A Substitute for Portland Cement: Experiments on Ecofriendly Reinforcement of Large-Scale Calcareous Sand by Microbial-Induced Carbonate Precipitation Spraying Method
Abstract
:1. Introduction
2. Experimental Setup
2.1. Experimental Equipment
2.2. Experimental Materials and Treatment Process of Spraying Method
2.3. Assessment Methods
- (1)
- Shear wave velocity
- (2)
- Penetration resistance
- (3)
- Equivalent calcium carbonate content
3. Experimental Results and Analysis
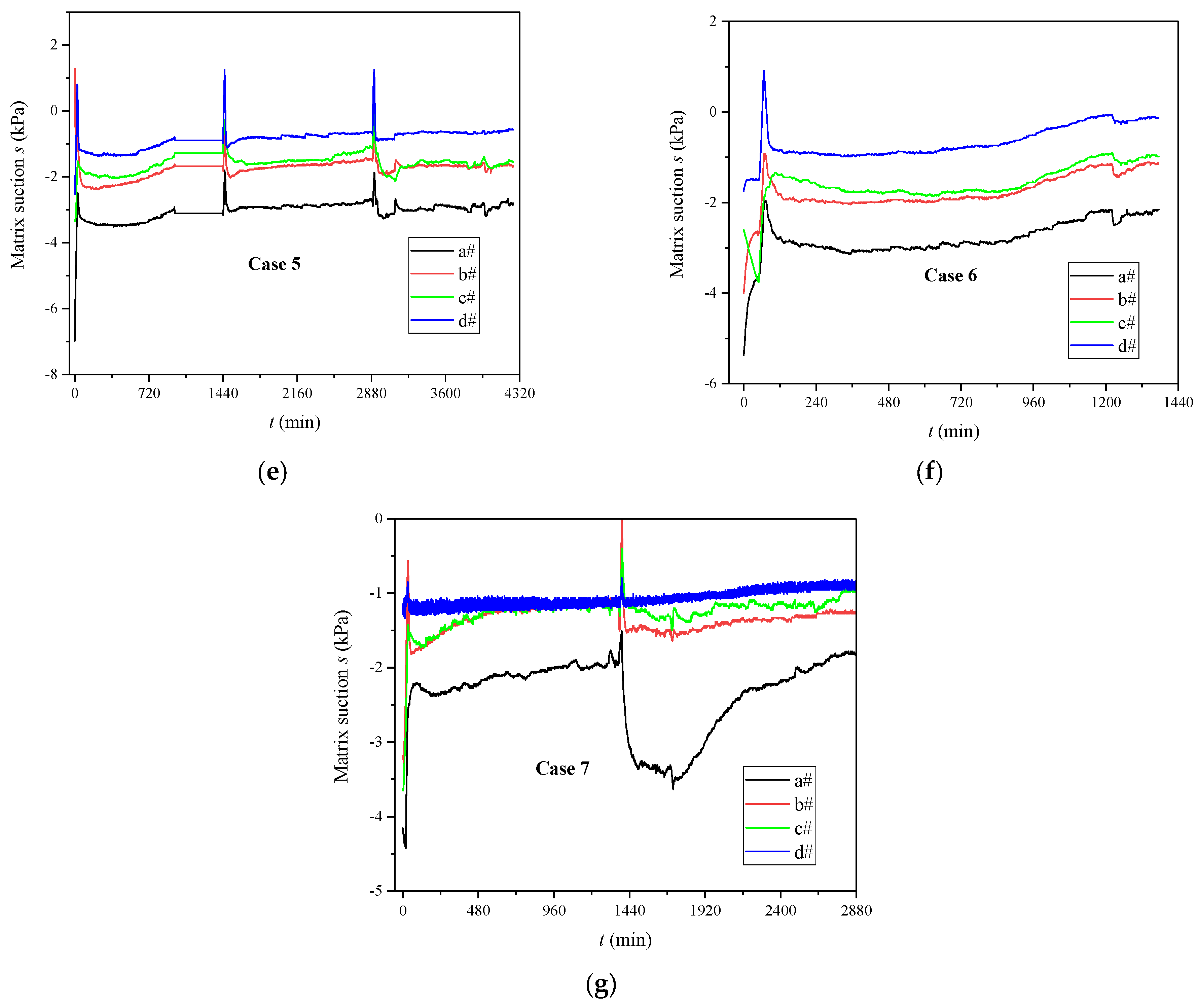
3.1. Evolutions of Volumetric Water Content and Matrix Suction
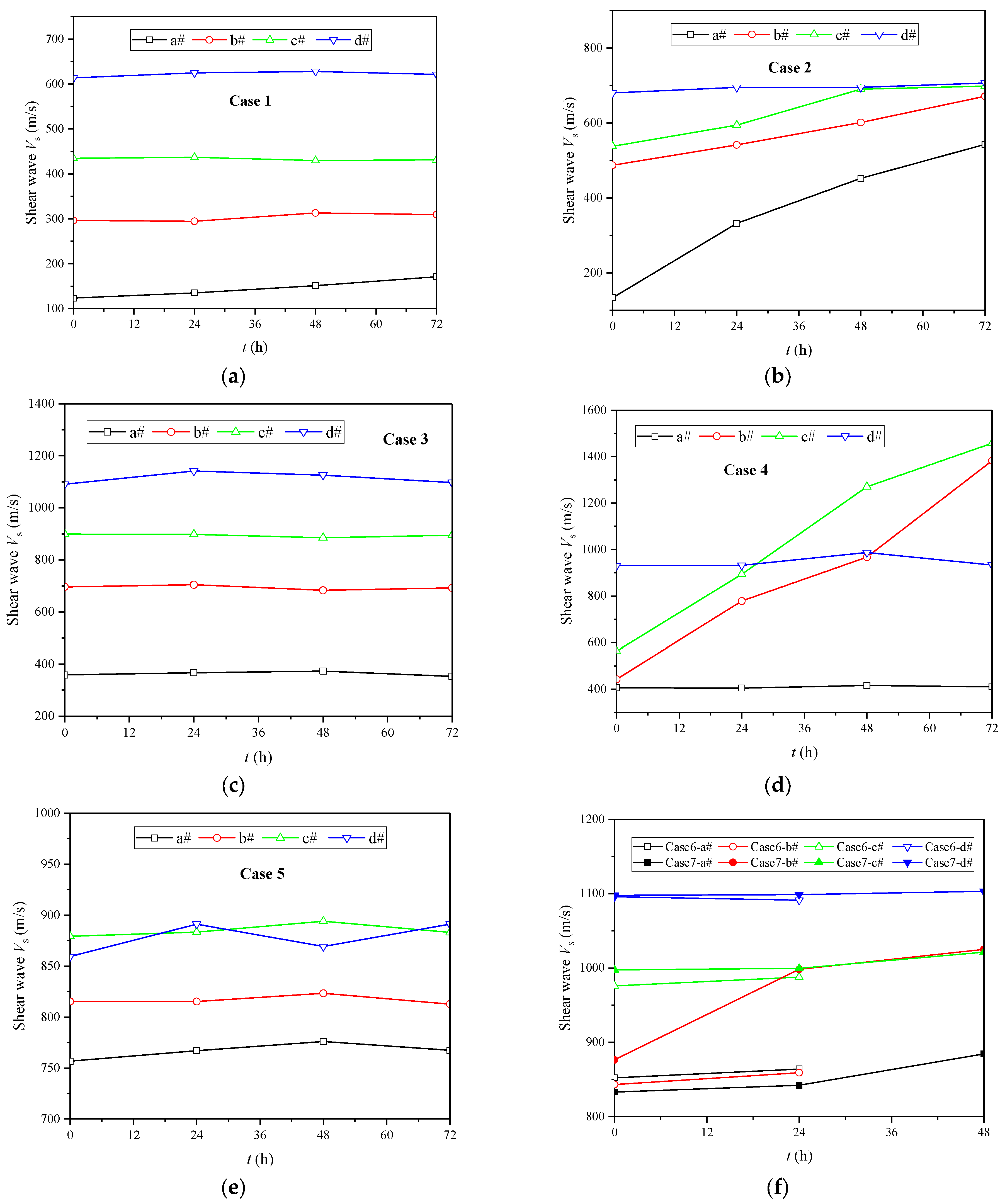
3.2. Evolution of Shear Wave Velocity
