Biosurfactants: Promising Biomolecules for Agricultural Applications
Abstract
:1. Introduction
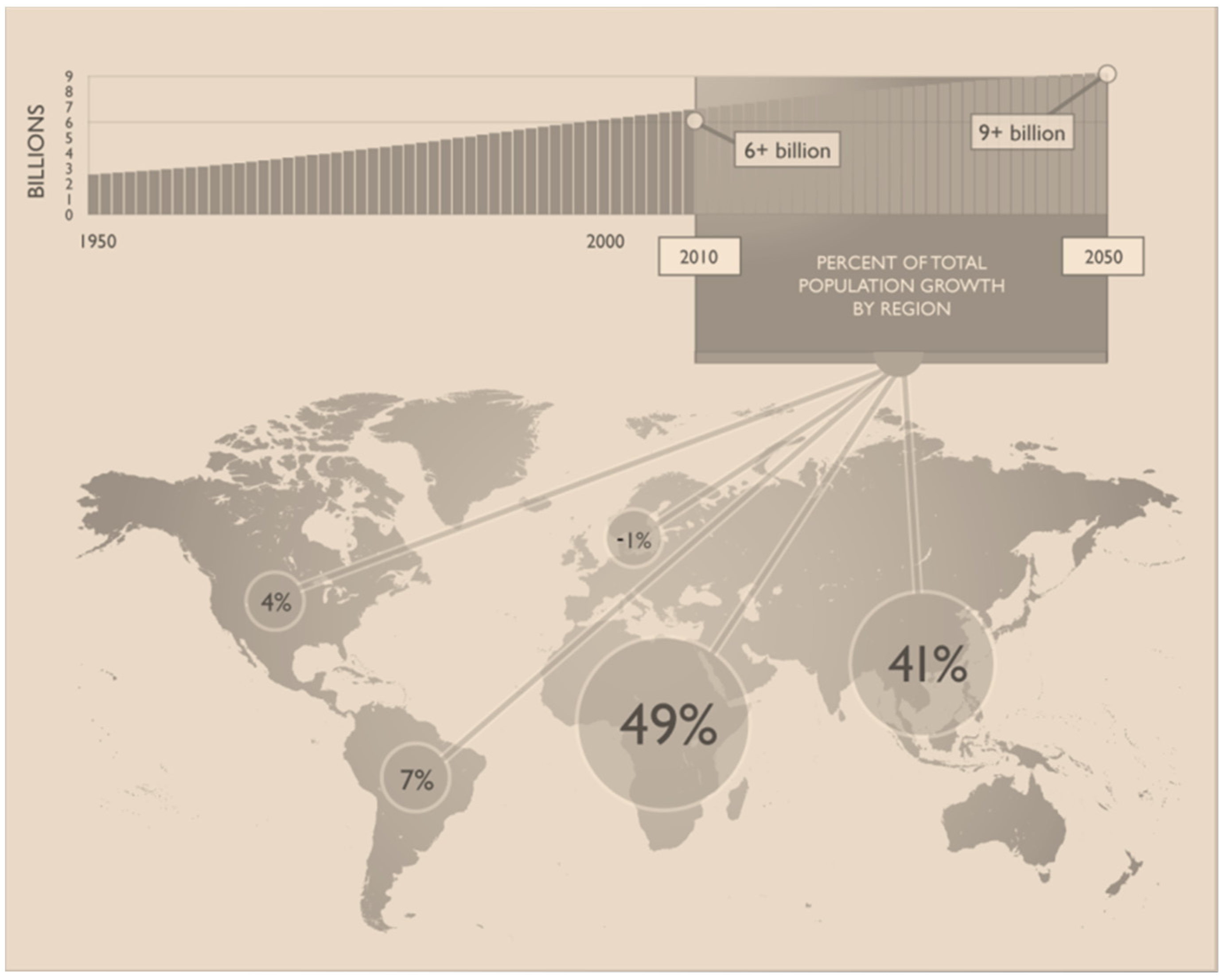
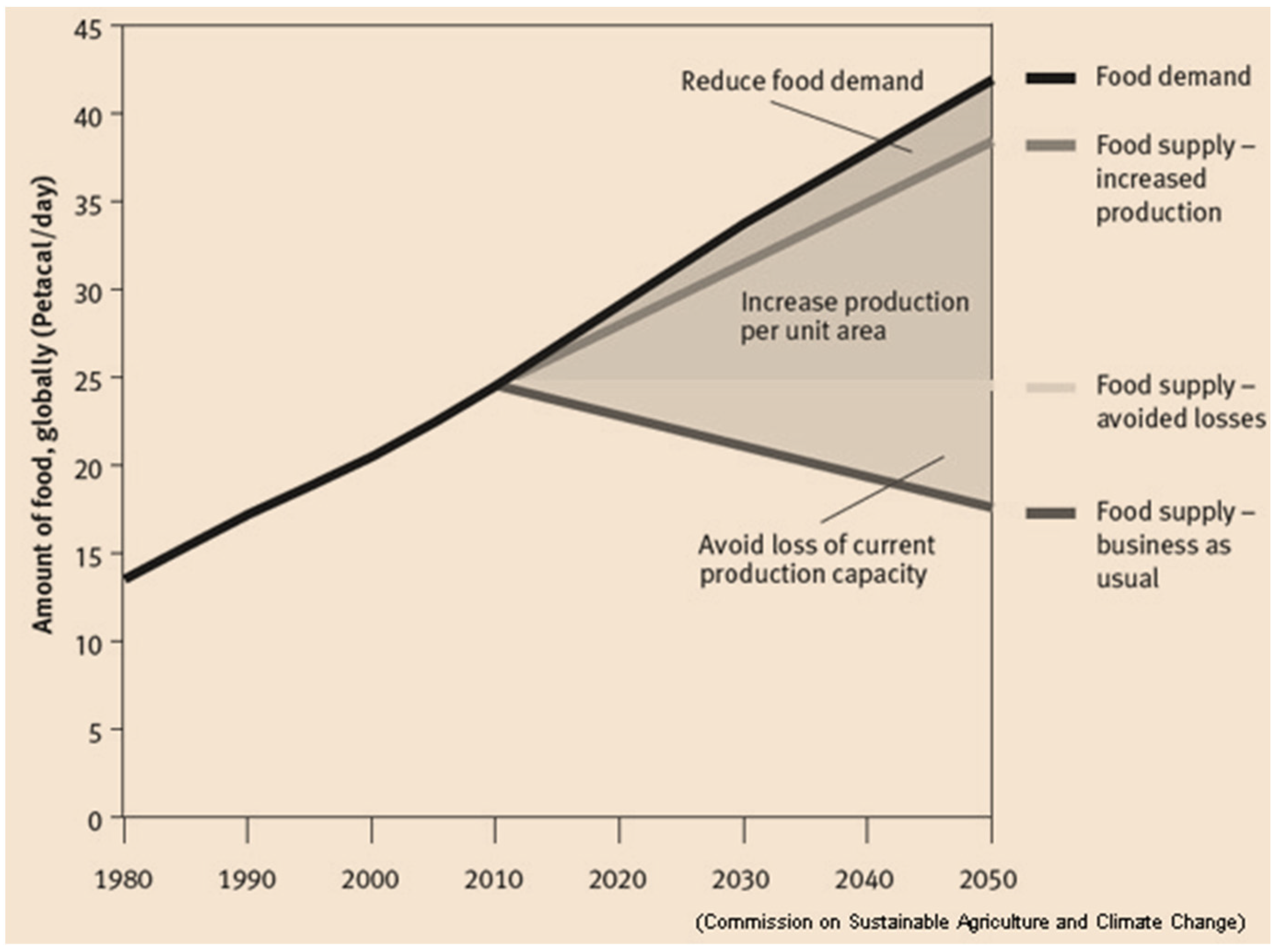
2. General Aspects of Global Agricultural Activity
3. Pesticides
3.1. Agricultural Defensives
3.2. Biological Control
3.3. Agricultural Biodefensives
3.3.1. Formulation of Agricultural Biodefensives
