Research on Greenhouse Gas Emission Reduction Methods of SBR and Anoxic Oxic Urban Sewage Treatment System
Abstract
1. Introduction
2. Related Works
3. Experimental Methods and Materials
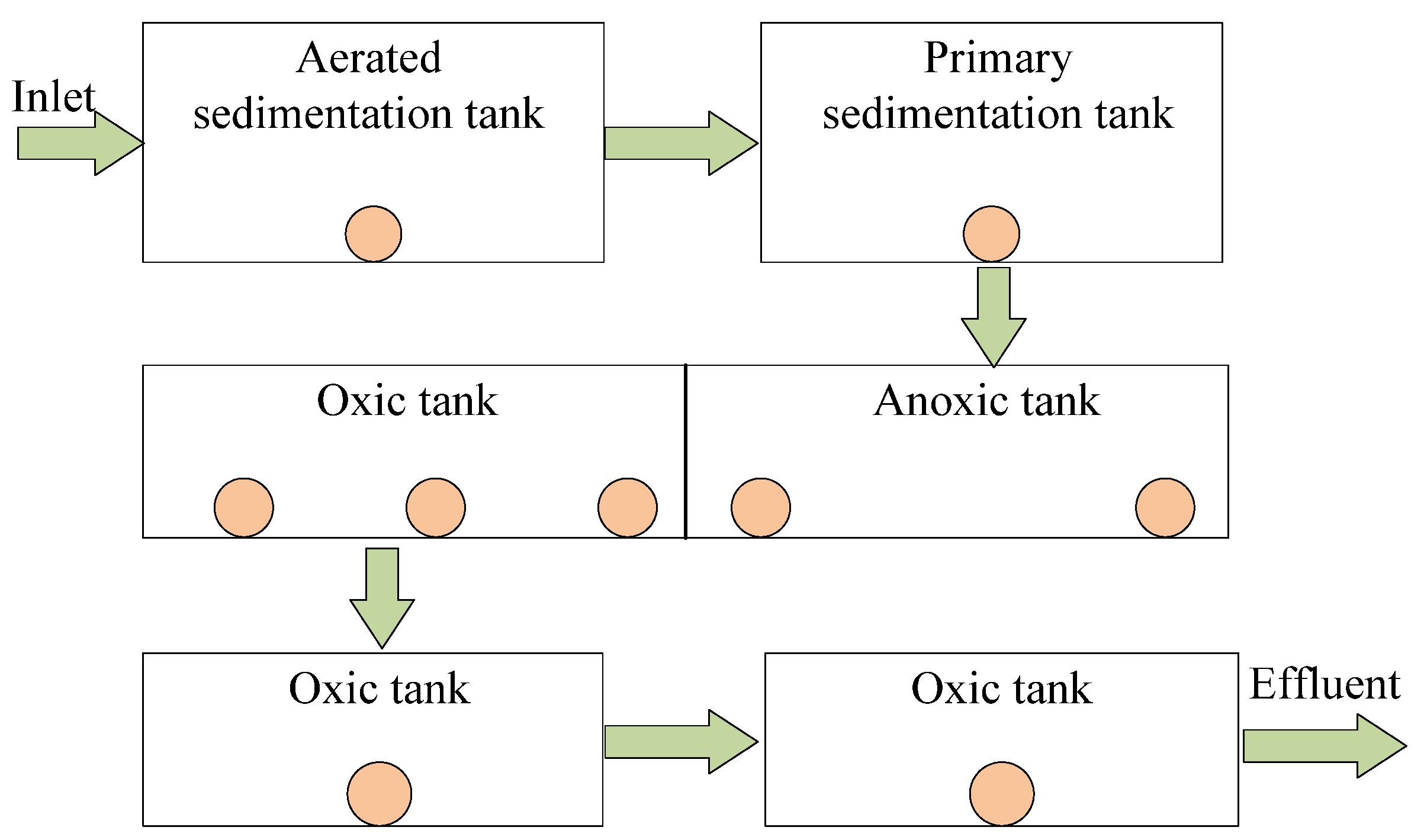
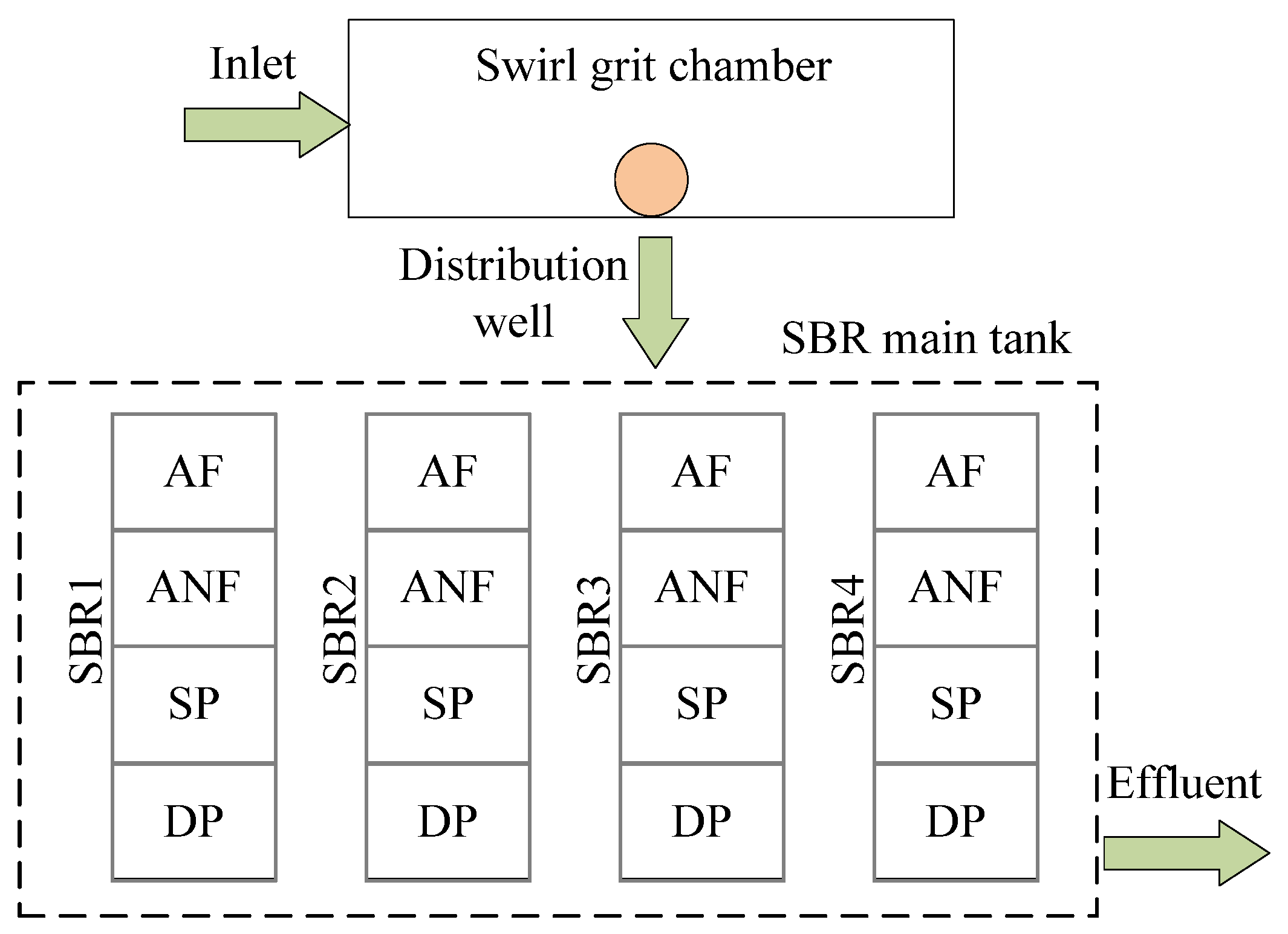
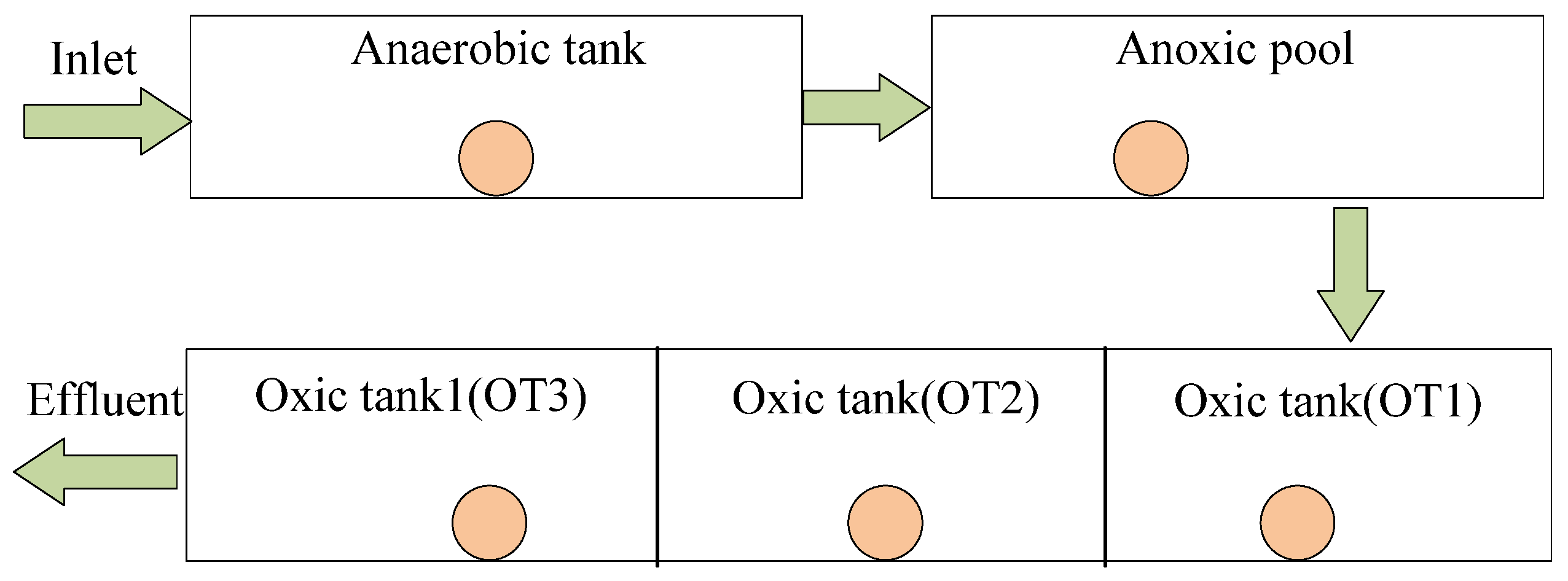
3.1. Environmental Setting of Sewage Treatment Plant
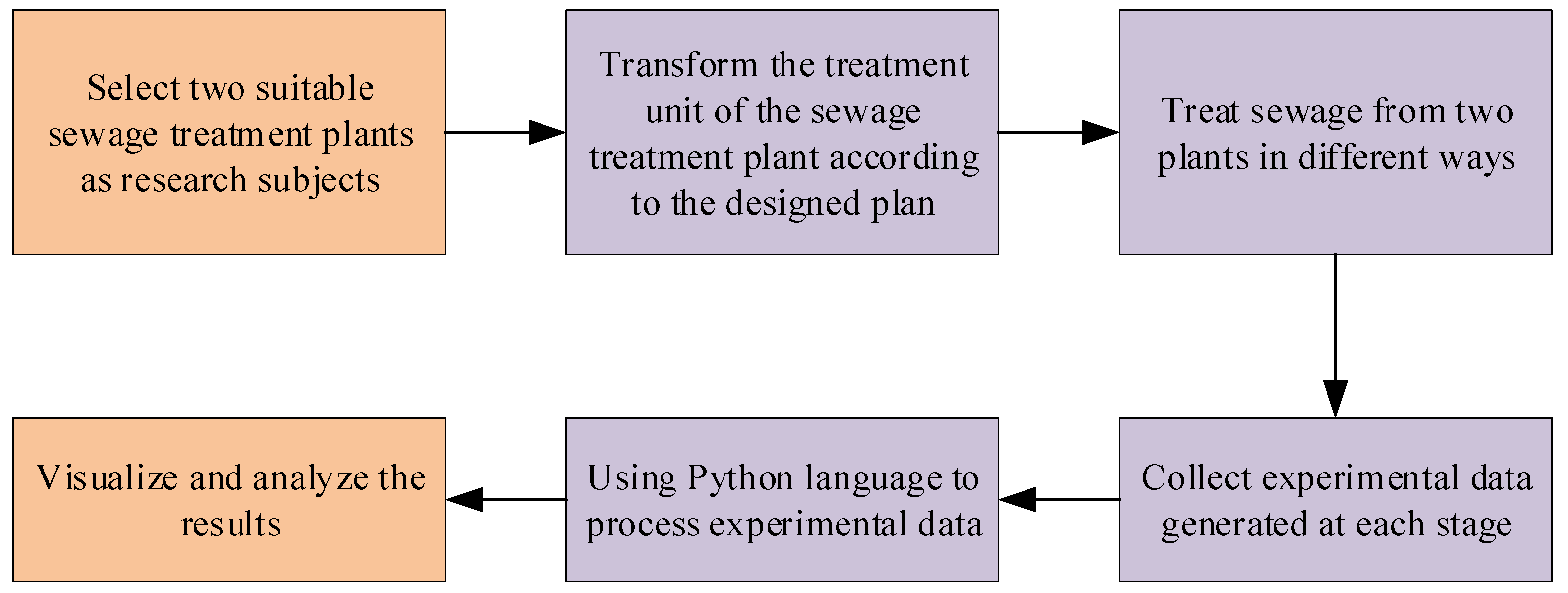
3.2. Experimental Scheme
3.3. Experimental Sampling Method
3.4. Experimental Instruments and Reagents
3.5. Calculation Method of Experimental Indices
4. Results
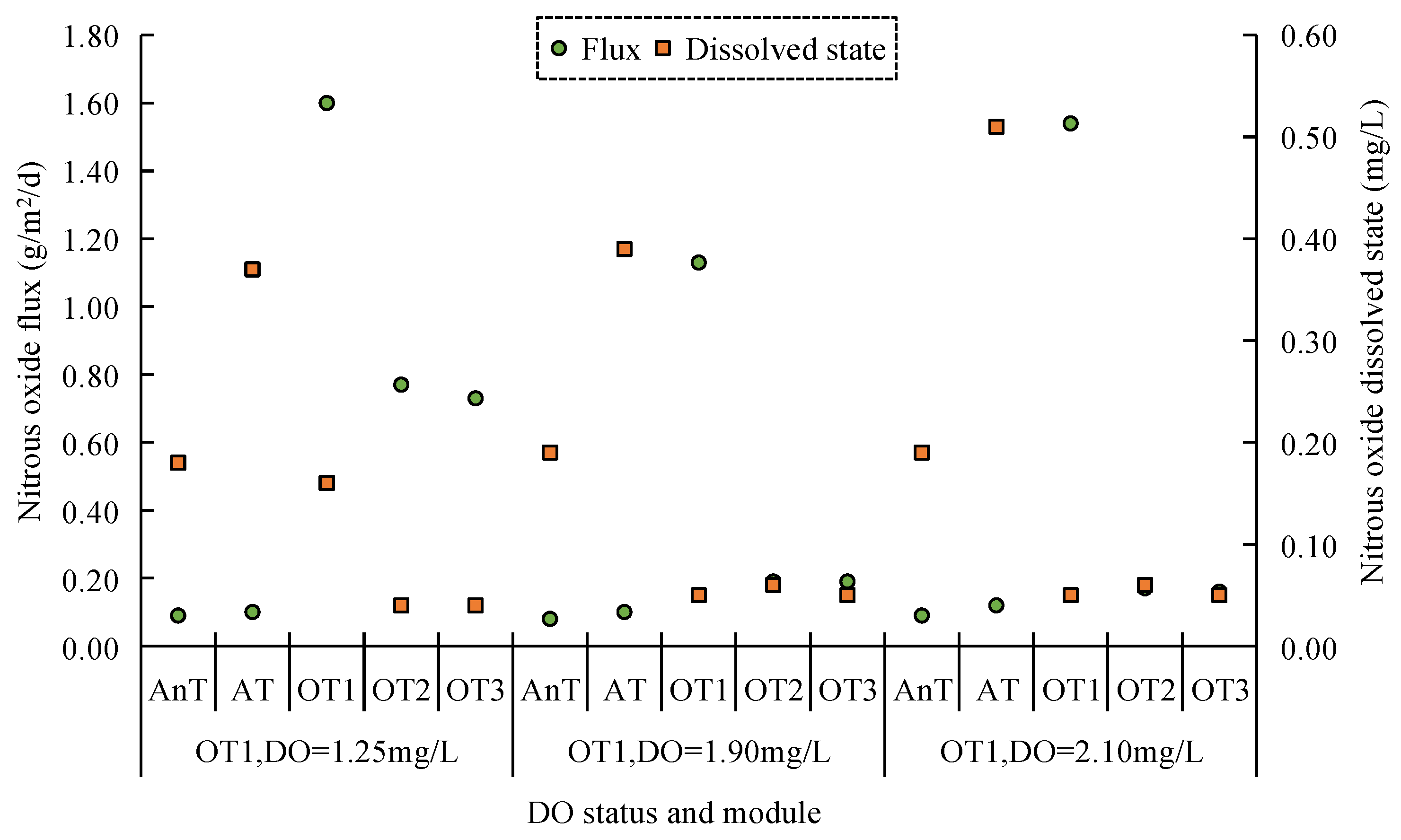
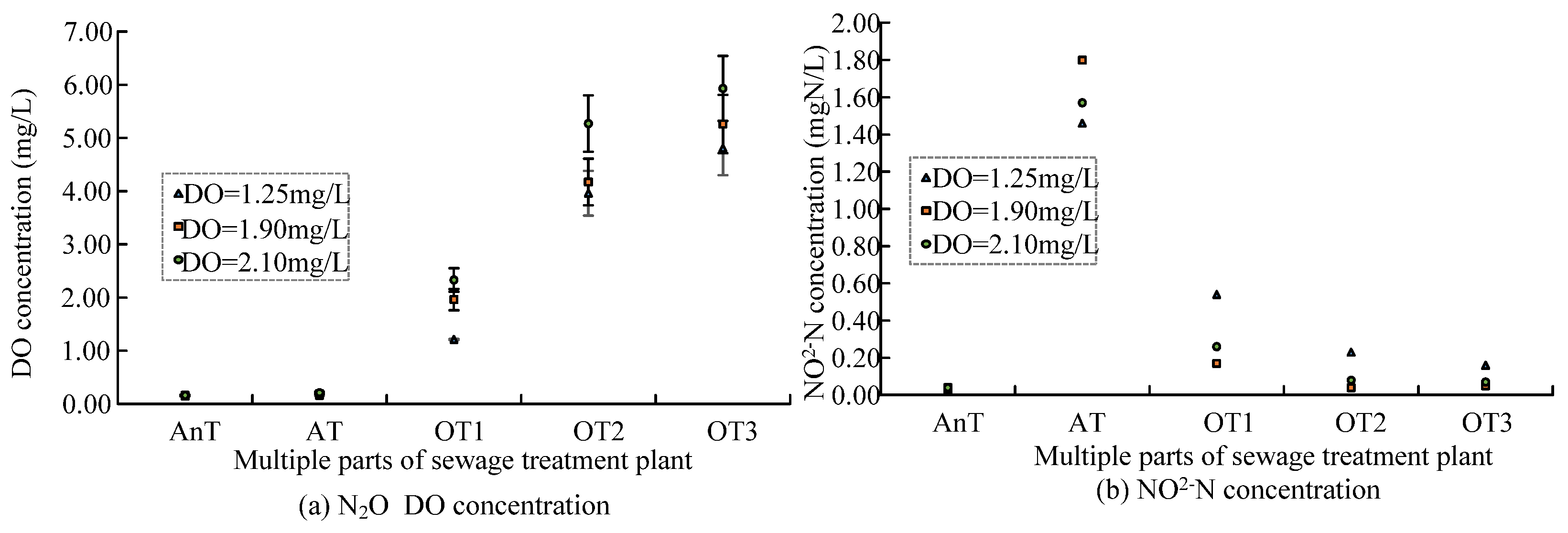
4.1. Impact of DO Concentration in A/O Sewage Treatment Method on Greenhouse
Gas Emissions
4.2. Effect of Temperature on Greenhouse Gas Emissions in PN-SBR Sewage Treatment Process
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murat-Blazejewska, S. Converting sewage holding tanks to rainwater harvesting tanks in Poland. Arch. Environ. Prot. 2020, 46, 121–131. [Google Scholar] [CrossRef]
- Marzec, I.; Bobiński, J.; Tejchman, J.; Schonnagel, J. Finite element analysis on failure of reinforced concrete corner in sewage tank under opening bending moment. Eng. Struct. 2020, 228, 111506. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Lindberg, L.; Ermolaev, E.; Vinners, B.; Lalander, C. Process efficiency and greenhouse gas emissions in black soldier fly larvae composting of fruit and vegetable waste with and without pre-treatment. J. Clean. Prod. 2022, 338, 130552. [Google Scholar] [CrossRef]
- Negi, H.; Agrawal, R.; Verma, A.; Goel, R. Municipal solid waste to bioenergy: Current status, opportunities, and challenges in Indian context. New Future Dev. Microb. Biotechnol. Bioeng. 2019, 191–203. [Google Scholar] [CrossRef]
- Agrawal, R.; Verma, A.; Verma, S.; Varma, A. Industrial methanogenesis: Biomethane production from organic wastes for energy supplementation. J. Recent Dev. Microb. Technol. 2021, 99–115. [Google Scholar] [CrossRef]
- Zhuang, H.; Guan, J.; Leu, S.Y.; Wang, Y.; Wang, H. Carbon footprint analysis of chemical enhanced primary treatment and sludge incineration for sewage treatment in Hong Kong. J. Clean. Prod. 2020, 272, 122630. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.T.; Park, Y.K. A comparative study on the reduction effect in greenhouse gas emissions between the combined heat and power plant and boiler. Sustainability 2020, 12, 5144. [Google Scholar] [CrossRef]
