Capacity and Mechanisms of Pb(II) and Cd(II) Sorption on Five Plant-Based Biochars
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Biochar Characterization
2.3. Adsorption Experiments
2.3.1. The Effect of Initial pH Value and Initial Dosage on the Adsorption
2.3.2. Isothermal Adsorption Experiment
2.3.3. Adsorption Kinetics Experiment
2.3.4. Thermodynamic Adsorption Experiment
2.3.5. Infrared Spectral Analysis
2.4. Data Analysis
3. Results
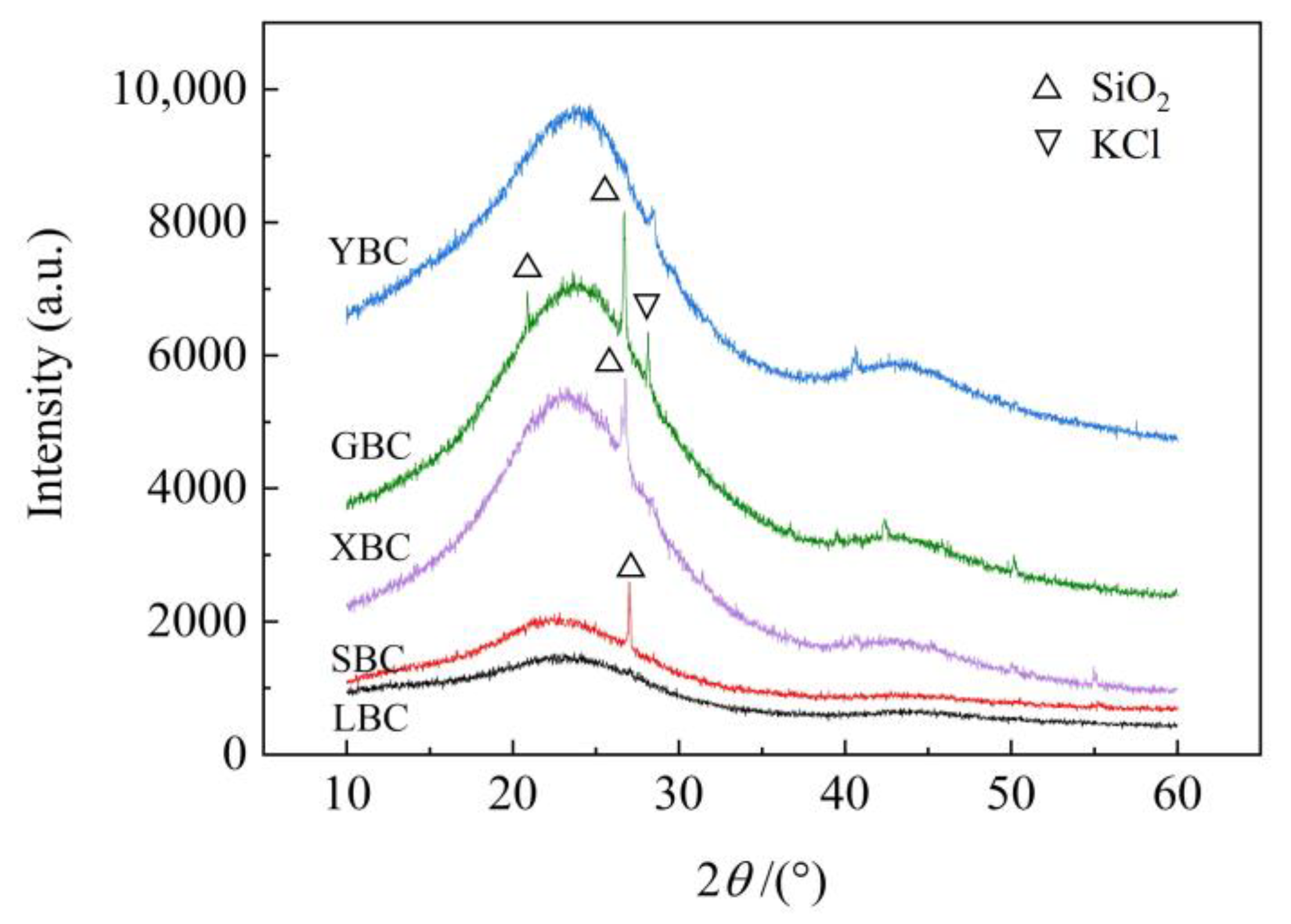
3.1. Biochar Characterization
3.2. Adsorption Studies
3.2.1. Effect of the Initial pH
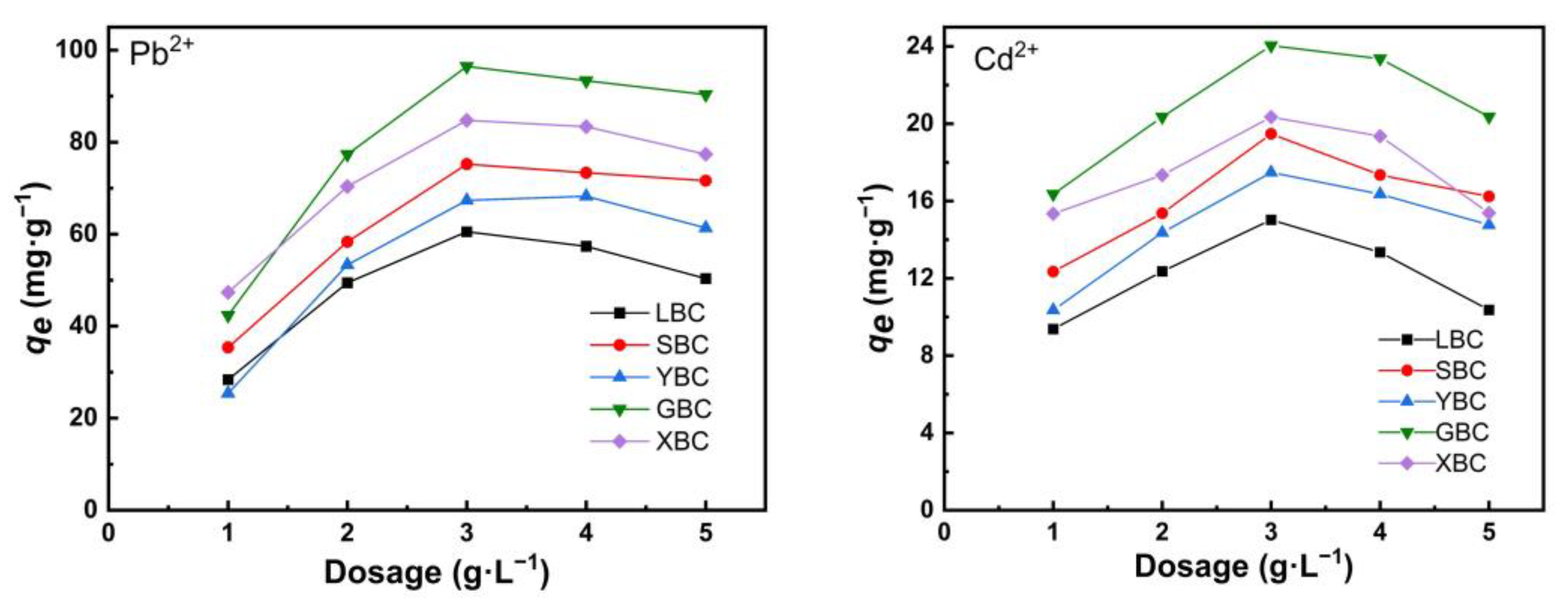
3.2.2. Effect of Initial Dosage
3.2.3. Adsorption Kinetics
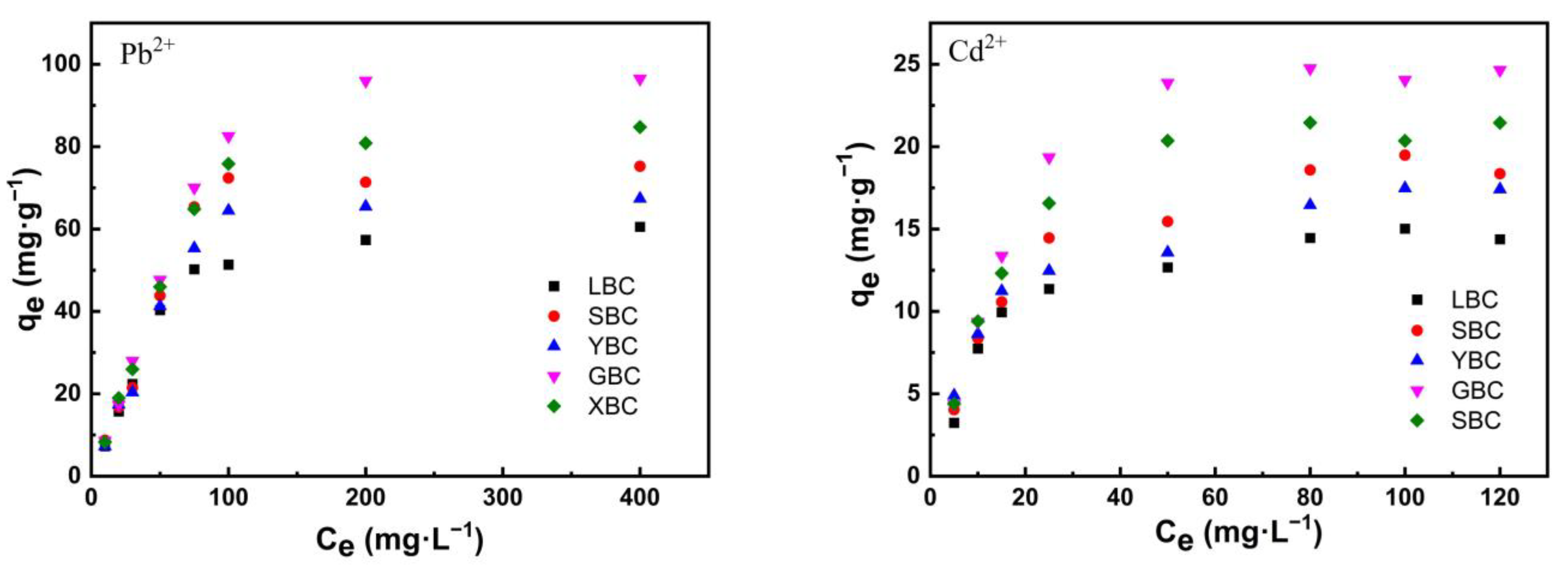
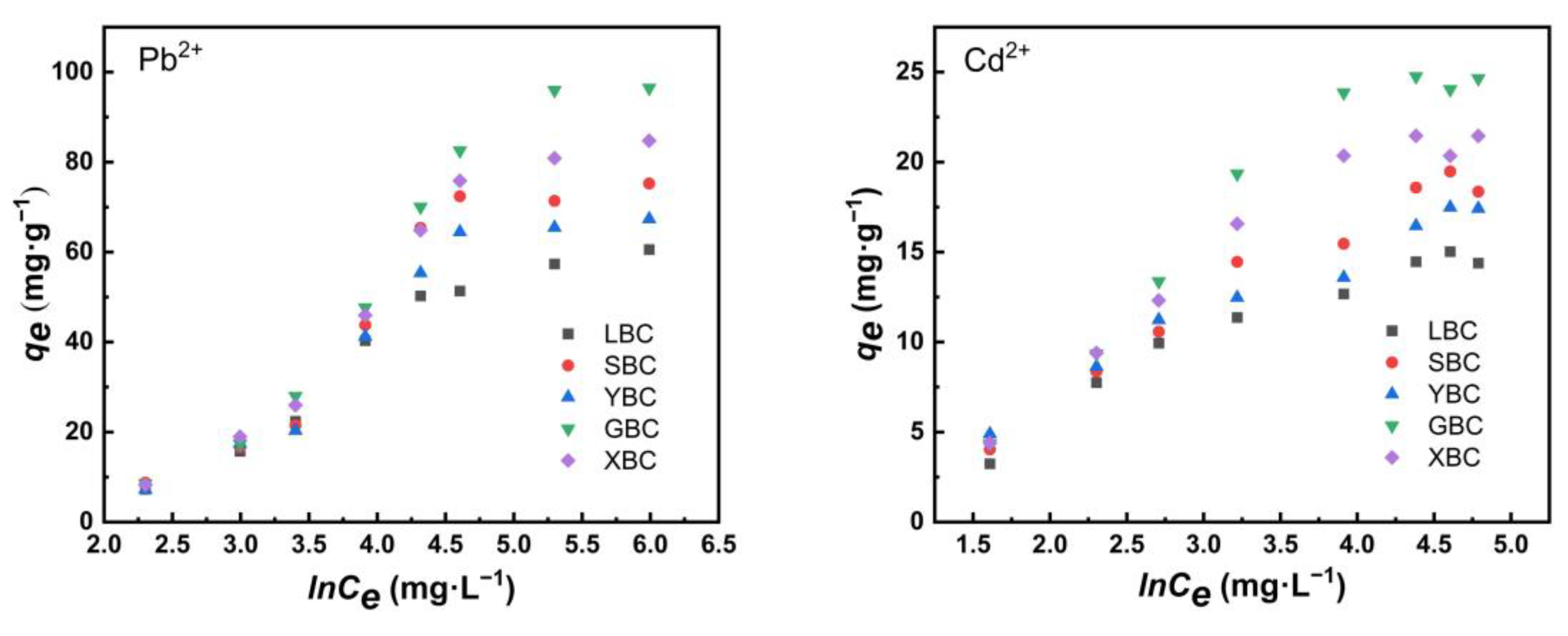
3.2.4. Isothermal Adsorption
3.2.5. Thermodynamics of Adsorption
3.2.6. FTIR Analysis before and after Adsorption
4. Discussion
4.1. Characteristics of the Five Plant-Based Biochars
4.2. Adsorption Characteristics of the Biochars for Pb2+ and Cd2+
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, X.; Hao, H.; Zhang, C.; He, Z.; Yang, X. Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars. Sci. Total Environ. 2016, 539, 566–575. [Google Scholar] [CrossRef]
- Nazari, S.; Rahimi, G.; Khademi Jolgeh Nezhad, A. Effectiveness of native and citric acid-enriched biochar of Chickpea straw in Cd and Pb sorption in an acidic soil. J. Environ. Chem. Eng. 2019, 7, 103064. [Google Scholar] [CrossRef]
- Yin, K.; Wang, J.; Zhai, S.; Xu, X.; Li, T.; Sun, S.; Xu, S.; Zhang, X.; Wang, C.; Hao, Y. Adsorption mechanisms for cadmium from aqueous solutions by oxidant-modified biochar derived from Platanus orientalis Linn leaves. J. Hazard. Mater. 2022, 428, 128261. [Google Scholar] [CrossRef] [PubMed]
