. Human Toxicity
3.1.3. Freshwater Eutrophication
3.1.4. Freshwater Ecotoxicity
3.1.5. Mineral, Fossil, and Renewable Resource Depletion
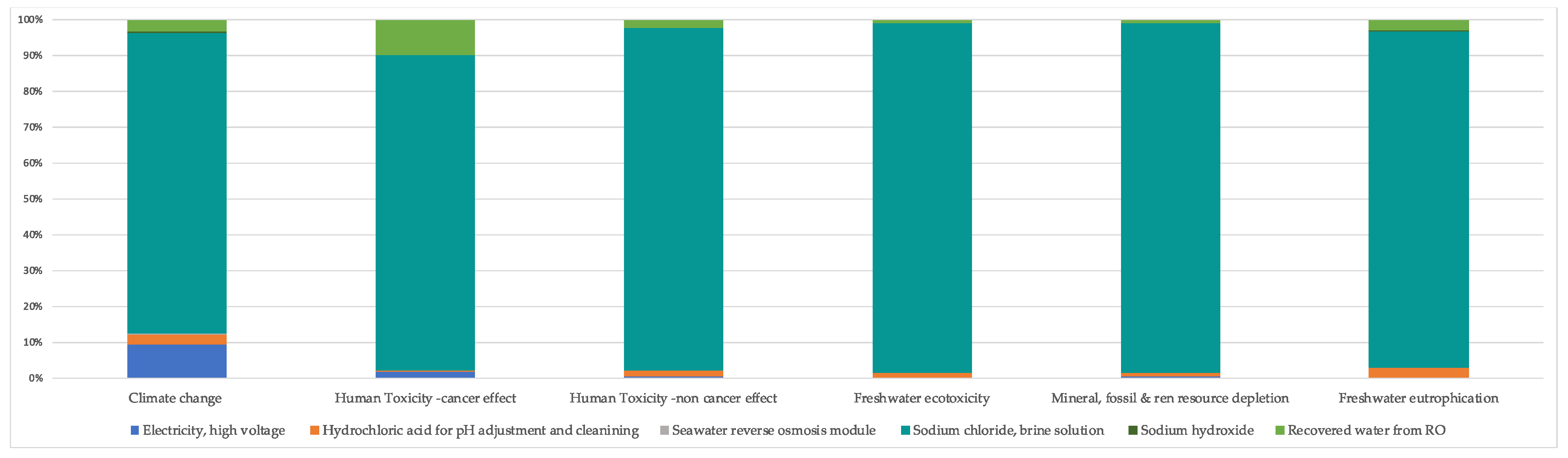
3.2. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piesse, M. Global Water Supply and Demand Trends Point towards Rising Water Insecurity. Future Directions International 2020. Available online: https://apo.org.au/node/276976 (accessed on 23 July 2023).
- UN-Water. The United Nations World Water Development Report 2020: Water and Climate Change 2020, 1st ed.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2020; pp. 1–219. [Google Scholar]
- UN-Water. Water in a Changing World, United Nations World Water Development, Report 3; World Water Assessment Program: Paris, France, 2009; pp. 1–429. [Google Scholar]
- EurEau. Europe’s Water in Figures–An Overview of the European Drinking Water and Waste Water Sectors. 2017. Available online: https://www.eureau.org/resources/publications/eureau-publications/5824-europe-s-water-in-figures-2021/file (accessed on 22 July 2023).
- FAO. Water Scarcity–One of the Greatest Challenges of Our Time. Available online: https://www.fao.org/zhc/detail-events/en/c/880881/ (accessed on 22 July 2023).
- Rachele, R. BRIEFING–Irrigation in EU Agriculture EN, European Asylum Support Office. Malta. 2019. Available online: https://policycommons.net/artifacts/2016252/briefing/2768694/ (accessed on 5 August 2023).