- Zaborowska, E.; Czerwionka, K.; Mkinia, J. Integrated plant-wide modelling for evaluation of the energy balance and greenhouse gas footprint in large wastewater treatment plants. Appl. Energy 2020, 282, 116126. [Google Scholar] [CrossRef]
- Jafri, Y.; Wetterlund, E.; Mesfun, S.; Radberg, H.; Mossberg, J.; Hulteberg, C.; Furusjo, E. Combining expansion in pulp capacity with production of sustainable biofuels—Techno-economic and greenhouse gas emissions assessment of drop-in fuels from black liquor part-streams. Appl. Energ. 2020, 279, 115879. [Google Scholar] [CrossRef]
- Kyung, D.; Jung, D.Y.; Lim, S.R. Estimation of greenhouse gas emissions from an underground wastewater treatment plant. Membr. Water Treat. 2020, 11, 173–177. [Google Scholar] [CrossRef]
- Lofty, J.; Muhawenimana, V.; Wilson, C.; Ouro, P. Microplastics removal from a primary settler tank in a wastewater treatment plant and estimations of contamination onto European agricultural land via sewage sludge recycling. Environ. Pollut. 2022, 304, 119198. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.D.; Park, D.G.; Hong, S.G.; Jeong, C.; Kim, H.; Chung, W. Influence of activated biochar pellet fertilizer application on greenhouse gas emissions and carbon sequestration in rice (Oryza sativa L.) production. Environ. Pollut. 2021, 285, 117457. [Google Scholar] [CrossRef]
- Puga, A.P.; Grutzmacher, P.; Cerri, C.; Ribeirinho, R.; Andrade, D. Biochar-based nitrogen fertilizers, Greenhouse gas emissions, use efficiency, and maize yield in tropical soils. Sci. Total Environ. 2020, 704, 135375. [Google Scholar] [CrossRef] [PubMed]
- Sperber, J.L.; Troyer, B.; Norman, M.; McPhillips, J.; Watson, K.; Erickson, E. PSIV-7 effect of biochar supplementation in beef cattle growing diets on greenhouse gas emissions. J. Anim. Sci. 2021, 99 (Suppl. 1), 211–219. [Google Scholar] [CrossRef]
- Rüdisüli, M.; Bach, C.; Bauer, C.; Beloin-Saint-Pierre, D.; Elbe, U.; Georges, G.; Limpach, R.; Pareschi, G.; Kannan, R.; Teske, L. Prospective life-cycle assessment of greenhouse gas emissions of electricity-based mobility options. Appl. Energy 2022, 306, 118065. [Google Scholar] [CrossRef]