- Rötting, T.S.; Mercado, M.; García, M.E.; Quintanilla, J. Environmental distribution and health impacts of As and Pb in crops and soils near Vinto smelter, Oruro, Bolivia. Int. J. Environ. Sci. Technol. 2013, 11, 935–948. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, G.; Huang, X.; Cao, X.; Ye, A.; Deng, Y.; Zhang, J.; Lin, C.; Zhang, R. Study on the physicochemical properties changes of field aging biochar and its effects on the immobilization mechanism for Cd(2+) and Pb(2). Ecotoxicol. Environ. Saf. 2021, 230, 113107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Selvaraj, M.; Bhaumik, A. Functionalized porous organic materials as efficient media for the adsorptive removal of Hg(ii) ions. Environ. Sci. Nano 2020, 7, 2887–2923. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, S.; Mondal, S.; Modak, A.; Chandra, B.K.; Das, S.; Nessim, G.D.; Majee, A.; Bhaumik, A. Thiadiazole containing N- and S-rich highly ordered periodic mesoporous organosilica for efficient removal of Hg(ii) from polluted water. Chem. Commun. 2020, 56, 3963–3966. [Google Scholar] [CrossRef]
- Modak, A.; Das, S.; Chanda, D.K.; Samanta, A.; Jana, S. Thiophene containing microporous and mesoporous nanoplates for separation of mercury from aqueous solution. New J. Chem. 2019, 43, 3341–3349. [Google Scholar] [CrossRef]
- Kaygusuz, M.K.; Isik, N.O.; Erden, K.E. The Removal of Pb(Ii) From Aqueous Solutions by Strong and Weak Acidic Cation Exchange Resins. Fresenius Environ. Bull. 2017, 26, 3448–3454. [Google Scholar]