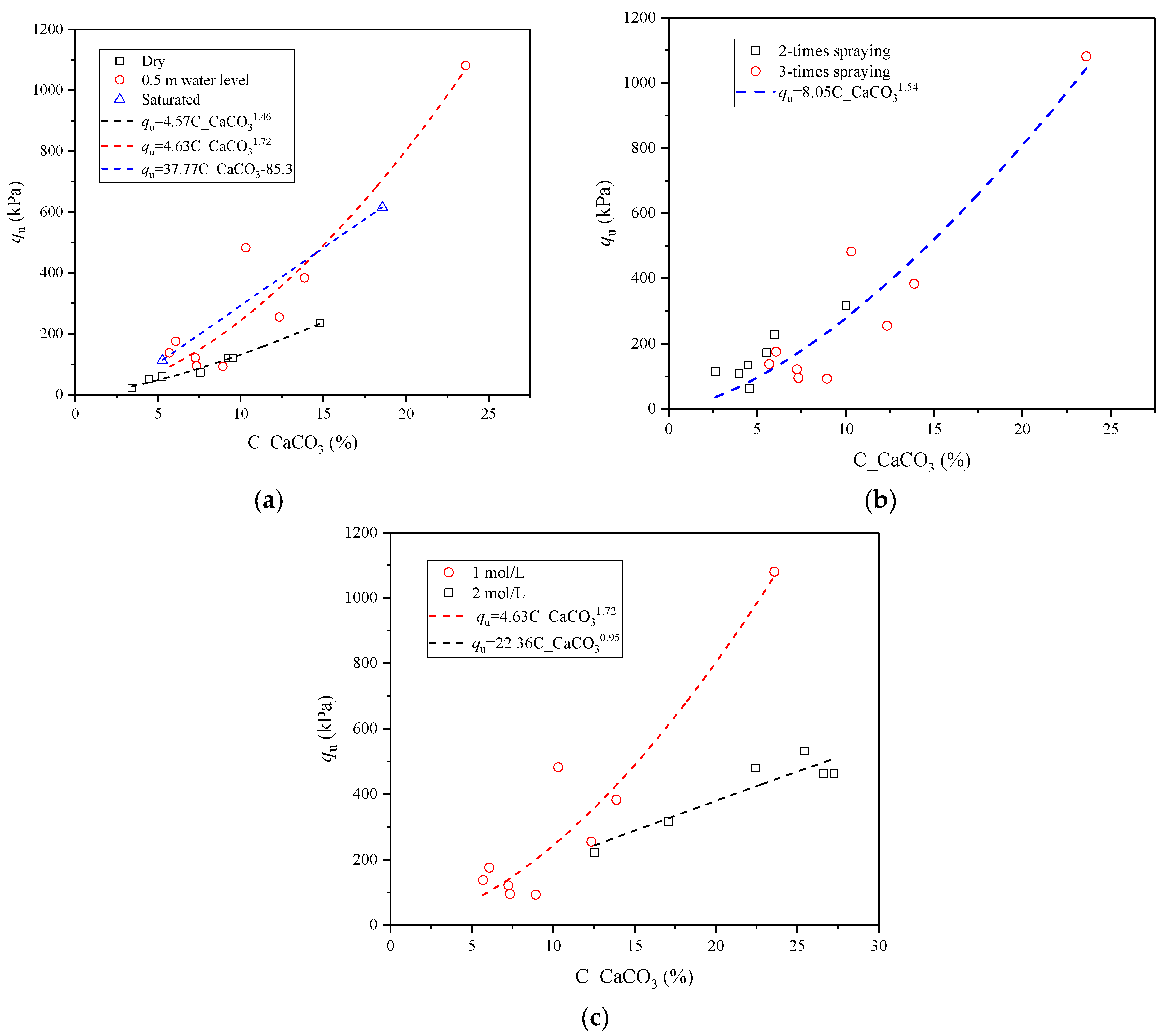
3.3. The Influence of Initial Water Level
3.4. The Influence of Spraying Times
3.5. The Influence of Concentration of Cementing Solution
4. Discussion
4.1. The Influence of MICP Reaction on SWRC
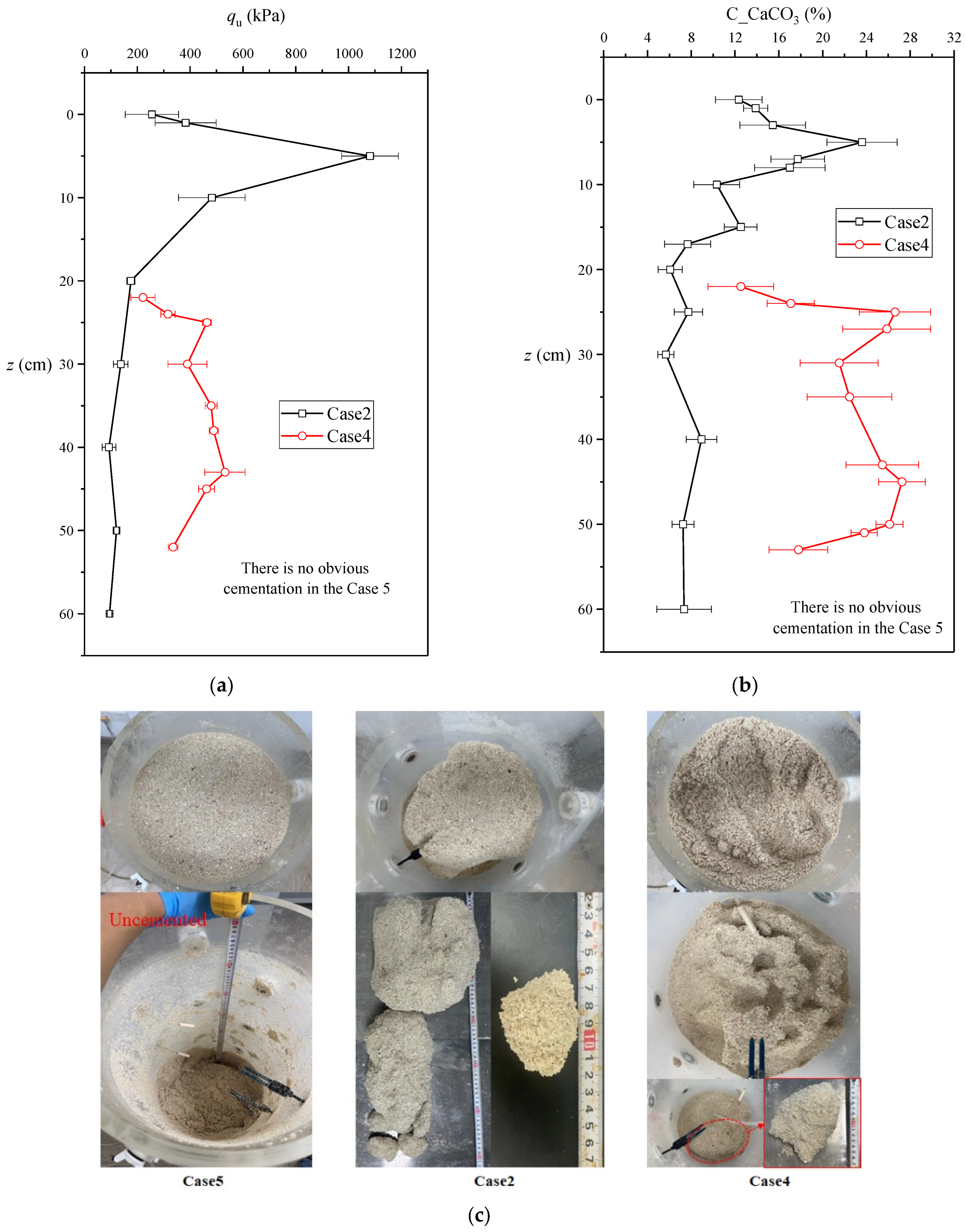
4.2. Relationship between Equivalent Calcium Carbonate Content, Penetration Resistance, and Shear Wave Velocity
4.3. Comparison of Reinforcement Methods for MICP Applications
5. Conclusions
- (1)
- The initial water level, spraying times, and CS concentration showed a significant impact on the equivalent calcium carbonate content, penetration resistance, and effective cementation range, involving the complex competition mechanism of infiltration and reaction. The distributions of penetration resistance and equivalent calcium carbonate content from top to bottom mainly included two patterns: monotonically decreasing, and initially increasing and then decreasing. The fully saturated case only showed a dense thin layer of calcium carbonate on the surface, and the completely dry case produced a middle cementation. When the initial water level was 0.5 m, the largest range of 60 cm effective cementation appeared from top to bottom because the microorganisms were more likely to migrate to the particle connection.
