Hydrochemical Evolution and Nitrate Source Identification of River Water and Groundwater in Huashan Watershed, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Analysis
2.4. Data Analysis
3. Results and Discussion
3.1. Hydrochemical Characteristics
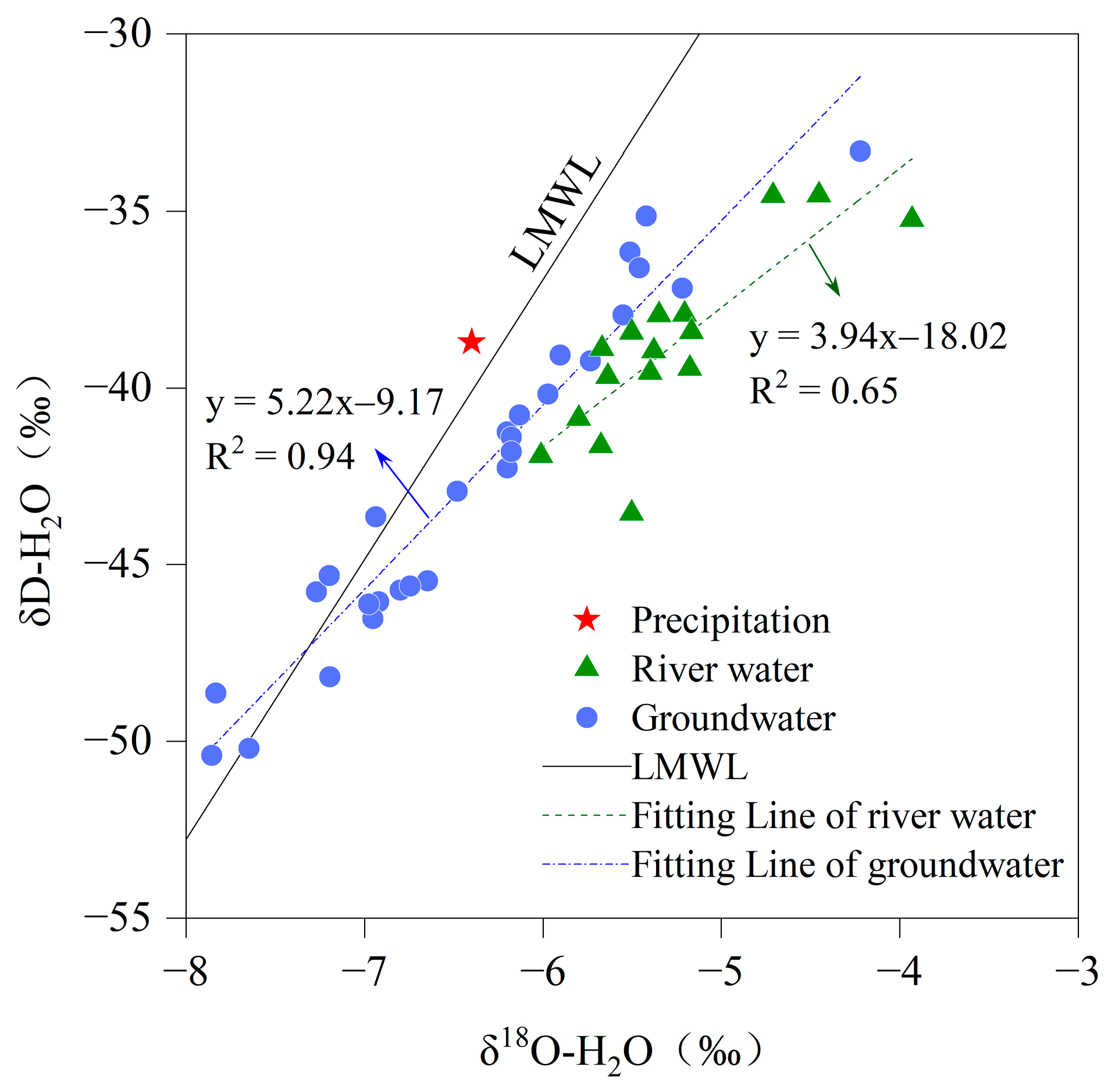
3.2. Origins of River Water and Groundwater
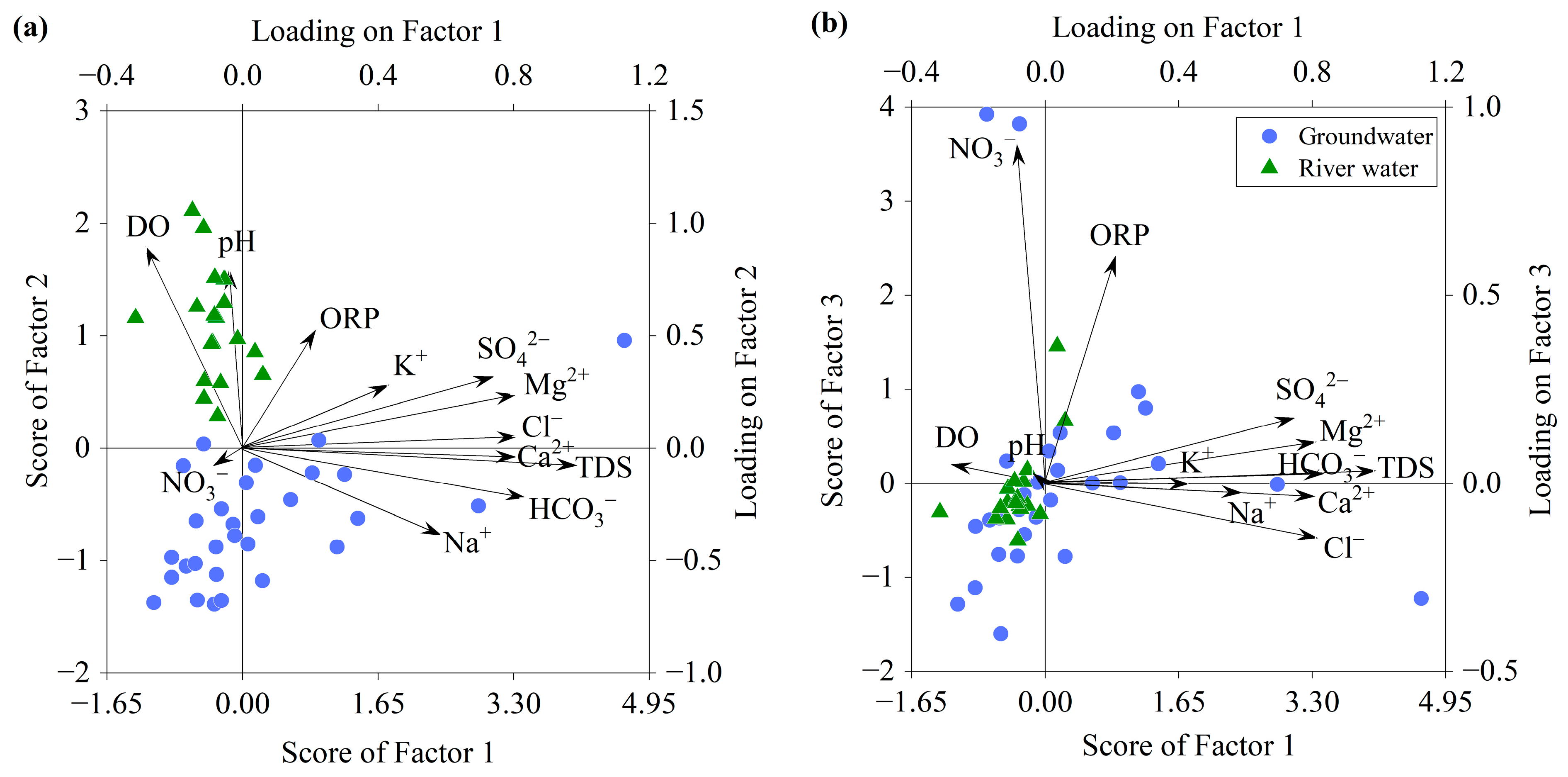
3.3. Factors Controlling Water Chemistry
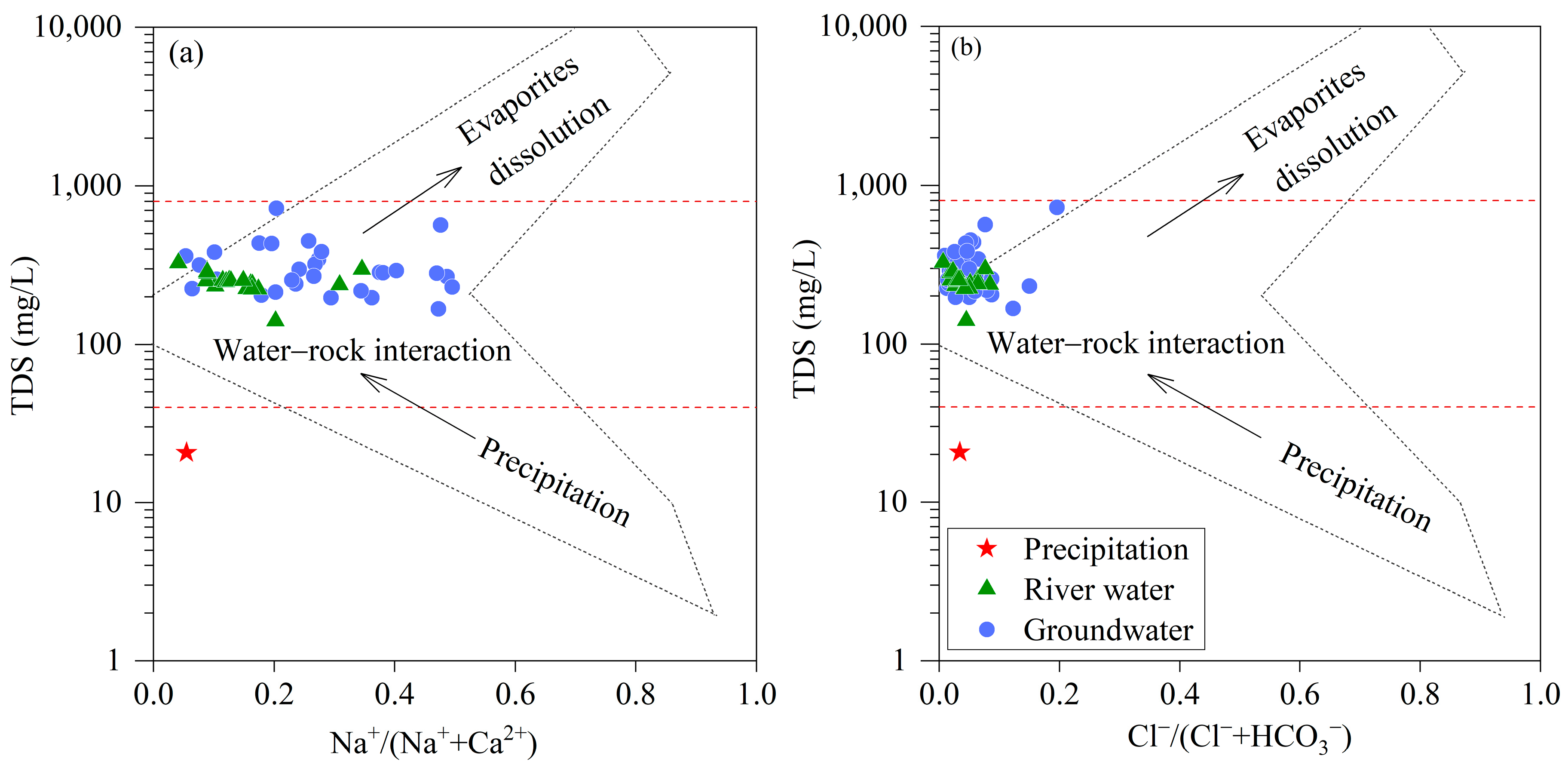
3.3.1. Water–Rock Interaction