3.3.2. Agricultural Adjuvants as Activators or Enhancers of Agricultural Defensives
3.3.3. Utility Adjuvants
3.3.4. Activator or Enhancer Adjuvants
4. Surfactants
4.1. Chemical Surfactants
4.2. Biobased Surfactants
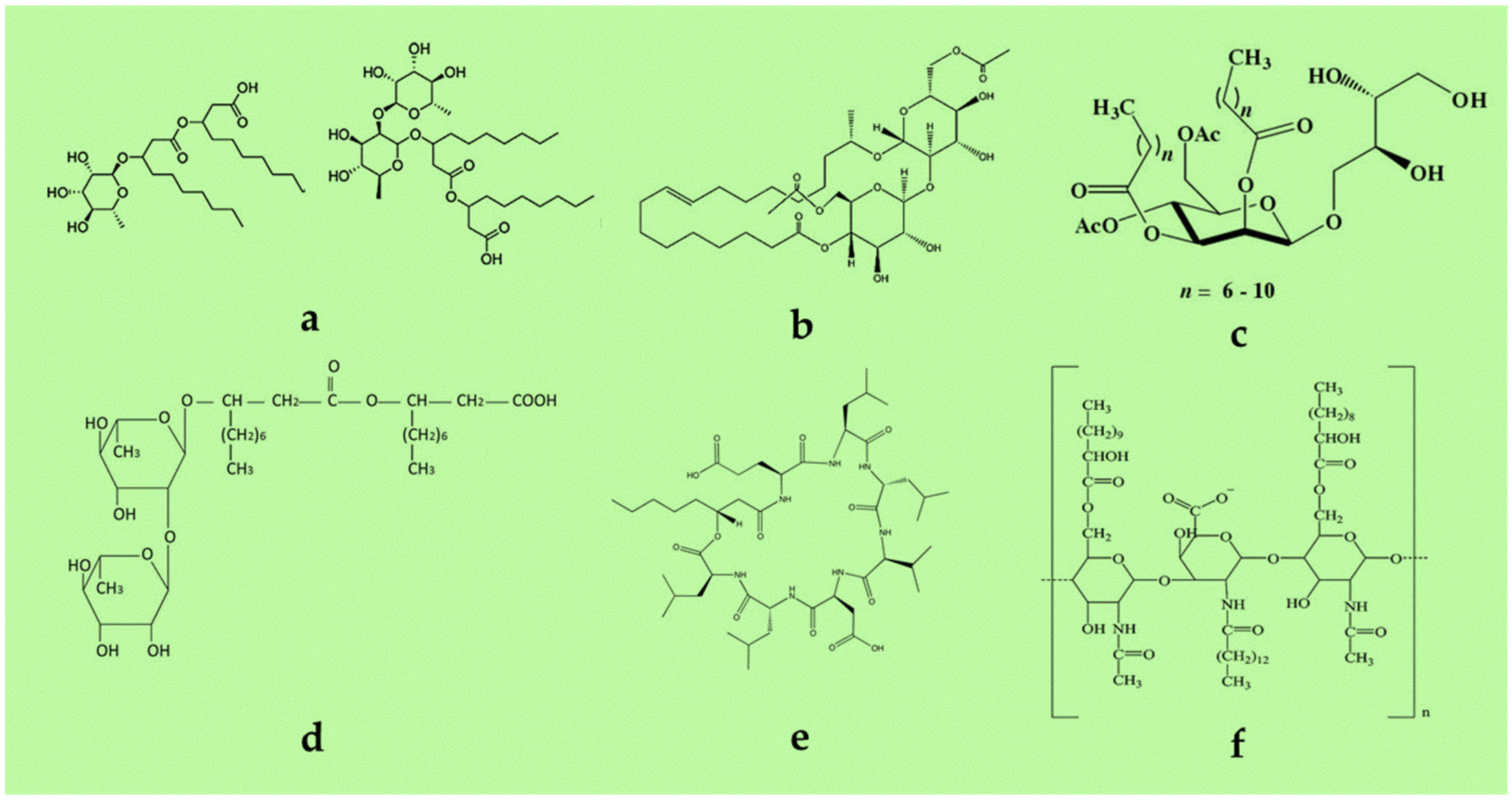
4.3. Biosurfactants
- Surface activity: Surfactant efficiency is measured with the CMC, which ranges from 1 to 2000 mg/L based on molecular structure, as discussed earlier [63]. An optimal biosurfactant can reduce the surface tension of water from 72 to 30–35 mN/m and the interfacial tension of oil and water from 40 to 1 mN/m [83]. Compared with synthetic surfactants, most microbial surfactants have lower surface and interfacial tensions and CMC values, making them more effective.