- (2)
- The calcium carbonate generated by the MICP reaction in the sand played a role in blocking pores and increasing the water retention capacity of the calcareous sand. As the degree of cementation increased, the SWRC curve gradually moved up, and the matrix suction corresponding to the same volume water content increased sequentially.
- (3)
- There was an obvious power function relationship between the equivalent calcium carbonate content and the penetration resistance. When the water level was 0.5 m, the equivalent calcium carbonate content and penetration resistance value were highest. Increasing the spraying time and the concentration of CS could generate larger calcium carbonate contents. The measured penetration resistance of 1 mol/L of CS concentration was higher than that of 2 mol/L of CS concentration with the same calcium carbonate content. There was a significant linear relationship between the normalized penetration resistance and the normalized shear wave velocity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Cementing solution |
| BS | Bacterial solution |
| MICP | Microbial-induced carbonate precipitation |
| SWRC | Soil–water characteristic curve |
| Cc | Coefficient of curvature |
| Cu | Coefficient of uniformity |
| Vs | Current shear wave velocity |
| Vs0 | Initial shear wave velocity |
| qu | Current penetration resistance |
| qu0 | Initial penetration resistance |
References
- Ismail, M.A.; Joer, H.A.; Sim, W.H.; Randolph, M.F. Effect of Cement Type on Shear Behavior of Cemented Calcareous Soil. J. Geotech. Geoenviron. Eng. 2002, 128, 520–529. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Hassanlourad, M.; Shahnazari, H. Shear behavior of chemically grouted carbonate sands. Int. J. Geotech. Eng. 2012, 6, 445–454. [Google Scholar] [CrossRef]
- Almajed, A.; Abbas, H.; Arab, M.; Alsabhan, A.; Hamid, W.; Al-Salloum, Y. Enzyme-Induced Carbonate Precipitation (EICP)-Based methods for ecofriendly stabilization of different types of natural sands. J. Clean. Prod. 2020, 274, 122627. