Bioremediation of Heavy Metals from Industrial Effluents Using Bacillus pakistanensis and Lysinibacillus composti
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Collection and Transportation
2.2. Collection of Industrial Effluents
2.3. Experimental Design
2.4. Water Sampling and Analysis
2.5. Heavy Metal Analysis
2.6. Bioremoval Efficiency (%)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of WW
3.2. Heavy Metals
3.2.1. Manganese (Mn)
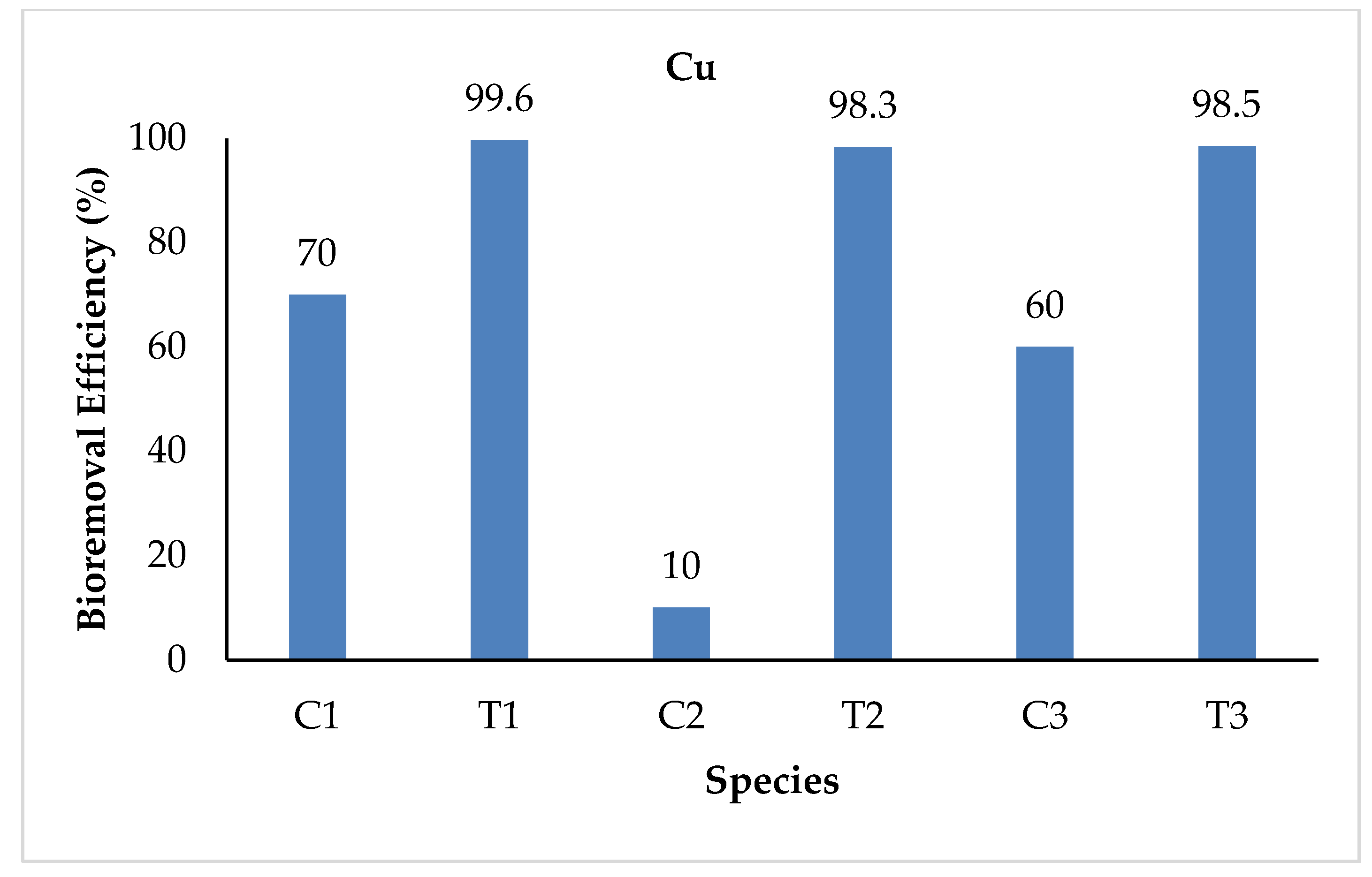
3.2.2. Copper (Cu)
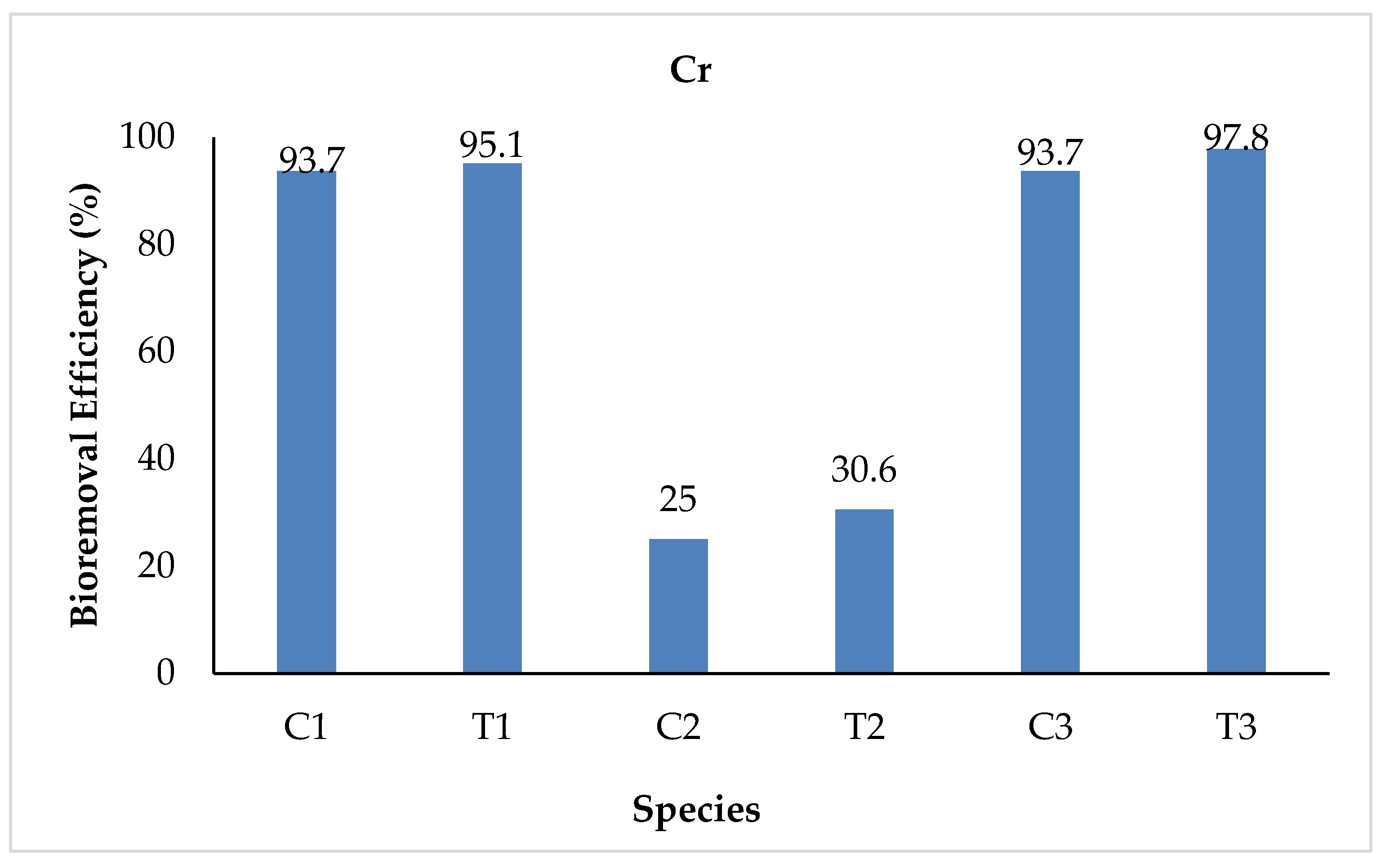
3.2.3. Chromium (Cr)
3.2.4. Cadmium (Cd)
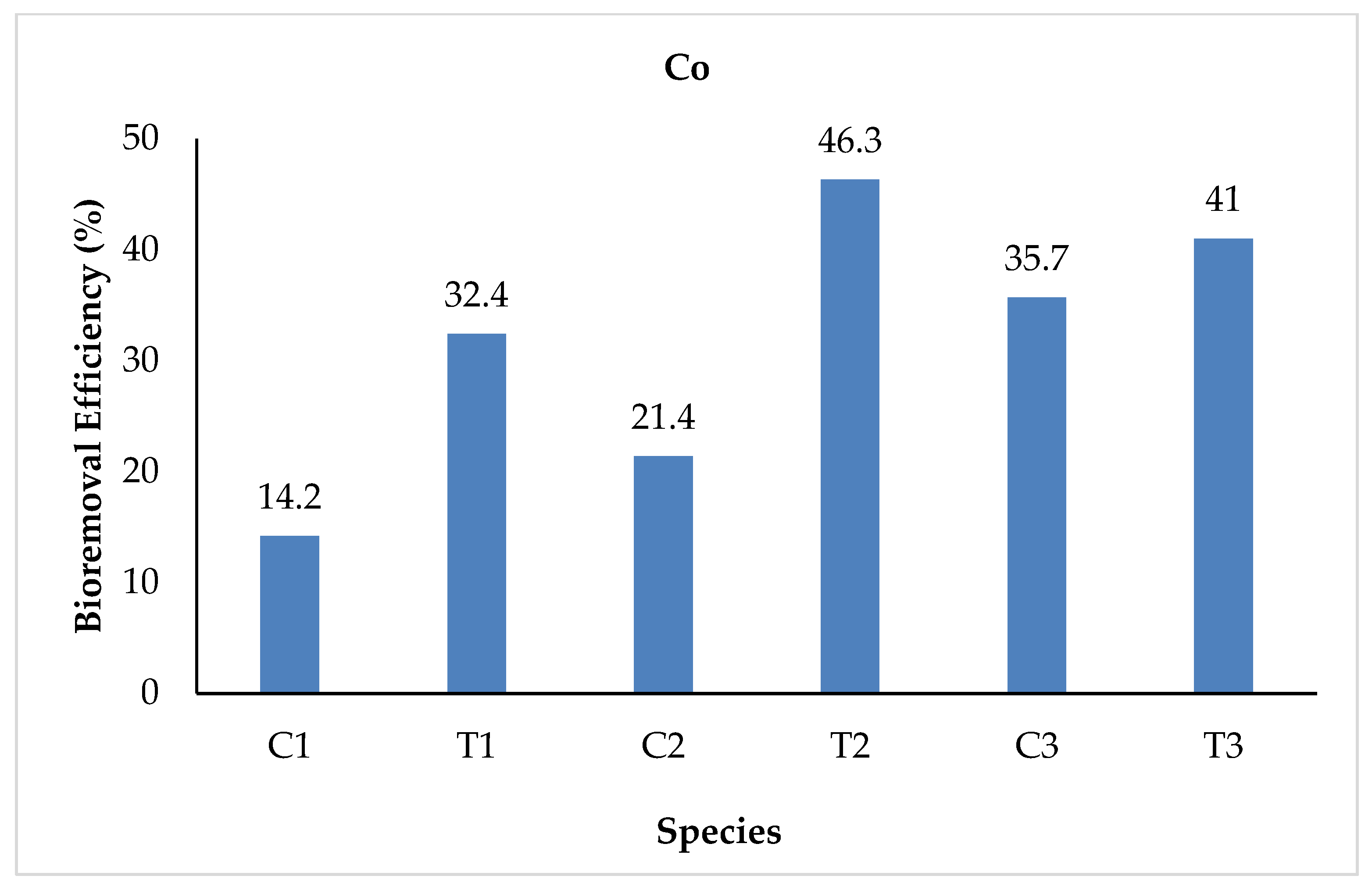
