Performance, Emission, and Spectroscopic Analysis of Diesel Engine Fuelled with Ternary Biofuel Blends
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Methods
2.2. Uncertainty Analysis
3. Result & Discussion
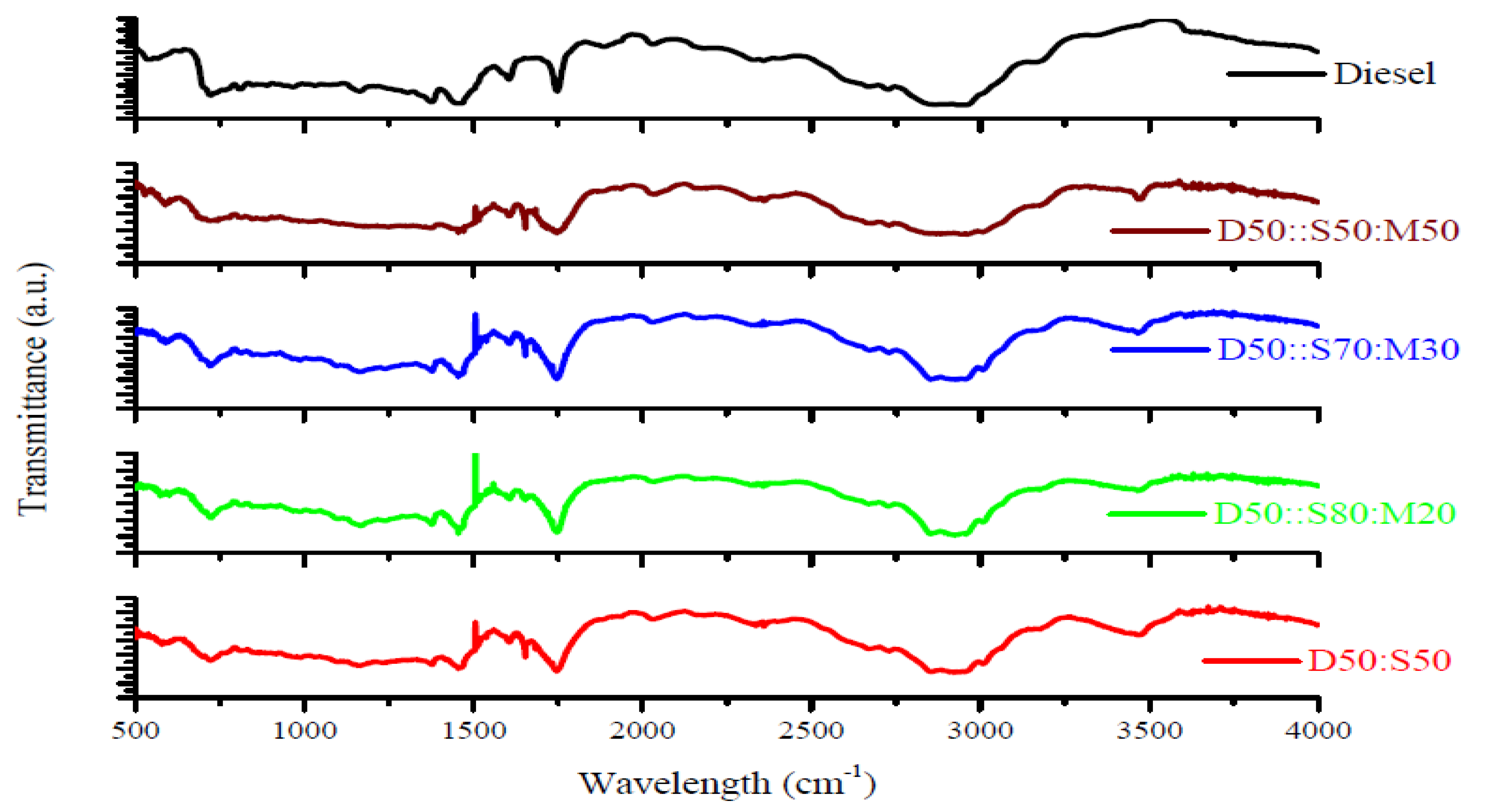