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, L.; Zhao, J.; Liu, L.; Zhong, S.; Sun, Y.; Yang, Y. Toward a better practice for estimating the CO2 emission factors of cement production: An experience from China. J. Clean. Prod. 2016, 139, 527–539. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Zeng, W.; Poon, C.S.; Lu, Z. Improvement in properties of concrete with modified RCA by microbial induced carbonate precipitation. Cem. Concr. Compos. 2021, 124, 104251. [Google Scholar] [CrossRef]
- Schuhmacher, M.; Domingo, J.L.; Garreta, J. Pollutants emitted by a cement plant: Health risks for the population living in the neighborhood. Environ. Res. 2004, 95, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Lyu, H.; Yang, J.; Zeng, C. Effects of cement content and curing period on geotechnical properties of cement-treated calcareous sands. Transp. Geotech. 2022, 33, 100732. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, A.; Li, N.; Wang, W.; Hu, J. Mechanical properties and microscopic characterization of cement stabilized calcareous sand modified by nano SiO2. Case Stud. Constr. Mater. 2022, 17, e01636. [Google Scholar] [CrossRef]
- Hu, J.; Xu, C.; Cheng, P.; Xiong, H.; Zhou, Y.; Ren, J. Mechanical properties and micromechanics of graphene oxide modified calcareous sand-cement mortar. Mar. Georesour. Geotechnol. 2023, 1–13. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 2012, 29, 544–549. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Stuedlein, A.W.; Chu, J.; Matthew Evans, T.; Van Paassen, L.A. Crystal growth of MICP through microfluidic chip tests. J. Geotech. Geoenviron. Eng. 2022, 148, 06022002. [Google Scholar] [CrossRef]
- Jiang, N.J.; Soga, K. The applicability of microbially induced calcite precipitation (MICP) for internal erosion control in gravel–sand mixtures. Géotechnique 2017, 67, 42–55. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Yang, H.; Li, Y.; Zhu, J. An innovative eco-friendly method for scour protection around monopile foundation. Appl. Ocean Res. 2022, 123, 103177. [Google Scholar] [CrossRef]
