Ex Situ Catalytic Pyrolysis of Invasive Pennisetum purpureum Grass with Activated Carbon for Upgrading Bio-Oil
Abstract
:1. Introduction
2. Experimental
2.1. Biomass Sample Preparation
2.2. Preparation of Activated Carbon (Catalyst)
2.3. Characterization of the Activated Carbon (SEM, BET, FTIR)
2.4. Regeneration of Activated Carbon (Catalyst)
2.5. Biomass to Catalyst Ratio
2.6. Non-Catalytic and Catalytic (Ex Situ) Pyrolysis Setup
2.7. Bio-Oils Analysis (GC–MS)
3. Results and Discussions
3.1. Characterizations
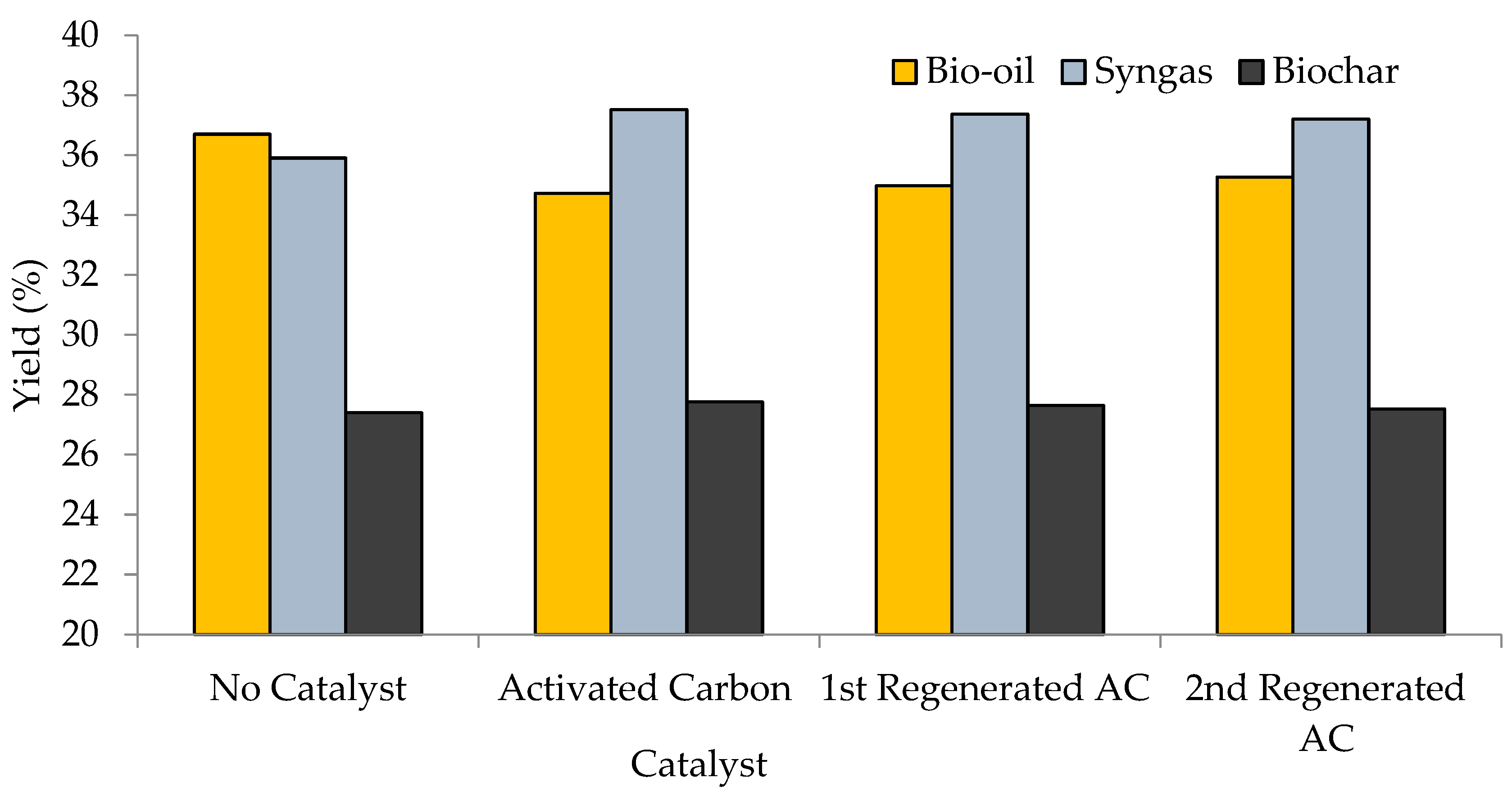
3.2. Product Yield (Catalytic and Non-Catalytic Pyrolysis)
3.3. Bio-Oil Analysis by GC–MS
3.3.1. Non-Catalytic Pyrolysis Process
3.3.2. Catalytic Pyrolysis Process (AC, RAC-1, and RAC-2)
3.4. Comparison of Major Bio-Oil Chemicals from Catalytic and Non-Catalytic Pyrolysis
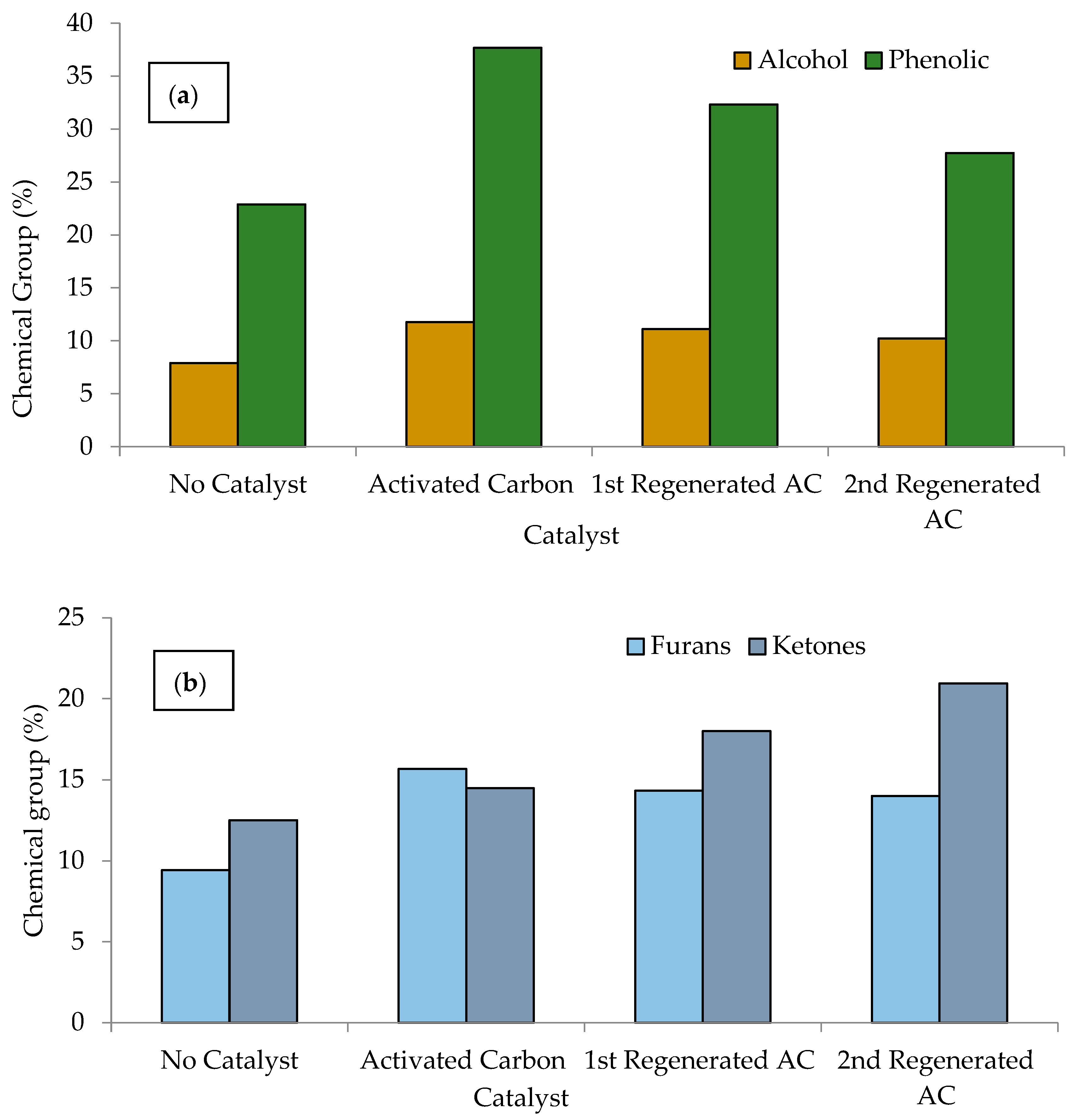
3.4.1. Comparison of Major Chemical Groups of Bio-Oils
3.4.2. Comparison of Some Specific Chemicals of Bio-Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afroze, S.; Reza, M.S.; Amin, M.R.; Taweekun, J.; Azad, A.K. Progress in Nanomaterials Fabrication and Their Prospects in Artificial Intelligence towards Solid Oxide Fuel Cells: A Review. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of Microalgae in Adjusted Wastewater to Enhance Biofuel Production and Reduce Environmental Impact: Pyrolysis Performances and Life Cycle Assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Reza, M.S.; Ahmad, N.B.H.; Afroze, S.; Taweekun, J.; Sharifpur, M.; Azad, A.K. Hydrogen Production from Water Splitting Through Photocatalytic Activity of Carbon-Based Materials, A Review. Chem. Eng. Technol. 2023, 46, 420–434. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing Bioenergy Production from the Raw and Defatted Microalgal Biomass Using Wastewater as the Cultivation Medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of Renewable Energy Sources in Environmental Protection: A Review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M.; Van Meerbeek, K.; Appels, L.; Dewil, R.; et al. Biomass of Invasive Plant Species as a Potential Feedstock for Bioenergy Production. Biofuels Bioprod. Biorefining 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Reza, M.S.; Islam, S.N.; Afroze, S.; Abu Bakar, M.S.; Sukri, R.S.; Rahman, S.; Azad, A.K.; Bakar, M.S.A.; Sukri, R.S.; Rahman, S.; et al. Evaluation of the Bioenergy Potential of Invasive Pennisetum Purpureum through Pyrolysis and Thermogravimetric Analysis. Energy Ecol. Environ. 2020, 5, 118–133. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R. Bin Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; InTech: London, UK, 2017; pp. 1–35. [Google Scholar]
- Reza, M.S.; Taweekun, J.; Afroze, S.; Siddique, S.A.; Islam, M.S.; Wang, C.; Azad, A.K. Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability 2023, 15, 1643. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical Developments in Hydroprocessing Bio-Oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Radenahmad, N.; Md Sumon, R.; Muhammad, S.; Abu, B.; Azad, A.K. Thermochemical Characterization of Rice Husk (Oryza Sativa Linn) for Power Generation. ASEAN J. Chem. Eng. 2020, 20, 184–195. [Google Scholar] [CrossRef]