- Eurostat. Irrigation: Number of Farms, Areas and Equipment by Size of Irrigated Area and NUTS 2 Regions. Available online: https://ec.europa.eu/eurostat/databrowser/view/ef_poirrig/default/table?lang=en (accessed on 25 July 2023).
- Zal, N.; Zachos, A.; Bariamis, G.; Baltas, E. ETC/ICM Report 1/2017: Use of Freshwater Resources in Europe 2002–2014–An Assessment Based on Water Quantity Accounts; European Topic Centre on Inland, Coastal and Marine Waters: Magdeburg, Germany, 2017. [Google Scholar]
- European Environment Agency. Use of Freshwater Resources in Europe. 2021. Available online: https://www.eea.europa.eu/data-and-maps/indicators/use-of-freshwater-resources-3/assessment-4 (accessed on 28 July 2023).
- ΕΕU. Water Scarcity Conditions as Measured Using the Water Exploitation Index Plus (WEI+), by Country, 2017. Available online: https://www.eea.europa.eu/data-and-maps/daviz/development-of-the-water-exploitation#tab-chart_1 (accessed on 2 April 2023).
- Cramer, W.; Guiot, J.; Marini, K. Climate and Environmental Change in the Mediterranean Basin–Current Situation and Risks for the Future; First Mediterranean Assessment Report; MedECC (Mediterranean Experts on Climate and Environmental Change); Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; ISBN 978-2-9577416-0-1. [Google Scholar]
- Ministry of Agriculture, Rural Development and Environment. Review of cumulative rainfall in Cyprus for the period 1901–2019. In The Farmer, 1st ed.; Ministry of Agriculture, Rural Development and Environment: Nicosia, Cyprus, 2020; p. 40. [Google Scholar]
- Koundouri, P. Water Resources Allocation: Policy and Socioeconomic Issues in Cyprus, 1st ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2010; Volume 1. [Google Scholar] [CrossRef]
- Sofroniou, A.; Bishop, S. Water scarcity in Cyprus: A review and call for integrated policy. Water 2014, 6, 2898–2928. [Google Scholar] [CrossRef]
- Water Development Department. Strategic Study for Water Management and Dealing with Drought. Available online: http://www.moa.gov.cy/moa/wdd/wdd.nsf/all/D075B511901214DAC225850D003E4200/$file/Stratigiki_Diaxirisis_Ydaton_March_2019.pdf?openelement (accessed on 2 April 2023).
- Water Development Department. Water Balance. Available online: http://www.moa.gov.cy/moa/wdd/wdd.nsf/page10_gr/page10_gr?opendocument (accessed on 20 June 2023).
- Water Development Department. Reservoir Storage. Available online: http://www.moa.gov.cy/moa/wdd/Wdd.nsf/page18_en/page18_en?opendocument (accessed on 22 June 2023).
- Water Development Department. Water Availability for Irrigation per Year (Irrigation Sources). Available online: https://www.data.gov.cy/dataset/%CE%B4%CE%B9%CE%AC%CE%B8%CE%B5%CF%83%CE%B7-%CE%BD%CE%B5%CF%81%CE%BF%CF%8D-%CE%B3%CE%B9%CE%B1-%CE%AC%CF%81%CE%B4%CE%B5%CF%85%CF%83%CE%B7-%CE%B1%CE%BD%CE%AC-%CE%AD%CF%84%CE%BF%CF%82-%CF%80%CE%B7%CE%B3%CE%AD%CF%82-%CE%AC%CF%81%CE%B4%CE%B5%CF%85%CF%83%CE%B7%CF%82 (accessed on 22 June 2023).
- Ministry of Agriculture, Rural Development and Environment. Dams of Cyprus. Available online: https://web.archive.org/web/20190310232250/http://www.moa.gov.cy/moa/wdd/wdd.nsf/All/5D3901C9FEF1181CC22581FE0022CE7F/$file/7_Page1_113.pdf?OpenElement (accessed on 22 June 2023).
- Council of European Union. Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31991L0271 (accessed on 22 June 2023).