3.1. Fourier Transform Infrared Spectroscopy (FTIR) Studies of Biodiesel
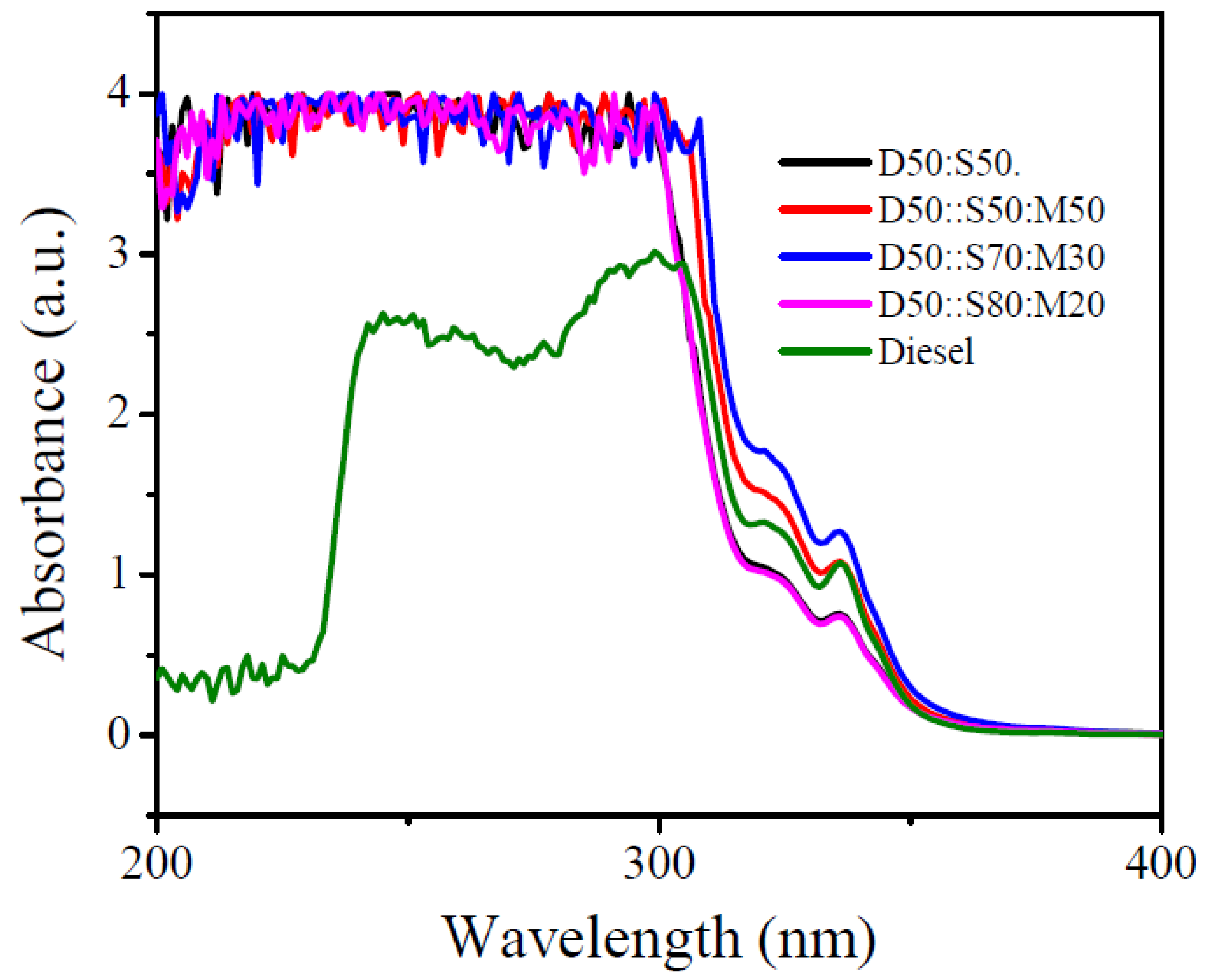
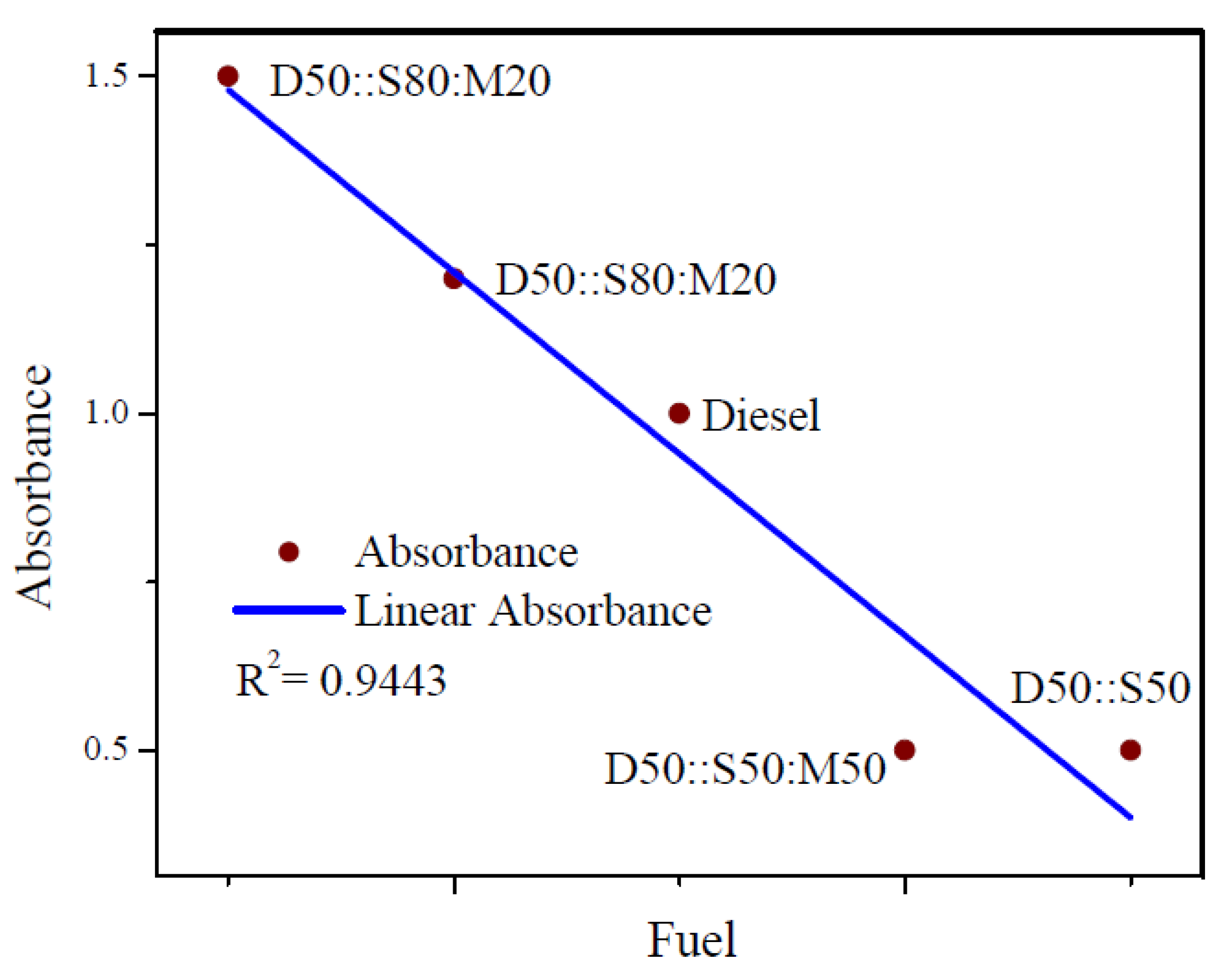
3.2. UV-vis Spectroscopy
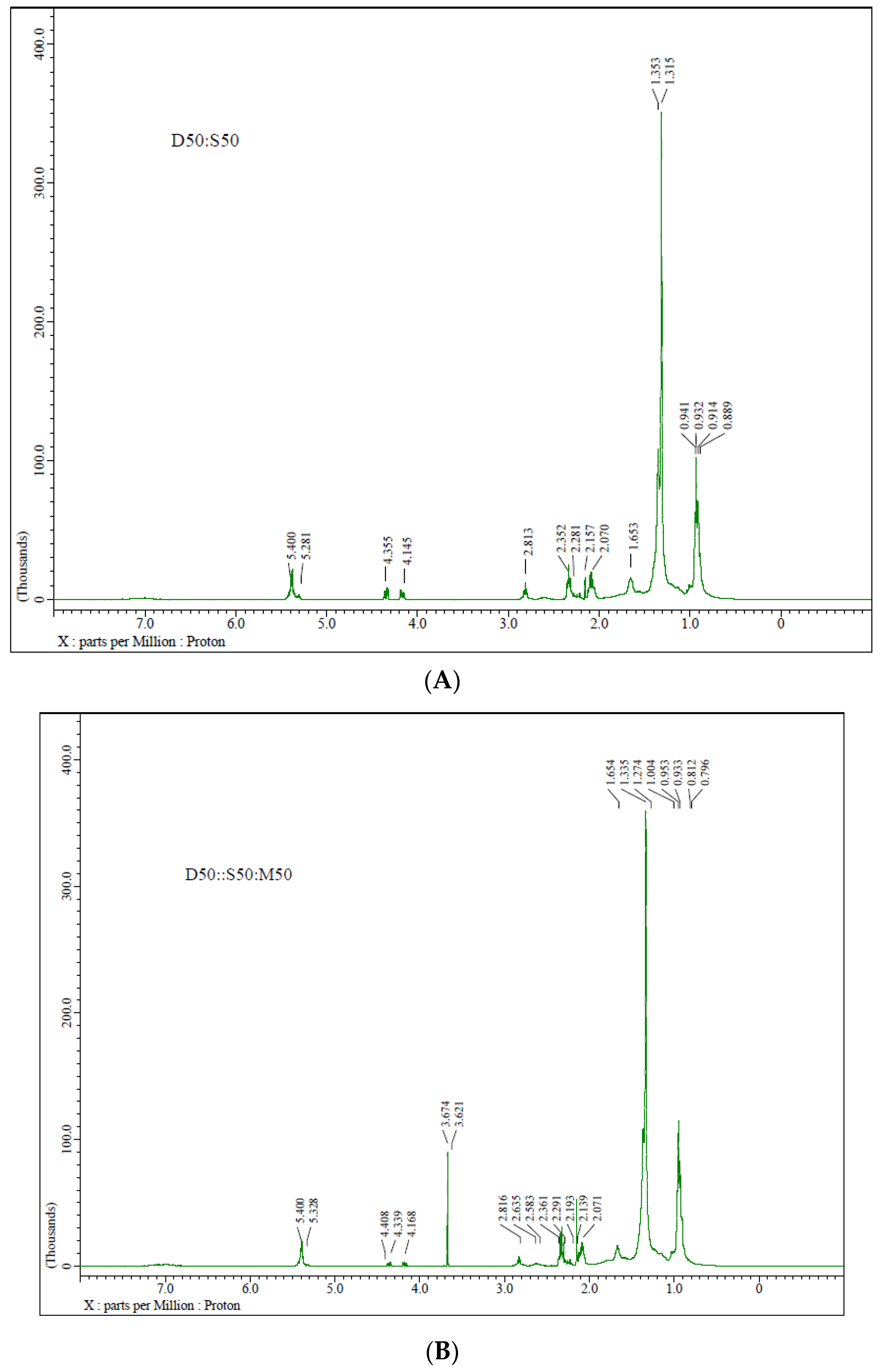