3.3.2. Ion-Exchange Processes
3.3.3. Influence of Human Activity
3.4. Nitrate Sources and Transformation Processes
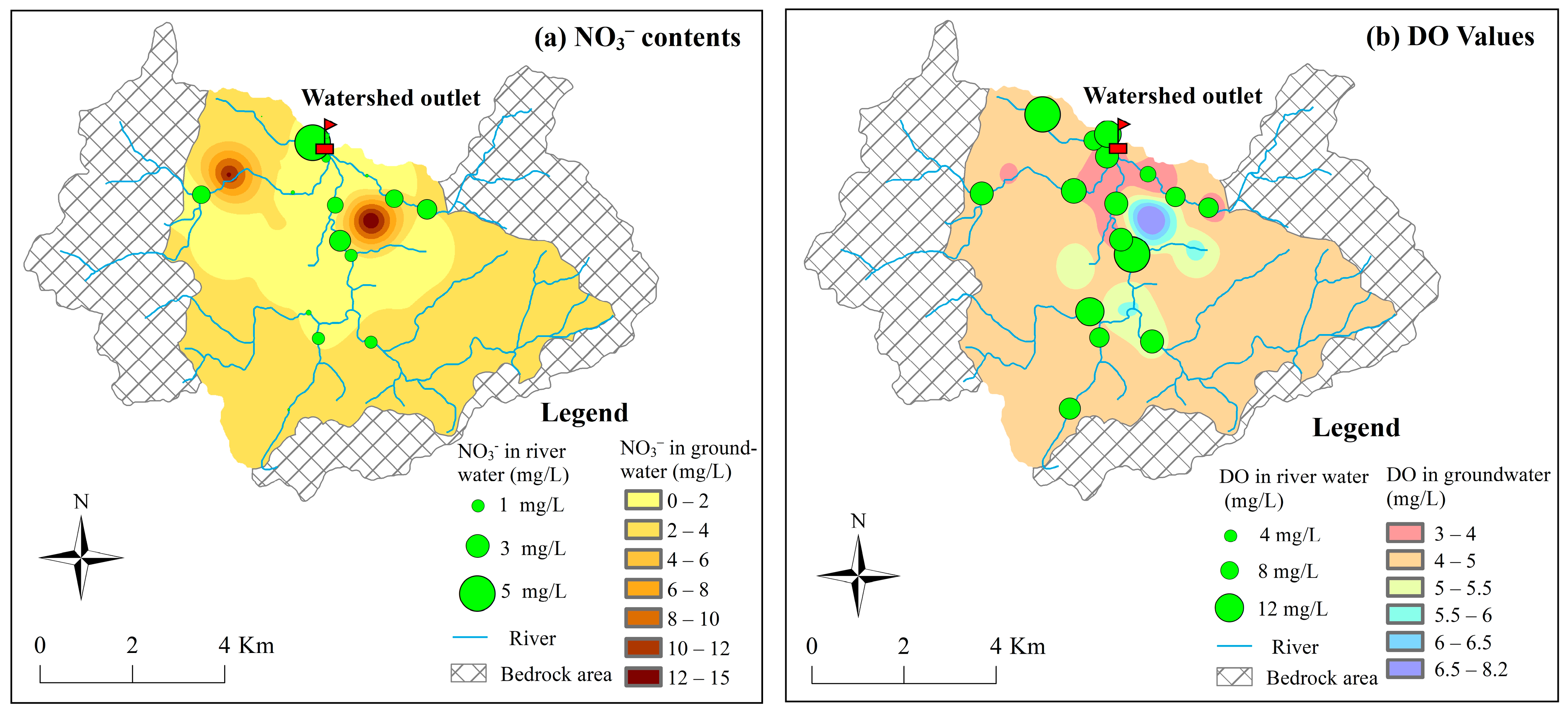
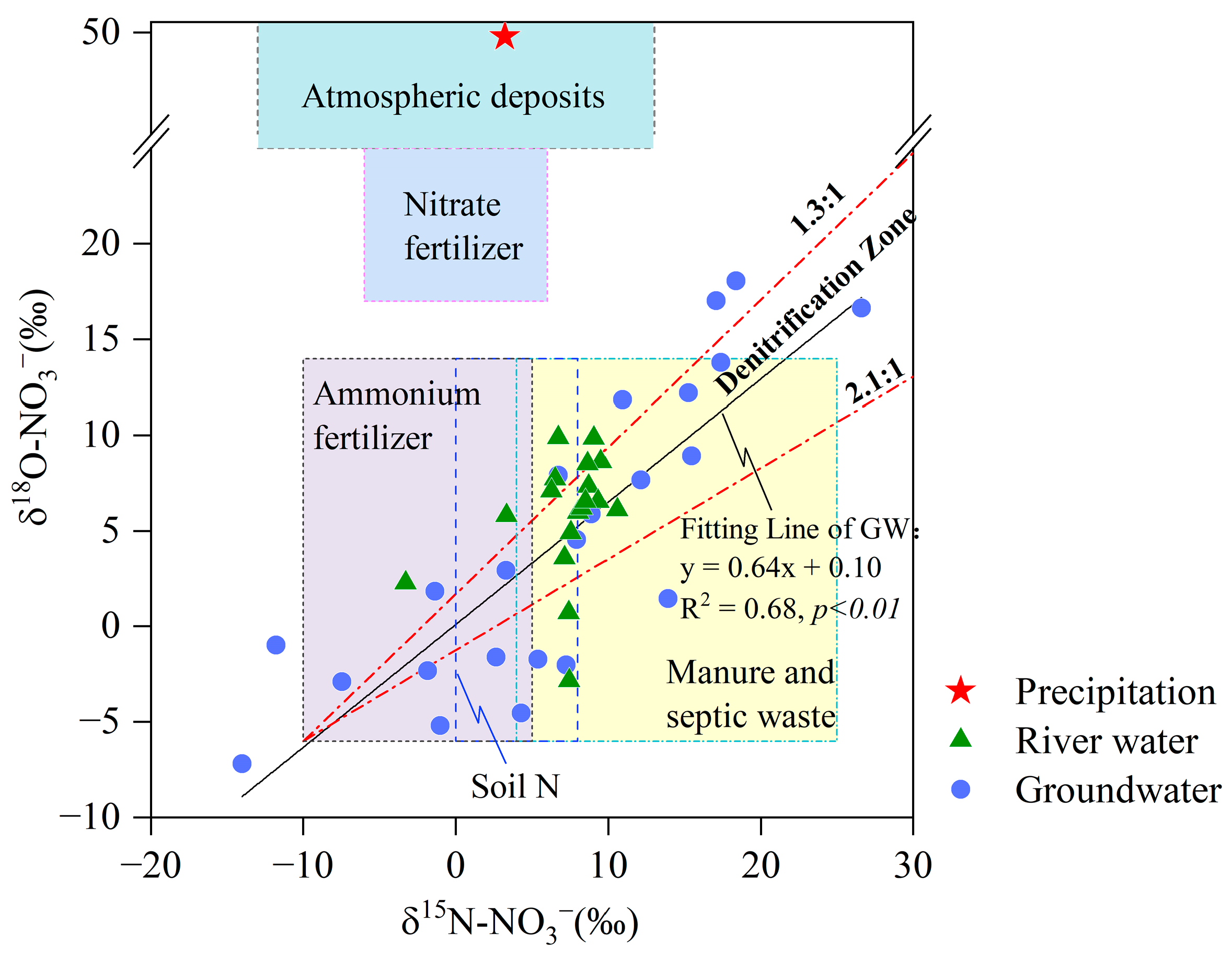
3.4.1. Qualitative Identification of Nitrate Sources