- European Parliament, Council of the European Union. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R0741 (accessed on 22 June 2023).
- Ministry of Agriculture, Rural Development and Environment. Water and Soil Pollution Control Law, 2002 (Law No. 106(I)/2002). Available online: http://www.cylaw.org/nomoi/enop/non-ind/2002_1_106/full.html (accessed on 22 June 2023).
- Ministry of Agriculture, Rural Development and Environment. Law No. 127 of 2018 Concerning the Environmental Impact Assessment of Certain Projects. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC211224/ (accessed on 22 June 2023).
- Water Development Department. Code of Good Agricultural Practice. Available online: https://www.moa.gov.cy/moa/environment/environmentnew.nsf/All/AF846B9CE574AB8EC2258021002B3B56?OpenDocument (accessed on 22 June 2023).
- Hadjigeorgiou, P. Reuse of Treated Effluent in Cyprus. In Proceedings of the 13th International Conference “EUROPE–INBO 2015”, Thessaloniki, Greece, 21–24 October 2015; Available online: https://www.riob.org/sites/default/files/IMG/pdf/TR1-9_Reuse_of_Treated_Effluent_in_Cyprus_-_Panayota_Hadjigeorgiou.pdf (accessed on 28 June 2023).
- Podmore, C. Irrigation salinity–Causes and impacts. Primefact 2009, 937, 1–4. [Google Scholar]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward Osmosis: Principles, Applications, and Recent Developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Eke, J.; Yusuf, A.; Giwa, A.; Sodiq, A. The Global Status of Desalination: An Assessment of Current Desalination Technologies, Plants and Capacity. Desalination 2020, 495, 114633. [Google Scholar] [CrossRef]
- Gonzalez, A.; Grágeda, M.; Usha, S. Assessment of Pilot-Scale Water Purification Module with Electrodialysis Technology and Solar Energy. Appl. Energy 2017, 206, 1643–1652. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Sane, M.G.; Parjane, S.B.; Guddad, M. Status of Membrane Distillation for Water and Wastewater Treatment–A Review. Desalination Water Treat. 2014, 52, 5199–5218. [Google Scholar] [CrossRef]
- Soroush, E.; Moravvej, Z.; Rahimpour, M.R. Achievements in Pervaporation Processes for Wastewater and Water Treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–186. [Google Scholar]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. Can Batch or Semi-Batch Processes Save Energy in Reverse-Osmosis Desalination? Desalination 2017, 402, 109–122. [Google Scholar] [CrossRef]
- Wei, X.; Sanders, K.T.; Childress, A.E. Reclaiming Wastewater with Increasing Salinity for Potable Water Reuse: Water Recovery and Energy Consumption during Reverse Osmosis Desalination. Desalination 2021, 520, 115316. [Google Scholar] [CrossRef]
- Panagopoulos, A. Brine Management (Saline Water & Wastewater Effluents): Sustainable Utilization and Resource Recovery Strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) Desalination Systems. Chem. Eng. Process.-Process Intensif. 2022, 176, 108944. [Google Scholar]
- Kayaci, S.; Tantekin-Ersolmaz, S.B.; Ahunbay, M.G.; Krantz, W.B. Technical and economic feasibility of the concurrent desalination and boron removal (CDBR) process. Desalination 2020, 486, 114474. [Google Scholar] [CrossRef]
- Kress, N. Theoretical analysis of the potential impacts of desalination on the marine environment. In Marine Impacts of Seawater Desalination, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–69. [Google Scholar]
- Mickley, M. Updated and Extended Survey of U.S. Municipal Desalination Plants; DWPR Report No. 207; U.S. Department of Interior, Bureau of Reclamation: Washington, DC, USA, 2018.