- Balat, M. An Overview of the Properties and Applications of Biomass Pyrolysis Oils. Energy Sources Part A Recover. Util. Environ. Eff. 2011, 33, 674–689. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research Progress in the Preparation of High-Quality Liquid Fuels and Chemicals by Catalytic Pyrolysis of Biomass: A Review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.; He, J.; Kumar, R.; Lu, Q. Catalytic Pyrolysis of Lignocellulosic Biomass: A Review of Variations in Process Factors and System Structure. Renew. Sustain. Energy Rev. 2020, 134, 110305. [Google Scholar] [CrossRef]
- Syazaidah, I.; Abu Bakar, M.S.; Reza, M.S.; Azad, A.K. Ex-Situ Catalytic Pyrolysis of Chicken Litter for Bio-Oil Production: Experiment and Characterization. J. Environ. Manag. 2021, 297, 113407. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and Challenges of Zeolite Chemistry in the Catalytic Conversion of Biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Yadavalli, G.; Wei, Y.; Zhang, X.; Zhu, L.; Liu, Y. Biofuel Production from Catalytic Microwave Pyrolysis of Douglas Fir Pellets over Ferrum-Modified Activated Carbon Catalyst. J. Anal. Appl. Pyrolysis 2015, 112, 74–79. [Google Scholar] [CrossRef]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation Processes: Advances on Catalytic Transformations of Biomass-Derived Platform Chemicals into Hydrocarbon Fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated Carbon from Lignocellulosic Biomass as Catalyst: A Review of the Applications in Fast Pyrolysis Process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Danquah, J.A.; Roberts, C.O.; Appiah, M. Elephant Grass (Pennisetum Purpureum): A Potential Source of Biomass for Power Generation in Ghana. Curr. J. Appl. Sci. Technol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Kendabie, P.; Nwachukwu, R.E.S. Bioenergy Potentials of Elephant Grass, Pennisetum Purpureum Schumach. Annu. Res. Rev. Biol. 2014, 4, 2215–2227. [Google Scholar] [CrossRef]
- Williams, C. Fertilizer Response of Napier Grass under Different Soil Conditions in Brunei. Exp. Agric. 1980, 16, 415–423. [Google Scholar] [CrossRef]
- Reza, M.S.; Azad, A.K.; Bakar, M.S.A.; Karim, M.R.; Sharifpur, M.; Taweekun, J. Evaluation of Thermochemical Characteristics and Pyrolysis of Fish Processing Waste for Renewable Energy Feedstock. Sustainability 2022, 14, 1203. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility Study on a New Pomelo Peel Derived Biochar for Tetracycline Antibiotics Removal in Swine Wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and Characterization of Activated Carbon from Reedy Grass Leaves by Chemical Activation with H3PO4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Reza, M.S.; Hasan, A.B.M.K.; Ahmed, A.S.; Afroze, S.; Bakar, M.S.A.; Islam, S.N.; Azad, A.K. COVID-19 Prevention: Role of Activated Carbon. J. Eng. Technol. Sci. 2021, 53, 210404. [Google Scholar] [CrossRef]
- Gaber, A.; Saif, H.; Ali, M.R.O. Sugarcane Bagasse Pyrolysis: Investigating the Effect of Process Parameters on the Product Yields. Mater. Sci. Forum 2020, 1008, 159–167. [Google Scholar] [CrossRef]
- NIST Standard Reference Database 1A NIST/EPA/NIH Mass Spectral Library (NIST 08) and NIST Mass Spectral Search Program (Version 2.0f). Available online: https://chemdata.nist.gov/mass-spc/ms-search/docs/Ver20Man.pdf (accessed on 5 March 2023).