- Hua, H.; Jiang, S.; Yuan, Z.; Liu, X.; Zhang, Y.; Cai, Z. Advancing greenhouse gas emission factors for municipal wastewater treatment plants in China. Environ. Pollut. 2022, 295, 118648. [Google Scholar] [CrossRef]
- Farkas, A.; Degiuli, N.; Marti, I.; Vujanovic, M. Greenhouse gas emissions reduction potential by using antifouling coatings in a maritime transport industry. J. Clean. Prod. 2021, 295, 126428. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, F.; Ma, X.; Guo, G. Greenhouse gas emissions from vegetables production in China. J. Clean. Prod. 2021, 317, 128449. [Google Scholar] [CrossRef]
- Kavehei, E.; Iram, N.; Rashti, M.R.; Jenkins, A.; Lemckert, C.; Adame, F. Greenhouse gas emissions from stormwater bioretention basins. Ecol. Eng. 2021, 159, 106120. [Google Scholar] [CrossRef]
- Wu, F.; Li, L.; Crandon, L.; Cao, Y.; Cheng, F.; Hicks, A.; Zeng, Y.; You, J. Environmental hotspots and greenhouse gas reduction potential for different lithium-ion battery recovery strategies. J. Clean. Prod. 2022, 2022, 130697. [Google Scholar] [CrossRef]
- Wich-Konrad, T.; Lüke, W.; Oles, M.; Deerberg, G. Assessment of industrial greenhouse gas reduction strategies within consistent system boundaries. Chem. Ing. Tech. 2020, 92, 1393–1402. [Google Scholar] [CrossRef]
- Ulrich, S.; Trench, A.; Hagemann, S. Gold mining greenhouse gas emissions, abatement measures, and the impact of a carbon price. J. Clean. Prod. 2022, 340, 130851. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, B.; Shao, S.; Nan, Z.; Dashan, W.; Zhenzhong, Z.; Junguo, L. Two-tier synergic governance of greenhouse gas emissions and air pollution in China’s megacity, Shenzhen: Impact evaluation and policy implication. Environ. Sci. Technol. 2021, 55, 7225–7236. [Google Scholar] [CrossRef]
- Lu, Y.; Schandl, H. Do sectoral material efficiency improvements add up to greenhouse gas emissions reduction on an economy-wide level? J. Ind. Ecol. 2021, 25, 145–149. [Google Scholar] [CrossRef]
- Gonzalez-Diaz, A.; Jiang, L.; Roskilly, A.P.; Roskilly, P.; Smallbone, J. The potential of decarbonising rice and wheat by incorporating carbon capture, utilisation and storage into fertiliser production. Green Chem. 2020, 22, 882–894. [Google Scholar] [CrossRef]
