A Study of Treatment of Reactive Red 45 Dye by Advanced Oxidation Processes and Toxicity Evaluation Using Bioassays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Radiation Treatment of Dye Samples
2.3. Determination of Chemical Oxygen Demand (COD)
2.4. Determination of G-Value, Dose Constant (k), D0.50, D0.90, and D0.99
2.5. Toxicity Evaluation
3. Results and Discussion
3.1. Effect of Hydrogen Peroxide on the Degradation of Reactive Red 45 Dye
3.2. Effect of Radiation on the Degradation
3.3. Effect of Radiation on Chemical Oxygen Demand (COD)
3.4. Effect of Radiation on pH of Reactive Red 45 Dye Aqueous Solutions
3.5. Dose Constant (k), Removal Efficiency (G-Value), D0.50, D0.90, and D0.99
3.6. Cytotoxicity of Reactive Red 45 Dye
3.7. Mutagenicity Evaluation of Reactive Red 45 Dye
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.-U.; Bhatti, H.N.; Iqbal, M.; Nazir, A.; Ain, H. Kinetic Study of Degradation of Basic Turquise Blue X-GB and Basic Blue X-GRRL using Advanced Oxidation Process. Z. Phys. Chem. 2019, 234, 1803–1817. [Google Scholar] [CrossRef]
- Ezzatzadeh, E. Green synthesis of α-aminophosphonates using ZnO nanoparticles as an efficient catalyst. Z. Naturforsch. B 2018, 73, 179–184. [Google Scholar] [CrossRef]
- Kanjal, M.I.; Muneer, M.; Bhatti, I.A.; Saeed, M.; Atta-Ul-Haq, A.-U.; Den, N.Z.U.; Haq, E.U.; Nisar, J.; Iqbal, M. Gamma and UV radiation induced degradation of methotrexate (anti-rheumatic drug) in aqueous solution and conditions optimization. Desalinat. Water Treat. 2020, 191, 332–341. [Google Scholar] [CrossRef]
- Ali, S.; Muneer, M.; Khosa, M.K.K.; Alfryyan, N.; Iqbal, M. Ionizing radiation based advanced oxidation process for reactive orange 122 dye degradation and kinetics studies. Z. Phys. Chem. 2022, 236, 1321–1338. [Google Scholar] [CrossRef]
- Irfan, A.; Abbas, G. Exploring the Photovoltaic Properties of Metal Bipyridine Complexes (Metal = Fe, Zn, Cr, and Ru) by Density Functional Theory. Z. Naturforsch. A 2018, 73, 337–344. [Google Scholar] [CrossRef]
- Rauf, M.; Marzouki, N.; Körbahti, B.K. Photolytic decolorization of Rose Bengal by UV/H2O2 and data optimization using response surface method. J. Hazard. Mater. 2008, 159, 602–609. [Google Scholar] [CrossRef]
- Zamouche, M.; Tahraoui, H.; Laggoun, Z.; Mechati, S.; Chemchmi, R.; Kanjal, M.I.; Amrane, A.; Hadadi, A.; Mouni, L. Optimization and Prediction of Stability of Emulsified Liquid Membrane (ELM): Artificial Neural Network. Processes 2023, 11, 364. [Google Scholar] [CrossRef]
- Kanjal, M.I.; Muneer, M.; Abdelhaleem, A.; Chu, W. Degradation of methotrexate by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism. Chin. J. Chem. Eng. 2020, 28, 2658–2667. [Google Scholar] [CrossRef]