- Reza, M.S.; Islam, S.N.; Afroze, S.; Bakar, M.S.A.; Taweekun, J.; Azad, A.K. Data on FTIR, TGA–DTG, DSC of Invasive Pennisetum Purpureum Grass. Data Br. 2020, 30, 105536. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin—From Natural Adsorbent to Activated Carbon: A Review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Ahmed, M.J. Preparation of Activated Carbons from Date (Phoenix Dactylifera L.) Palm Stones and Application for Wastewater Treatments: Review. Process Saf. Environ. Prot. 2016, 102, 168–182. [Google Scholar] [CrossRef]
- Aslam, Z.; Anait, U.; Abbas, A.; Ihsanullah, I.; Irshad, U.; Mahmood, N. Adsorption of Carbon Dioxide onto Activated Carbon Prepared from Lawn Grass. Biomass Convers. Biorefin. 2020, 12, 3121–3131. [Google Scholar] [CrossRef]
- Lü, L.; Lu, D.; Chen, L.; Luo, F. Removal of Cd(II) by Modified Lawny Grass Cellulose Adsorbent. Desalination 2010, 259, 120–130. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Kerré, B.; Kopittke, P.M.; Horemans, B.; Smolders, E. Biochar Affects Carbon Composition and Stability in Soil: A Combined Spectroscopy-Microscopy Study. Sci. Rep. 2016, 6, 25127. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality Variations of Poultry Litter Biochar Generated at Different Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Popescu, C.; Popescu, M.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral Characterization of Eucalyptus Wood. Soc. Appl. Spectrosc. 2018, 61, 1168–1177. [Google Scholar] [CrossRef]
- Mopoung, S.; Moonsri, P.; Palas, W.; Khumpai, S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Sci. World J. 2015, 2015, 415961. [Google Scholar] [CrossRef]
- Özçimen, D.; Ersoy-Meriçboyu, A. Characterization of Biochar and Bio-Oil Samples Obtained from Carbonization of Various Biomass Materials. Renew. Energy 2010, 35, 1319–1324. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Shao, S.; Xiao, R. Catalytic Conversion of Lignin Pyrolysis Model Compound- Guaiacol and Its Kinetic Model Including Coke Formation. Sci. Rep. 2016, 6, 37513. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave Assisted Preparation of Activated Carbon from Biomass: A Review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Abu Bakar, M. Catalytic Intermediate Pyrolysis of Brunei Rice Husk for Bio-Oil Production. Ph.D. Thesis, Aston University, Birmingham, UK, 2013. [Google Scholar]
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-Assisted Catalytic Pyrolysis of Lignocellulosic Biomass for Production of Phenolic-Rich Bio-Oil. Bioresour. Technol. 2016, 211, 382–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, H.; Yang, Z.; Duan, D.; Villota, E.; Ruan, R. From Glucose-Based Carbohydrates to Phenol-Rich Bio-Oils Integrated with Syngas Production via Catalytic Pyrolysis over an Activated Carbon Catalyst. Green Chem. 2018, 20, 3346–3358. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Yang, W.; Jönsson, P.G. Catalytic Pyrolysis of Lignocellulosic Biomass: The Influence of the Catalyst Regeneration Sequence on the Composition of Upgraded Pyrolysis Oils over a H-ZSM-5/Al-MCM-41 Catalyst Mixture. ACS Omega 2020, 5, 28992–29001. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Characterization of Bio-Oils in Chemical Families. Biomass Bioenergy 2007, 31, 222–242. [Google Scholar] [CrossRef]
- Ingram, L.; Mohan, D.; Bricka, M.; Steele, P.; Strobel, D.; Crocker, D.; Mitchell, B.; Mohammad, J.; Cantrell, K.; Pittman, C.U. Pyrolysis of Wood and Bark in an Auger Reactor: Physical Properties and Chemical Analysis of the Produced Bio-Oils. Energy Fuels 2008, 22, 614–625. [Google Scholar] [CrossRef]
- Moens, L.; Black, S.K.; Myers, M.D.; Czernik, S. Study of the Neutralization and Stabilization of a Mixed Hardwooc Bio-Oil. Energy Fuels 2009, 23, 2695–2699. [Google Scholar] [CrossRef]