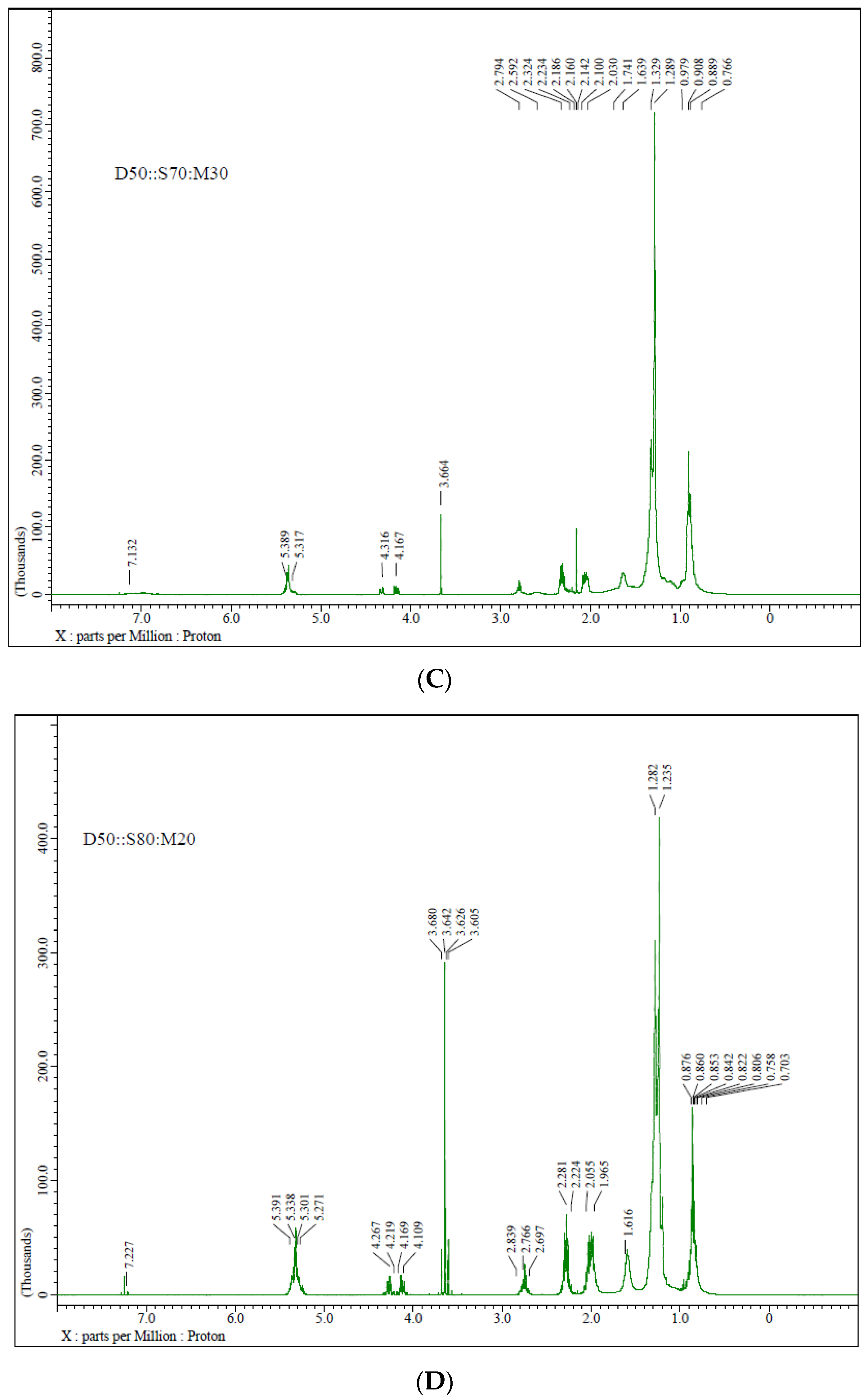
3.3. NMR Spectrum Characterization
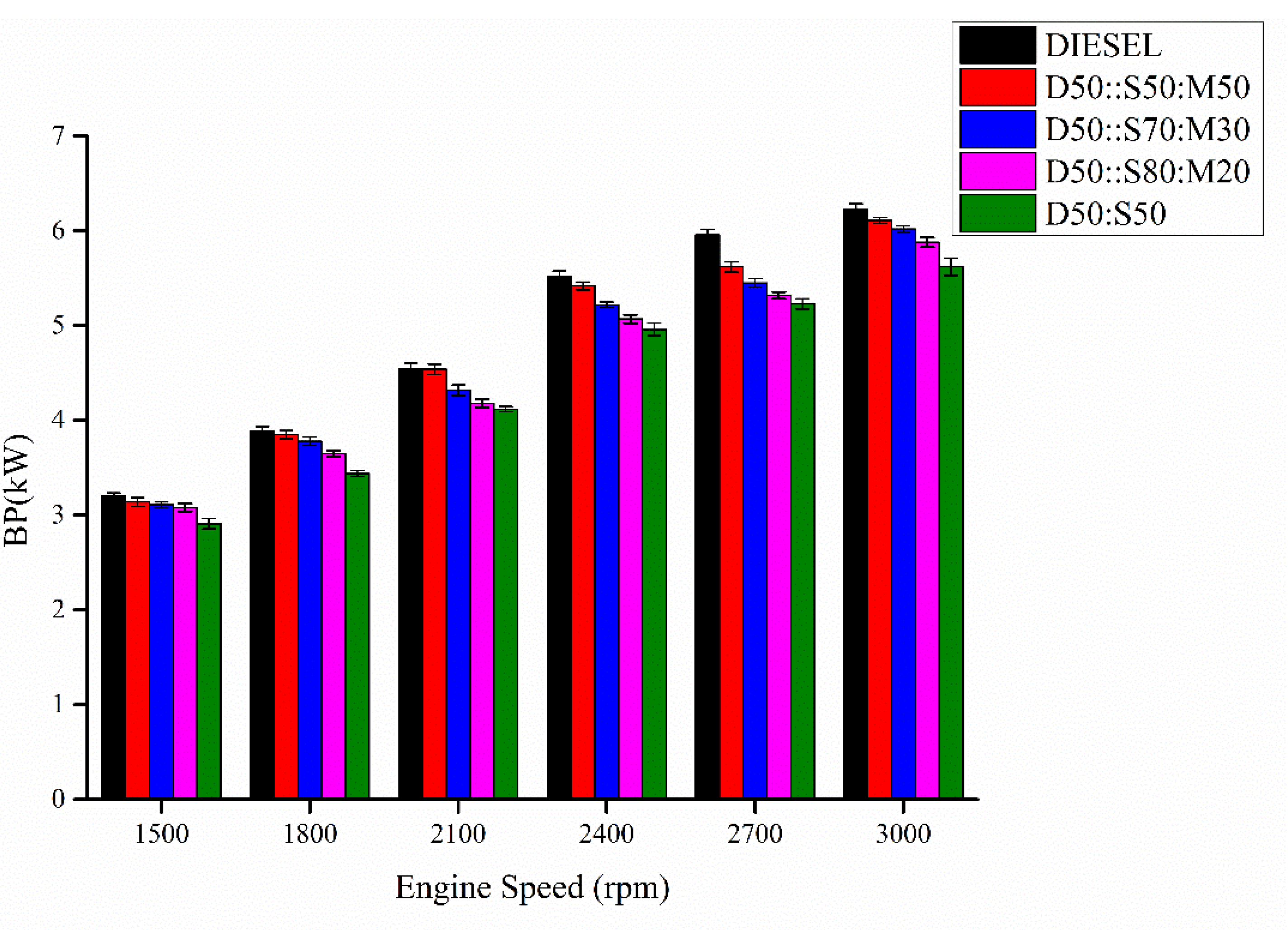
3.4. Brake Power (BP)
3.5. Brake Thermal Efficiency (BTE)
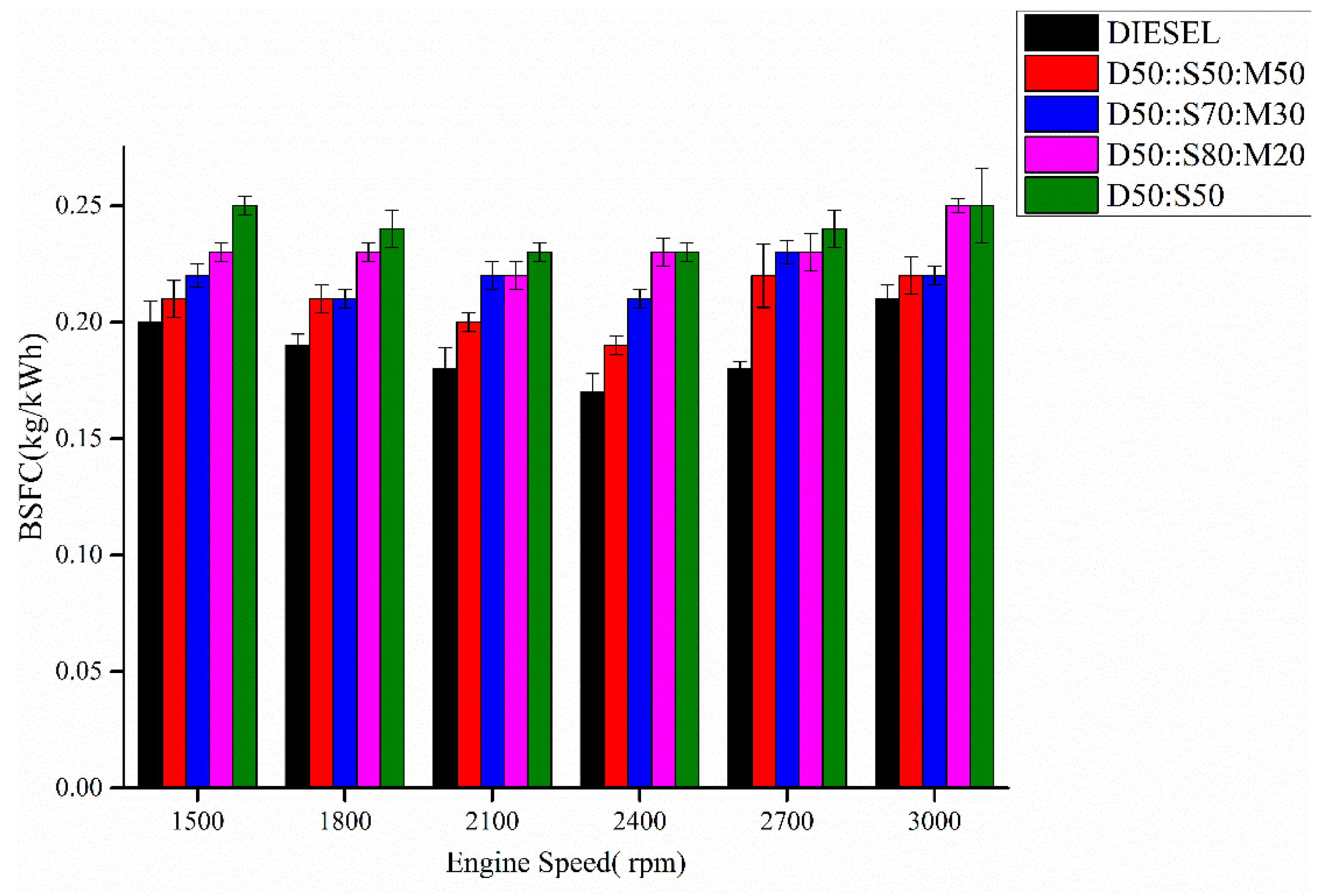
3.6. Brake Specific Fuel Consumption (BSFC)