- Ojstrsek, A.; Gorjanc, N.; Fakin, D. Reduction of Lead and Antimony Ions from the Crystal Glass Wastewaters Utilising Adsorption. Sustainability 2021, 13, 11156. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Chandraiah, M.R. Facile synthesis of zero valent iron magnetic biochar composites for Pb(II) removal from the aqueous medium. Alex. Eng. J. 2016, 55, 619–625. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef]
- Abdul, G.; Zhu, X.; Chen, B. Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 2017, 319, 9–20. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gomez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid. Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, J.; Xu, R. Adsorption of Cr(III) from acidic solutions by crop straw derived biochars. J. Environ. Sci. 2013, 25, 1957–1965. [Google Scholar] [CrossRef]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The relationship between properties of plant-based biochars and sorption of Cd(II), Pb(II) and Zn(II) in soil model systems. Heliyon 2020, 6, e05388. [Google Scholar] [CrossRef]
- Nie, T.H.; Yang, X.; Chen, H.B.; Muller, K.; Shaheen, S.M.; Rinklebe, J.; Song, H.; Xu, S.; Wu, F.C.; Wang, H.L. Effect of biochar aging and co-existence of diethyl phthalate on the mono-sorption of cadmium and zinc to biochar-treated soils. J. Hazard. Mater. 2021, 408, 124850. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.H.; Gao, M.L.; Qiu, W.W.; Islam, M.S.; Song, Z.G. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere 2020, 246, 125701. [Google Scholar] [CrossRef] [PubMed]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef]