- Belkin, N.; Rahav, E.; Elifantz, H.; Kress, N.; Berman-Frank, I. The effect of coagulants and antiscalants discharged with seawater desalination brines on coastal microbial communities: A laboratory and in situ study from the southeastern Mediterranean. Water Res. 2017, 110, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Date, M.; Patyal, V.; Jaspal, D.; Malviya, A.; Khare, K. Zero liquid discharge technology for recovery, reuse, and reclamation of wastewater: A critical review. J. Water Process Eng. 2022, 49, 103129. [Google Scholar] [CrossRef]
- Tahir, F.; Al-Ghamdi, S.G. Integrated MED and HDH desalination systems for an energy-efficient zero liquid discharge (ZLD) system. Energy Rep. 2022, 8, 29–34. [Google Scholar] [CrossRef]
- Pala, J.K.; Moulik, S.; Ghosh, A.K.; Kamesh, R.; Roy, A. A Zero Liquid Discharge Strategy with MSF Coupled with Crystallizer. In Sustainable Water Treatment: Advances and Technological Interventions, 1st ed.; Moulik, S., Mullick, A., Roy, A., Eds.; Scrivener Publishing LLC: Salem, MA, USA, 2020; pp. 487–515. [Google Scholar]
- Schwantes, R.; Chavan, K.; Winter, D.; Felsmann, C.; Pfafferott, J. Techno-economic comparison of membrane distillation and MVC in a zero liquid discharge application. Desalination 2018, 428, 50–68. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef]
- Jim, K.M.; Kim, K.J.; Jang, Y.N. Effect of supersaturation on the particle size of ammonium sulfate in semibatch evaporative crystallization. Ind. Eng. Chem. Res. 2013, 52, 11151–11158. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management–Life Cycle Assessment–Principles and Framework. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/37456.html (accessed on 10 July 2023).
- Ecoinvent. System Models. Available online: https://ecoinvent.org/the-ecoinvent-database/system-models/ (accessed on 18 June 2023).
- Ecoinvent. Geographies. Available online: https://ecoinvent.org/the-ecoinvent-database/geographies/ (accessed on 18 June 2023).
- Dow Water Solutions. Filmtec™ Reverse Osmosis Membranes. Technical Manual. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-NF-FilmTec-Manual-45-D01504-en.pdf (accessed on 3 March 2023).
- Mavhungu, A.; Masindi, V.; Foteinis, S.; Mbaya, R.; Tekere, M.; Kortidis, I.; Chatzisymeon, E. Advocating circular economy in wastewater treatment: Struvite formation and drinking water reclamation from real municipal effluents. J. Environ. Chem. Eng. 2020, 8, 103957. [Google Scholar] [CrossRef]