- Runge, E.; Langille, J.; Schentag, C.; Bourassa, A.; Letros, D.; Loewen, P.; Lloyd, N.; Degenstein, D.; Grandmont, F. A balloon-borne imaging Fourier transform spectrometer for atmospheric trace gas profiling. Rev. Sci. Instr. 2021, 92, 94502. [Google Scholar] [CrossRef]
- Yaman, C.; Anil, I.; Alagha, O. Potential for greenhouse gas reduction and energy recovery from MSW through different waste management technologies. J. Clean. Prod. 2020, 264, 121432. [Google Scholar] [CrossRef]
- Al-Douri, A.; Alsuhaibani, A.S.; Moore, M.; Nielsen, B.; El-Baz, A.; El-Halwagi, M. Greenhouse gases emissions in liquified natural gas as a marine fuel: Life cycle analysis and reduction potential. Can. J. Chem. Eng. 2021, 100, 1178–1186. [Google Scholar] [CrossRef]
- Jaworski, A.; Mdziel, M.; Kuszewski, H. Sustainable public transport strategies—Decomposition of the bus fleet and its influence on the decrease in greenhouse gas emissions. Energies 2022, 15, 2238. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Bi, X.; Clift, R. Greenhouse gas emission reduction potential and cost of bioenergy in British Columbia, Canada. Energy Policy 2020, 138, 111285. [Google Scholar] [CrossRef]
- Aurea, A.; Da, S.; Bertelli, F.L.; Andrade, R.A.; Eduardo, F.L.; Yuri, R.G. PSVIII-19 Greenhouse gas emissions from beef cattle production in Brazil: How we can mitigate from animal operations? J. Anim. Sci. 2021, 99 (Suppl. 3), 430–436. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Cao, R.; Zeng, W. Refrigerant alternative and optimization under the constraint of the greenhouse gas emissions reduction target. J. Clean. Prod. 2021, 296, 126580. [Google Scholar] [CrossRef]
- Lemma, B.; Ararso, K.; Evangelista, P.H. Attitude towards biogas technology, use and prospects for greenhouse gas emission reduction in southern Ethiopia. J. Clean. Prod. 2020, 283, 124608. [Google Scholar] [CrossRef]
- Malav, M.K.; Prasad, S.; Jain, N.; Dinesh, K.; Kanojiya, S. Effect of organic rice (Oryza sativa) cultivation on greenhouse gas emission. Indian J. Agric. Sci. 2020, 90, 1769–1775. [Google Scholar] [CrossRef]
- Guo, X.; Broeze, J.; Groot, J.J.; Axmann, H. A worldwide hotspot analysis on food loss and waste, associated greenhouse gas emissions, and protein losses. Sustainability 2020, 12, 7488. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, S.; Peng, T.; Ou, X. Greenhouse gas life cycle analysis of China’s fuel cell medium- and heavy-duty trucks under segmented usage scenarios and vehicle types. Energy 2022, 249, 123628. [Google Scholar] [CrossRef]