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Sci. Total Env. 2021, 795, 148722. [Google Scholar] [CrossRef]
- Paranavithana, G.N.; Kawamoto, K.; Inoue, Y.; Saito, T.; Vithanage, M.; Kalpage, C.S.; Herath, G.B.B. Adsorption of Cd2+ and Pb2+ onto coconut shell biochar and biochar-mixed soil. Environ. Earth Sci. 2016, 75, 484. [Google Scholar] [CrossRef]
- Aschale, M.; Tsegaye, F.; Amde, M. Potato peels as promising low-cost adsorbent for the removal of lead, cadmium, chromium and copper from wastewater. Desalination Water Treat. 2021, 222, 405–415. [Google Scholar] [CrossRef]
- Ni, B.-J.; Huang, Q.-S.; Wang, C.; Ni, T.-Y.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Medha, I.; Chandra, S.; Vanapalli, K.R.; Samal, B.; Bhattacharya, J.; Das, B.K. (3-Aminopropyl)triethoxysilane and iron rice straw biochar composites for the sorption of Cr (VI) and Zn (II) using the extract of heavy metals contaminated soil. Sci. Total Environ. 2021, 771, 144764. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Conversion of crop, weed and tree biomass into biochar for heavy metal removal and wastewater treatment. Biomass Convers. Biorefinery 2021, 13, 4901–4914. [Google Scholar] [CrossRef]
- Ma, S.; Wang, X.; Wang, S.; Feng, K. Effects of temperature on physicochemical properties of rice straw biochar and its passivation ability to Cu2+ in soil. J. Soils Sediments 2022, 22, 1418–1430. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Cation-Exchange Capacity of Soils (Sodium Acetate); Method 9081; EPA: Washington, DC, USA, 1986.