- Qashqoosh, M.T.A.; Alahdal, F.A.M.; Kadaf Manea, Y.; Zakariya, S.M.; Naqvi, S. Synthesis, characterization, spectroscopic and docking studies of antiulcer and nanoantiulcer drugs (LPZ and NLPZ) with bovine serum albumin. Chem. Phys. 2019, 527, 110462. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, S.; Znad, H.; Hasan, N. Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J. Mol. Liq. 2021, 329, 115541. [Google Scholar] [CrossRef]
- Noreen, S.; Ismail, S.; Ibrahim, S.M.; Kusuma, H.S.; Nazir, A.; Yaseen, M.; Iqbal, M. ZnO, CuO and Fe2O3 green synthesis for the adsorptive removal of direct golden yellow dye adsorption: Kinetics, equilibrium and thermodynamics studies. Z. Phys. Chem. 2021, 235, 1055–1075. [Google Scholar] [CrossRef]
- Muneer, M.; Kanjal, M.I.; Saeed, M.; Jamal, M.A.; Haq, A.U.; Iqbal, M.; Haq, E.U.; Ali, S. Degradation of moxifloxacin by ionizing radiation and toxicity assessment. Z. Phys. Chem. 2021, 235, 1629–1643. [Google Scholar] [CrossRef]
- Shaheen, M.; Bhatti, I.A.; Ashar, A.; Mohsin, M.; Nisar, J.; Almoneef, M.M.; Iqbal, M. Synthesis of Cu-doped MgO and its enhanced photocatalytic activity for the solar-driven degradation of disperse red F3BS with condition optimization. Z. Phys. Chem. 2021, 235, 1395–1412. [Google Scholar] [CrossRef]
- Verma, P.; Samanta, S.K. Microwave-enhanced advanced oxidation processes for the degradation of dyes in water. Environ. Chem. Lett. 2018, 16, 969–1007. [Google Scholar] [CrossRef]
- Shafiquea, A.; Bhattia, I.A.; Ashara, A.; Mohsina, M.; Ahmadc, S.A.; Nisard, J.; Iqbal, M. nanoparticles synthesis, characterization and photocatalytic activity evaluation for the degradation of 2-chlorophenol. Desalinat. Water Treat. 2020, 187, 399–409. [Google Scholar] [CrossRef]
- Mohsin, M.; Bhatti, I.A.; Ashar, A.; Mahmood, A.; ul Hassan, Q.; Iqbal, M. Fe/ZnO@ ceramic fabrication for the enhanced photocatalytic performance under solar light irradiation for dye degradation. J. Mater. Res. Technol. 2020, 9, 4218–4229. [Google Scholar] [CrossRef]
- Bokhari, T.H.; Ahmad, N.; Jilani, M.I.; Saeed, M.; Usman, M.; Haq, A.U.; Javed, T. UV/H2O2, UV/H2O2/SnO2 and Fe/H2O2 based advanced oxidation processes for the degradation of disperse violet 63 in aqueous medium. Mater. Res. Express 2020, 7, 015531. [Google Scholar] [CrossRef]