- Park, G.L.; Schäfer, A.I.; Richards, B.S. The effect of intermittent operation on a wind-powered membrane system for brackish water desalination. Water Sci. Technol. 2012, 65, 867–874. [Google Scholar] [CrossRef]
- Ghermandi, A.; Messalem, R. The advantages of NF desalination of brackish water for sustainable irrigation: The case of the Arava Valley in Israel. Desalination Water Treat. 2009, 10, 101–107. [Google Scholar] [CrossRef]
- Panagopoulos, A. Techno-economic assessment of minimal liquid discharge (MLD) treatment systems for saline wastewater (brine) management and treatment. Process Saf. Environ. Prot. 2019, 146, 656–669. [Google Scholar] [CrossRef]
- Cipolletta, G.; Lancioni, N.; Akyol, Ç.; Eusebi, A.L.; Fatone, F. Brine treatment technologies towards minimum/zero liquid discharge and resource recovery: State of the art and techno-economic assessment. J. Environ. Manag. 2021, 300, 113681. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, S.; Masjedi, S.K.; Kazemi, A.; Khaki, E.; Moeinaddini, M.; Olsen, S.I. Life cycle assessment of reverse osmosis for high-salinity seawater desalination process: Potable and industrial water production. J. Clean. Prod. 2023, 382, 135299. [Google Scholar] [CrossRef]
- Borghesi, G.; Stefanini, R.; Vignali, G. Life cycle assessment of packaged organic dairy product: A comparison of different methods for the environmental assessment of alternative scenarios. J. Food Eng. 2022, 318, 110902. [Google Scholar] [CrossRef]
- Clavreul, J.; Guyonnet, D.; Christensen, T.H. Quantifying uncertainty in LCA modelling of waste management systems. Waste Manag. 2012, 32, 2482–2495. [Google Scholar] [CrossRef] [PubMed]
- Elginoz, N.; Papadaskalopoulou, C.; Harris, S. Using life cycle assessment at an early stage of design and development of zero discharge brine treatment and recovery. Water Resour. Ind. 2022, 28, 100184. [Google Scholar] [CrossRef]
- Niero, M.; Pizzol, M.; Bruun, H.G.; Thomsen, M. Comparative life cycle assessment of wastewater treatment in Denmark including sensitivity and uncertainty analysis. J. Clean. Prod. 2014, 68, 25–35. [Google Scholar] [CrossRef]
- Mannan, M.; Alhaj, M.; Mabrouk, A.N.; Al-Ghamdi, S.G. Examining the life-cycle environmental impacts of desalination: A case study in the State of Qatar. Desalination 2019, 452, 238–246. [Google Scholar] [CrossRef]
- Harris, S.; Tsalidis, G.; Corbera, J.B.; Gallart, J.J.E.; Tegstedt, F. Application of LCA and LCC in the early stages of wastewater treatment design: A multiple case study of brine effluents. J. Clean. Prod. 2021, 307, 127298. [Google Scholar] [CrossRef]