- Choi, S.-G.; Chang, I.; Lee, M.; Lee, J.-H.; Han, J.-T.; Kwon, T.-H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Zamani, A.; Xiao, P.; Baumer, T.; Carey, T.J.; Sawyer, B.; DeJong, J.T.; Boulanger, R.W. Mitigation of liquefaction triggering and foundation settlement by MICP treatment. J. Geotech. Geoenviron. Eng. 2021, 147, 04021099. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Liu, H.; Zhang, Z.; Ding, X. Performance evaluation of a MICP-treated calcareous sandy foundation using shake table tests. Soil Dyn. Earthq. Eng. 2020, 129, 105959. [Google Scholar] [CrossRef]
- O’Donnell, S.T.; Kavazanjian, E., Jr.; Rittmann, B.E. MIDP: Liquefaction mitigation via microbial denitrification as a two-stage process. II: MICP. J. Geotech. Geoenviron. Eng. 2017, 143, 04017095. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Xiao, Y.; Stuedlein, A.W.; Evans, T.M. Liquefaction resistance of bio-cemented calcareous sand. Soil Dyn. Earthq. Eng. 2018, 107, 9–19. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R.; Wang, H.; Wu, L.; Xia, J. Liquefaction resistance of biocemented loess soil. J. Geotech. Geoenviron. Eng. 2021, 147, 04021117. [Google Scholar] [CrossRef]
- Zamani, A.; Montoya, B.M. Undrained monotonic shear response of MICP-treated silty sands. J. Geotech. Geoenviron. Eng. 2018, 144, 04018029. [Google Scholar] [CrossRef]
- Feng, K.; Montoya, B.M. Influence of confinement and cementation level on the behavior of microbial-induced calcite precipitated sands under monotonic drained loading. J. Geotech. Geoenviron. Eng. 2016, 142, 04015057. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Ye, Z.; Shen, C.; Zhou, W. Interface shear behavior between MICP-treated calcareous sand and steel. J. Mater. Civ. Eng. 2021, 33, 04020455. [Google Scholar] [CrossRef]
- Ghasemi, P.; Montoya, B.M. Field implementation of microbially induced calcium carbonate precipitation for surface erosion reduction of a coastal plain sandy slope. J. Geotech. Geoenviron. Eng. 2022, 148, 04022071. [Google Scholar] [CrossRef]
- Liu, S.; Gao, X. Evaluation of the anti-erosion characteristics of an MICP coating on the surface of tabia. J. Mater. Civ. Eng. 2020, 32, 04020304. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.-H.; Tang, C.-S.; Pan, X.-H.; Jiang, N.-J.; Singh, D.N.; Cheng, Y.-J.; Shi, B. Bio-mediated method for improving surface erosion resistance of clayey soils. Eng. Geol. 2021, 293, 106295. [Google Scholar] [CrossRef]