3.4.2. Nitrate Transformation Processes
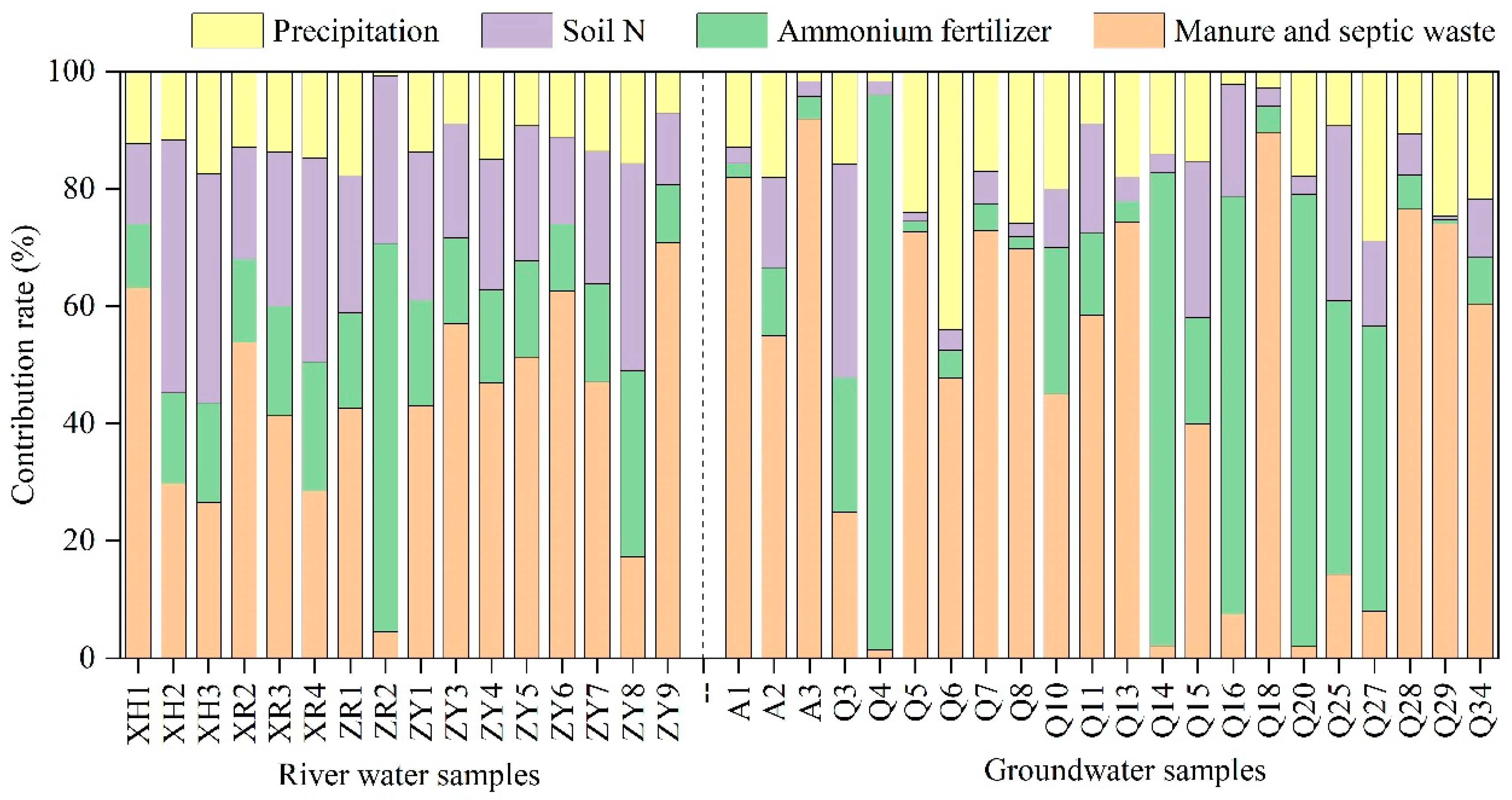
3.4.3. Quantitative Identification of Nitrate Sources
4. Conclusions
- (1)
- The river water is in a slightly alkaline and oxidizing environment, while the groundwater shows a slightly acidic to neutral environment, and both oxidizing and reducing environments were present in it. Both the surface water and groundwater were freshwater and were dominated by HCO3-Ca and HCO3-Ca·Mg types.