- Zeng, W.; Yin, Z.; Gao, M.; Wang, X.; Feng, J.; Ren, Y.; Wei, T.; Fan, Z. In-situ growth of magnesium peroxide on the edge of magnesium oxide nanosheets: Ultrahigh photocatalytic efficiency based on synergistic catalysis. J. Colloid Interface Sci. 2020, 561, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Stojadinović, S.; Radić, N.; Vasilić, R. ZnO Particles Modified MgAl Coatings with Improved Photocatalytic Activity Formed by Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy in Aluminate Electrolyte. Catalysts 2022, 12, 1503. [Google Scholar] [CrossRef]
- Hasan, N.; Salman, S.; Hasan, M.; Kubra, K.T.; Sheikh, C.; Rehan, A.I.; Rasee, A.I.; Awual, E.; Waliullah, R.; Hossain, M.S.; et al. Assessing sustainable Lutetium(III) ions adsorption and recovery using novel composite hybrid nanomaterials. J. Mol. Struct. 2023, 1276, 134795. [Google Scholar] [CrossRef]
- Muneer, M.; Bhatti, I.A.; Bhatti, H.N.; Khalil-Ur-Rehman. Treatment of Dyes Industrial Effluents by Ionizing Radiation. Asian J. Chem. 2011, 23, 2392–2394. [Google Scholar]
- USEPA. Lettuce seed germination (Lactuca sativa). In Protocol for Short-Term Toxicity Screening of Hazardous Waste Sites; EPA/600/3-88/029; Environmental Research Laboratory: Corvallis, OR, USA, 1989. [Google Scholar]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. Mol. Mech. Mutagen. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Hasan, M.; Kubra, K.T.; Hasan, N.; Awual, E.; Salman, S.; Sheikh, C.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.; Islam, S.; et al. Sustainable ligand-modified based composite material for the selective and effective cadmium(II) capturing from wastewater. J. Mol. Liq. 2023, 371, 121125. [Google Scholar] [CrossRef]
- Ata, S.; Shaheen, I.; Ayne, Q.U.; Ghafoor, S.; Sultan, M.; Majid, F.; Bibi, I.; Iqbal, M. Graphene and silver decorated ZnO composite synthesis, characterization and photocatalytic activity evaluation. Diam. Relat. Mater. 2018, 90, 26–31. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Environ. Mutagen. Relat. Subj. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Costa, F.A.; dos Reis, E.M.; Azevedo, J.C.; Nozaki, J. Bleaching and photodegradation of textile dyes by H2O2 and solar or ultraviolet radiation. Sol. Energy 2004, 77, 29–35. [Google Scholar] [CrossRef]
- Muneer, M.; Kanjal, M.I.; Saeed, M.; Javed, T.; Haq, A.U.; Ud Den, N.Z.; Jamal, M.A.; Ali, S.; Iqbal, M. High energy radiation induced degradation of reactive yellow 145 dye: A mechanistic study. Radiat. Phys. Chem. 2020, 177, 109115. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Yang, L. The use of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Kanjal, M.I.; Muneer, M.; Saeed, M.; Chu, W.; Alwadai, N.; Iqbal, M.; Abdelhaleem, A. Oxone activated TiO2 in presence of UV-LED light for the degradation of moxifloxacin: A mechanistic study. Arab. J. Chem. 2022, 15, 104061. [Google Scholar] [CrossRef]