- Rashid, M.I.; Benhelal, E.; Rafiq, S. Reduction of greenhouse gas emissions from gas, oil, and coal power plants in pakistan by carbon capture and storage (CCS): A review. Chem. Eng. Technol. 2020, 43, 2140–2148. [Google Scholar] [CrossRef]
- Vahidi, E.; Kirchain, R.; Burek, J.; Gregory, J. Regional variation of greenhouse gas mitigation strategies for the United States building sector. Appl. Energ. 2021, 302, 117527. [Google Scholar] [CrossRef]
- Elobeid, A.; Carriquiry, M.; Dumortier, J.; Swenson, D.; Hayes, D. China-U.S.trade dispute and its impact on global agricultural markets, the U.S. economy, and greenhouse gas emissions. J. Agric. Econ. 2021, 72, 647–672. [Google Scholar] [CrossRef]
- Sapkota, K.; Gemechu, E.; Oni, A.O.; Ma, L.; Kumar, A. Greenhouse gas emissions from Canadian oil sands supply chains to China. Energy 2022, 251, 123850. [Google Scholar] [CrossRef]
- Müller, R.C.; Schiessl, A.; Volk, R.; Schultmann, F. Assessment of site-specific greenhouse gas emissions of chemical producers: Case studies of propylene and toluene diisocyanate. J. Clean. Prod. 2021, 317, 128086. [Google Scholar] [CrossRef]
- Bo, Y.; Jgermeyr, J.; Yin, Z.; Jiang, Y.; Xu, J.; Liang, H.; Zhou, F. Global benefits of non-continuous flooding to reduce greenhouse gases and irrigation water use without rice yield penalty. J. Glob. Chang. Biol. 2022, 28, 3636–3650. [Google Scholar] [CrossRef] [PubMed]
- Bakkaloglu, S.; Lowry, D.; Fisher, R.E.; Menoud, M.; Lanoisellé, M.; Chen, H.; Rckmann, T.; Nisbet, E.G. Stable isotopic signatures of methane from waste sources through atmospheric measurements. J. Atmos. Environ. 2022, 276, 119021. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Li, Y.; Kumar, A.; Kleeman, M.J. Future emissions of particles and gases that cause regional air pollution in California under different greenhouse gas mitigation strategies. J. Atmos. Environ. 2022, 273, 118960. [Google Scholar] [CrossRef]
- Iqbal, A.; Ekama, G.A.; Zan, F.; Liu, X.; Chui, H.K.; Chen, G.H. Potential for co-disposal and treatment of food waste with sewage: A plant-wide steady-state model evaluation. Water Res. 2020, 184, 116175. [Google Scholar] [CrossRef]