- (2)
- Both the river water and groundwater originate from precipitation and are influenced by evaporation, with the river water experiencing a stronger influence of evaporation. The main constituent ions in the river water and groundwater primarily originated from carbonate and silicate dissolution, with the presence of cation exchange in the groundwater where the displacement of Na+/K+ in the groundwater by Ca2+/Mg2+ on mineral surfaces is the dominant process. The water chemistry of the river water was greatly influenced by physicochemical factors, while that of the groundwater was mainly controlled by water–rock interactions.
- (3)
- The nitrate isotopic analysis revealed that the NO3− in surface water was mainly influenced by soil nitrogen and manure and septic wastes, while the NO3− in groundwater was jointly affected by ammonium fertilizers, soil nitrogen, and manure and septic wastes. With the exception of denitrification observed in the groundwater at the watershed outlet, denitrification was absent in both the groundwater in the piedmont area and surface water.
- (4)
- SIAR model analysis results demonstrated that the contribution rates of atmospheric precipitation, ammonium fertilizers, soil nitrogen, and manure and septic wastes to nitrate in the river water were 12%, 21%, 25%, and 42%, respectively, while contribution rates to nitrate in the groundwater were 16%, 27%, 10%, and 47%, respectively. Overall, manure and septic wastes were the main sources of nitrate in the river water and groundwater in the study area. It is necessary to prevent the leakage of manure and septic wastes when managing water resources.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Zhang, F.; Ding, W.; Wang, K.; Peng, J.; Cao, N.; He, C. Native forests transformed into cash crops reduced soil multi-functionality by modifying the microbial community composition and keystone species’ abundance in the Jianghuai Hilly Region. Environ. Sci. Pollut. Res. 2023, 30, 113747–113757. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Wang, N.; Zhou, Y. Impact of rainfall-runoff on phosphorus export from an agriculture watershed in the hilly region of Yangtze-Huaihe zone, China. J. Agro-Environ. Sci. 2019, 38, 2165–2173. (In Chinese) [Google Scholar]
- Chen, X.; Ren, M.; Li, G.; Zhang, J.; Xie, F.; Zheng, L. Identification of nitrate accumulation mechanism of surface water in a mining-rural-urban agglomeration area based on multiple isotopic evidence. Sci. Total Environ. 2023, 912, 169123. [Google Scholar] [CrossRef] [PubMed]
- Iori, V.; Muzzini, V.G.; Venditti, I.; Casentini, B.; Iannelli, M.A. Phytotoxic impact of bifunctionalized silver nanoparticles (AgNPs-Cit-L-Cys) and silver nitrate (AgNO3) on chronically exposed callus cultures of Populus nigra L. Environ. Sci. Pollut. Res. 2023, 30, 116175–116185. [Google Scholar] [CrossRef] [PubMed]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.H.; Hassan, G.; Sarhan, A.-H. Hydrochemistry of shallow groundwater and its assessment for drinking and irrigation purposes in Tarmiah district, Baghdad governorate, Iraq. Groundw. Sustain. Dev. 2020, 10, 100300. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Efremova, T.A. Microplastic Concentrations in Bottom Sediments of the Lakes of Specially Protected Natural Territories: Case Study of the Kizhskie Skerries, Lake Onega and Vodlozero Lake. Water Resour. 2023, 50, 901–912. [Google Scholar] [CrossRef]
- Fitts, C.R. (Ed.) Groundwater Science, 2nd ed.; Academic Press: Boston, MA, USA, 2013; pp. 421–497. [Google Scholar]
- Abdessamed, D.; Jodar-Abellan, A.; Ghoneim, S.S.; Almaliki, A.; Hussein, E.E.; Pardo, M.Á. Groundwater quality assessment for sustainable human consumption in arid areas based on GIS and water quality index in the watershed of Ain Sefra (SW of Algeria). Environ. Earth Sci. 2023, 82, 510. [Google Scholar] [CrossRef]
- Balcerowska-Czerniak, G.; Gorczyca, B. Rapid assessment of surface water quality using statistical multivariate analysis approach: Oder River system case study. Sci. Total Environ. 2023, 912, 168754. [Google Scholar] [CrossRef]