- Yorgun, S.; Yildiz, D. Slow Pyrolysis of Paulownia Wood: Effects of Pyrolysis Parameters on Product Yields and Bio-Oil Characterization. J. Anal. Appl. Pyrolysis 2015, 114, 68–78. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S. Analytical Pyrolysis Studies of Corn Stalk and Its Three Main Components by TG-MS and Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 11–18. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Tang, Z.; Li, W.Z.; Zhu, X.F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Titania and Zirconia/titania Based Catalysts. Fuel 2010, 89, 2096–2103. [Google Scholar] [CrossRef]
- Kim, T.-S.; Kim, J.-Y.; Kim, K.-H.; Lee, S.; Choi, D.; Choi, I.-G.; Choi, J.W. The Effect of Storage Duration on Bio-Oil Properties. J. Anal. Appl. Pyrolysis 2012, 95, 118–125. [Google Scholar] [CrossRef]
- Williams, P.T.; Horne, P.A. Characterisation of Oils from the Fluidised Bed Pyrolysis of Biomass with Zeolite Catalyst Upgrading. Biomass Bioenergy 1994, 7, 223–236. [Google Scholar] [CrossRef]
- Mullen, C.A.; Tarves, P.C.; Boateng, A.A. Role of Potassium Exchange in Catalytic Pyrolysis of Biomass over ZSM-5: Formation of Alkyl Phenols and Furans. ACS Sustain. Chem. Eng. 2017, 5, 2154–2162. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of Biomass Pyrolysis Oil Properties and Upgrading Research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, W.W.; Jiang, H.; Yu, H.Q. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Prado, G.H.C.; Rao, Y.; De Klerk, A. Nitrogen Removal from Oil: A Review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Stefanidis, S.D.; Kalogiannis, K.G.; Delimitis, A.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic Upgrading of Biomass Pyrolysis Vapors Using Transition Metal-Modified ZSM-5 Zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A Study of Lignocellulosic Biomass Pyrolysis via the Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Sadare, O.O.; Obazu, F.; Daramola, M.O. Biodesulfurization of Petroleum Distillates—Current Status, Opportunities and Future Challenges. Environments 2017, 4, 85. [Google Scholar] [CrossRef]
- Thomas, V.M.; Bedford, J.A.; Cicerone, R.J. Bromine Emissions from Leaded Gasoline. Geophys. Res. Lett. 1997, 24, 1371–1374. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, Y.; Wang, Y.; Lei, H.; Wang, Q.; Ruan, R. Production of Renewable Jet Fuel and Gasoline Range Hydrocarbons from Catalytic Pyrolysis of Soapstock over Corn Cob-Derived Activated Carbons. Energy 2020, 209, 118454. [Google Scholar] [CrossRef]
- Shan Ahamed, T.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of Bio-Oil from Thermochemical Conversion of Various Biomass—Mechanism, Challenges and Opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Upreti, G.K.; Awad, S.; Burnens, G.; Kassargy, C.; Tazerout, M. Experimental Investigation on the Reduction of Catalyst Costs in the Polyethylene Pyrolysis Process. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 12122. [Google Scholar] [CrossRef]
- Huo, E.; Duan, D.; Lei, H.; Liu, C.; Zhang, Y.; Wu, J.; Zhao, Y.; Huang, Z.; Qian, M.; Zhang, Q.; et al. Phenols Production Form Douglas Fir Catalytic Pyrolysis with MgO and Biomass-Derived Activated Carbon Catalysts. Energy 2020, 199, 117459. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Z.; Sun, J.; Wang, Q. Recent Progress of Catalytic Pyrolysis of Biomass by HZSM-5. Cuihua Xuebao/Chin. J. Catal. 2013, 34, 641–650. [Google Scholar] [CrossRef]