- Shi, Z.; Ma, A.; Chen, Y.; Zhang, M.; Zhang, Y.; Zhou, N.; Fan, S.; Wang, Y. The Removal of Tetracycline from Aqueous Solutions Using Peanut Shell Biochars Prepared at Different Pyrolysis Temperatures. Sustainability 2023, 15, 874. [Google Scholar] [CrossRef]
- Chen, B.L.; Johnson, E.J.; Chefetz, B.; Zhu, L.Z.; Xing, B.S. Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: Role of polarity and accessibility. Environ. Sci. Technol. 2005, 39, 6138–6146. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil. Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, L.; Lei, S.; Liu, M.; Hong, C.; Che, L.; Wang, J.; Qiu, Y. Lead competition alters the zinc adsorption mechanism on animal-derived biochar. Sci. Total Environ. 2020, 713, 136395. [Google Scholar] [CrossRef]
- Ozbay, N.; Putun, A.E.; Putun, E. Bio-oil production from rapid pyrolysis of cottonseed cake: Product yields and compositions. Int. J. Energy Res. 2006, 30, 501–510. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, X.; Gu, Y.; Liu, S.; Liu, Y.; Hu, X.; Li, J.; Zhou, Y.; Liu, S.; He, Y. Rice waste biochars produced at different pyrolysis temperatures for arsenic and cadmium abatement and detoxification in sediment. Chemosphere 2020, 250, 126268. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.; Sinha, K. Morpho-mineralogical exploration of crop, weed and tree derived biochar. J. Hazard. Mater. 2021, 407, 124370. [Google Scholar] [CrossRef]
- Su, Y.; Wen, Y.; Yang, W.; Zhang, X.; Xia, M.; Zhou, N.; Xiong, Y.; Zhou, Z. The mechanism transformation of ramie biochar’s cadmium adsorption by aging. Bioresour. Technol. 2021, 330, 124947. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Maszkowska, J.; Wagil, M.; Mioduszewska, K.; Kumirska, J.; Stepnowski, P.; Bialk-Bielinska, A. Thermodynamic studies for adsorption of ionizable pharmaceuticals onto soil. Chemosphere 2014, 111, 568–574. [Google Scholar] [CrossRef]
- Ding, L.; Wu, C.; Deng, H.; Zhang, X. Adsorptive characteristics of phosphate from aqueous solutions by MIEX resin. J. Colloid Interface Sci. 2012, 376, 224–232. [Google Scholar] [CrossRef]
- Chakravarty, S.; Mohanty, A.; Sudha, T.N.; Upadhyay, A.K.; Konar, J.; Sircar, J.K.; Madhukar, A.; Gupta, K.K. Removal of Pb(II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). J. Hazard. Mater. 2010, 173, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Li, L.; Long, A.; Fossum, B.; Kaiser, M. Effects of pyrolysis temperature and feedstock type on biochar characteristics pertinent to soil carbon and soil health: A meta-analysis. Soil. Use Manag. 2022, 39, 43–52. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Liu, Y.; Bi, D.; Xiao, L.; Lu, J.; Theng, B.K.G.; Wang, H.; Zhang, L.; et al. Assessing the effect of pyrolysis temperature on the molecular properties and copper sorption capacity of a halophyte biochar. Environ. Pollut. 2019, 251, 56–65. [Google Scholar] [CrossRef]
- Huang, F.; Gao, L.-Y.; Deng, J.-H.; Chen, S.-H.; Cai, K.-Z. Quantitative contribution of Cd2+ adsorption mechanisms by chicken-manure-derived biochars. Environ. Sci. Pollut. Res. 2018, 25, 28322–28334. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Gao, K.; Yu, H. Effects of different pyrolysis temperatures on the preparation and characteristics of bio-char from rice straw. Acta Sci. Circumstantiae 2016, 36, 1757–1765. [Google Scholar]