3.2.5. Cobalt
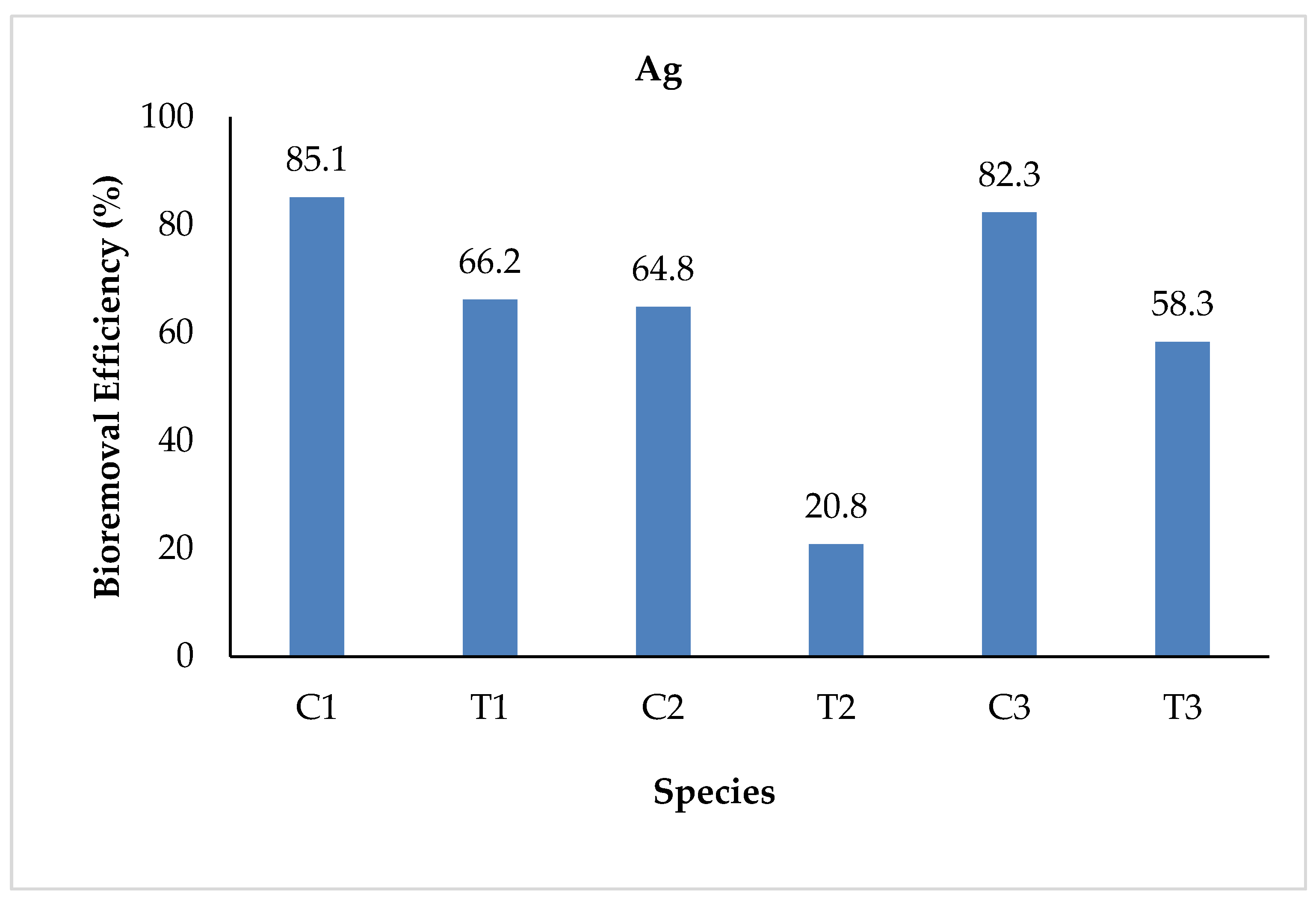
3.2.6. Silver (Ag)
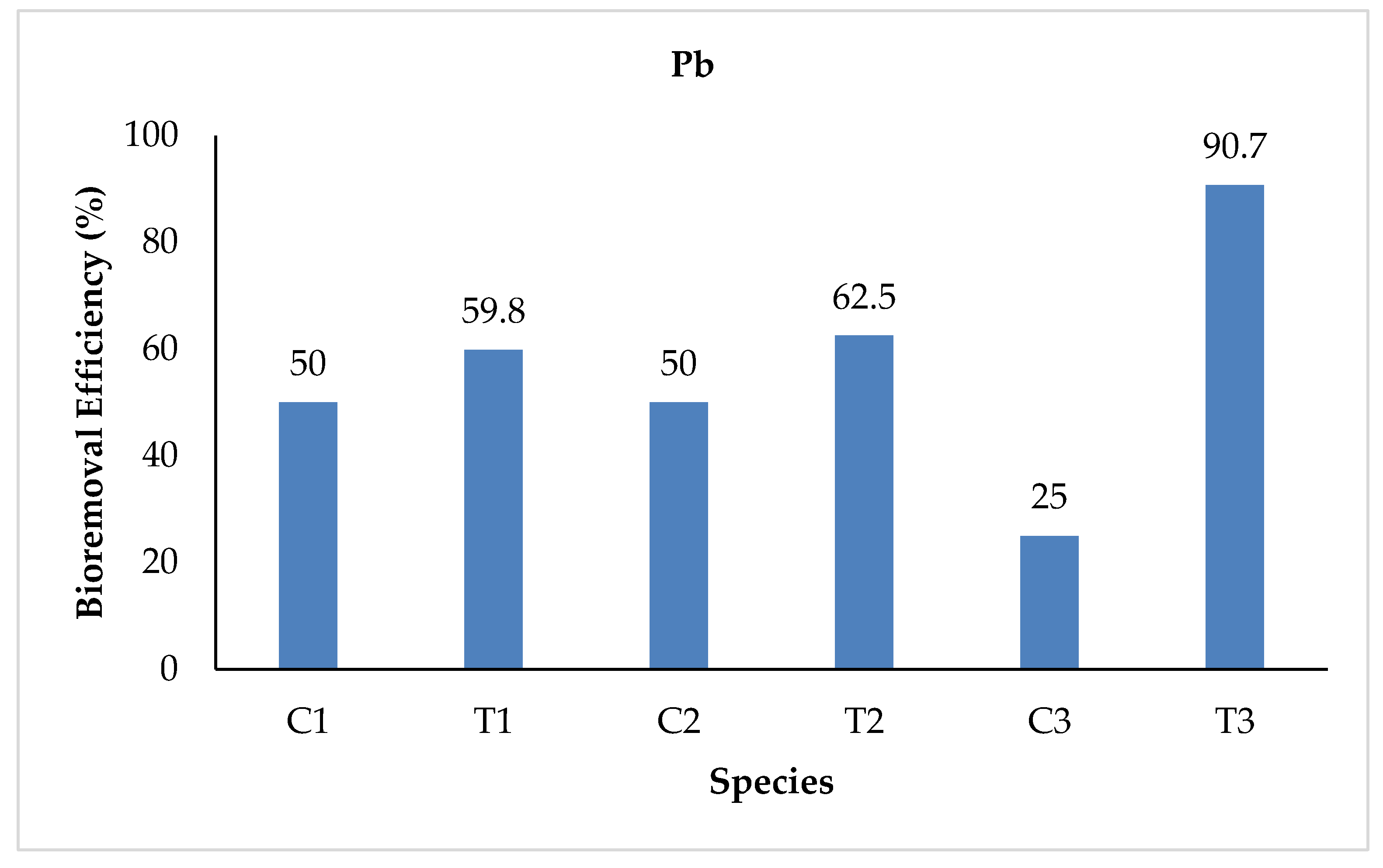
3.2.7. Lead (Pb)
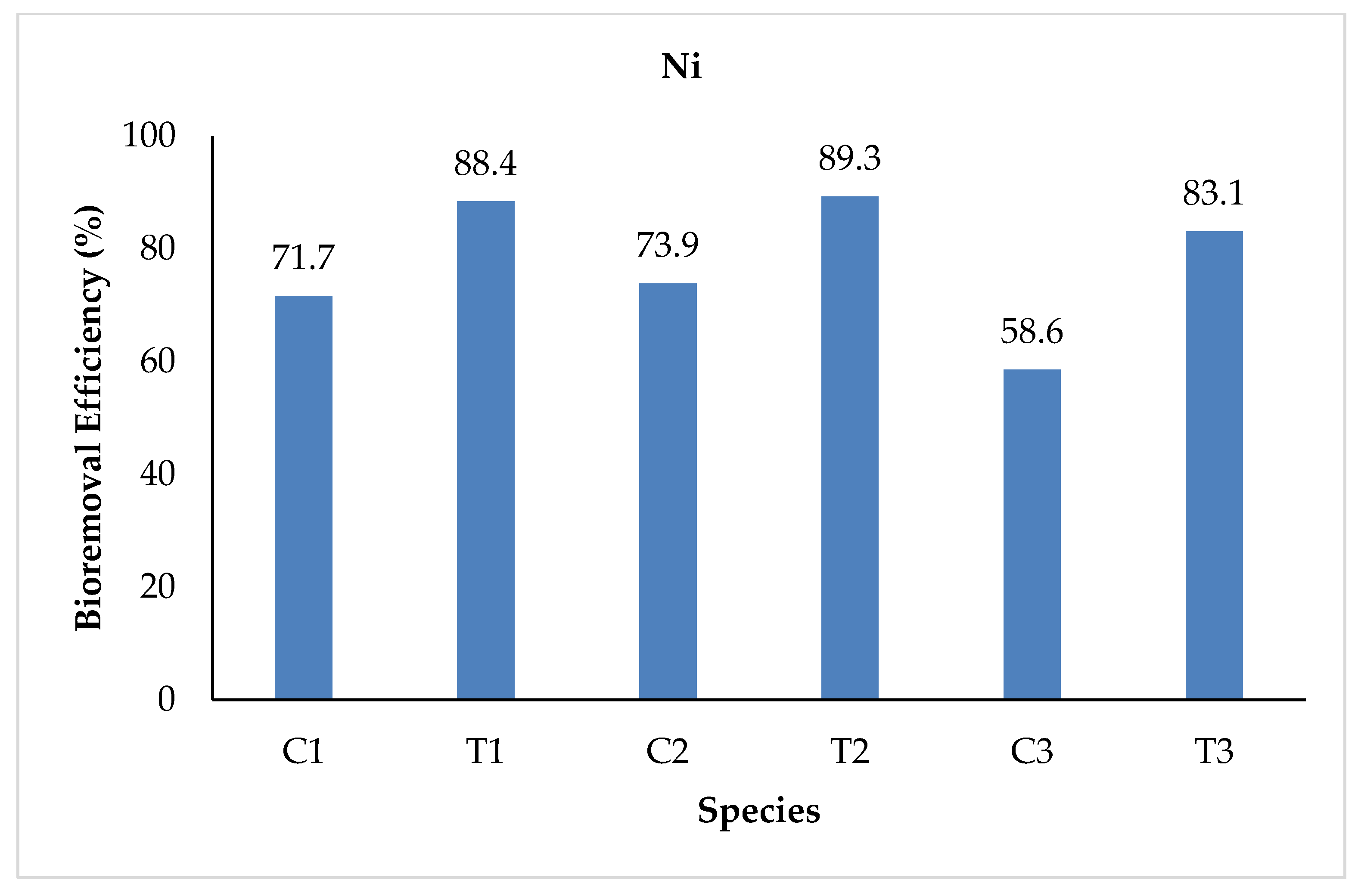
3.2.8. Nickel (Ni)
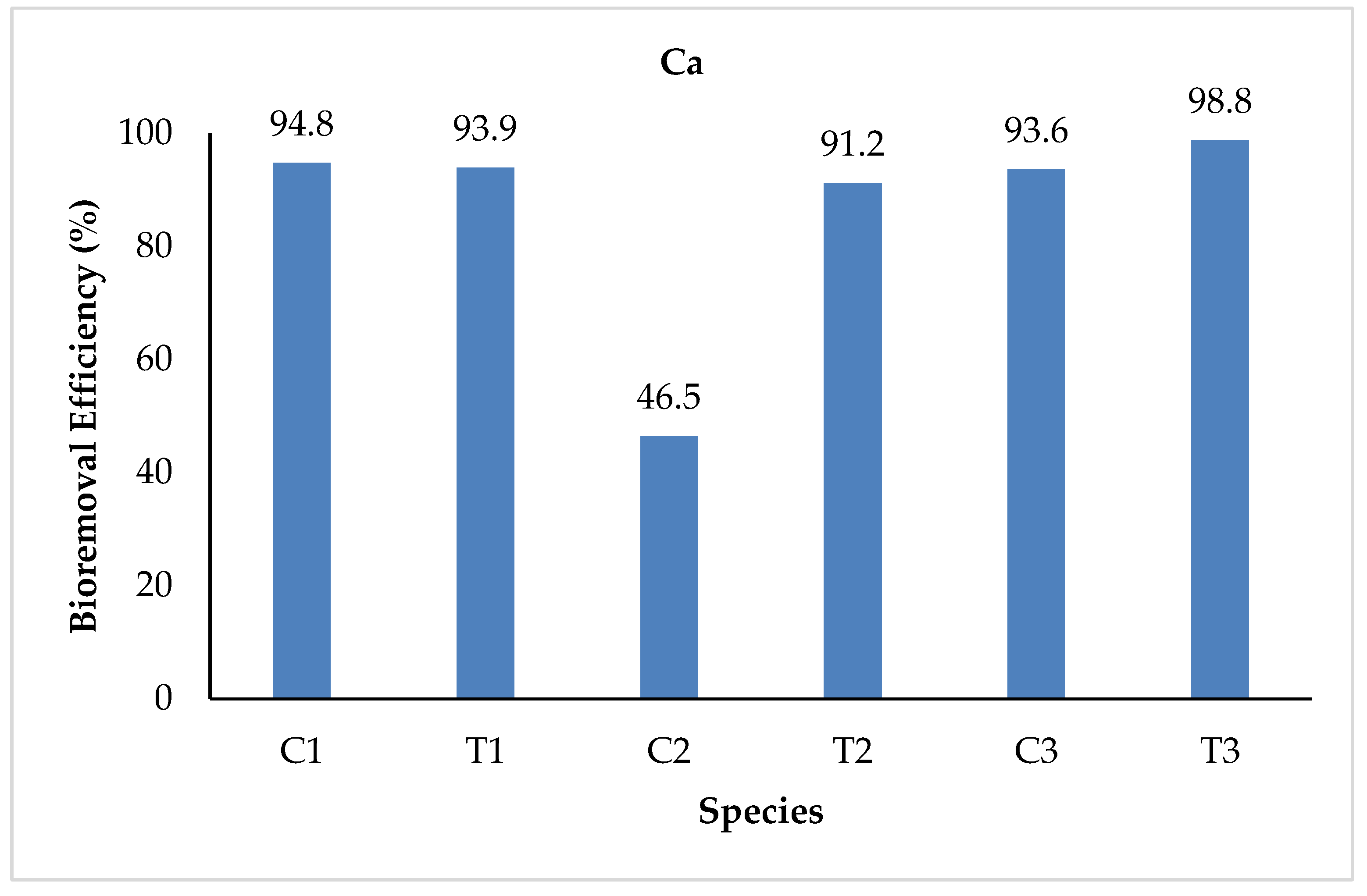
3.2.9. Calcium (Ca)
3.2.10. Magnesium (Mg)
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption potential of brown algae, Sargassum polycystum, for the removal of toxic metals, cadmium and zinc. Environ. Sci. Pollut. Res. 2022, 29, 41909–41922. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Schröder, P.; Bilen, S.; Insam, H.; Juárez, M.F.-D. Co-inoculation effect of Rhizobium and Achillea millefolium L. oil extracts on growth of common bean (Phaseolus vulgaris L.) and soil microbial-chemical properties. Sci. Rep. 2019, 9, 15178. [Google Scholar] [CrossRef] [PubMed]
- UN Water. 2010. Available online: http://www.unwater.org/wwd10/flashindex.html (accessed on 5 January 2023).