- Vitolo, S.; Bresci, B.; Seggiani, M.; Gallo, M.G. Catalytic Upgrading of Pyrolytic Oils over HZSM-5 Zeolite: Behaviour of the Catalyst When Used in Repeated Upgrading-Regenerating Cycles. Fuel 2001, 80, 17–26. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Liu, Y.; Liang, J.; Tang, J. Renewable Phenols Production by Catalytic Microwave Pyrolysis of Douglas Fir Sawdust Pellets with Activated Carbon Catalysts. Bioresour. Technol. 2013, 142, 546–552. [Google Scholar] [CrossRef]
- Yang, C.; Jia, L.; Chen, C.; Liu, G.; Fang, W. Bio-Oil from Hydro-Liquefaction of Dunaliella Salina over Ni/REHY Catalyst. Bioresour. Technol. 2011, 102, 4580–4584. [Google Scholar] [CrossRef]
- Obeid, F.; Chu Van, T.; Brown, R.; Rainey, T. Nitrogen and Sulphur in Algal Biocrude: A Review of the HTL Process, Upgrading, Engine Performance and Emissions. Energy Convers. Manag. 2019, 181, 105–119. [Google Scholar] [CrossRef]
- Cigno, E.; Magagnoli, C.; Pierce, M.S.; Iglesias, P. Lubricating Ability of Two Phosphonium-Based Ionic Liquids as Additives of a Bio-Oil for Use in Wind Turbines Gearboxes. Wear 2017, 376–377, 756–765. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Konstas, K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Catalytic Upgradation of Bio-Oil over Metal Supported Activated Carbon Catalysts in Sub-Supercritical Ethanol. J. Environ. Chem. Eng. 2021, 9, 105059. [Google Scholar] [CrossRef]
- Pham, T.N.; Sooknoi, T.; Crossley, S.P.; Resasco, D.E. Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion. ACS Catal. 2013, 3, 2456–2473. [Google Scholar] [CrossRef]
- Dinh Ngo, S.; Tuong Vi Tran, T.; Kongparakul, S.; Reubroycharoen, P.; Kidkhuntod, P.; Chanlek, N.; Wang, J.; Guan, G.; Samart, C. Catalytic Pyrolysis of Napier Grass with Nickel-Copper Core-Shell Bi-Functional Catalyst. J. Anal. Appl. Pyrolysis 2020, 145, 104745. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. Synergistic Effects of Catalyst Mixtures on Biomass Catalytic Pyrolysis. Front. Bioeng. Biotechnol. 2020, 8, 1391. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Weber, M.; Weber, V. Phenol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2020; pp. 1–20. [Google Scholar]
- Fiege, H. Cresols and Xylenols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Müller, H. Tetrahydrofuran. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Peng, J.; Chen, P.; Lou, H.; Zheng, X. Catalytic Upgrading of Bio-Oil by HZSM-5 in Sub- and Super-Critical Ethanol. Bioresour. Technol. 2009, 100, 3415–3418. [Google Scholar] [CrossRef]
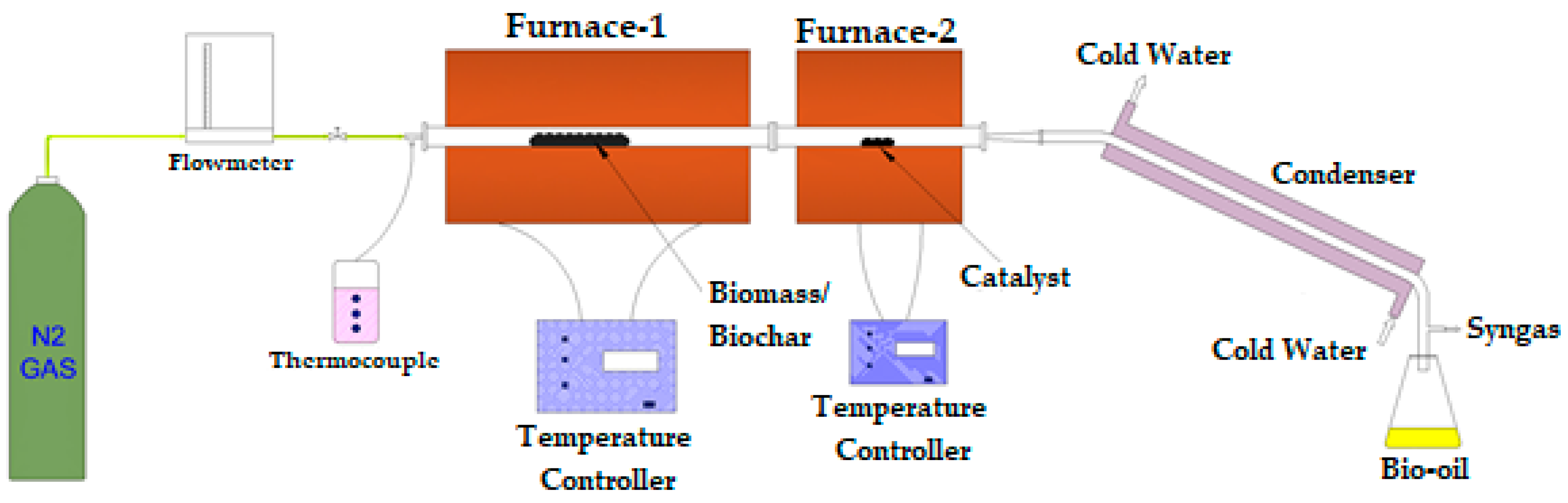


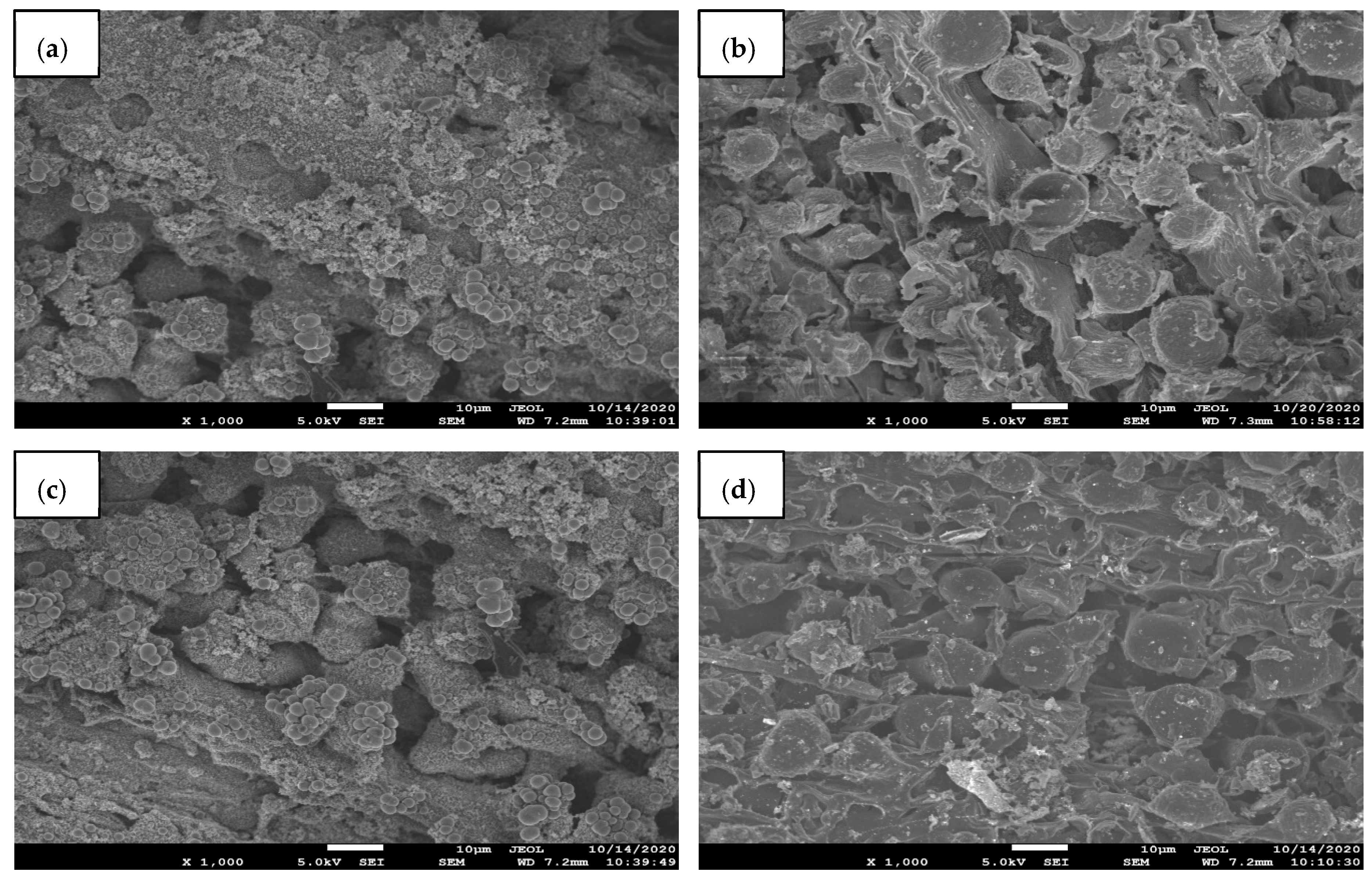


| Catalyst Name | Catalyst Code | Biomass (wt.):Catalyst (wt.) |
|---|---|---|
| No catalyst | -- | 25 (g):0 (g) |
| Activated Carbon | AC | 25 (g):1 (g) |
| First-Time Regenerated Activated Carbon | RAC-1 | 25 (g):1 (g) |
| Second-Time Regenerated Activated Carbon | RAC-2 | 25 (g):1 (g) |
| Catalyst | Biochar (wt.%) | Bio-Oil (wt.%) | Syngas (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | σ | Mean | σ | Mean | σ | ||||
| No catalyst | 27.40 | 0.35 | 0.25 | 36.70 | 0.61 | 0.43 | 35.90 | 0.67 | 0.47 |
| AC | 27.76 | 0.46 | 0.33 | 34.72 | 0.29 | 0.21 | 37.52 | 0.81 | 0.57 |
| RAC-1 | 27.65 | 0.51 | 0.36 | 34.98 | 0.53 | 0.37 | 37.37 | 0.34 | 0.24 |
| RAC-2 | 27.53 | 0.39 | 0.28 | 35.27 | 0.48 | 0.34 | 37.20 | 0.57 | 0.40 |
| Ret. Time (min) | Chemical Name | MW | Formula | Peak Area (%) | Chemical Group |
|---|---|---|---|---|---|
| 3.027 | Acetic acid, ethoxy- | 104 | C4H8O3 | 0.61 | Acid |
| 3.172 | 1H-Pyrazole, 3,5-dimethyl- | 96 | C5H8N2 | 4.45 | Nitrogenous |
| 3.34 | 2-Furanmethanol | 98 | C5H6O2 | 3.01 | Furans |
| 3.391 | 2-Propanone, 1-(acetyloxy)- | 116 | C5H8O3 | 4.29 | Misc. Oxygenated |
| 3.758 | 2-Cyclopenten-1-one, 3-methyl- | 96 | C6H8O | 0.82 | Ketones |
| 3.841 | Butanoic acid, 4-hydroxy- | 104 | C4H8O3 | 2.52 | Acid |
| 3.977 | Cyclohexanone | 98 | C6H10O | 2.82 | Ketones |
| 4.067 | 2-Hexanone, 6-hydroxy- | 116 | C6H12O2 | 1.13 | Misc. Oxygenated |