- Ren, X.H.; He, J.Y.; Chen, Q.; He, F.; Wei, T.; Jia, H.L.; Guo, J.K. Marked changes in biochar’s ability to directly immobilize Cd in soil with aging: Implication for biochar remediation of Cd-contaminated soil. Environ. Sci. Pollut. Res. 2022, 29, 73856–73864. [Google Scholar] [CrossRef]
- Lee, J.W.; Kidder, M.; Evans, B.R.; Paik, S.; Buchanan, A.C., III; Garten, C.T.; Brown, R.C. Characterization of Biochars Produced from Cornstovers for Soil Amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Wei, W. Formaldehyde and VOC emissions at different manufacturing stages of wood-based panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Xu, J.; He, Y. Compound-specific stable isotope analysis for characterization of the transformation of gamma-HCH induced by biochar. Chemosphere 2023, 314, 137729. [Google Scholar] [CrossRef]
- Machado, F.M.; Bergmann, C.P.; Fernandes, T.H.M.; Lima, E.C.; Royer, B.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon. J. Hazard. Mater. 2011, 192, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Borchard, N.; Sarmiento, C.; Atkinson, R.; Ladd, B. Key factors determining biochar sorption capacity for metal contaminants: A literature synthesis. Biochar 2020, 2, 151–163. [Google Scholar] [CrossRef]
- Kocaoba, S.; Arisoy, M. Biosorption of cadmium(II) and lead(II) from aqueous solutions using Pleurotus ostreatus immobilized on bentonite. Sep. Sci. Technol. 2018, 53, 1703–1710. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, S.; Chen, H.; Huang, L.; Qiu, R. Pb(II) and Cr(VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour. Technol. 2013, 147, 545–552. [Google Scholar] [CrossRef]
- Wang, X.; Xue, Y.; Cheng, X.; Liu, Y. An overview of heavy metal removal using biochar. China Rural. Water Hydropower 2013, 12, 51–56. [Google Scholar]
- Mehellou, A.; Delimi, R.; Benredjem, Z.; Innocent, C. Affinity of Cation-Exchange Membranes Towards Metallic Cations: Application in Continuous Electropermutation. Sep. Sci. Technol. 2015, 50, 495–504. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Qiu, W.; Wang, P.-P.; Liu, Y.-L.; Zou, J.; Wang, L.; Ma, J. Mechanism study about the adsorption of Pb(II) and Cd(II) with iron-trimesic metal-organic frameworks. Chem. Eng. J. 2020, 385, 123507. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, R.; Xia, B.; Ying, R.; Hu, Z.; Tao, X.; Yu, H.; Xiao, F.; Chu, Q.; Chen, H.; et al. Effect of Pyrolysis Temperature on Removal Efficiency and Mechanisms of Hg(II), Cd(II), and Pb (II) by Maize Straw Biochar. Sustainability 2022, 14, 9022. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Comparison of the lead and copper adsorption capacities of plant source materials and their biochars. J. Env. Manag. 2019, 236, 118–124. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Plant-Derived Biochar | ||||
|---|---|---|---|---|---|
| YBC | GBC | XBC | LBC | SBC | |
| pH | 9.23 | 9.86 | 9.51 | 8.21 | 9.64 |
| Ash (%) | 21.3 | 29.7 | 24.1 | 19.6 | 27.2 |
| BET surface area (m2·g−1) | 98 | 245 | 181 | 96 | 101 |
| Average pore diameter (nm) | 4.517 | 3.501 | 3.068 | 5.747 | 5.455 |
| Micropore volume (cm3·g−1) | 0.004 | 0.080 | 0.055 | 0.003 | 0.006 |
| Total pore volume (cm3·g−1) | 0.110 | 0.215 | 0.139 | 0.103 | 0.137 |