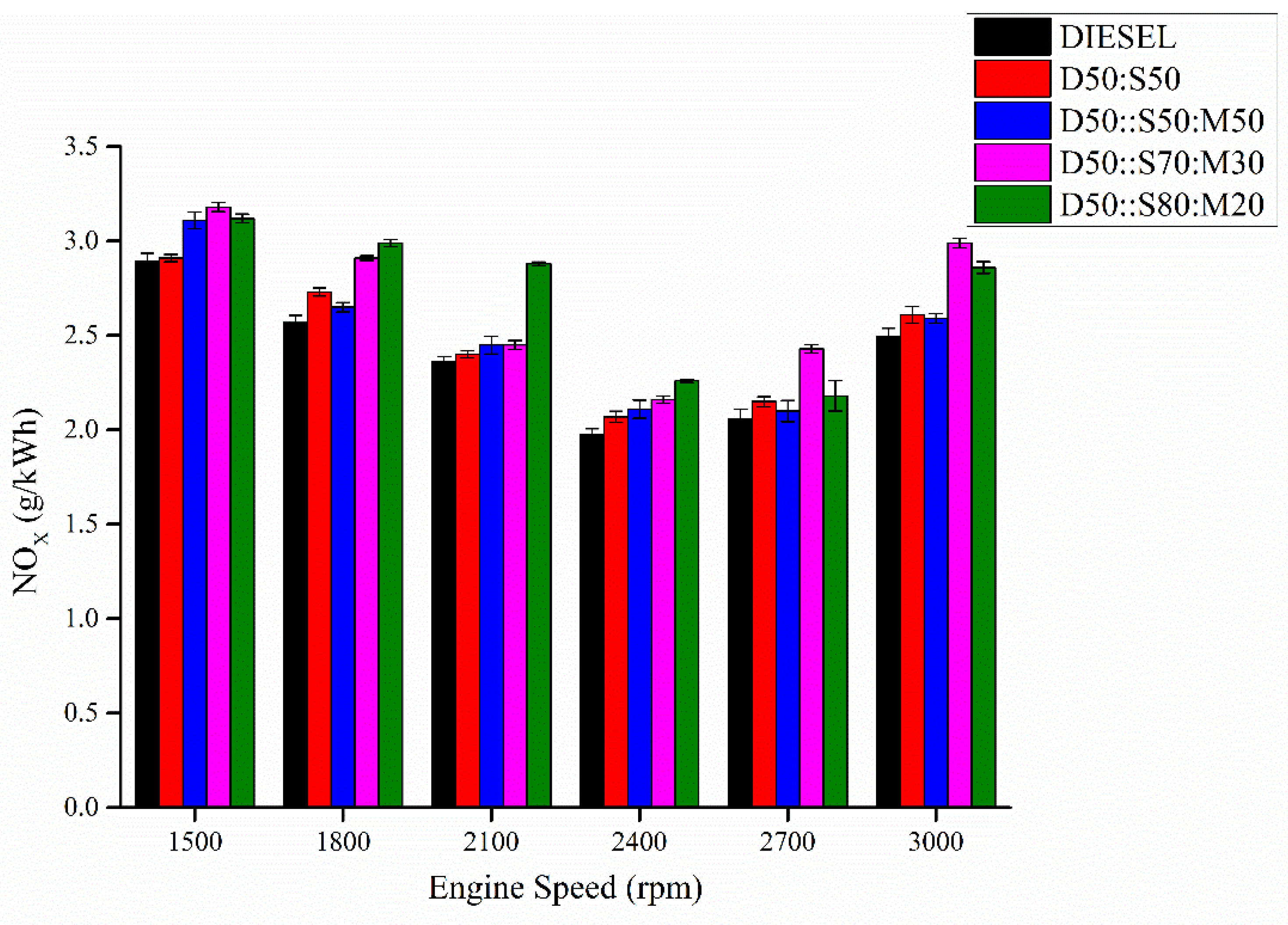
3.7. Nitrous Oxide (NOx)
3.8. Carbon Monoxide (CO)
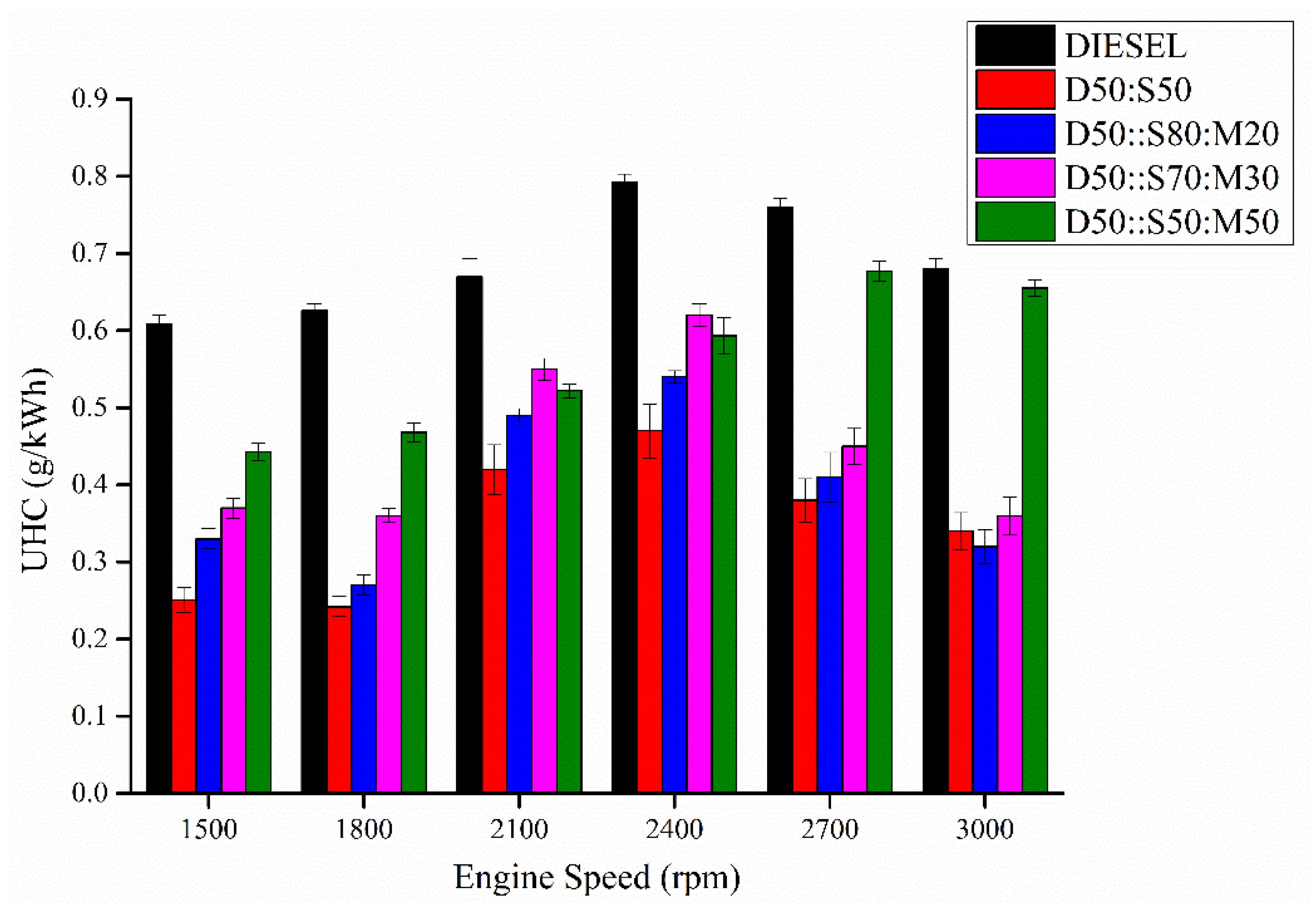
3.9. Unburnt Hydrocarbon (UHC)
4. Conclusions
- The characterizations of blended-biodiesel were performed by using FTIR, and UV-vis. FTIR spectra showed the existence of the strong ester carbonyl bond (C=O), indicating the presence of FAME at 1745 cm−1. Again, the presence of conjugated dienes in the oxidized-biodiesel was detected using UV-vis spectroscopy.