| 4.266 | 2-Furancarboxaldehyde, 5-methyl- | 110 | C6H6O2 | 0.88 | Furans |
| 4.308 | 2-Cyclopenten-1-one, 3-methyl- | 96 | C6H8O | 0.83 | Ketones |
| 4.47 | Phenol | 94 | C6H6O | 3.02 | Phenolic |
| 4.629 | Octanoic acid, 2-amino- | 159 | C8H17NO2 | 2.54 | Nitrogenous |
| 4.775 | Triethylenediamine | 112 | C6H12N2 | 0.64 | Nitrogenous |
| 4.916 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 112 | C6H8O2 | 2.58 | Ketones |
| 5.038 | 2-Propenoic acid, 2-methyl-, 1,2-ethanediyl Ester | 142 | C8H14O2 | 0.86 | Ester |
| 5.112 | Phenol, 2-methyl- | 108 | C7H8O | 1.80 | Phenolic |
| 5.293 | Phenol, 4-methyl- | 108 | C7H8O | 2.56 | Phenolic |
| 5.384 | Butanoic acid, 2-propenyl ester | 128 | C7H12O2 | 0.93 | Ester |
| 5.428 | Phenol, 2-methoxy- | 124 | C7H8O2 | 1.62 | Phenolic |
| 5.606 | Cyclopropyl carbinol | 72 | C4H8O | 7.24 | Alcohol |
| 5.683 | Maltol | 126 | C6H6O3 | 0.80 | Acid |
| 5.724 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 126 | C7H10O2 | 1.90 | Ketones |
| 5.929 | Phenol, 2,5-dimethyl- | 122 | C8H10O | 1.23 | Phenolic |
| 5.999 | Pentanoic acid, 5-bromo- | 181 | C5H9BrO2 | 0.32 | Bromide |
| 6.095 | Phenol, 4-ethyl- | 122 | C8H10O | 1.04 | Phenolic |
| 6.152 | Benzamide, N-hydroxy- | 137 | C7H7NO2 | 0.23 | Nitrogenous |
| 6.206 | n-Decanoic acid | 172 | C10H20O2 | 0.46 | Acid |
| 6.276 | Benzyl alcohol | 108 | C7H8O | 0.34 | Alcohol |
| 6.346 | Phenol, 2-methoxy-4-methyl- | 138 | C8H10O2 | 0.56 | Phenolic |
| 6.506 | 1,2-Benzenediol | 110 | C6H6O2 | 3.98 | Phenolic |
| 6.578 | Benzofuran, 2,3-dihydro- | 120 | C8H8O | 3.68 | Furans |
| 6.748 | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 126 | C6H6O3 | 1.63 | Furans |
| 6.874 | Furan, 2,5-dimethyl- | 96 | C6H8O | 0.22 | Furans |
| 6.945 | 2,5-Hexanedione | 114 | C6H10O2 | 0.52 | Ketones |
| 7.061 | 1,2-Benzenediol, 3-methoxy- | 140 | C7H8O3 | 1.38 | Misc. Oxygenated |
| 7.151 | Phenol, 4-ethyl-2-methoxy- | 152 | C9H12O2 | 1.00 | Phenolic |
| 7.19 | Hydroquinone | 110 | C6H6O2 | 1.66 | Phenolic |
| 7.295 | 3,3,5,5-Tetramethylcyclohexanol | 156 | C10H20O | 0.34 | Alcohol |
| 7.348 | 1,2-Benzenediol, 3-methyl- | 124 | C7H8O2 | 0.89 | Phenolic |
| 7.393 | 1,5-Diacetoxypentane | 188 | C9H16O4 | 0.51 | Misc. Oxygenated |
| 7.502 | 2-Propenal, 3-phenyl- | 132 | C9H8O | 1.29 | Aldehyde |
| 7.743 | 2H-Pyran, tetrahydro-2-methyl- | 100 | C6H12O | 0.85 | Misc. Oxygenated |
| 7.86 | Phenol, 2,6-dimethoxy- | 154 | C8H10O3 | 1.92 | Phenolic |
| 7.922 | Benzene, 1-methyl-2-(methylthio)- | 138 | C8H10S | 0.73 | Sulfide |
| 8.02 | 2-Heptanone, 6-methyl- | 128 | C8H16O | 1.00 | Ketones |
| 8.088 | 1-Pentyn-3-ol, 3,4-dimethyl- | 112 | C7H12O | 0.56 | Misc. Oxygenated |
| 8.172 | 3-Decanone | 156 | C10H20O | 0.48 | Ketones |
| 8.229 | 1,3-Benzenediol, 4-ethyl- | 138 | C8H10O2 | 1.11 | Phenolic |
| 8.362 | 3-Buten-2-one, 4-(2,5,6,6-tetramethyl-2-cyclohexen-1-yl)- | 206 | C14H22O | 0.31 | Ketones |
| 8.413 | 2-Propenoic acid, 2-methyl-, 1,2-ethanediyl ester | 142 | C8H14O2 | 0.67 | Ester |
| 8.486 | 1,3-Benzenediol, 4,5-dimethyl- | 138 | C8H10O2 | 0.26 | Phenolic |
| 8.636 | Acetic acid, ethoxy- | 104 | C4H8O3 | 1.62 | Acid |
| 8.832 | 3,4-Dimethylanisole | 136 | C9H12O | 0.62 | Ether |
| 9.139 | Bicyclo [2.2.1]heptan-2-one, 5-(acetyloxy)-4,7,7-trimethyl-, endo- | 210 | C12H18O3 | 0.21 | Ketones |
| 9.667 | 5-tert-Butylpyrogallol | 182 | C10H14O3 | 1.39 | Misc. Oxygenated |
| 9.743 | Benzene, 1,2-dimethoxy- | 138 | C8H10O2 | 1.61 | Ether |
| 9.788 | 2(3H)-Naphthalenone,4,4a,5,6,7,8-hexahydro-4a-methyl- | 164 | C11H16O | 3.16 | Misc. Oxygenated |
| 10.002 | 11-Heneicosanone | 310 | C21H42O | 0.82 | Ketones |
| 10.099 | 3’,5’-Dimethoxyacetophenone | 180 | C10H12O3 | 0.22 | Phenolic |