| C (%) | 64.00 | 66.72 | 59.77 | 65.54 | 58.61 |
| H (%) | 2.07 | 2.21 | 1.92 | 1.89 | 1.91 |
| O (%) | 31.91 | 29.90 | 37.12 | 30.61 | 37.44 |
| N (%) | 1.81 | 1.03 | 0.90 | 1.73 | 1.92 |
| H/C | 0.032 | 0.033 | 0.032 | 0.029 | 0.033 |
| O/C | 0.499 | 0.448 | 0.621 | 0.467 | 0.639 |
| (N+O)/C | 0.527 | 0.464 | 0.636 | 0.493 | 0.672 |
| CEC (cmol·kg−1) | 9.79 | 9.89 | 22.50 | 4.60 | 12.50 |
| Biochar | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|
| qe (mg·g−1) | k1 (min−1) | R2 | qe (mg·g−1) | k2 (10−3·g·mg−1·min−1) | R2 | |
| Pb2+ | ||||||
| LBC | 52.566 | 0.052 | 0.851 | 56.77 | 1.337 | 0.961 |
| SBC | 77.234 | 0.065 | 0.839 | 82.44 | 1.192 | 0.963 |
| YBC | 63.937 | 0.064 | 0.766 | 68.77 | 1.345 | 0.924 |
| GBC | 89.962 | 0.068 | 0.846 | 96.02 | 1.072 | 0.967 |
| XBC | 83.468 | 0.043 | 0.852 | 90.44 | 0.696 | 0.952 |
| Cd2+ | ||||||
| LBC | 14.331 | 0.068 | 0.935 | 15.27 | 6.687 | 0.996 |
| SBC | 18.916 | 0.105 | 0.934 | 19.828 | 8.827 | 0.993 |
| YBC | 16.503 | 0.089 | 0.904 | 17.409 | 8.172 | 0.986 |
| GBC | 24.499 | 0.058 | 0.788 | 26.241 | 3.368 | 0.935 |
| XBC | 22.052 | 0.089 | 0.685 | 23.321 | 6.119 | 0.900 |
| Biochar | Intraparticle Diffusion Model | |||||
|---|---|---|---|---|---|---|
| k1 | C1 | R2 | k2 | C2 | R2 | |
| Pb2+ | ||||||
| LBC | 1.893 | 1.435 | 0.976 | 0.104 | 12.795 | 0.632 |
| SBC | 2.422 | 4.253 | 0.986 | 0.075 | 17.961 | 0.733 |
| YBC | 2.026 | 3.351 | 0.991 | 0.111 | 14.978 | 0.888 |
| GBC | 1.943 | 6.793 | 0.999 | 0.305 | 19.939 | 0.726 |
| XBC | 1.639 | 8.521 | 0.946 | 0.225 | 19.059 | 0.780 |
| Cd2+ | ||||||
| LBC | 5.470 | 8.177 | 0.996 | 0.689 | 41.878 | 0.652 |
| SBC | 7.648 | 17.127 | 0.979 | 0.772 | 65.910 | 0.661 |
| YBC | 6.347 | 14.272 | 0.964 | 0.967 | 49.514 | 0.670 |
| GBC | 9.677 | 18.084 | 0.997 | 0.947 | 76.119 | 0.685 |
| XBC | 7.823 | 13.266 | 0.912 | 1.138 | 64.897 | 0.590 |
| Biochar | Temperature (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | n | KF (mg·g−1) | R2 | ||
| LBC | 25 | 72.575 | 0.0199 | 0.936 | 2.849 | 8.481 | 0.763 |
| 35 | 74.522 | 0.0207 | 0.934 | 2.892 | 9.033 | 0.760 | |
| 45 | 78.694 | 0.0239 | 0.934 | 2.845 | 9.176 | 0.760 | |
| XBC | 25 | 106.263 | 0.0156 | 0.932 | 2.558 | 9.427 | 0.776 |
| 35 | 109.028 | 0.0153 | 0.935 | 2.537 | 9.457 | 0.783 | |
| 45 | 111.289 | 0.0187 | 0.940 | 2.568 | 9.979 | 0.789 | |
| YBC | 25 | 84.346 | 0.0178 | 0.904 | 2.726 | 8.785 | 0.726 |
| 35 | 86.522 | 0.0181 | 0.898 | 2.749 | 9.193 | 0.716 | |
| 45 | 91.523 | 0.0190 | 0.915 | 2.661 | 8.998 | 0.750 | |
| SBC | 25 | 94.475 | 0.0174 | 0.879 | 2.692 | 9.532 | 0.701 |
| 35 | 96.932 | 0.0180 | 0.883 | 2.735 | 10.186 | 0.701 | |
| 45 | 102.297 | 0.0204 | 0.895 | 2.693 | 10.334 | 0.713 | |
| GBC | 25 | 125.483 | 0.0132 | 0.937 | 2.386 | 9.144 | 0.794 |
| 35 | 126.386 | 0.0137 | 0.931 | 2.403 | 9.4121 | 0.783 | |
| 45 | 132.312 | 0.0152 | 0.941 | 2.370 | 9.484 | 0.840 | |
| Biochar | Temperature (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | n | KF (mg·g−1) | R2 | ||
| LBC | 25 | 16.463 | 0.081 | 0.957 | 3.388 | 3.844 | 0.846 |
| 35 | 18.300 | 0.086 | 0.971 | 3.259 | 4.026 | 0.885 | |
| 45 | 20.112 | 0.096 | 0.988 | 3.294 | 4.272 | 0.926 | |
| XBC | 25 | 24.987 | 0.064 | 0.966 | 3.031 | 4.800 | 0.846 |
| 35 | 27.472 | 0.057 | 0.983 | 2.850 | 4.720 | 0.888 | |
| 45 | 31.073 | 0.079 | 0.992 | 2.642 | 4.582 | 0.919 | |