- Dagliya, M.; Satyam, N.; Sharma, M.; Garg, A. Experimental study on mitigating wind erosion of calcareous desert sand using spray method for microbially induced calcium carbonate precipitation. J. Rock Mech. Geotech. Eng. 2022, 14, 1556–1567. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Shi, B.; Tang, C.-S.; Pan, X.-H.; Cheng, Y.-J.; Xu, J.-J. Potential drought mitigation through microbial induced calcite precipitation-MICP. Water Resour. Res. 2021, 57, e2020WR029434. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Tang, C.-S.; Pan, X.-H.; Liu, B.; Xie, Y.-H.; Cheng, Q.; Shi, B. Application of microbial induced carbonate precipitation for loess surface erosion control. Eng. Geol. 2021, 294, 106387. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Chu, J.; He, J. Microbially induced calcite precipitation for seepage control in sandy soil. Geomicrobiol. J. 2019, 36, 366–375. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Doctoral Dissertation, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Ogino, T.; Kawaguchi, T.; Yamashita, S.; Kawajiri, S. Measurement deviations for shear wave velocity of bender element test using time domain, cross-correlation, and frequency domain approaches. Soils Found. 2015, 55, 329–342. [Google Scholar] [CrossRef]
- Kou, H.-L.; Wu, C.-Z.; Ni, P.-P.; Jang, B.-A. Assessment of erosion resistance of biocemented sandy slope subjected to wave actions. Appl. Ocean Res. 2020, 105, 102401. [Google Scholar] [CrossRef]
- Kou, H.-L.; Wu, C.; Jang, B.-A.; Wang, D. Spatial distribution of CaCO3 in biocemented sandy slope using surface percolation. J. Mater. Civ. Eng. 2021, 33, 06021004. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, R.; Wang, J. The influence of environmental factors and precipitation precursors on enzyme-induced carbonate precipitation (EICP) process and its application on modification of recycled concrete aggregates. J. Clean. Prod. 2023, 395, 136444. [Google Scholar] [CrossRef]