- Iqbal, M.; Nisar, J. Cytotoxicity and mutagenicity evaluation of gamma radiation and hydrogen peroxide treated textile effluents using bioassays. J. Environ. Chem. Eng. 2015, 3, 1912–1917. [Google Scholar] [CrossRef]
- Iqbal, M. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Janssens, R.; Cristovao, M.; Bronze, M.; Crespo, J.; Pereira, V.; Luis, P. Coupling of nanofiltration and UV, UV/TiO2 and UV/H2O2 processes for the removal of anti-cancer drugs from real secondary wastewater effluent. J. Environ. Chem. Eng. 2019, 7, 103351. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, M.; Xu, Z.; Zhang, D.; Li, L. Catalytic ozonation of organic contaminants in petrochemical wastewater with iron-nickel foam as catalyst. Sep. Purif. Technol. 2019, 211, 269–278. [Google Scholar] [CrossRef]
- Jadhav, J.; Kalyani, D.; Telke, A.; Phugare, S.; Govindwar, S. Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour. Technol. 2010, 101, 165–173. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan beads immobilized manganese peroxidase catalytic potential for detoxification and decolorization of textile effluent. Int. J. Biol. Macromol. 2016, 89, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Abbas, M.; Arshad, M.; Hussain, T.; Ullah Khan, A.; Masood, N.; Ahmad Khera, R. Short Communication Gamma Radiation Treatment for Reducing Cytotoxicity and Mutagenicity in Industrial Wastewater. Pol. J. Environ. Stud. 2015, 24, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M.; Kanjal, M.I.; Iqbal, M.; Saeed, M.; Khosa, M.K.; Den, N.Z.U.; Ali, S.; Nazir, A. Gamma and UV radiations induced treatment of anti-cancer methotrexate drug in aqueous medium: Effect of process variables on radiation efficiency evaluated using bioassays. Appl. Radiat. Isot. 2020, 166, 109371. [Google Scholar] [CrossRef]
- Iqbal, M.; Muneer, M.; Iqbal, M.; Kanjal, M.I. Tannery Wastewater Treatment Using Gamma Radiation: Kasur Tanneries Waste Management Agency, Pakistan. Phys. Chem. 2015, 17, 41–46. [Google Scholar]
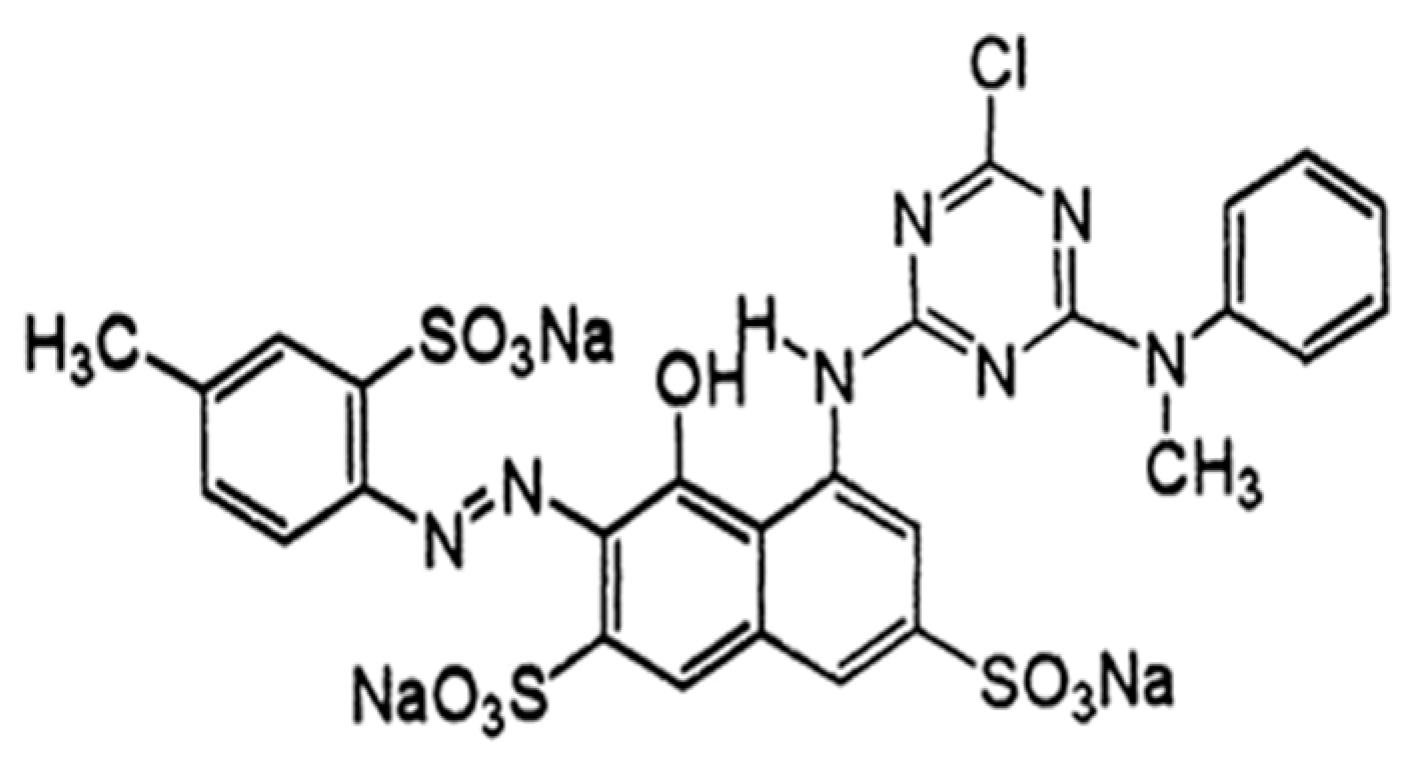








| Dye Name | Reactive Red 45 |
|---|---|
| Molecular formula | Na3C27H19ClN7O10S3 |
| Molecular weight (g/mol) | 802.107 |
| Chemical nature | Anionic red 45 |
| Color index name | Red |
| λmax (nm) | 512 |
| Reactive group | Azo group |
| Treatment Dye | Absorbed Dose | G-Value | Dose Constant | D0.50 | D0.90 | D0.99 | |
|---|---|---|---|---|---|---|---|
| (ppm) | (kGy) | (µmol/J) | (µM/kGy) | (kGy) | (kGy) | (kGy) | |
| Gamma | 50 | 0.25–2 | 0.9814–0.0228 | 0.944 | 0.734 | 2.439 | 4.878 |