- Foam capacity: Biosurfactants are compounds that can reduce the surface tension of liquids, making it easier to create foam, or improve their colloidal stability by preventing bubbles from merging. They are particularly effective at the gas–liquid interface, where they form bubbles that move through the liquid, creating foam. In short, biosurfactants are substances that promote the production of foam [84].
- Emulsification and demulsification: Biosurfactants have emulsifying and demulsifying properties. Emulsions are a colloidal system of two immiscible liquids, wherein a liquid phase is dispersed and suspended in the form of small droplets, the dimensions of which range from 1 nm to 1 μm, in a second liquid (continuous phase). The two types of emulsions are water-in-oil (W/O) and oil-in-water (O/W). Biosurfactants signify the solubilization of large particles with micellar structures by assisting the dispersion of one liquid into another and making it easier for two immiscible liquids to be mixed. Demulsification is a process that occurs in two steps. Firstly, flocculation takes place when droplets come together to form flocs. Then, coalescence occurs when water droplets combine to form larger droplets. This reduction in the quantity of water droplets leads to demulsification. During the demulsification process, the stable interface between the internal and bulk levels is disturbed, causing the emulsions to split. Biosurfactants help to make the demulsification process easier [83].
- Solubilization: When the concentration of biosurfactants in a liquid surpasses a certain point known as the CMC, they spontaneously group together and form small nano-sized aggregates. These aggregates have a hydrophobic core and a hydrophilic surface that is exposed to water. This unique structure enhances the bioavailability of water-insoluble substances, such as chemical agents or molecules, by enabling their transportation and confinement within the aqueous phase [63,84].
- Wetting: Wetting capability refers to a liquid’s ability to connect with another surface and spread evenly over it. When a liquid with a high wetting capacity comes in contact with a surface, it creates a thin and continuous film. Biosurfactants are effective wetting agents because they can lower liquid surface tension by reducing attractive forces, which increases their affinity toward different surfaces. Instead of being connected to surface tension, they penetrate through the pores [84].
- Dispersion: Dispersion occurs when the cohesive attraction between similar particles decreases. A small amount of dispersing agent (such as BS) is added to a suspension to prevent insoluble particles from aggregating. For example, BS can remove hydrophobic molecules from rock surfaces, making them more mobile and easier to recover during oil extraction. Dispersion also plays a role in reducing or completely preventing the formation of biofilms by unwanted microbes [63,71].
- Temperature, pH, and ionic strength tolerance: Several biosurfactants remain effective in adverse conditions, such as high temperatures, a pH range of 3–12, and up to a 10% saline concentration, while synthetic surfactants are inactivated by ≥2% NaCl [71].
- Specificity: The high diversity of molecules, each with its own complexity and specific functional groups, confers particular/specific activities to biosurfactants. Similar to synthetic surfactants, biosurfactants show the ability to self-aggregate and form micelles, which increase their specificity and allow them to have different morphological structures. In addition, their ability to create spherical, rod-shaped, and vesicle-like structures has caught the attention of various industries like food, cosmetics, and pharmaceuticals. They also have the potential to detoxify pollutants and demulsify industrial emulsions [71].
- Biocompatibility and digestibility: The composition of biosurfactants makes them more biodegradable and biocompatible than their chemical counterparts under variations in temperature, pH, and degradation time [85].