- Poblete, I.; Araújo, M. Sewage-water treatment with bio-energy production and carbon capture and storage. Chemosphere 2021, 286, 131763. [Google Scholar] [CrossRef]
- Díaz, I.; Fdz-Polanco, F.; Mutsvene, B.; Fdz-Polanco, M. Effect of operating pressure on direct biomethane production from carbon dioxide and exogenous hydrogen in the anaerobic digestion of sewage sludge. Appl. Energy 2020, 280, 115915. [Google Scholar] [CrossRef]
- Vane, C.H.; Kim, A.W.; Lopes, D.; Moss, H.V. Contrasting sewage, emerging and persistent organic pollutants in sediment cores from the River Thames estuary, London, England, UK. Mar. Pollut. Bull. 2022, 175, 113340. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, G.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S. Effects of the lignite bioorganic fertilizer on greenhouse gas emissions and pathways of nitrogen and carbon cycling in saline-sodic farmlands at Northwest China. J. Clean. Prod. 2022, 334, 130080. [Google Scholar] [CrossRef]
- Kavanagh, I.; Fenton, O.; Healy, M.G. Mitigating ammonia and greenhouse gas emissions from stored cattle slurry using agricultural waste, commercially available products and a chemical acidifier. J. Clean. Prod. 2021, 294, 126251. [Google Scholar] [CrossRef]
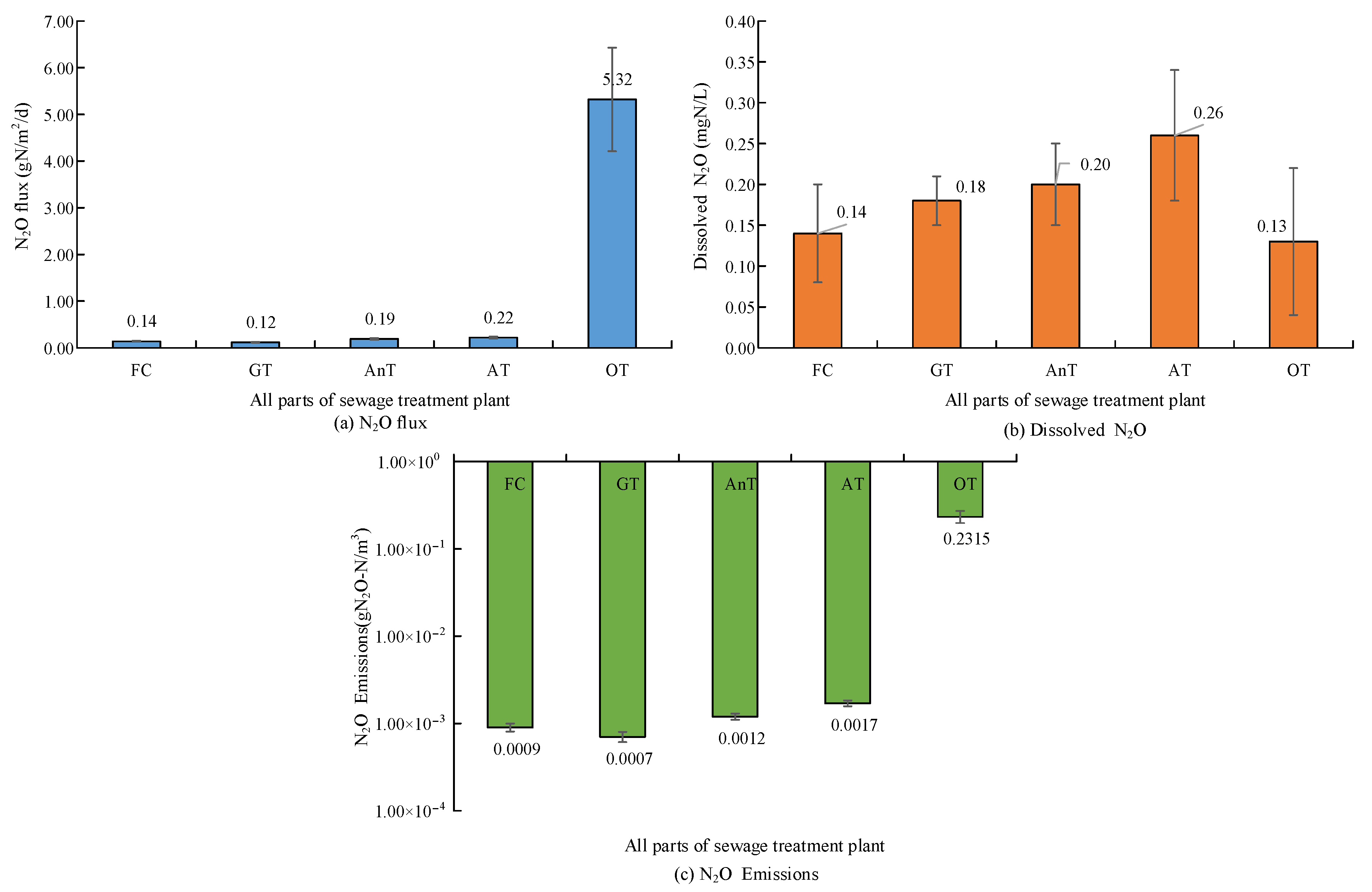
| Reference Number | Author | Title | Contribution |
|---|---|---|---|
| [7] | Zhuang, H., Guan, J., Leu, S.Y., Wang, Y., Wang, H | Carbon footprint analysis of chemical-enhanced primary treatment and sludge incineration for sewage treatment in Hong Kong | Developed nine different sewage treatment processes, some of which can reduce greenhouse gas emissions from sewage treatment |
| [8] | Kim, D., Kim, K.T., Park, Y.K | A comparative study on the reduction effect in greenhouse gas emissions between the combined heat and power plant and boiler | An experiment was conducted, and it was found that boilers emit higher greenhouse gases throughout their entire lifecycle than cogeneration plants |
| [9] | Zaborowska, E., Czerwionka, K., Mkinia, J | Integrated plant-wide modeling for evaluation of the energy balance and greenhouse gas footprint in large wastewater treatment plants | Designed a comprehensive model of the entire sewage treatment plant |