- Xiang, X.; De, K.; Lin, W.; Feng, T.; Li, F.; Wei, X.; Wang, W. Different fates and retention of deposited NH4+ and NO3− in the alpine grasslands of the Qinghai-Tibet plateau. Ecol. Indic. 2024, 158, 111415. [Google Scholar] [CrossRef]
- Nikolenko, O.; Jurado, A.; Borges, A.V.; Knller, K.; Brouyre, S. Isotopic composition of nitrogen species in groundwater under agricultural areas: A review. Sci. Total Environ. 2018, 621, 1415–1432. [Google Scholar] [CrossRef]
- Hosono, T.; Tokunaga, T.; Kagabu, M.; Nakata, H.; Orishikida, T.; Lin, I.T.; Shimada, J. The use of delta N-15 and delta O-18 tracers with an understanding of groundwater flow dynamics for evaluating the origins and attenuation mechanisms of nitrate pollution. Water Res. 2013, 47, 2661–2675. [Google Scholar] [CrossRef]
- Xuan, Y.; Mai, Y.; Xu, Y.; Zheng, J.; He, Z.; Shu, L.; Cao, Y. Enhanced microbial nitrification-denitrification processes in a subtropical metropolitan river network. Water Res. 2022, 222, 118857. [Google Scholar] [CrossRef]
- Parkinson, E.W.; Stoddart, S.; Sparacello, V.; Bertoldi, F.; Fonzo, O.; Malone, C.; Marini, E.; Martinet, F.; Moggi-Cecchi, J.; Pacciani, E.; et al. Multiproxy bioarchaeological data reveals interplay between growth, diet and population dynamics across the transition to farming in the central Mediterranean. Sci. Rep. 2023, 13, 21965. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, S.; Niu, R.; Hu, M.; Huang, G.; Pan, D.; Yan, S.; Liu, T.; Li, X.; Li, F. Insights into nitrate-reducing Fe (II) oxidation by Diaphorobacter caeni LI3T through kinetic, nitrogen isotope fractionation, and genome analyses. Sci. Total Environ. 2023, 912, 168720. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yue, F.J.; Tang, J.; Li, C.; Li, S.L. Nitrate transformation and source tracking of rivers draining into the Bohai Sea using a multi-tracer approach combined with an optimized Bayesian stable isotope mixing model. J. Hazard. Mater. 2024, 463, 132901. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martinez, J.A.; Mora, A.; Knappett, P.S.K.; Ornelas-Soto, N.; Mahlknecht, J. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef]
- Ren, K.; Pan, X.; Yuan, D.; Zeng, J.; Liang, J.; Peng, C. Nitrate sources and nitrogen dynamics in a karst aquifer with mixed nitrogen inputs (Southwest China): Revealed by multiple stable isotopic and hydro-chemical proxies. Water Res. 2022, 210, 118000. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yun, S.-T.; Mayer, B.; Lee, J.-H.; Kim, T.-S.; Kim, H.-K. Quantification of nitrate sources in groundwater using hydrochemical and dual isotopic data combined with a Bayesian mixing model. Agric. Ecosyst. Environ. 2015, 199, 369–381. [Google Scholar] [CrossRef]
- Weizu, G.U.; Jiaju, L.U.; Haixing, T.; Quanjiu, W. Challenges of basin study to traditional hydrological conceptions: The 50 years anniversary of hydrological basin study of PRC and the 20 years anniversary of Chuzhou Hydrological Laboratory. Adv. Water Sci. 2003, 14, 368–378. [Google Scholar]
- Buda, A.R.; Veith, T.L.; Folmar, G.J.; Feyereisen, G.W.; Bryant, R.B.; Church, C.D.; Schmidt, J.P.; Dell, C.J.; Kleinman, P.J.A. U.S. Department of Agriculture Agricultural Research Service Mahantango Creek Watershed, Pennsylvania, United States: Long-Term Precipitation Database. Water Resour. Res. 2011, 47, W08702. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Guo, K.; Liu, S. Coupled Simulation of Runoff and Nitrogen in Huashan Hydrological Experimental Watershed. J. China Hydrol. 2015, 35, 6. (In Chinese) [Google Scholar]
- Gao, J.; Yu, L. Hydrological model for the Transformation of “three waters” in the Wudaogou Region. Groundwater 1996, 18, 40–44+81. (In Chinese) [Google Scholar]
- Li, J.; Zhang, L.; Li, H. Output Time Characteristics of Source Phosphorus from Agricultural Small Watershed in Jianghuai Hilly Area. J. China Hydrol. 2017, 37, 7. (In Chinese) [Google Scholar]