4.3.1. Application of Biosurfactants in the Agricultural Industry and Trends
Soil Quality Enhancement with Soil Amendments
Adjuvants for Plant Pathogen Elimination
Adjuvants for Seed Germination and Plant Growth
Adjuvants for Beneficial Microbe Interactions
4.3.2. Producing Biosurfactant-Based Biopesticides for the Agricultural Industry
4.3.3. Nanotechnology for Delivering Pesticides
4.3.4. Metagenomics of Biosurfactants Applied in the Agricultural Industry
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mrabet, R. Sustainable agriculture for food and nutritional security. In Sustainable Agriculture and the Environment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–90. [Google Scholar]
- Rebolledo-Leiva, R.; Moreira, M.T.; González-García, S. Progress of social assessment in the framework of bioeconomy under a life cycle perspective. Renew. Sustain. Energy Rev. 2023, 175, 113162. [Google Scholar] [CrossRef]
- Castro, M.J.L.; Ojeda, C.; Cirelli, A.F. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 2014, 12, 85–95. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Dehnert, G.K.; Freitas, M.B.; Sharma, P.P.; Barry, T.P.; Karasov, W.H. Impacts of subchronic exposure to a commercial 2,4-D herbicide on developmental stages of multiple freshwater fish species. Chemosphere 2021, 263, 127638. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, T.; Xu, Y.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Zhu, L. Ecotoxicology of strobilurin fungicides. Sci. Total Environ. 2020, 742, 140611. [Google Scholar] [CrossRef]
- Calista, N.; Haikael, M.D.; Athanasia, M.O.; Neema, K.; Judith, K. Does Pesticide exposure contribute to the growing burden of non—Communicable diseases in Tanzania. Sci. Afr. 2022, 17, e01276. [Google Scholar] [CrossRef]
- Carrança Thaís Agrotóxico Mais Usado do Brasil Está Associado a 503 Mortes Infantis por ano, Revela Estudo—BBC News Brasil. Available online: https://www.bbc.com/portuguese/brasil-57209799 (accessed on 18 May 2023).
- Campanhola, C.; Bettiol, W. (Eds.) Métodos Alternativos de Controle Fitossanitário; Embrapa Meio Ambiente: Jaguariúna, SP, Brazil, 2003; ISBN 85-85771-22-4. [Google Scholar]
- Ward, L.T.; Hladik, M.L.; Guzman, A.; Winsemius, S.; Bautista, A.; Kremen, C.; Mills, N.J. Pesticide exposure of wild bees and honey bees foraging from field border flowers in intensively managed agriculture areas. Sci. Total Environ. 2022, 831, 154697. [Google Scholar] [CrossRef]
- Rigotto, R.M.; Vasconcelos, D.P.E.; Rocha, M.M. Pesticide use in Brazil and problems for public health. Cad. Saude Publica 2014, 30, 1360–1362. [Google Scholar] [CrossRef]