- Aziz, N.I.H.A.; Hanafiah, M.M. Application of life cycle assessment for desalination: Progress, challenges and future directions. Environ. Pollut. 2021, 268, 115948. [Google Scholar] [CrossRef]
- Larsen, H.F. LCA of wastewater treatment. In Life Cycle Assessment: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2018; pp. 861–886. [Google Scholar]
- Munoz, I.; Rodriguez, A.; Rosal, R.; Fernandez-Alba, A.R. Life Cycle Assessment of Urban Wastewater Reuse with Ozonation as Tertiary Treatment: A Focus on Toxicity-Related Impacts. Sci. Total Environ. 2009, 407, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.; Fussmann, K.E.; Rahav, E.; Zeev, E.B. Chronic Effects of Brine Discharge from Large-Scale Seawater Reverse Osmosis Desalination Facilities on Benthic Bacteria. Water Res. 2019, 151, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. Mitigation of Eutrophication Caused by Wastewater Discharge: A Simulation-Based Approach. Ambio 2021, 50, 413–424. [Google Scholar] [CrossRef] [PubMed]
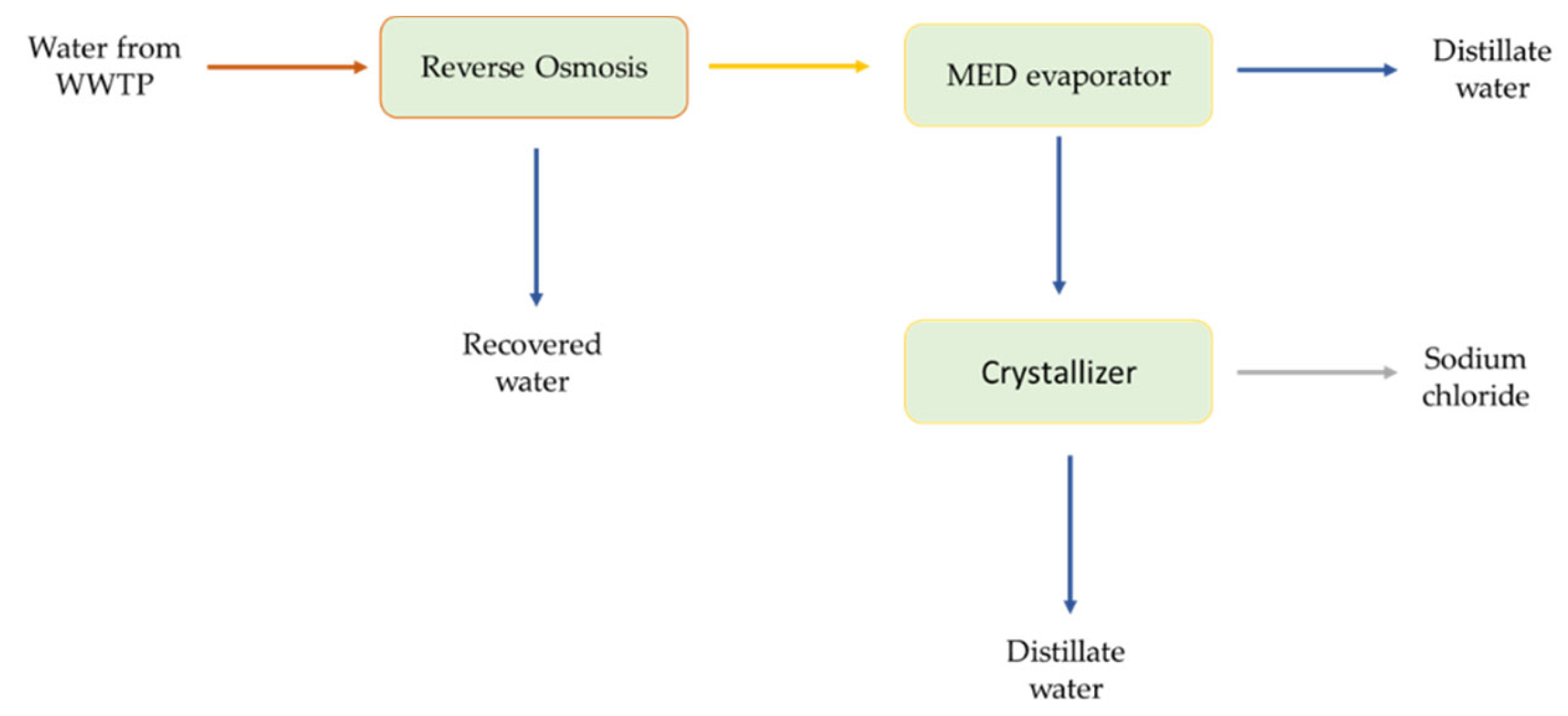

| MLD System | |||||||
|---|---|---|---|---|---|---|---|
| Reverse Osmosis Unit | MED Evaporator | Crystallizer | |||||
| WWTP Effluent | Recovered Water | Generated Brine | Recovered Water | Concentrate Stream | Recovered Water | Saturated in NaCl Stream | |
| K+ (mg/L) | 48.77 | 1.31 | 238.61 | 2.49 | 1193 | 0.43 | 7158 |
| Na+ (mg/L) | 555.7 | 14.81 | 2719 | 8.11 | 13,596 | 1.06 | 81,577 |
| Ca+2 (mg/L) | 169 | 1.66 | 838.36 | 2.64 | 4191 | 0.69 | 25,150 |
| Mg+2 (mg/L) | 69.18 | 0.7 | 343.1 | 0.25 | 1715 | 1.67 | 10,293 |
| Cl− (mg/L) | 784.8 | 20.27 | 3842 | 11 | 19,214 | 0.77 | 115,287 |
| SO4−2 (mg/L) | 634.9 | 6.08 | 3150 | 7.8 | 15,750 | 0.04 | 94,505 |
| HCO3− (mg/L) | 249.2 | 7.334 | 1216 | 0.09 | 6083 | 0.96 | 36,499 |
| pH | 7.12 | 7.72 | 8.25 | 7.72 | 7.54 | 6.12 | 8.12 |
| Inputs to LCA | Unit | 1 m3 of Inlet |
|---|---|---|
| For the first process | ||
| HCl (30%) for the pH adjustment of RO | kg | 0.4025 |
| HCl (30%) for the membrane cleaning of RO | kg | 0.00335 |
| NaOH (50%) membrane cleaning of RO | kg | 0.00289 |
| Seawater reverse osmosis module | m2 | 0.00057 |
| Recovered water from RO | kg | 800 |
| Sodium chloride, brine solution | kg | 2.46 |
| Electric energy of RO | kWh/m3 | 0.8 |
| For the second process | ||
| HCl (30%) for the pH adjustment of RO | kg | 0.4025 |
| HCl (30%) for the membrane cleaning of RO | Kg | 0.00335 |
| NaOH (50%) membrane cleaning of RO | kg | 0.00289 |
| Seawater reverse osmosis module | m2 | 0.00057 |
| Recovered water from RO | kg | 800 |