- Zhang, L.; Dai, Y.; Lin, J.; Han, J.; Sun, X.; Li, X.; Liu, P.; Liao, A. Evaluating Spatiotemporal Variations of Groundwater–Surface Water Interaction Using an Integrated Hydrological Model in Huashan Basin, China. Sustainability 2022, 14, 14325. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; Mu, D.; Liu, W.; Chen, Y.; Fida, M. Unveiling the biogeochemical mechanism of nitrate in the vadose zone-groundwater system: Insights from integrated microbiology, isotope techniques, and hydrogeochemistry. Sci. Total Environ. 2024, 906, 167481. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, L.; Song, X.; Guo, M.; Li, B.; Cui, X. Sources and hydrogeochemical processes of groundwater under multiple water source recharge condition. Sci. Total Environ. 2023, 903, 166660. [Google Scholar] [CrossRef]
- Dawson, T.E.; Pausch, R.C.; Parker, H.M. The role of hydrogen and oxygen stable isotopes in understanding water movement along the soil-plant-atmospheric continuum. In Stable Isotopes; Garland Science: New York, NY, USA, 2020; pp. 169–183. [Google Scholar]
- Barbeta, A.; Jones, S.P.; Clavé, L.; Wingate, L.; Gimeno, T.E.; Fréjaville, B.; Wohl, S.; Ogée, J. Unexplained hydrogen isotope offsets complicate the identification and quantification of tree water sources in a riparian forest. Hydrol. Earth Syst. Sci. 2019, 23, 2129–2146. [Google Scholar] [CrossRef]
- Moine, B.N.; Bolfan-Casanova, N.; Radu, I.B.; Ionov, D.A.; Costin, G.; Korsakov, A.V.; Golovin, A.V.; Oleinikov, O.B.; Deloule, E.; Cottin, J.Y. Molecular hydrogen in minerals as a clue to interpret∂ D variations in the mantle. Nat. Commun. 2020, 11, 3604. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil-water separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, Z.; Song, Z.; Chen, W.; Tian, D.; Tang, S.; Wang, X.; Wang, J.; Liu, W.; Wang, Y.; et al. Variations and controlling factors of soil denitrification rate. Glob. Chang. Biol. 2022, 28, 2133–2145. [Google Scholar] [CrossRef]




| T (°C) | pH | DO (mg/L) | ORP (mV) | Na+ (mg/L) | K+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | CO32− (mg/L) | NO3− (mg/L) | Si (mg/L) | TDS (mg/L) | δD-H2O (‰) | δ18O-H2O (‰) | δ15N-NO3− (‰) | δ18O-NO3− (‰) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Precipitation (n = 1) | |||||||||||||||||||
| / | / | / | / | 0.37 | 0.81 | 6.33 | 0.45 | 0.45 | 1.31 | 12.81 | 2.74 | 20.66 | −38.70 | −6.40 | 3.23 | 49.79 | |||
| River water samples (n = 16) | |||||||||||||||||||
| Min | 10.80 | 6.31 | 5.29 | 125.90 | 7.17 | 0.99 | 3.65 | 4.06 | 5.05 | 0.22 | 18.32 | 0.00 | 0.10 | 5.19 | 98.08 | −47.84 | −7.59 | −3.29 | −2.84 |
| Mean | 14.38 | 7.67 | 9.28 | 158.51 | 14.64 | 2.28 | 55.34 | 14.41 | 10.49 | 15.00 | 220.66 | 6.46 | 0.98 | 12.82 | 230.10 | −39.51 | −5.50 | 6.82 | 4.92 |
| Max | 18.60 | 9.46 | 14.72 | 211.30 | 37.22 | 3.45 | 88.26 | 25.29 | 24.18 | 29.55 | 329.84 | 42.06 | 2.95 | 16.68 | 298.03 | −34.55 | −3.93 | 10.58 | 9.89 |
| Groundwater samples (n = 29) | |||||||||||||||||||
| Min | 16.50 | 6.43 | 3.08 | −91.70 | 5.13 | 0.17 | 28.40 | 5.40 | 3.28 | 0.09 | 164.92 | 0.00 | 0.07 | 0.31 | 167.07 | −50.39 | −7.86 | −14.04 | −7.18 |
| Mean | 18.58 | 6.82 | 4.62 | 117.11 | 27.07 | 1.06 | 74.79 | 16.97 | 18.90 | 16.79 | 312.78 | 0.00 | 2.47 | 4.03 | 313.89 | −42.60 | −6.41 | 6.78 | 4.45 |
| Max | 20.80 | 7.16 | 8.19 | 229.20 | 84.45 | 4.94 | 188.76 | 56.23 | 124.86 | 64.47 | 513.08 | 0.00 | 15.12 | 6.02 | 725.74 | −33.30 | −4.22 | 26.60 | 18.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lin, J.; Zhang, L.; Han, J.; Dai, Y.; Min, X.; Wang, H. Hydrochemical Evolution and Nitrate Source Identification of River Water and Groundwater in Huashan Watershed, China. Sustainability 2024, 16, 423. https://doi.org/10.3390/su16010423
Li X, Lin J, Zhang L, Han J, Dai Y, Min X, Wang H. Hydrochemical Evolution and Nitrate Source Identification of River Water and Groundwater in Huashan Watershed, China. Sustainability. 2024; 16(1):423. https://doi.org/10.3390/su16010423
Chicago/Turabian StyleLi, Xue, Jin Lin, Lu Zhang, Jiangbo Han, Yunfeng Dai, Xing Min, and Huirong Wang. 2024. "Hydrochemical Evolution and Nitrate Source Identification of River Water and Groundwater in Huashan Watershed, China" Sustainability 16, no. 1: 423. https://doi.org/10.3390/su16010423