| 10.598 | Pentanoic acid, 4-oxo-, ethyl ester | 144 | C7H12O3 | 0.45 | Ester |
| 10.978 | 4-Acetylbutyric acid | 130 | C6H10O3 | 0.19 | Acid |
| 11.522 | Glucitol, 6-O-nonyl- | 308 | C15H32O6 | 0.19 | Misc. Oxygenated |
| 12.356 | 2-Propanone, 1,1-diphenyl- | 210 | C15H14O | 0.23 | Ketones |
| 12.45 | 4-Methyldaphnetin | 192 | C10H8O4 | 0.19 | Misc. Oxygenated |
| 14.161 | n-Hexadecanoic acid | 256 | C16H32O2 | 0.21 | Acid |
| 15.951 | cis-13-Octadecenoic acid | 282 | C18H34O2 | 1.59 | Acid |
| 16.129 | Octadecanoic acid | 284 | C18H36O2 | 0.35 | Acid |
| Ret. Time (min) | Chemical Name | MW | Chemical Formula | AC | RAC-1 | RAC-2 | Chemical Group |
|---|---|---|---|---|---|---|---|
| Area (%) | Area (%) | Area (%) | |||||
| 3.029 | Propanoic acid | 74 | C3H6O2 | 1.43 | 5.40 | 1.42 | Acid |
| 3.146 | 2-Cyclopenten-1-one | 82 | C5H6O | 5.05 | 1.88 | 0.92 | Ketones |
| 3.245 | Furan, tetrahydro- | 72 | C4H8O | 1.26 | 2.70 | 2.85 | Furans |
| 3.337 | 2-Furanmethanol | 98 | C5H6O2 | 3.36 | 2.40 | 3.99 | Furans |
| 3.407 | 2-Butene-1,4-diol | 88 | C4H8O2 | 2.84 | 2.51 | 0.00 | alcohol |
| 3.524 | 3-Hepten-1-ol | 114 | C7H14O | 1.32 | 4.42 | 3.85 | alcohol |
| 3.618 | Furan, 2-methyl- | 82 | C5H6O | 0.10 | 3.44 | 0.00 | Furans |
| 3.675 | 2-Furanmethanol | 98 | C5H6O2 | 0.33 | 1.85 | 2.06 | Furans |
| 3.781 | 2-Cyclopenten-1-one, 3-methyl- | 96 | C6H8O | 0.86 | 0.89 | 3.37 | Ketones |
| 3.847 | Phenol, 2-methyl- | 108 | C7H8O | 2.34 | 2.04 | 1.84 | Phenolic |
| 3.917 | Phenol | 94 | C6H6O | 0.87 | 1.51 | 0.00 | Phenolic |
| 3.962 | 2-Cyclopenten-1-one, 2-methyl- | 96 | C6H8O | 1.94 | 1.48 | 1.80 | Ketones |
| 4.141 | Butyrolactone | 86 | C4H6O2 | 0.36 | 2.69 | 1.98 | Ketones |
| 4.276 | Cyclohexanone | 98 | C6H10O | 0.85 | 1.01 | 3.53 | Ketones |
| 4.313 | 2-Cyclopenten-1-one, 3-methyl- | 96 | C6H8O | 0.93 | 2.24 | 1.51 | Ketones |
| 4.449 | Phenol | 94 | C6H6O | 12.83 | 12.19 | 2.68 | Phenolic |
| 4.664 | 2-Propanone, 1-(acetyloxy)- | 116 | C5H8O3 | 0.59 | 3.37 | 2.83 | Misc. Oxygenated |
| 4.883 | 1,2-Cyclopentanedione, 3-methyl- | 112 | C6H8O2 | 2.01 | 0.89 | 1.03 | Ketones |
| 4.984 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 112 | C6H8O2 | 0.20 | 2.13 | 1.91 | Ketones |
| 5.025 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 110 | C7H10O | 0.12 | 0.68 | 1.03 | Ketones |
| 5.091 | Benzyl alcohol | 108 | C7H8O | 2.32 | 2.19 | 2.44 | Alcohol |
| 5.339 | Phenol, 2-methyl- | 108 | C7H8O | 4.86 | 4.15 | 2.55 | Phenolic |
| 5.408 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 126 | C7H10O2 | 0.11 | 2.40 | 1.04 | Ketones |
| 5.466 | Phenol, 2-ethyl- | 122 | C8H10O | 0.28 | 1.45 | 3.27 | Phenolic |
| 5.671 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 126 | C7H10O2 | 1.89 | 1.23 | 2.25 | Ketones |
| 5.798 | Phenol, 2-ethyl- | 122 | C8H10O | 0.20 | 0.80 | 0.80 | Phenolic |
| 5.907 | Phenol, 2,5-dimethyl- | 122 | C8H10O | 0.95 | 1.96 | 2.25 | Phenolic |
| 6.059 | Phenol, 4-ethyl- | 122 | C8H10O | 2.54 | 1.85 | 1.73 | Phenolic |
| 6.177 | Phenol, 2,6-dimethyl- | 122 | C8H10O | 0.13 | 1.04 | 0.85 | Phenolic |
| 6.243 | 2,4-Imidazolidinedione, 5,5-dimethyl- | 128 | C5H8N2O2 | 0.10 | 0.78 | 0.62 | Nitrogenous |
| 6.39 | Benzofuran, 2,3-dihydro- | 120 | C8H8O | 10.06 | 2.57 | 3.89 | Furans |
| 6.533 | 1,2-Benzenediol | 110 | C6H6O2 | 4.62 | 1.84 | 2.09 | Phenolic |
| 6.685 | Resorcinol monoacetate | 152 | C8H8O3 | 2.40 | 1.22 | 1.74 | Misc. Oxygenated |
| 6.852 | 2-Coumaranone | 134 | C8H6O2 | 0.27 | 0.57 | 0.56 | Furans |
| 6.95 | 1,2-Benzenediol, 4-methyl- | 124 | C7H8O2 | 1.89 | 1.34 | 0.92 | Phenolic |
| 7.01 | Caprolactam | 113 | C6H11NO | 1.40 | 1.56 | 2.27 | Nitrogenous |
| 7.068 | Hydroquinone | 110 | C6H6O2 | 2.61 | 0.84 | 1.39 | Phenolic |
| 7.243 | 4-Methylcatechol | 124 | C7H8O2 | 3.63 | 1.45 | 1.99 | Alcohol |