- Ayaz, T.; Khan, S.; Khan, A.Z.; Lei, M.; Alam, M. Remediation of industrial wastewater using four hydrophyte species: A comparison of individual (pot experiments) and mix plants (constructed wetland). J. Environ. Manag. 2020, 255, 109833. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Shah, A.H.; Saeed, F.; Ali, M.; Qaisrani, S.A.; Dumat, C. Heavy metal contamination and exposure risk assessment via drinking groundwater in Vehari, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 39852–39864. [Google Scholar] [CrossRef]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Miscu, V.; Djur, S.; Strelkova, L.; Vergel, K.; Nekhoroshkov, P. Growth and heavy metals accumulation by Spirulina platensis biomass from multicomponent copper containing synthetic effluents during repeated cultivation cycles. Ecol. Eng. 2020, 142, 105637. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant. 2021, 173, 148–166. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Kosek, K.; Luczkiewicz, A.; Fudala-Książek, S.; Jankowska, K.; Szopińska, M.; Svahn, O.; Tränckner, J.; Kaiser, A.; Langas, V.; Björklund, E. Implementation of advanced micropollutants removal technologies in wastewater treatment plants (WWTPs)-Examples and challenges based on selected EU countries. Environ. Sci. Policy 2020, 112, 213–226. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Acquafredda, P.; De Vietro, N.; Ranieri, E.; Cosma, P.; Rizzi, V.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention (Pb (II), Cd (II), Ni (II)) from single and multimetal solutions by natural biosorbents from the olive oil milling operations. Process Saf. Environ. Prot. 2018, 114, 79–90. [Google Scholar] [CrossRef]
- Huang, D.; Li, B.; Ou, J.; Xue, W.; Li, J.; Li, Z.; Li, T.; Chen, S.; Deng, R.; Guo, X. Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: Preparation, final disposal, mechanism and influencing factors. J. Environ. Manag. 2020, 261, 109879. [Google Scholar] [CrossRef]
- Sunder, G.S.S.; Adhikari, S.; Rohanifar, A.; Poudel, A.; Kirchhoff, J.R. Evolution of environmentally friendly strategies for metal extraction. Separations 2020, 7, 4. [Google Scholar] [CrossRef]
- Chu, W.-L.; Phang, S.-M. Biosorption of heavy metals and dyes from industrial effluents by microalgae. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Singapore, 2019; pp. 599–634. [Google Scholar] [CrossRef]
- Hubbard, A.T. Encyclopedia of Surface and Colloid Science; CRC Press: Boca Raton, FL, USA, 2002; Volume 1. [Google Scholar]
- Ojha, N.; Karn, R.; Abbas, S.; Bhugra, S. Bioremediation of Industrial Wastewater: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 796, 012012. [Google Scholar] [CrossRef]
- Kashyap, S.; Chandra, R.; Kumar, B.; Verma, P. Biosorption efficiency of nickel by various endophytic bacterial strains for removal of nickel from electroplating industry effluents: An operational study. Ecotoxicology 2022, 31, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, I.; Khan, S.; Waqas, M.; Asma, M.; Nawab, J.; Gul, N.; Raiz, A.; Li, G. Heavy metal uptake capacity of fresh water algae (Oedogonium westti) from aqueous solution: A mesocosm research. Int. J. Phytoremediat. 2016, 18, 393–398. [Google Scholar] [CrossRef]
- Khan, S.; Shamshad, I.; Waqas, M.; Nawab, J.; Ming, L. Remediating industrial wastewater containing potentially toxic elements with four freshwater algae. Ecol. Eng. 2017, 102, 536–541. [Google Scholar] [CrossRef]
- APHA. Compendium of Methods for the Microbiological Examination, 3rd ed.; American Public Health Association: Washington, DC, USA, 1992; pp. 105–119, 325–367, 371–415, 451–469, 637–658. [Google Scholar]
- Shamshad, I.; Khan, S.; Waqas, M.; Ahmad, N.; Ur-Rehman, K.; Khan, K. Removal and bioaccumulation of heavy metals from aqueous solutions using freshwater algae. Water Sci. Technol. 2015, 71, 38–44. [Google Scholar] [CrossRef]