- The signal at 3.6 δ in the 1HNMR corresponds to methyl ester moiety, authenticating biodiesel production.
- The addition of MO:SME improved the BSFC of enriched biodiesels near to pure diesel due to decreased kinematic viscosity with increase CV.
- BTE of the engine enhanced by increasing the quantity of MO in the blend of SME and diesel due to high CV and low viscosity. BTE of all biodiesels remained lower than that of pure diesel. Increasing the content of MO in SME leads to an increase in CO emissions by the incomplete combustion process can be facilitated due to certain factors. For the above reason, the HC emission increased by increasing the quantity of MO in blends of SME and diesel.
- The low cetane number and low oxygen content are the reasons. Invigorating D50:S50 with MO resulted in escalated UHC due to the reduced oxygen concentration, which led to insufficient combustion. The blended biodiesels emit more NOx than that of pure diesel and D50:S50 as the unsaturation degree increases with the addition of MO. NOx emissions can be reduced by incorporating EGR system in the experimental set-up, i.e., connecting CRDI diesel engine with the EGR. The inclusion of 20–40% alcohol, preferably butanol, in the diesel-biodiesel blends. The high latent heat of vaporization of alcohol present in the blend will have cooling effect inside the engine cylinder which will reduce the in-cylinder temperature and ultimately NOx formation will decrease.
- Further studies on EGR, and performance, emissions studies on inclusion of alcohol in blended biodiesels with diesel. More concrete repercussions will come from how IP and timing affect the efficiency and emissions of enriched biodiesel. Applications of the research could result in the creation of green alternative fuels with lower exhaust emissions and better engine efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altaie, M.A.H.; Janius, R.B.; Rashid, U.; Yap, Y.H.T.; Yunus, R.; Zakaria, R. Cold flow and fuel properties of methyl oleate and palm-oil methyl ester blends. Fuel 2015, 160, 238–244. [Google Scholar] [CrossRef]
- Suresh, M.; Jawahar, C.P.; Richard, A. A review on biodiesel production, combustion, performance, and emission characteristics of non-edible oils in variable compression ratio diesel engine using biodiesel and its blends. Renew. Sustain. Energy Rev. 2018, 92, 38–49. [Google Scholar] [CrossRef]
- Mohd Noor, C.W.; Noor, M.M.; Mamat, R. Biodiesel as alternative fuel for marine diesel engine applications: A review. Renew. Sustain. Energy Rev. 2018, 94, 127–142. [Google Scholar] [CrossRef]
- Soloiu, V.; Weaver, J.; Ochieng, H.; Duggan, M.; Davoud, S.; Vlcek, B.; Jenkins, C.; Butts, C. Experimental Study of Combustion and Emissions Characteristics of Methyl Oleate, as a Surrogate for Biodiesel, in a Direct Injection Diesel Engine; No. 2013-01-1142, SAE Technical Paper; SAE: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Zhang, Z.; E, J.; Deng, Y.; Pham, M.; Zuo, W.; Peng, Q.; Yin, Z. Effects of fatty acid methyl esters proportion on combustion and emission characteristics of a biodiesel fueled marine diesel engine. Energy Convers. Manag. 2018, 159, 244–253. [Google Scholar] [CrossRef]
- Altaie, M.A.H.; Janius, R.B.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R.; Zakaria, R.; Adam, N.M. Performance and exhaust emission characteristics of direct-injection diesel engine fueled with enriched biodiesel. Energy Convers. Manag. 2015, 106, 365–372. [Google Scholar] [CrossRef]
- Soloiu, V.; Moncada, J.D.; Gaubert, R.; Knowles, A.; Molina, G.; Ilie, M.; Harp, S.; Wiley, J.T. Reactivity Controlled Compression Ignition combustion and emissions using n-butanol and methyl oleate. Energy 2018, 165, 911–924. [Google Scholar] [CrossRef]
- Soloiu, V.; Lewis, J.; Covington, A.; Nelson, D.; Schmidt, N. Oleic methyl ester investigations in an indirect injection diesel engine; stage one: Combustion investigations. SAE Int. J. Fuels Lubr. 2011, 4, 58–75. [Google Scholar] [CrossRef]
- Bax, S.; Hakka, M.H.; Glaude, P.-A.; Herbinet, O.; Battin-Leclerc, F. Experimental study of the oxidation of methyl oleate in a jet-stirred reactor. Combust. Flame 2010, 157, 1220–1229. [Google Scholar] [CrossRef]
- Lata, D.B.; Ahmad, A.; Prakash, O.; Khan, M.; Chatterjee, R.; Hasnain, S.M.M. Impact of Exhaust Gas Recirculation (EGR) on the Emission of the Dual-Fuel Diesel Engine with Hydrogen as a Secondary Fuel. J. Inst. Eng. Ser. C 2021, 102, 1489–1502. [Google Scholar] [CrossRef]
- Sener, R.; Giil, M.Z. Optimization of the combustion chamber geometry and injection parameters on a light-duty diesel engine for emission minimization using multi-objective genetic algorithm. Fuel 2021, 304, 121379. [Google Scholar] [CrossRef]
- Kadian, A.K.; Khan, M.; Sharma, R.; Hasnain, S.M. Performance enhancement and emissions mitigation of DI-CI engine fuelled with ternary blends of jatropha biodiesel-diesel-heptanol. Mater. Sci. Energy Technol. 2022, 5, 145–154. [Google Scholar] [CrossRef]
- Khan, M.; Sharma, R.; Kadian, A.K.; Hasnain, S.M.M. An assessment of alcohol inclusion in various combinations of biodiesel-diesel on the performance and exhaust emission of modern-day compression ignition engines—A review. Mater. Sci. Energy Technol. 2022, 5, 81–98. [Google Scholar] [CrossRef]