| [10] | Jafri, Y., Wetterlund, E., Mesfun, S., Radberg, H., Mossberg, J., Hulteberg, C., Furusjo, E | Combining expansion in pulp capacity with production of sustainable biofuels—techno-economic and greenhouse gas emissions assessment of drop-in fuels from black liquor part-streams | An improved pyrolysis bio-oil method was analyzed as an energy source for transportation vehicles; experiments showed that this method can also provide sufficient power for transportation vehicles and lower greenhouse gas emissions |
| [11] | Kyung, D., Jung, D.Y., Lim, S.R | Estimation of greenhouse gas emissions from an underground wastewater treatment plant | Several strategies have been proposed to help reduce wastewater discharge |
| [12] | Lofty, J., Muhawenimana, V., Wilson, C., Ouro, P | Microplastics removal from a primary settler tank in a wastewater treatment plant and estimations of contamination onto European agricultural land via sewage sludge recycling | Research has found that appropriately reducing the operating environment temperature of sewage treatment plants can help reduce the rate of greenhouse gas emissions, but too low an environmental temperature can lead to a decrease in the quality of sewage treatment |
| Module Number | Modular | Module Abbreviation | Volume (m3) | Surface Area (m2) | HRT (h) |
|---|---|---|---|---|---|
| 1 | Aerated grit chambers | AGT | 2256 | 498 | 0.02 |
| 2 | Primary sedimentation tank | PST | 149,709 | 24,910 | / |
| 3 | Anoxic pool | AT | 31,988 | 5279 | 1.55 |
| 4 | Aerobic tank | OT | 159,624 | 27,061 | 7.68 |
| 5 | Secondary sedimentation tank | FC | 93,785 | 24,073 | 2.50–4.10 |
| Module Number | Modular | Module Abbreviation | HRT (h) | Total Surface Area |
|---|---|---|---|---|
| 1 | Grit chamber | GT | 0.12 | 119.6 |
| 2 | Anaerobic tank | AnT | 1.10 | 1120.0 |
| 3 | Anoxic pool | AT | 1.10 | 1120.0 |
| 4 | Aerobic tank | OT | 5.97 | 6500.0 |
| 5 | Secondary sedimentation tank | FC | 3.14 | 1910.0 |
| Index No | Evaluating Indicator | Index Value (mg/L) | Index No | Evaluating Indicator | Index Value (mg/L) |
|---|---|---|---|---|---|
| 1 | Inlet water TN | 33.8 ± 3.5 | 07 | Outlet water TN | 15.26 ± 1.88 |
| 2 | Inlet water SS | 98.4 ± 14.2 | 08 | Outlet water TSS | 12.98 ± 4.10 |
| 3 | Inlet water COD/N | 5.91 ± 0.97 L | 09 | Temperature | 25.8 ± 2.5 |
| 4 | COD of inlet water | 189.7 ± 25.1 | 10 | COD of outlet water | 22.4 ± 4.1 |