- Younas, U.; Iqbal, S.; Saleem, A.; Iqbal, M.; Nazir, A.; Noureen, S.; Mehmood, K.; Nisar, N. Fertilizer industrial effluents: Physico-chemical characterization and water quality parameters evaluation. Acta Ecol. Sin. 2017, 37, 236–239. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Kloos, H.; Van Hulle, S.W.H. Physicochemical properties of the sugar industry and ethanol distillery wastewater and their impact on the environment. Sugar Tech 2019, 21, 265–277. [Google Scholar] [CrossRef]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hossain, B.; Islam, N.; Alam, M.S.; Hossen, Z. Industrialisation scenario at Sreepur of Gazipur, Bangladesh and physico-chemical properties of wastewater discharged from industries. Asian J. Environ. Ecol. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Chapman, D. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef]
- Aniyikaiye, T.E.; Oluseyi, T.; Odiyo, J.O.; Edokpayi, J.N. Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2019, 16, 1235. [Google Scholar] [CrossRef]
- Tariq, M.; Anayat, A.; Waseem, M.; Rasool, M.H.; Zahoor, M.A.; Ali, S.; Rizwan, M.; Abdel-Daim, M.M.; Alkahtani, S. Physicochemical and bacteriological characterization of industrial wastewater being discharged to surface water bodies: Significant threat to environmental pollution and human health. J. Chem. 2020, 2020, 9067436. [Google Scholar] [CrossRef]
- Paul, S.A.; Chavan, S.K.; Khambe, S.D. Studies on characterization of textile industrial waste water in Solapur city. Int. J. Chem. Sci. 2012, 10, 635–642. [Google Scholar]
- Momeni, M.M.; Kahforoushan, D.; Abbasi, F.; Ghanbarian, S. Using chitosan/CHPATC as coagulant to remove color and turbidity of industrial wastewater: Optimization through RSM design. J. Environ. Manag. 2018, 211, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Marsetty, S.K.; Xu, P. Assessing groundwater quality and health risks of fluoride pollution in the Shasler Vagu (SV) watershed of Nalgonda, India. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 1569–1588. [Google Scholar] [CrossRef]
- Stone, N.M.; Thomforde, H.K. Understanding Your Fish Pond Water Analysis Report; Cooperative Extension Program, University of Arkansas at Pine Bluff, US Department of Agriculture and County Governments Cooperating: Pine Bluff, AR, USA, 2004. [Google Scholar]
- Ali, J.; Khan, M.A.; Nazneen, S.; Muhammad, J.; Nasir, M.J.; Shah, M.T.; Khan, S. Assessment of heavy metals and physico-chemical characteristics of water and sediments, Kurram River (Pakistan). J. Himal. Earth Sci. 2018, 51, 113–126. [Google Scholar]
- Gokalp, Z.; Mohammed, D. Assessment of heavy metal pollution in Heshkaro stream of Duhok city, Iraq. J. Clean. Prod. 2019, 237, 117681. [Google Scholar] [CrossRef]
- Kamika, I.; Momba, M.N.B. Assessing the resistance and bioremediation ability of selected bacterial and protozoan species to heavy metals in metal-rich industrial wastewater. BMC Microbiol. 2013, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Nirgude, N.T.; Shukla, S.; Venkatachalam, A. Physico-chemical analysis of some industrial effluents from Vapi industrial area, Gujarat, India. Rasayan J. Chem. 2013, 6, 68–72. [Google Scholar]



| Physiochemical Parameters | C1 | T1 | C2 | T2 | C3 | T3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Eff. % | Mean | Eff. % | Mean | Eff. % | Mean | Eff. % | Mean | Eff. % | Mean | Eff. (%) | ||
| pH | I | 7.3 | 6.83 | 7.3 | 6.83 | 7.3 | 6.83 | −20.2 | |||||
| F | 7.98 | 8.02 | 8.23 | 8.10 | 8.27 | 8.21 | |||||||
| EC (mS/cm) | I | 0.44 | 45.45 | 1.195 | 11.9 | 0.44 | 15.9 | 1.195 | 16.0 | 0.436 | 46.5 | 1.195 | 15.8 |
| F | 0.24 | 1.052 | 0.37 | 1.003 | 0.23 | 1.006 | |||||||
| Temperature (°C) | I | 26.6 | 45.1 | 26.8 | 45.1 | 26.6 | 45.8 | 26.8 | 45.1 | 26.6 | 45.1 | 26.8 | 45.1 |
| F | 14.6 | 14.7 | 14.4 | 14.7 | 14.6 | 14.7 | |||||||
| BOD (mg/L) | I | 04 | 50 | 85.5 | 75.4 | 04 | 75 | 85.5 | 96.4 | 04 | 25 | 85.5 | 43.8 |
| F | 02 | 21 | 01 | 03 | 03 | 48 | |||||||
| COD (mg/L) | I | 05 | 60 | 921 | 80.5 | 05 | 80 | 921 | 86.5 | 05 | 20 | 921 | 84.7 |
| F | 02 | 179 | 01 | 124 | 04 | 140 | |||||||
| TSS (mg/L) | I | 02 | 9.5 | 232 | 83.6 | 02 | 39 | 232 | 90.0 | 02 | 2.0 | 232 | 84.4 |