- Jhansi Rani, S.; Usha, R. Transgenic plants: Types, benefits, public concerns and future. J. Pharm. Res. 2013, 6, 879–883. [Google Scholar] [CrossRef]
- Rauchecker, M. The territorial and sectoral dimensions of advocacy—The conflicts about pesticide use in Argentina. Polit. Geogr. 2019, 75, 102067. [Google Scholar] [CrossRef]
- Bailey, K.L.; Boyetchko, S.M.; Längle, T. Social and economic drivers shaping the future of biological control: A Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol. Control 2010, 52, 221–229. [Google Scholar] [CrossRef]
- Cavalcante, B.D.A.M.; Scapini, T.; Camargo, A.F.; Ulrich, A.; Bonatto, C.; Dalastra, C.; Mossi, A.J.; Fongaro, G.; Di Piero, R.M.; Treichel, H. Orange peels and shrimp shell used in a fermentation process to produce an aqueous extract with bioherbicide potential to weed control. Biocatal. Agric. Biotechnol. 2021, 32, 101947. [Google Scholar] [CrossRef]
- Ulrich, A.; Lerin, L.A.; Camargo, A.F.; Scapini, T.; Diering, N.L.; Bonafin, F.; Gasparetto, I.G.; Confortin, T.C.; Sansonovicz, P.F.; Fabian, R.L.; et al. Alternative bioherbicide based on Trichoderma koningiopsis: Enzymatic characterization and its effect on cucumber plants and soil organism. Biocatal. Agric. Biotechnol. 2021, 36, 102127. [Google Scholar] [CrossRef]
- Mann, R.; Bidwell, J. The acute toxicity of agricultural surfactants to the tadpoles of four Australian and two exotic frogs. Environ. Pollut. 2001, 114, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, S.; Kaur, H.; Sharma, P.; Sindhu, M.; Wati, L. Routing microbial biosurfactants to agriculture for revitalization of soil and plant growth. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 313–338. [Google Scholar]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.; de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Sivapathasekaran, C.; Sen, R. Origin, properties, production and purification of microbial surfactants as molecules with immense commercial potential. Tenside Surfactants Deterg. 2017, 54, 92–104. [Google Scholar]
- Maldaner, J.; Oliveira, M.N.; De Alexandria Santos, D.; Simote Silva, S.Y.; Da Cruz Silva, S.; Da Costa Lima, T.; Da Silva, M.L.; Lima Silva, H.T.; Siqueira-Silva, D.H.; Pauli kist Steffen, G.; et al. Bioherbicide and anesthetic potential of Aniba canelilla essential oil, a contribution to the demands of the agricultural sector. Biocatal. Agric. Biotechnol. 2022, 42, 102353. [Google Scholar] [CrossRef]
- Serrano-Carreón, L.; Aranda-Ocampo, S.; Balderas-Ruíz, K.A.; Juárez, A.M.; Leyva, E.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Galindo, E. A case study of a profitable mid-tech greenhouse for the sustainable production of tomato, using a biofertilizer and a biofungicide. Electron. J. Biotechnol. 2022, 59, 13–24. [Google Scholar] [CrossRef]