| YBC | 25 | 18.955 | 0.079 | 0.965 | 3.322 | 4.315 | 0.925 |
| 35 | 20.726 | 0.080 | 0.966 | 3.356 | 4.778 | 0.878 | |
| 45 | 22.302 | 0.098 | 0.925 | 3.541 | 5.565 | 0.806 | |
| SBC | 25 | 21.823 | 0.061 | 0.971 | 2.939 | 3.989 | 0.891 |
| 35 | 24.497 | 0.069 | 0.965 | 2.860 | 4.269 | 0.883 | |
| 45 | 27.947 | 0.082 | 0.959 | 2.684 | 4.291 | 0.883 | |
| GBC | 25 | 29.802 | 0.055 | 0.954 | 2.800 | 4.933 | 0.834 |
| 35 | 31.584 | 0.053 | 0.954 | 2.755 | 5.068 | 0.840 | |
| 45 | 33.305 | 0.063 | 0.963 | 2.759 | 5.368 | 0.854 | |
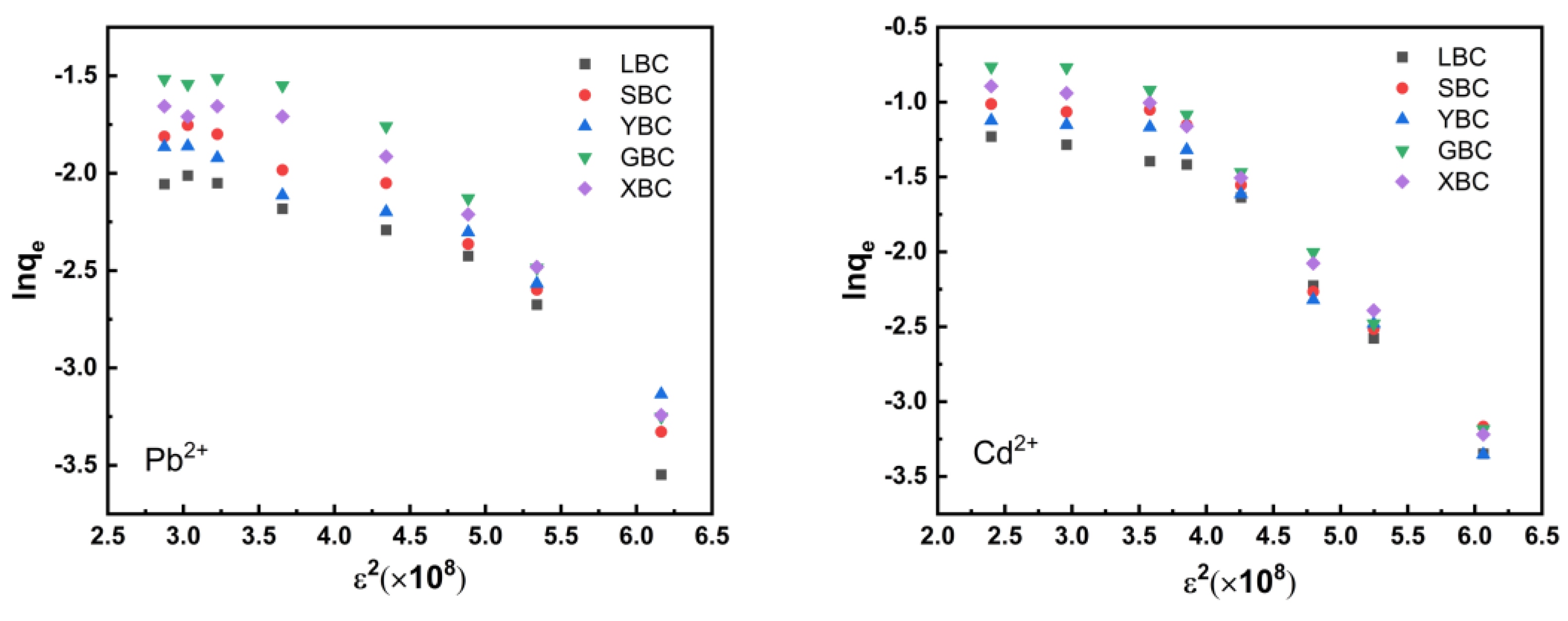
| Biochar | Temkin Model | D–R Model | ||||
|---|---|---|---|---|---|---|
| A | B | R2 | Q0/mmol·g−1 | E/kJ·mol−1 | R2 | |
| Pb2+ | ||||||
| LCB | 0.177 | 16.084 | 0.904 | 1.758 | 9.191 | 0.863 |
| SCB | 0.148 | 21.384 | 0.847 | 2.553 | 8.826 | 0.865 |
| YBC | 0.153 | 18.977 | 0.872 | 2.282 | 8.858 | 0.843 |
| GBC | 0.119 | 28.181 | 0.924 | 3.890 | 8.392 | 0.908 |
| XBC | 0.137 | 23.885 | 0.913 | 3.001 | 8.684 | 0.890 |
| Cd2+ | ||||||
| LBC | 0.877 | 3.354 | 0.924 | 0.469 | 11.278 | 0.813 |
| SBC | 0.600 | 4.660 | 0.957 | 0.664 | 10.784 | 0.894 |
| YBC | 0.942 | 3.776 | 0.966 | 0.455 | 11.983 | 0.911 |
| GBC | 0.482 | 6.625 | 0.928 | 1.111 | 10.051 | 0.874 |
| XBC | 0.610 | 5.355 | 0.933 | 0.799 | 10.647 | 0.870 |
| Biochar | Temperature (K) | ΔG0 (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0 (J·mol−1·K−1) |
|---|---|---|---|---|
| LBC | 298 | −4.680 | 7.194 | 39.6902 |
| 308 | −4.930 | |||
| 318 | −5.480 | |||
| XBC | 298 | −4.072 | 7.035 | 36.9715 |
| 308 | −4.159 | |||
| 318 | −4.824 | |||
| YBC | 298 | −4.403 | 2.486 | 23.0746 |
| 308 | −4.592 | |||
| 318 | −4.866 | |||
| SCB | 298 | −4.340 | 6.284 | 35.527 |
| 308 | −4.579 | |||
| 318 | −5.055 | |||
| GBC | 298 | −3.668 | 5.283 | 29.914 |
| 308 | −3.850 | |||
| 318 | −4.272 |
| Biochar | Temperature (K) | ΔG0 (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0 (J·mol−1·K−1) |
|---|---|---|---|---|
| LBC | 298 | −7.333 | 6.926 | 47.728 |
| 308 | −7.738 | |||
| 318 | −8.292 | |||
| XBC | 298 | −6.748 | 8.050 | 49.029 |
| 308 | −6.685 | |||
| 318 | −7.754 | |||
| YBC | 298 | −7.279 | 8.345 | 52.123 |
| 308 | −7.553 | |||
| 318 | −8.334 | |||
| SCB | 298 | −6.644 | 11.391 | 60.399 |
| 308 | −7.170 | |||
| 318 | −7.856 | |||
| GBC | 298 | −6.370 | 5.769 | 40.424 |
| 308 | −6.517 | |||
| 318 | −7.191 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; He, J.; Sun, J.; Pei, Z.; Wu, Q.; Yu, R. Capacity and Mechanisms of Pb(II) and Cd(II) Sorption on Five Plant-Based Biochars. Sustainability 2023, 15, 7627. https://doi.org/10.3390/su15097627
Yu Y, He J, Sun J, Pei Z, Wu Q, Yu R. Capacity and Mechanisms of Pb(II) and Cd(II) Sorption on Five Plant-Based Biochars. Sustainability. 2023; 15(9):7627. https://doi.org/10.3390/su15097627
Chicago/Turabian StyleYu, Yan, Jiangtao He, Jingyang Sun, Zixuan Pei, Qidong Wu, and Rui Yu. 2023. "Capacity and Mechanisms of Pb(II) and Cd(II) Sorption on Five Plant-Based Biochars" Sustainability 15, no. 9: 7627. https://doi.org/10.3390/su15097627
APA StyleYu, Y., He, J., Sun, J., Pei, Z., Wu, Q., & Yu, R. (2023). Capacity and Mechanisms of Pb(II) and Cd(II) Sorption on Five Plant-Based Biochars. Sustainability, 15(9), 7627. https://doi.org/10.3390/su15097627