| Electric energy of RO | kWh/m3 | 0.8 |
| Electric energy of the MED evaporator | kWh/m3 | 50 |
| Distillate water from MED | kg | 160 |
| NaCl salt | kg | 2.52 |
| Distillate water from crystallizer | kg | 33.2 |
| Electric energy of the crystallizer unit | kWh/m3 | 40 |
| Impact Categories | Reference Case | MLD System | |
|---|---|---|---|
| Climate change | kg CO2 eq | 8.111801582 | 76.93662 |
| Ozone depletion | kg CFC-11 eq | 8.74162 × 10−6 | 2.12 × 10−5 |
| Human toxicity, non-cancer effects | CTUh | 8.96984 × 10−6 | −1.3409 × 10−5 |
| Human toxicity, cancer effects | CTUh | 1.13618 × 10−6 | −1.9511 × 10−6 |
| Particulate matter | kg PM2.5 eq | 0.009104561 | 0.057195 |
| Ionizing radiation HH | kBq U235 eq | 1.144598178 | 0.468890 |
| Ionizing radiation E (interim) | CTUe | 3.64172 × 10−6 | 2.28 × 10−5 |
| Photochemical ozone formation | kg NMVOC eq | 0.029059083 | 0.332728 |
| Acidification | molc H+ eq | 0.074622896 | 0.771287 |
| Terrestrial eutrophication | molc N eq | 0.107720009 | 1.13852496 |
| Freshwater eutrophication | kg P eq | 0.00641044 | −0.013632318 |
| Marine eutrophication | kg N eq | 0.009861996 | 0.09536647 |
| Freshwater ecotoxicity | CTUe | 900.8927124 | −1278.14923 |
| Land use | kg C deficit | 21.93650427 | 161.34076 |
| Water resource depletion | m3 water eq | −0.132457986 | −0.2211319 |
| Mineral, fossil, and renewable resource depletion | kg Sb eq | 0.00122319 | −0.001539 |
| Climate Change (kg CO2 eq) | Human Toxicity—Cancer Effect (CTUh) | Human Toxicity—Non-Cancer Effect (CTUh) | Freshwater Ecotoxicity (CTUe) | Mineral, Fossil, and Renewable Resource Depletion (kg Sb eq) | Freshwater Eutrophication (kg P eq) | |
|---|---|---|---|---|---|---|
| Electricity, high voltage | 0.8047 | 2.44535 × 10−8 | 2.66747 × 10−8 | 1.4315 | 3.85123 × 10−6 | 1.16798 × 10−5 |
| Hydrochloric acid for pH adjustment | 0.2368 | 5.2337 × 10−9 | 1.69088 × 10−7 | 10.7306 | 1.50923 × 10−5 | 0.0002 |
| Hydrochloric acid for cleaning | 0.0081 | 8.38407 × 10−10 | 5.79731 × 10−9 | 0.3679 | 5.17449 × 10−7 | 6.52903 × 10−6 |
| Seawater reverse osmosis module | 0.0499 | 7.21336 × 10−11 | 3.42506 × 10−10 | 0.0190 | 6.99749 × 10−8 | 3.80574 × 10−7 |
| Sodium chloride. Brine solution | 7.2769 | 1.23892 × 10−6 | 8.95226 × 10−6 | 893.8007 | 0.001211313 | 0.0064 |
| Sodium hydroxide | 0.0157 | 1.11977 × 10−9 | 5.88437 × 10−9 | 0.3538 | 5.34199 × 10−7 | 8.36219 × 10−6 |
| Recovered water from RO | −0.28041 | −1.34463 × 10−7 | −1.90206 × 10−7 | −5.8109 | −8.18755 × 10−6 | −0.0002 |