| F | 1.81 | 38 | 1.22 | 23 | 1.96 | 36 | |||||||
| TDS (mg/L) | I | 288 | 14.2 | 794 | 16.6 | 288 | 9.7 | 794 | 45.0 | 288 | 17.0 | 794 | 18.1 |
| F | 247 | 662 | 260 | 436 | 239 | 650 | |||||||
| Sulfide (mg/L) | I | 0.40 | 20.0 | 5.6 | 76.7 | 0.4 | 10.0 | 5.6 | 87.5 | 0.4 | 25.0 | 5.6 | 83.9 |
| F | 0.32 | 1.3 | 0.36 | 0.7 | 0.30 | 0.9 | |||||||
| Color (TCU) | I | 0.34 | 50.0 | 440 | 85.6 | 0.34 | 23.5 | 440 | 85.5 | 0.34 | 79.4 | 440 | 87.3 |
| F | 0.17 | 63.13 | 0.26 | 63.72 | 0.07 | 55.45 | |||||||
| Turbidity (NTU) | I | 1.08 | 3.7 | 54.65 | 81.06 | 1.08 | 3.7 | 54.65 | 84.1 | 1.080 | 4.6 | 54.65 | 73.9 |
| F | 1.04 | 10.35 | 1.04 | 8.642 | 1.03 | 14.23 | |||||||
| Fluoride (mg/L) | I | 0.13 | 7.6 | 3.6 | 25 | 0.13 | 23.0 | 3.6 | 11.1 | 0.13 | 15.4 | 3.6 | 11.1 |
| F | 0.12 | 2.7 | 0.10 | 3.2 | 0.11 | 3.2 | |||||||
| Chloride (mg/L) | I | 54 | 24.0 | 162 | 32.0 | 54 | 40.7 | 162 | 19.7 | 54 | 33.3 | 162 | 91.3 |
| F | 41 | 110 | 32 | 130 | 36 | 14 | |||||||
| Calcium Hardness (mg/L) | I | 280 | 28.5 | 590 | 1.6 | 280 | 25.0 | 590 | 18.6 | 280 | 35.7 | 590 | 42.3 |
| F | 200 | 580 | 210 | 480 | 180 | 340 | |||||||
| Magnesium Hardness (mg/L) | I | 180 | 5.5 | 395 | 46.8 | 180 | 14.6 | 395 | 77.2 | 180 | 10.5 | 395 | 49.3 |
| F | 170 | 210 | 157 | 90 | 161 | 200 | |||||||
| Total Hardness (mg/L) | I | 460 | 19.5 | 985 | 52.2 | 460 | 20.2 | 985 | 42.1 | 460 | 25.8 | 985 | 45.1 |
| F | 370 | 470 | 367 | 570 | 341 | 540 | |||||||
| HMs | C1 | T1 | C2 | T2 | C3 | T3 | |
|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | ||
| Mn | Initial | 0.013 | 1.230 | 0.013 | 1.230 | 0.013 | 1.230 |
| Final | 0.004 | 0.049 | 0.011 | 0.011 | 0.008 | 0.008 | |
| Cu | Initial | 0.010 | 0.810 | 0.010 | 0.810 | 0.010 | 0.810 |
| Final | 0.003 | 0.003 | 0.009 | 0.013 | 0.004 | 0.012 | |
| Cr | Initial | 0.016 | 2.120 | 0.016 | 2.120 | 0.016 | 2.120 |
| Final | 0.001 | 0.102 | 0.012 | 1.470 | 0.001 | 0.046 | |
| Cd | Initial | 0.012 | 0.180 | 0.012 | 0.180 | 0.012 | 0.180 |
| Final | 0.010 | 0.010 | 0.005 | 0.055 | 0.001 | 0.081 | |
| Co | Initial | 0.014 | 0.151 | 0.014 | 0.151 | 0.014 | 0.151 |
| Final | 0.012 | 0.102 | 0.011 | 0.081 | 0.009 | 0.089 | |
| Ag | Initial | 0.054 | 0.240 | 0.054 | 0.240 | 0.054 | 0.240 |
| Final | 0.008 | 0.080 | 0.019 | 0.190 | 0.012 | 0.100 | |
| Pb | Initial | 0.002 | 1.120 | 0.002 | 1.120 | 0.002 | 1.120 |
| Final | 0.001 | 0.450 | 0.001 | 0.420 | 0.0017 | 0.104 | |
| Ni | Initial | 0.046 | 0.113 | 0.046 | 0.113 | 0.046 | 0.113 |
| Final | 0.013 | 0.013 | 0.012 | 0.012 | 0.019 | 0.019 | |
| Ca | Initial | 1.720 | 14.50 | 1.720 | 14.50 | 1.720 | 14.50 |
| Final | 0.088 | 0.880 | 0.920 | 1.265 | 0.110 | 0.160 | |
| Mg | Initial | 0.040 | 0.190 | 0.040 | 0.190 | 0.040 | 0.190 |
| Final | 0.017 | 0.047 | 0.007 | 0.097 | 0.016 | 0.016 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, R.; Bashir, K.; Ahmad, S.; Ullah, A.; Shah, S.F.; Ali, Q.; Yasmin, H.; Ahmad, A. Bioremediation of Heavy Metals from Industrial Effluents Using Bacillus pakistanensis and Lysinibacillus composti. Sustainability 2023, 15, 7591. https://doi.org/10.3390/su15097591
Ali R, Bashir K, Ahmad S, Ullah A, Shah SF, Ali Q, Yasmin H, Ahmad A. Bioremediation of Heavy Metals from Industrial Effluents Using Bacillus pakistanensis and Lysinibacillus composti. Sustainability. 2023; 15(9):7591. https://doi.org/10.3390/su15097591
Chicago/Turabian StyleAli, Ramzan, Kashif Bashir, Saeed Ahmad, Amin Ullah, Said Farooq Shah, Qurban Ali, Humaira Yasmin, and Ajaz Ahmad. 2023. "Bioremediation of Heavy Metals from Industrial Effluents Using Bacillus pakistanensis and Lysinibacillus composti" Sustainability 15, no. 9: 7591. https://doi.org/10.3390/su15097591
APA StyleAli, R., Bashir, K., Ahmad, S., Ullah, A., Shah, S. F., Ali, Q., Yasmin, H., & Ahmad, A. (2023). Bioremediation of Heavy Metals from Industrial Effluents Using Bacillus pakistanensis and Lysinibacillus composti. Sustainability, 15(9), 7591. https://doi.org/10.3390/su15097591