- Koç, M.A.; Şener, R. Prediction of emission and performance characteristics of reactivity-controlled compression ignition engine with the intelligent software based on adaptive neural-fuzzy and neural-network. J. Clean. Prod. 2021, 318, 128642. [Google Scholar] [CrossRef]
- Khan, M.M.; Kadian, A.K.; Sharma, R.P. An investigation of performance and emission of diesel engine by using quaternary blends of neem biodiesel–neem oil–decanol–diesel. Sādhanā 2023, 48, 28. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Yan, B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 2014, 171, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Furlan, P.Y.; Wetzel, P.; Johnson, S.; Wedin, J.; Och, A. Investigating the Oxidation of Biodiesel from Used Vegetable Oil by FTIR Spectroscopy: Used Vegetable Oil Biodiesel Oxidation Study by FTIR. Spectrosc. Lett. 2010, 43, 580–585. [Google Scholar] [CrossRef]
- Chatterjee, R.; Mukherjee, S.K.; Paul, B.; Chattopadhyaya, S. Evaluation of spectroscopic analysis, performance and emissions of enriched Jatropha and Madhuca methyl ester for clean environment. Clean Technol. Environ. Policy 2022, 24, 2295–2312. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution nmr spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Lankhorst, P.P.; Chang, A.N. The application of NMR in compositional and quantitative analysis of oils and lipids. In Modern Magnetic Resonance; Webb, G., Ed.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1743–1764. [Google Scholar]
- Faraguna, F.; Racar, M.; Jukić, A. Test method for determination of different biodiesels (fatty acid alkyl esters) content in diesel fuel using FTIR-ATR. Renew. Energy 2018, 133, 1231–1235. [Google Scholar] [CrossRef]
- Acevedo, J.C.; Hernández, J.A.; Valdés, C.F.; Khanal, S.K. Analysis of operating costs for producing biodiesel from palm oil at pilot-scale in Colombia. Bioresour. Technol. 2015, 188, 117–123. [Google Scholar] [CrossRef]
- Avinash, A.; Murugesan, A. Economic analysis of biodiesel production from waste cooking oil. Energy Sources Part B Econ. Plan. Policy 2017, 12, 890–894. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Romagnoli, F.; Cabulis, U. Environmental Life Cycle Assessment of Rapeseed and Rapeseed Oil Produced in Northern Europe: A Latvian Case Study. Sustainability 2020, 12, 5699. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sharma, V.; Mukherjee, S. The environmental impacts and allocation methods used in lca studies of vegetable oil-based bio-diesels. Waste Biomass-Valorization 2015, 6, 579–603. [Google Scholar] [CrossRef]
- Arguelles-Arguelles, A.; Amezcua-Allieri, M.A.; Ramírez-Verduzco, L.F. Life cycle assessment of green diesel production by hydrodeoxygenation of palm oil. Front. Energy Res. 2021, 9, 690725. [Google Scholar] [CrossRef]
- Gupta, R.; McRoberts, R.; Yu, Z.; Smith, C.; Sloan, W.; You, S. Life cycle assessment of biodiesel production from rapeseed oil: Influence of process parameters and scale. Bioresour. Technol. 2022, 360, 127532. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Romagnoli, F.; Kirsanovs, V.; Cabulis, U. Life Cycle Assessment of vegetable oil based polyols for polyurethane production. J. Clean. Prod. 2020, 266, 121403. [Google Scholar] [CrossRef]
- Bhonsle, A.K.; Singh, J.; Trivedi, J.; Atray, N. Comparative LCA studies of biodiesel produced from used cooking oil using conventional and novel room temperature processes. Bioresour. Technol. Rep. 2022, 18, 101072. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Aydin, M.; Yilbasi, Z.; Arslan, M. Investigation on the structural effects of the addition of alcohols having various chain lengths into the vegetable oil-biodiesel-diesel fuel blends: An attempt for improving the performance, combustion, and exhaust emission characteristics of a compression ignition engine. Fuel 2020, 269, 117455. [Google Scholar] [CrossRef]
- Shah, S.N.; Joshi, A.; Patel, A.; Brahmkhatri, V.P. Synthesis of jatropha oil based biodiesel using environmentally friendly catalyst and their blending studies with diesel. Energy Power 2013, 3, 7–11. [Google Scholar]
- Chatterjee, R.; Mukherjee, S.K.; Paul, B.; Chattopadhyaya, S. Comparative spectroscopic analysis, performance and emissions evaluation of Madhuca longifolia and Jatropha curcas produced biodiesel. Environ. Sci. Pollut. Res. 2021, 28, 62444–62460. [Google Scholar] [CrossRef]
- Chatterjee, R.; Mukherjee, S. Spectroscopic analysis and performance studies of jatropha extracted bio-diesel. Waste Biomass- Valorization 2017, 9, 1579–1585. [Google Scholar] [CrossRef]
- Berdeaux, O.; Fontagné, S.; Sémon, E.; Velasco, J.; Sébédio, J.L.; Dobarganes, C. A detailed identification study on high-temperature degradation products of oleic and linoleic acid methyl esters by GC–MS and GC–FTIR. Chem. Phys. Lipids 2012, 165, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M. Biodiesel (FAME) Analysis by FT-IR. 2007. Available online: http://www.thermo.com/eThermo/CMA/PDFs/Articles/articlesFile_2448.pdf (accessed on 25 November 2020).