- Saffari, R.; Nikooee, E.; Habibagahi, G.; van Genuchten, M.T. Effects of biological stabilization on the water retention properties of unsaturated soils. J. Geotech. Geoenviron. Eng. 2019, 145, 04019028. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Miao, L.; Wang, H.; Wu, L.; Shi, W.; Kawasaki, S. State-of-the-art review of soil erosion control by MICP and EICP techniques: Problems, applications, and prospects. Sci. Total Environ. 2023, 169016. [Google Scholar] [CrossRef]

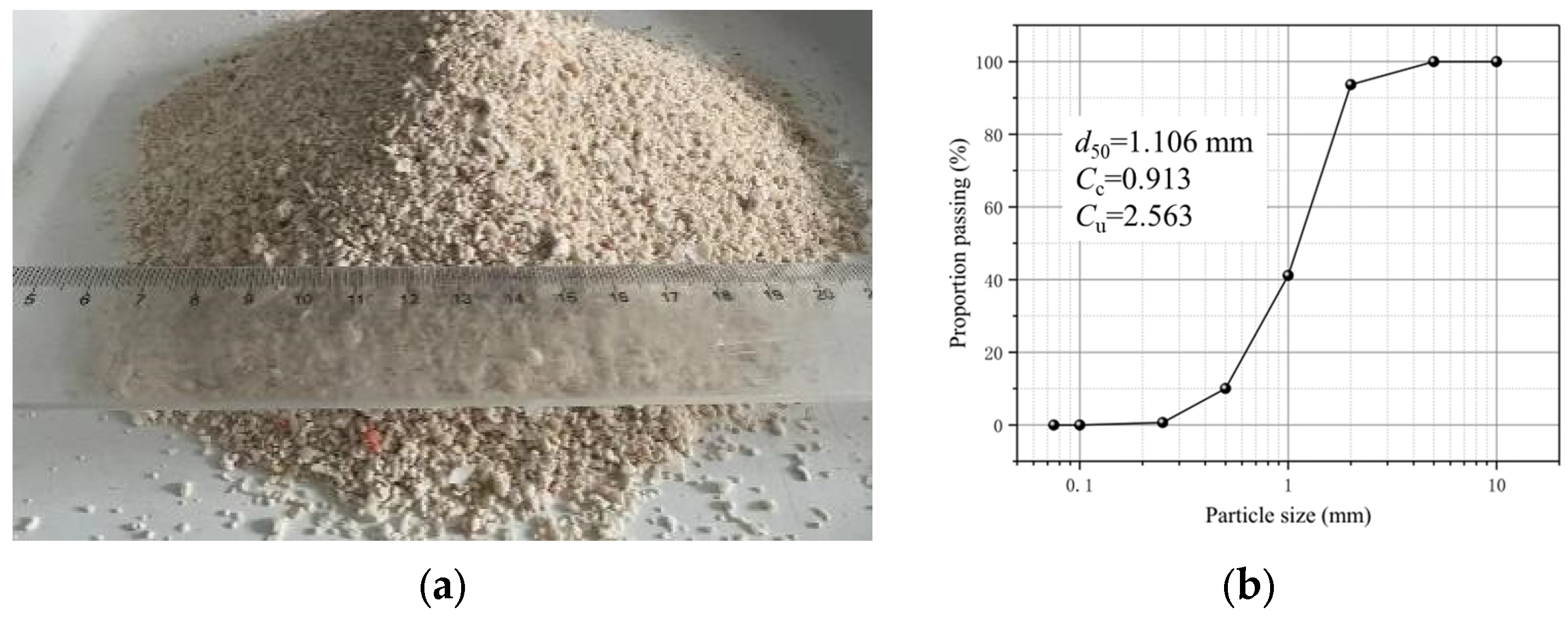
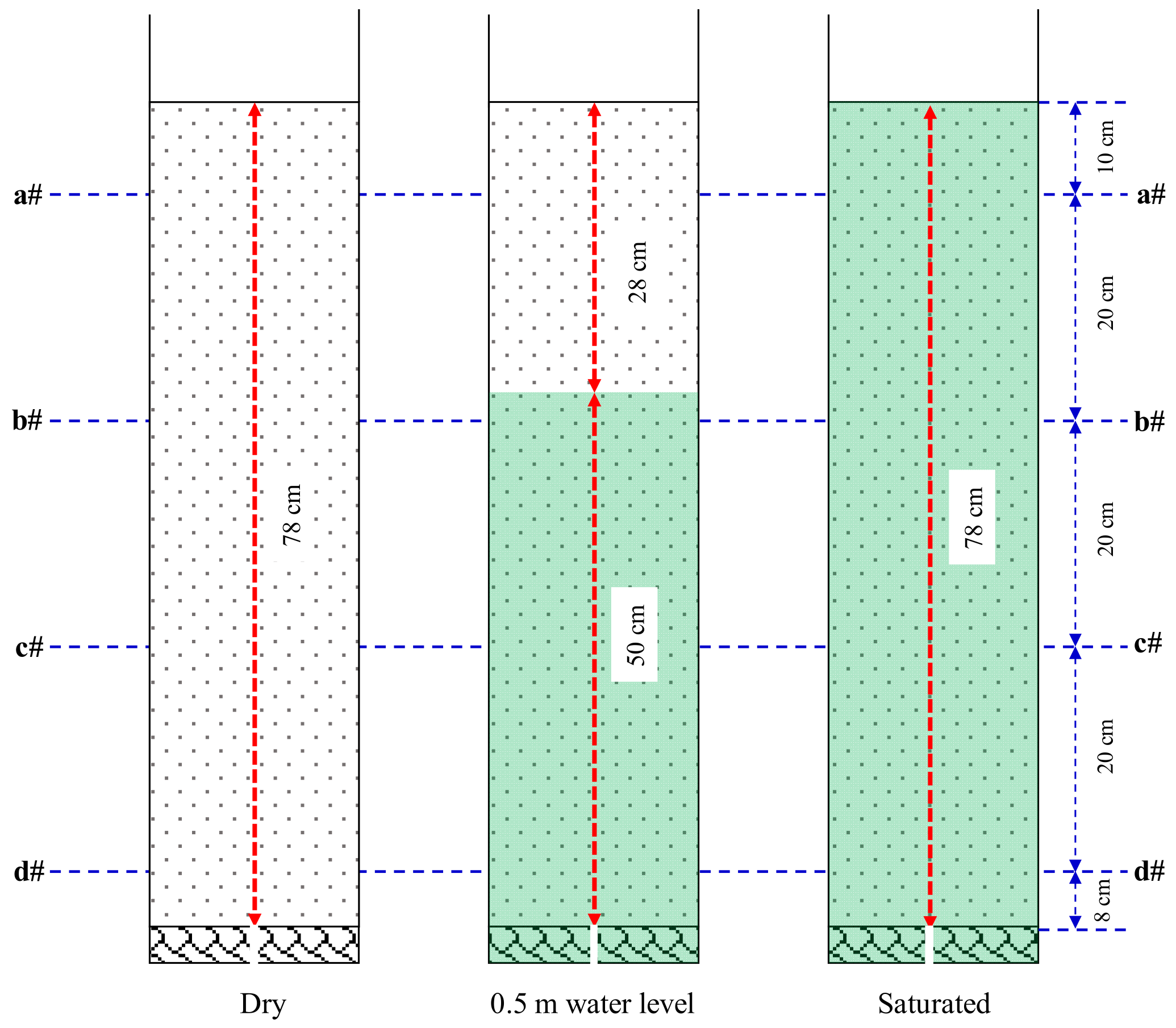

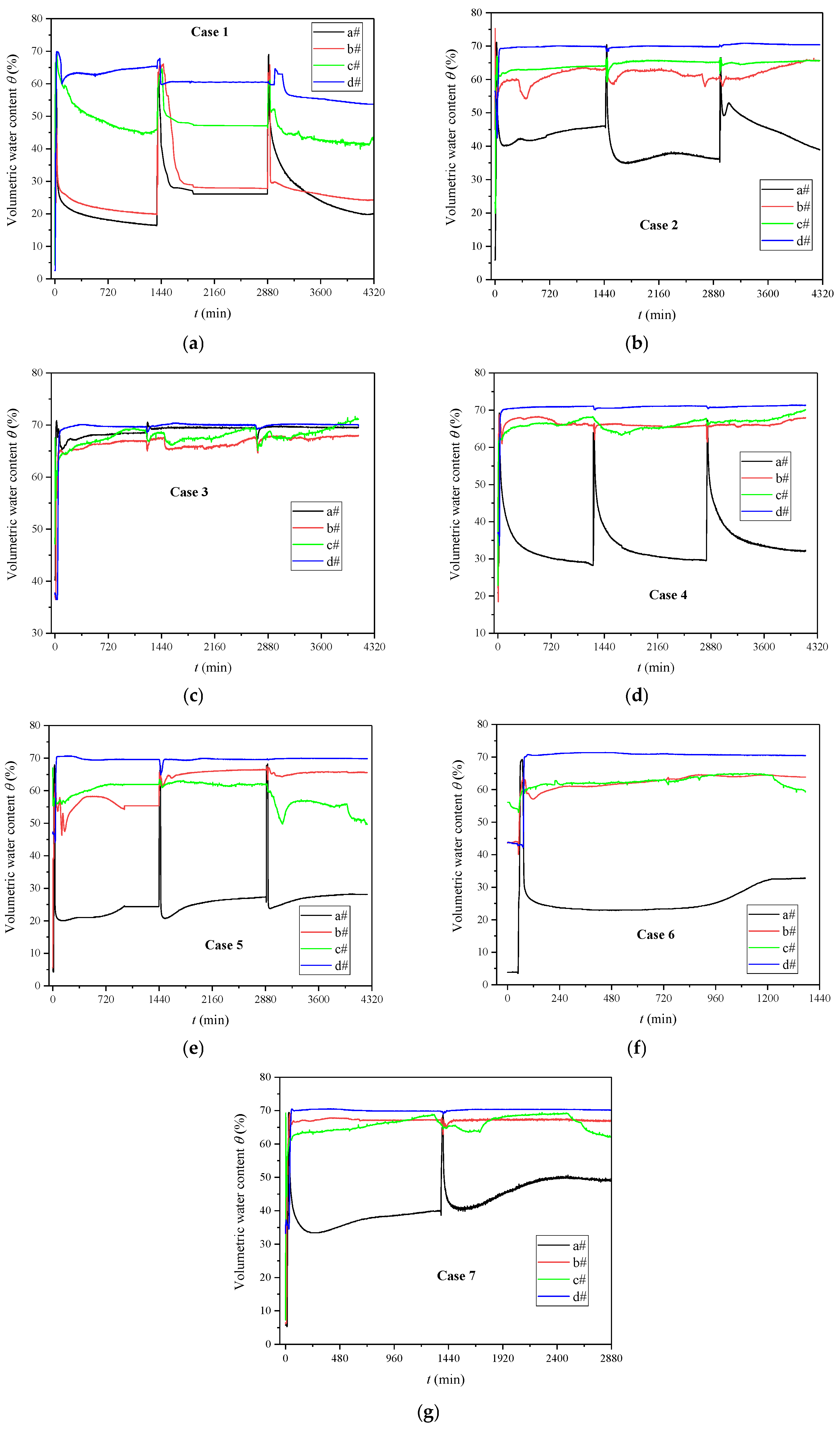
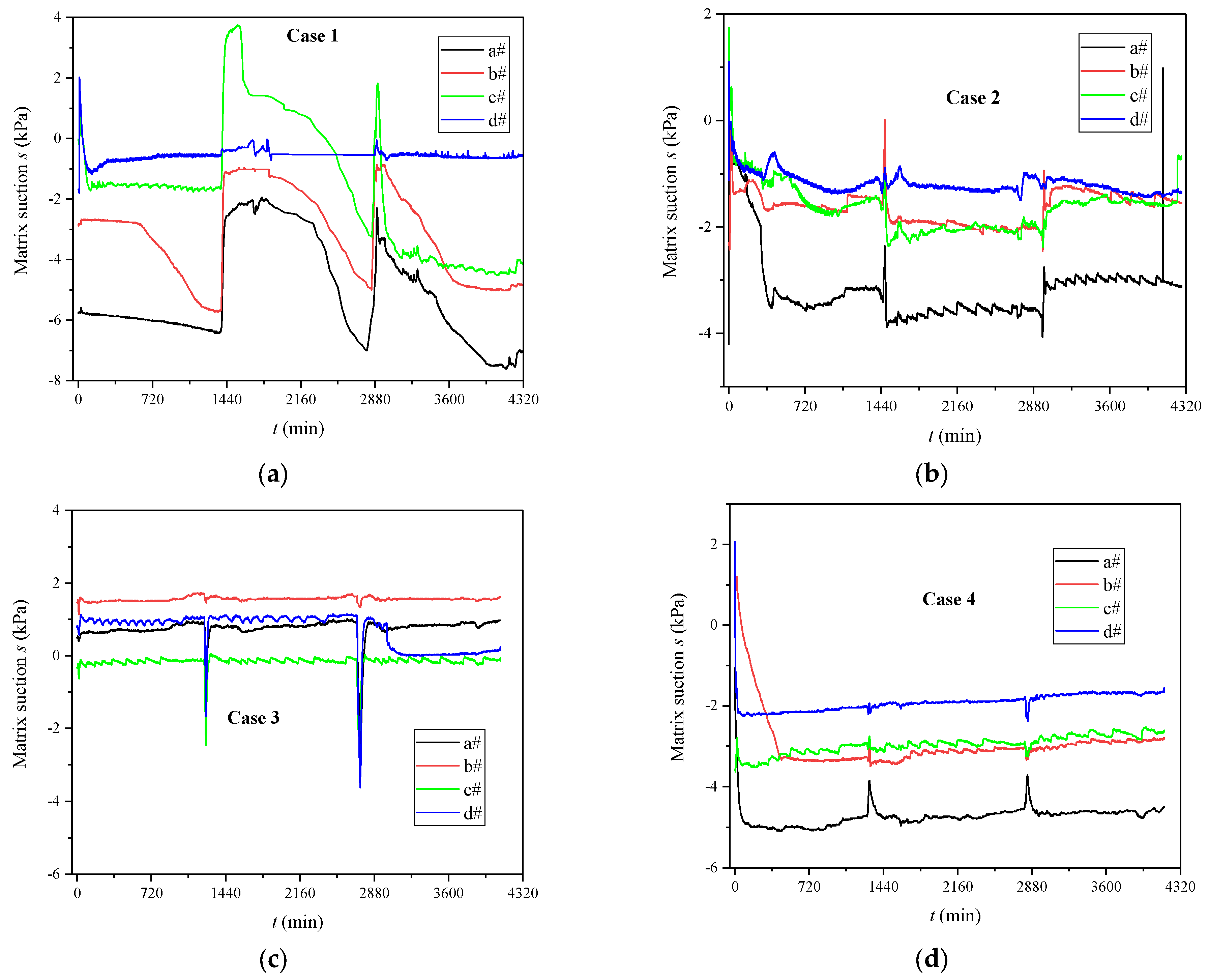




| No | Initial Water Level (m) | OD600 | UA (mM Urea/min) | Concentration of CS (mol/L) | Spraying Times |
|---|---|---|---|---|---|
| Case 1 | 0 | 1.894/1.919/2.070 | 7.78/8.44/7.78 | 1.0/1.0/1.0 | 1-2-3 |
| Case 2 | 0.5 | 1.950/2.050/1.986 | 7.77/8.66/7.11 | 1.0/1.0/1.0 | 1-2-3 |
| Case 3 | 0.78 | 1.970/1.930/2.090 | 7.67/8.13/7.22 | 1.0/1.0/1.0 | 1-2-3 |
| Case 4 | 0.5 | 1.809/1.950/1.850 | 8.23/7.56/8.11 | 2.0/2.0/2.0 | 1-2-3 |
| Case 5 | 0.5 | 1.725/1.823/1.924 | 7.11/8.67/7.55 | 0.5/0.5/0.5 | 1-2-3 |
| Case 6 | 0.5 | 1.740 | 7.44 | 1.0 | 1 |
| Case 7 | 0.5 | 1.914/2.012 | 7.45/9.11 | 1.0/1.0 | 1-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Rui, S.; Li, L.; Guo, Z.; Sun, X. A Substitute for Portland Cement: Experiments on Ecofriendly Reinforcement of Large-Scale Calcareous Sand by Microbial-Induced Carbonate Precipitation Spraying Method. Sustainability 2024, 16, 225. https://doi.org/10.3390/su16010225
Li Y, Rui S, Li L, Guo Z, Sun X. A Substitute for Portland Cement: Experiments on Ecofriendly Reinforcement of Large-Scale Calcareous Sand by Microbial-Induced Carbonate Precipitation Spraying Method. Sustainability. 2024; 16(1):225. https://doi.org/10.3390/su16010225
Chicago/Turabian StyleLi, Yujie, Shengjie Rui, Lingling Li, Zhen Guo, and Xingye Sun. 2024. "A Substitute for Portland Cement: Experiments on Ecofriendly Reinforcement of Large-Scale Calcareous Sand by Microbial-Induced Carbonate Precipitation Spraying Method" Sustainability 16, no. 1: 225. https://doi.org/10.3390/su16010225