| 100 | 0.25–2 | 1.8098–0.0181 | 0.714 | 0.969 | 3.221 | 6.443 | |
| Gamma/H2O2 | 50 | 0.25–2 | 1.2577–0.0117 | 2.028 | 0.034 | 1.135 | 2.270 |
| 100 | 0.02–2 | 2.3119–0.0263 | 1.415 | 0.489 | 1.626 | 3.252 | |
| Sample | Exposure Time (min) | A. cepa Test | |||||
|---|---|---|---|---|---|---|---|
| Root Length (cm) | Root Count | Mitotic Index | |||||
| Untreated | 4.2 ± 0.52 | 14 ± 0.63 | 12 ± 0.28 | ||||
| Positive control | 9.3 ± 0.77 | 29 ± 0.45 | 32 ± 0.74 | ||||
| Negative control | 3.5 ± 0.63 | 11 ± 0.23 | 14 ± 0.66 | ||||
| UV alone | UV + H2O2 | UV alone | UV + H2O2 | UV alone | UV + H2O2 | ||
| Sample 1 | 30 | 4.9 ± 0.68 | 5.4 ± 0.44 | 15 ± 0.44 | 17 ± 0.68 | 15 ± 0.47 | 18 ± 0.69 |
| Sample 2 | 90 | 5.5 ± 0.31 | 6.2 ± 0.65 | 18 ± 0.31 | 20 ± 0.74 | 19 ± 0.31 | 21 ± 0.74 |
| Sample 3 | 180 | 6.3 ± 0.67 | 6.9 ± 0.57 | 20 ± 0.44 | 22 ± 0.76 | 22 ± 0.44 | 23 ± 0.13 |
| Sample | Absorbed Dose (kGy) | A. cepa Test | |||||
|---|---|---|---|---|---|---|---|
| Root Length (cm) | Root Count | Mitotic Index | |||||
| Untreated | 4.2 ± 0.52 | 14 ± 0.63 | 12 ± 0.28 | ||||
| Positive control | 9.3 ± 0.77 | 29 ± 0.45 | 32 ± 0.74 | ||||
| Negative control | 3.5 ± 0.63 | 11 ± 0.23 | 14 ± 0.66 | ||||
| Gamma alone | Gamma +H2O2 | Gamma alone | Gamma +H2O2 | Gamma alone | Gamma +H2O2 | ||
| Sample 1 | 0.25 | 5.9 ± 0.62 | 6.5 ± 0.44 | 19 ± 0.42 | 21 ± 0.61 | 20 ± 0.41 | 21 ± 0.62 |
| Sample 2 | 0.75 | 6.7 ± 0.31 | 7.4 ± 0.65 | 21 ± 0.51 | 25 ± 0.24 | 23 ± 0.61 | 24 ± 0.44 |
| Sample 3 | 2 | 7.8 ± 0.65 | 8.2 ± 0.55 | 24 ± 0.43 | 27 ± 0.73 | 25 ± 0.42 | 28 ± 0.12 |
| Sample | Exposure Time (min) | Brine Shrimp Test | |
|---|---|---|---|
| % Age Death (after 24 h) | |||
| Untreated | 88 ± 0.27 | ||
| Positive control | 100 ± 0.0 | ||
| Negative control | 0 | ||
| UV alone | UV + H2O2 | ||
| Sample 1 | 30 | 26 ± 0.67 | 20 ± 0.85 |
| Sample 2 | 90 | 22 ± 0.53 | 16 ± 0.35 |
| Sample 3 | 180 | 19 ± 0.81 | 14 ± 1.04 |
| Sample | Absorbed Dose (kGy) | Brine Shrimp (Artemia salina) Test | |
|---|---|---|---|
| % Age Death (after 24 h) | |||
| Untreated | 88 ± 0.27 | ||
| Positive control | 100 ± 0.0 | ||
| Negative control | 0 | ||
| Gamma alone | Gamma + H2O2 | ||
| Sample 1 | 0.25 | 23 ± 0.68 | 17 ± 0.85 |
| Sample 2 | 0.75 | 28 ± 0.63 | 12 ± 0.35 |
| Sample 3 | 2 | 25 ± 0.82 | 8 ± 1.01 |
| Sample | Exposure Time (min) | Hemolytic Test (% Age Hemolysis) | |
|---|---|---|---|
| Positive control | 96.23 ± 0.82 | 96.23 ± 0.82 | |
| Negative control | 2.11 ± 0.32 | 2.11 ± 0.32 | |
| UV alone | UV + H2O2 | ||
| Sample 1 | 30 | 16.32 ± 0.55 | 18.52 ± 0.28 |
| Sample 2 | 120 | 17.56 ± 0.75 | 19.36 ± 0.76 |