| Climate Change (kg CO2 eq) | Human Toxicity—Cancer Effect (CTUh) | Human Toxicity—Non-Cancer Effect (CTUh) | Freshwater Ecotoxicity (CTUe) | Mineral, Fossil, and Renewable Resource Depletion (kg Sb eq) | Freshwater Eutrophication (kg P eq) | |
|---|---|---|---|---|---|---|
| Electricity, high voltage | 91.3480 | 5.94025 × 10−7 | 3.02758 × 10−6 | 162.4729 | 0.00044 | 0.0013 |
| Hydrochloric acid for pH adjustment and cleaning | 0.3907 | 4.11799 × 10−8 | 2.62978 × 10−7 | 15.9803 | 2.2037 × 10−5 | 0.0004 |
| Seawater reverse osmosis module | 0.0499 | 7.21336 × 10−11 | 3.42506 × 10−10 | 0.0190 | 6.99749 × 10−8 | 3.80574 × 10−7 |
| Sodium hydroxide | 0.0101 | 9.98341 × 10−10 | 5.13713 × 10−9 | 0.3437 | 5.04259 × 10−7 | 8.94393 × 10−6 |
| Deionized water | 0.0431 | −8.14836 × 10−9 | −3.16215 × 10−8 | −1.8489 | −2.39396 × 10−6 | −2.84081 × 10−5 |
| Recovered water from RO | −0.2804 | −1.34463 × 10−7 | −1.90206 × 10−7 | −5.8109 | −8.18756 × 10−6 | −0.0002 |
| Sodium chloride | −14.6247 | −2.44509 × 10−6 | −1.64832 × 10−5 | −1449.3054 | −0.00199 | −0.0151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramidi, M.; Spyropoulou, C.; Loizou, C.; Kyriazi, M.; Novakovic, J.; Moustakas, K.; Malamis, D.; Loizidou, M. Adding Value to Reclaimed Water from Wastewater Treatment Plants: The Environmental Feasibility of a Minimal Liquid Discharge System for the Case Study of Larnaca. Sustainability 2023, 15, 14305. https://doi.org/10.3390/su151914305
Avramidi M, Spyropoulou C, Loizou C, Kyriazi M, Novakovic J, Moustakas K, Malamis D, Loizidou M. Adding Value to Reclaimed Water from Wastewater Treatment Plants: The Environmental Feasibility of a Minimal Liquid Discharge System for the Case Study of Larnaca. Sustainability. 2023; 15(19):14305. https://doi.org/10.3390/su151914305
Chicago/Turabian StyleAvramidi, Maria, Christina Spyropoulou, Constantinos Loizou, Maria Kyriazi, Jelica Novakovic, Konstantinos Moustakas, Dimitris Malamis, and Maria Loizidou. 2023. "Adding Value to Reclaimed Water from Wastewater Treatment Plants: The Environmental Feasibility of a Minimal Liquid Discharge System for the Case Study of Larnaca" Sustainability 15, no. 19: 14305. https://doi.org/10.3390/su151914305
APA StyleAvramidi, M., Spyropoulou, C., Loizou, C., Kyriazi, M., Novakovic, J., Moustakas, K., Malamis, D., & Loizidou, M. (2023). Adding Value to Reclaimed Water from Wastewater Treatment Plants: The Environmental Feasibility of a Minimal Liquid Discharge System for the Case Study of Larnaca. Sustainability, 15(19), 14305. https://doi.org/10.3390/su151914305