| 7.395 | Phenol, 4-ethyl-2-methoxy- | 152 | C9H12O2 | 0.48 | 0.46 | 1.02 | Phenolic |
| 7.535 | Benzoic acid, 2-hydroxy-5-methyl-, methyl ester | 166 | C9H10O3 | 0.34 | 0.97 | 0.71 | Ester |
| 7.602 | 2-Nonen-1-ol | 142 | C9H18O | 0.18 | 0.27 | 0.99 | Alcohol |
| 7.648 | 1,3-Benzenediol, 2-methyl- | 124 | C7H8O2 | 0.57 | 0.27 | 0.35 | Phenolic |
| 7.74 | 3-Methoxybenzyl alcohol | 138 | C8H10O2 | 1.31 | 0.26 | 0.44 | alcohol |
| 7.849 | 1,4-Benzenediol, 2-methyl- | 124 | C7H8O2 | 0.39 | 0.43 | 2.10 | Phenolic |
| 7.899 | Benzofuran, 2-methyl- | 132 | C9H8O | 0.26 | 0.80 | 0.66 | Furans |
| 7.938 | Benzaldehyde, 4-hydroxy- | 122 | C7H6O2 | 0.21 | 0.40 | 0.51 | Aldehyde |
| 8.153 | 1,3-Benzenediol, 4-ethyl- | 138 | C8H10O2 | 2.15 | 0.24 | 1.84 | Phenolic |
| 8.281 | Ethanone, 1-(3-hydroxyphenyl)- | 136 | C8H8O2 | 0.18 | 0.46 | 0.57 | Ketones |
| 8.413 | 1,3-Benzenediol, 4,5-dimethyl- | 138 | C8H10O2 | 0.07 | 0.22 | 0.53 | Phenolic |
| 8.558 | Benzoic acid, ethyl ester | 150 | C9H10O2 | 0.44 | 0.15 | 1.70 | Ester |
| 8.752 | Phenol, 2,3,6-trimethyl- | 136 | C9H12O | 0.76 | 0.10 | 0.47 | Phenolic |
| 8.846 | Isoborneol | 154 | C8H10O2 | 0.17 | 0.01 | 0.53 | Alcohol |
| 8.942 | Phenol, 2,3,6-trimethyl- | 136 | C9H12O | 0.13 | 0.00 | 1.19 | Phenolic |
| 9.155 | Benzeneacetic acid, 3,4-dihydroxy- | 168 | C8H8O4 | 0.10 | 0.00 | 1.03 | Acid |
| 9.256 | Nonanoic acid | 158 | C9H18O2 | 1.70 | 0.00 | 2.72 | Acid |
| 9.668 | 2(3H)-Naphthalenone,4,4a,5,6,7,8-hexahydro-4a-methyl- | 164 | C11H16O | 0.48 | 0.01 | 0.84 | Misc. Oxygenated |
| 10.18 | 4-Acetylbutyric acid | 130 | C6H10O3 | 0.23 | 0.00 | 0.58 | Acid |
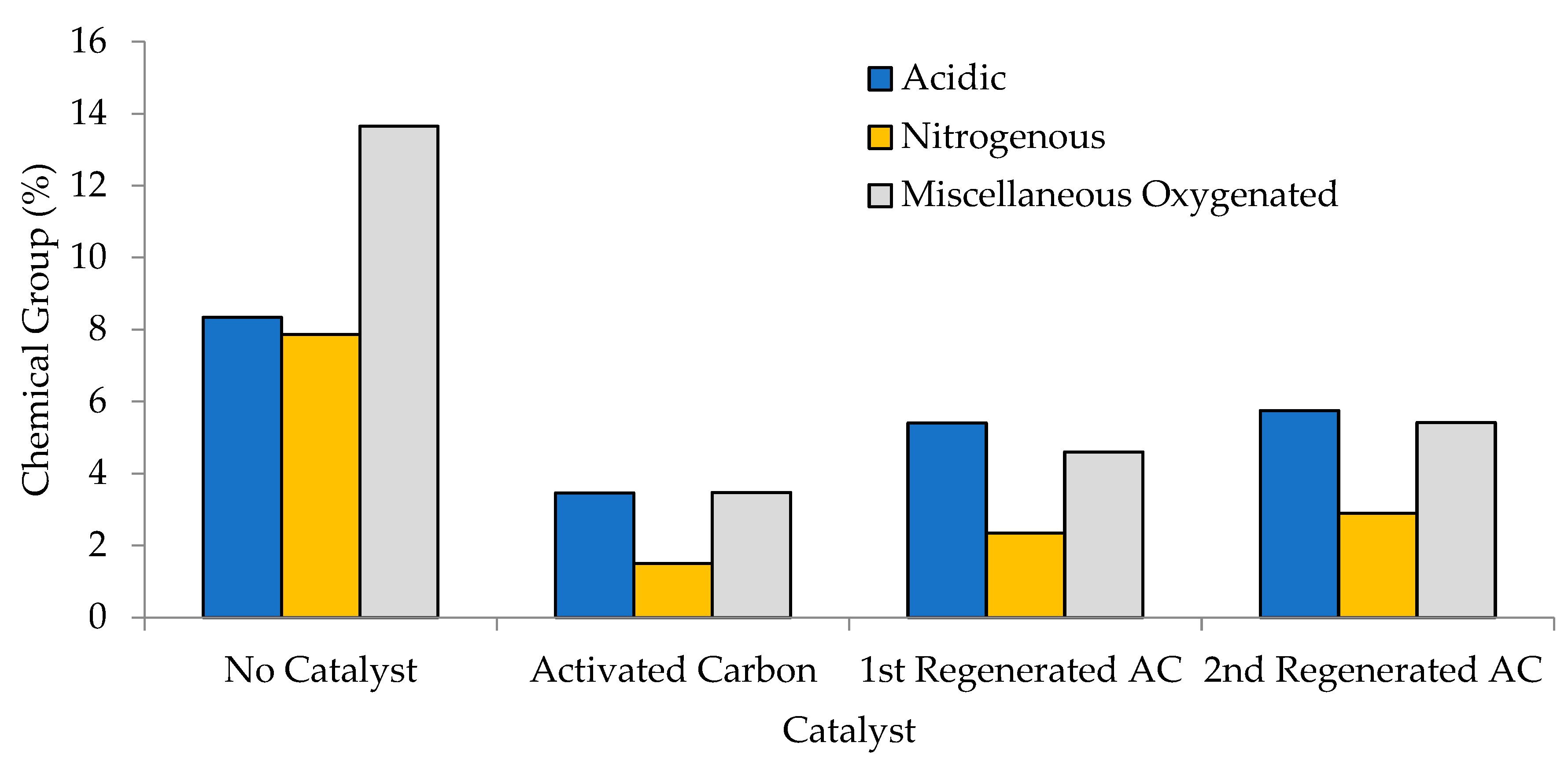
| Chemical Groups | No Catalyst | Activated Carbon (AC) | 1st Regenerated AC (RAC-1) | 2nd Regenerated AC (RAC-2) |
|---|---|---|---|---|
| Acid | 8.34 | 3.46 | 5.40 | 5.75 |
| Alcohol | 7.91 | 11.78 | 11.10 | 10.23 |
| aldehyde | 1.29 | 0.21 | 0.40 | 0.51 |
| Ester | 2.91 | 0.78 | 1.12 | 2.41 |
| Ether | 2.23 | - | - | - |
| Furans | 9.42 | 15.65 | 14.33 | 14.01 |
| Ketones | 12.51 | 14.49 | 17.99 | 20.94 |
| Nitrogenous | 7.86 | 1.50 | 2.34 | 2.90 |
| Misc. Oxygenated | 13.65 | 3.47 | 4.60 | 5.41 |
| Phenolic | 22.88 | 38.66 | 32.72 | 27.84 |
| Bromide | 0.32 | - | - | - |
| Sulfide | 0.73 | - | - | - |
| Total (%) | 90.05 | 90.00 | 90.00 | 90.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Zh. Bekmyrza, K.; Taweekun, J.; Ja’afar, F.; Saifullah Abu Bakar, M.; Azad, A.K.; Roy, H.; et al. Ex Situ Catalytic Pyrolysis of Invasive Pennisetum purpureum Grass with Activated Carbon for Upgrading Bio-Oil. Sustainability 2023, 15, 7628. https://doi.org/10.3390/su15097628
Reza MS, Afroze S, Kuterbekov K, Kabyshev A, Zh. Bekmyrza K, Taweekun J, Ja’afar F, Saifullah Abu Bakar M, Azad AK, Roy H, et al. Ex Situ Catalytic Pyrolysis of Invasive Pennisetum purpureum Grass with Activated Carbon for Upgrading Bio-Oil. Sustainability. 2023; 15(9):7628. https://doi.org/10.3390/su15097628
Chicago/Turabian StyleReza, Md Sumon, Shammya Afroze, Kairat Kuterbekov, Asset Kabyshev, Kenzhebatyr Zh. Bekmyrza, Juntakan Taweekun, Fairuzeta Ja’afar, Muhammad Saifullah Abu Bakar, Abul K. Azad, Hridoy Roy, and et al. 2023. "Ex Situ Catalytic Pyrolysis of Invasive Pennisetum purpureum Grass with Activated Carbon for Upgrading Bio-Oil" Sustainability 15, no. 9: 7628. https://doi.org/10.3390/su15097628
APA StyleReza, M. S., Afroze, S., Kuterbekov, K., Kabyshev, A., Zh. Bekmyrza, K., Taweekun, J., Ja’afar, F., Saifullah Abu Bakar, M., Azad, A. K., Roy, H., & Islam, M. S. (2023). Ex Situ Catalytic Pyrolysis of Invasive Pennisetum purpureum Grass with Activated Carbon for Upgrading Bio-Oil. Sustainability, 15(9), 7628. https://doi.org/10.3390/su15097628