| Sample 3 | 180 | 19.23 ± 0.56 | 21.25 ± 0.53 |
| Sample | Absorbed Dose (kGy) | Hemolytic Test (% Age Hemolysis) | |
|---|---|---|---|
| Positive control | 96.23 ± 0.82 | 96.23 ± 0.82 | |
| Negative control | 2.11 ± 0.32 | 2.11 ± 0.32 | |
| Gamma alone | Gamma + H2O2 | ||
| Sample 1 | 0.25 | 17.57 ± 0.28 | 19.56 ± 0.58 |
| Sample 2 | 0.75 | 18.62 ± 0.66 | 21.34 ± 0.37 |
| Sample 3 | 2 | 20.25 ± 0.51 | 23.21 ± 0.69 |
| Sample | Exposure Time (min) | AMES Test (% Age Mutagenicity) | |||
|---|---|---|---|---|---|
| - | TA98 Count | TA100 Count | |||
| Standard | 77.08 ± 0.46 | 70.83 ± 0.91 | |||
| Background | 33 ± 0.71 | 29.17 ± 0.85 | |||
| - | UV alone | UV + H2O2 | UV alone | UV + H2O2 | |
| Blank | - | 39.58 ± 0.33 | 31.08 ± 0.65 | 35.42 ± 0.55 | 32.24 ± 0.41 |
| Sample 1 | 30 | 31.25 ± 0.19 | 28.13 ± 0.48 | 33.33 ± 0.42 | 29.12 ± 0.25 |
| Sample 2 | 120 | 27.08 ± 0.43 | 16.67 ± 0.47 | 28.13 ± 0.18 | 19.79 ± 0.81 |
| Sample 3 | 180 | 20.83 ± 0.45 | 12.5 ± 0.58 | 21.88 ± 0.66 | 15.67 ± 0.76 |
| Sample | Radiation Dose (kGy) | AMES Test (% Age Mutagenicity) | |||
|---|---|---|---|---|---|
| - | TA98 Count | TA100 Count | |||
| Standard | 77.08 ± 0.46 | 70.83 ± 0.91 | |||
| Background | 33 ± 0.71 | 29.17 ± 0.85 | |||
| - | Gamma alone | Gamma + H2O2 | Gamma alone | Gamma + H2O2 | |
| Blank | - | 39.58 ± 0.35 | 31.08 ± 0.82 | 35.42 ± 0.29 | 32.24 ± 0.41 |
| Sample 1 | 0.25 | 29.17 ± 0.25 | 19.79 ± 0.51 | 30.24 ± 0.25 | 23.96 ± 0.35 |
| Sample 2 | 0.75 | 23.96 ± 0.91 | 13.54 ± 0.26 | 25.32 ± 0.79 | 18.75 ± 0.48 |
| Sample 3 | 2 | 17.71 ± 0.34 | 9.38 ± 0.85 | 20.83 ± 0.34 | 13.54 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanjal, M.I.; Muneer, M.; Jamal, M.A.; Bokhari, T.H.; Wahid, A.; Ullah, S.; Amrane, A.; Hadadi, A.; Tahraoui, H.; Mouni, L. A Study of Treatment of Reactive Red 45 Dye by Advanced Oxidation Processes and Toxicity Evaluation Using Bioassays. Sustainability 2023, 15, 7256. https://doi.org/10.3390/su15097256
Kanjal MI, Muneer M, Jamal MA, Bokhari TH, Wahid A, Ullah S, Amrane A, Hadadi A, Tahraoui H, Mouni L. A Study of Treatment of Reactive Red 45 Dye by Advanced Oxidation Processes and Toxicity Evaluation Using Bioassays. Sustainability. 2023; 15(9):7256. https://doi.org/10.3390/su15097256
Chicago/Turabian StyleKanjal, Muhammad Imran, Majid Muneer, Muhammad Asghar Jamal, Tanveer Hussain Bokhari, Abdul Wahid, Shafqat Ullah, Abdeltif Amrane, Amina Hadadi, Hichem Tahraoui, and Lotfi Mouni. 2023. "A Study of Treatment of Reactive Red 45 Dye by Advanced Oxidation Processes and Toxicity Evaluation Using Bioassays" Sustainability 15, no. 9: 7256. https://doi.org/10.3390/su15097256