- Vega-Lizama, T.; Díaz-Ballote, L.; Hernández-Mézquita, E.; May-Crespo, F.; Castro-Borges, P.; Castillo-Atoche, A.; González-García, G.; Maldonado, L. Thermogravimetric analysis as a rapid and simple method to determine the degradation degree of soy biodiesel. Fuel 2015, 156, 158–162. [Google Scholar] [CrossRef]
- Pereira, G.G.; Alberici, R.M.; Fernandes, G.D.; Cunha, I.B.; Eberlin, M.N.; Dobarganes, M.C.; Daroda, R.J.; Barrera-Arellano, D. Ambient sonic-spray ionization mass spectrometry for rapid monitoring of secondary oxidation products in biodiesel. Eur. J. Lipid Sci. Technol. 2014, 116, 952–960. [Google Scholar] [CrossRef]
- Fang, H.L.; McCormick, R.L. Spectroscopic Study of Biodiesel Degradation Pathways; No. 2006-01-3300, SAE Technical Paper; SAE: Warrendale, PA, USA, 2006. [Google Scholar] [CrossRef]
- Zawadzki, A.; Shrestha, D.S.; He, B. Biodiesel blend level detection using ultraviolet absorption spectra. Trans. ASABE 2007, 50, 1349–1353. [Google Scholar] [CrossRef]
- Hasnain, S.M.M.; Chatterjee, R.; Sharma, R.P. Spectroscopic performance and emission analysis of glycine max biodiesel. J. Inst. Eng. Ser. C 2020, 101, 587–594. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Ambrozin, A.R.P.; Lião, L.M.; Ferreira, A.G. Determination of biodiesel blend levels in different diesel samples by 1H NMR. Fuel 2009, 88, 691–696. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.; Kalam, M.; Atabani, A. Evaluation of biodiesel blending, engine performance and emissions characteristics of Jatropha curcas methyl ester: Malaysian perspective. Energy 2013, 55, 879–887. [Google Scholar] [CrossRef]
- Devan, P.; Mahalakshmi, N. Study of the performance, emission and combustion characteristics of a diesel engine using poon oil-based fuels. Fuel Process. Technol. 2009, 90, 513–519. [Google Scholar] [CrossRef]
- McCormick, R.L.; Graboski, M.S.; Alleman, T.L.; Herring, A.M.; Tyson, K.S. Impact of Biodiesel Source Material and Chemical Structure on Emissions of Criteria Pollutants from a Heavy-Duty Engine. Environ. Sci. Technol. 2001, 35, 1742–1747. [Google Scholar] [CrossRef]
- Hirkude, J.B.; Padalkar, A. Performance and emission analysis of a compression ignition: Engine operated on waste fried oil methyl esters. Appl. Energy 2012, 90, 68–72. [Google Scholar] [CrossRef]
- Kivevele, T.T.; Kristóf, L.; Bereczky, A.; Mbarawa, M.M. Engine performance, exhaust emissions and combustion characteristics of a CI engine fuelled with croton megalocarpus methyl ester with antioxidant. Fuel 2011, 90, 2782–2789. [Google Scholar] [CrossRef]
- Ozsezen, A.N.; Canakci, M.; Turkcan, A.; Sayin, C. Performance and combustion characteristics of a DI diesel engine fueled with waste palm oil and canola oil methyl esters. Fuel 2009, 88, 629–636. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M.; Velraj, R. Mitigation of NOx emissions from a jatropha biodiesel fuelled DI diesel engine using antioxidant additives. Fuel 2011, 90, 2721–2725. [Google Scholar] [CrossRef]


| Properties | Test Method | D50:S50 | D50::S80:M20 | D50::S70:M30 | D50::S50:M50 | Diesel |
|---|---|---|---|---|---|---|
| Density (Kg/m3) | ASTM D1298 | 855.12 | 844.72 | 830.5 | 824.33 | 810.00 |
| Viscosity (mm2/s) | ASTM D445 | 4.11 | 3.90 | 3.84 | 3.90 | 3.18 |
| Lower Calorific Value (MJ/Kg) | ASTM D240 | 36.61 | 37.46 | 38.17 | 38.90 | 42.50 |
| Pour Point (°C) | ASTM D97 | −11 | −14 | −14 | −13 | −20 |
| Cloud Point (°C) | ASTM D2500 | 12 | 5 | 3 | −1 | −5 |
| Flash Point (°C) | ASTM D93 | 75 | 78 | 84 | 91 | 51 |
| Fire Point (°C) | ASTM D93 | 78 | 81 | 87 | 100 | 56 |
| Carbon (%) | - | 77.14 | 76.77 | 77.11 | 77.23 | 86.23 |
| Hydrogen (%) | - | 11.02 | 11.32 | 11.10 | 11.12 | 13.14 |
| Oxygen (%) | - | 11.84 | 11.73 | 11.79 | 11.65 | - |
| Sl No. | Item | Specification |
|---|---|---|
| 1. | Engine Capacity | 625 cc |
| 2. | No. of Cylinder | 1 |
| 3. | No. of Stroke | 4 |
| 4. | Compression Ratio | 18:1 |
| 5. | Bore | 93 +0.018 mm |
| 6. | Stroke | 92 mm |
| 7. | Max. Power | 6.7 kW@ 3000 rpm |
| 8. | Number of holes | 6 |
| 9. | Normal/Standard fuel injection pressure | 270 bars |
| 10. | Increased fuel injection pressure | 300 bars |
| 11. | Injection timing | 230 CA bTDC |
| 12. | Injection pressure | 300 bars |
| 13. | No. of Valves/Cylinder | 2 |
| 14. | No. of injection nozzles | 6 |
| 15. | Diameter of fuel injector nozzle | 145 micron |
| Sr. No. | Instrument | Uncertainty (%) |
|---|---|---|
| 1. | Pressure Sensor | ±0.4 |
| 2. | Temperature Sensor | ±0.9 |
| 3. | Load Indicator | ±0.5 |
| 4. | Digital Stopwatch | ±0.1 |
| 5. | Speed Indicator | ±0.2 |
| 6. | AVL Exhaust Gas Analyzer | |
| CO | ±0.2 | |
| CO2 | ±0.4 | |
| UHC | ±0.5 | |
| NO | ±1.0 | |
| 7. | BP | ±0.2 |
| 8. | BTE | ±1.2 |
| 9. | BSFC | ±1.1 |
| Sr. No. | Equipment | Measured Quantity | Range | Accuracy |
|---|---|---|---|---|
| 1. | AVL Exhaust Gas Analyzer | CO | 0–15% | ±0.03% |
| UHC | 0–30,000 ppm | ±10 rpm | ||
| NO | 0–5000 ppm | ±10 rpm | ||
| 2. | Speed Sensor | Engine speed | 0–10,000 rpm | ±10 rpm |
| 3. | Burette | Fuel Quantity | 0–1000 cc | ±0.1 cc |
| The Following Samples Contain Peaks | Functional-Group | ppm |
|---|---|---|
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 Diesel | -CH2-CH3 | 0.84–0.90 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 Diesel | -CH2-CH3 | 0.93–1.29 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 Diesel | -CH2-C=C- | 1.96–2.15 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 Diesel | -OC=O-CH2 | 2.28 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 Diesel | C=C-CH2-C=C | 2.75–2.83 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20 | -COOCH3 | 3.64–3.68 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 | -CH2OCOR | 4.0–4.5 |
| D50::S50:M50, D50::S70:M30, D50::S80:M20, D50:S50 | -CH=CH- | 5.24–5.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasnain, S.M.M.; Chatterjee, R.; Ranjan, P.; Kumar, G.; Sharma, S.; Kumar, A.; Salah, B.; Ullah, S.S. Performance, Emission, and Spectroscopic Analysis of Diesel Engine Fuelled with Ternary Biofuel Blends. Sustainability 2023, 15, 7415. https://doi.org/10.3390/su15097415
Hasnain SMM, Chatterjee R, Ranjan P, Kumar G, Sharma S, Kumar A, Salah B, Ullah SS. Performance, Emission, and Spectroscopic Analysis of Diesel Engine Fuelled with Ternary Biofuel Blends. Sustainability. 2023; 15(9):7415. https://doi.org/10.3390/su15097415
Chicago/Turabian StyleHasnain, S M Mozammil, Rajeshwari Chatterjee, Prabhat Ranjan, Gaurav Kumar, Shubham Sharma, Abhinav Kumar, Bashir Salah, and Syed Sajid Ullah. 2023. "Performance, Emission, and Spectroscopic Analysis of Diesel Engine Fuelled with Ternary Biofuel Blends" Sustainability 15, no. 9: 7415. https://doi.org/10.3390/su15097415