| 5 | Inlet water BOD | 76.2 ± 10.3 L | 11 | Outlet water BOD | 11.62 ± 2.40 |
| 6 | Inlet water NH4+-N | 22.5 ± 4.1 | 12 | Outlet water NH4+-N | 0.81 ± 0.13 |
| Scheme No | Aeration Rate m3air/m3water/h (DO Concentration mg/L) | NH4+ Removal Rate % | TN Removal Rate % | COD Removal Rate % | N2O Emission Coefficient % (Nitrogen Element of Influent Water) |
|---|---|---|---|---|---|
| 1 | 2.00 | 96.37 ± 0.48 | 52.25 ± 4.42 | 87.54 ± 3.59 | 0.75 ± 0.20 |
| 2 | 3.00 (1.25) | 96.86 ± 0.77 | 56.69 ± 3.67 | 88.51 ± 3.82 | 0.67 ± 0.14 |
| 3 | 4.00 (1.90) | 97.52 ± 0.82 | 52.79 ± 4.09 | 87.07 ± 3.41 | 0.31 ± 0.07 |
| 4 | 5.00 (2.10) | 97.54 ± 0.88 | 49.57 ± 4.08 | 87.86 ± 3.75 | 0.38 ± 0.05 |
| 5 | 6.00 (2.20) | 98.95 ± 0.92 | 47.31 ± 3.77 | 87.51 ± 3.56 | 0.42 ± 0.06 |
| / | / | AER1-N2O | AER2-N2O | AER1-NO | AER2-NO | sAOR |
|---|---|---|---|---|---|---|
| Temperature | Correlation coefficient | 0.946 | 0.651 | 0.850 | 0.844 | 0.927 |
| P | 0.0230 | 0.684 | 0.379 | 0.326 | 0.014 | |
| AER1-N2O | Correlation coefficient | 1 | 0.784 | 0.745 | 0.681 | 0.953 |
| P | / | 0.602 | 0.559 | 0.513 | 0.012 | |
| AER2-N2O | Correlation coefficient | / | 1 | 0.158 | 0.159 | 0.792 |
| P | / | / | 0.886 | 0.745 | 0.358 | |
| AER1-NO | Correlation coefficient | / | / | 1 | 1.000 | 0.663 |
| P | / | / | / | 0.000 | 0.485 | |
| AER2-NO | Correlation coefficient | / | / | / | 1 | 0.715 |
| P | / | / | / | / | 0.483 | |
| sAOR | Correlation coefficient | / | / | / | / | 1 |
| P | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Li, W.; Xu, J. Research on Greenhouse Gas Emission Reduction Methods of SBR and Anoxic Oxic Urban Sewage Treatment System. Sustainability 2023, 15, 7234. https://doi.org/10.3390/su15097234
Bai M, Li W, Xu J. Research on Greenhouse Gas Emission Reduction Methods of SBR and Anoxic Oxic Urban Sewage Treatment System. Sustainability. 2023; 15(9):7234. https://doi.org/10.3390/su15097234
Chicago/Turabian StyleBai, Mei, Wen Li, and Jin Xu. 2023. "Research on Greenhouse Gas Emission Reduction Methods of SBR and Anoxic Oxic Urban Sewage Treatment System" Sustainability 15, no. 9: 7234. https://doi.org/10.3390/su15097234
APA StyleBai, M., Li, W., & Xu, J. (2023). Research on Greenhouse Gas Emission Reduction Methods of SBR and Anoxic Oxic Urban Sewage Treatment System. Sustainability, 15(9), 7234. https://doi.org/10.3390/su15097234