- Shokouhi, D.; Seifi, A. Organic extracts of seeds of Iranian Moringa peregrina as promising selective biofungicide to control Mycogone perniciosa. Biocatal. Agric. Biotechnol. 2020, 30, 101848. [Google Scholar] [CrossRef]
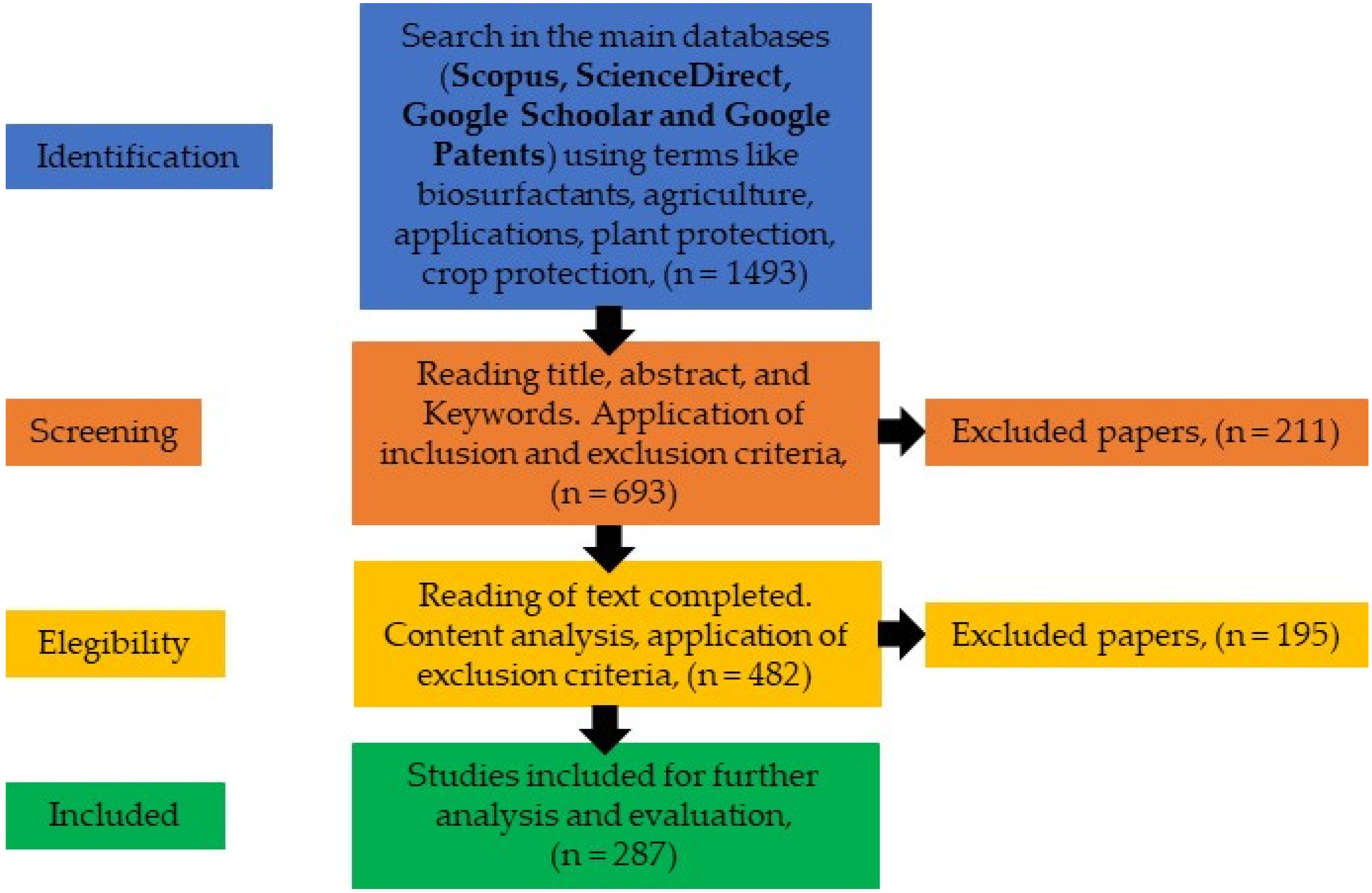
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Mertens, M. Genetic Engineering: Modified Crops, More Pesticides. Available online: https://eu.boell.org/en/PesticideAtlas-genetic-engineering (accessed on 23 May 2023).
- Benbrook, C.M. Impacts of genetically engineered crops on pesticide use in the U.S.—The first sixteen years. Environ. Sci. Eur. 2012, 24, 24. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Fuchs, B.; Nissinen, R.; Puigbò, P.; Rainio, M.; Saikkonen, K.; Helander, M. Ecosystem consequences of herbicides: The role of microbiome. Trends Ecol. Evol. 2023, 38, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, S.; Raine, N.E. Fungicides and bees: A review of exposure and risk. Environ. Int. 2022, 165, 107311. [Google Scholar] [CrossRef] [PubMed]
- Treviño, M.J.S.; Pereira-Coelho, M.; López, A.G.R.; Zarazúa, S.; dos Santos Madureira, L.A.; Majchrzak, T.; Płotka-Wasylka, J. How pesticides affect neonates?—Exposure, health implications and determination of metabolites. Sci. Total Environ. 2023, 856, 158859. [Google Scholar] [CrossRef] [PubMed]
- Roltsch, W.J.; Bürgi, L.P.; Tomic-Carruthers, N.; Rugman-Jones, P.F.; Stouthamer, R.; Mills, N.J. Mortality of light brown apple moth egg masses in coastal California: Impact of resident Trichogramma parasitism and predation. Biol. Control 2021, 152, 104465. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Ramírez-Camejo, L.A.; Bayman, P.; Mejía, L.C. Drosophila melanogaster as an emerging model host for entomopathogenic fungi. Fungal Biol. Rev. 2022, 42, 85–97. [Google Scholar] [CrossRef]
- dos Santos Gomes, A.C.; da Silva, R.R.; Moreira, S.I.; Vicentini, S.N.C.; Ceresini, P.C. Biofungicides: An eco-friendly approach for plant disease management. Encycl. Mycol. 2021, 2, 641–649. [Google Scholar] [CrossRef]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Gouli, V.V.; Marcelino, J.A.P.; Gouli, S.Y. Microbial formulations for control of noxious organisms. In Microbial Pesticides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–247. [Google Scholar]
- do Nascimento, J.; Cristina Goncalves, K.; Pinto Dias, N.; Luiz de Oliveira, J.; Bravo, A.; Antonio Polanczyk, R. Adoption of Bacillus thuringiensis-based biopesticides in agricultural systems and new approaches to improve their use in Brazil. Biol. Control 2022, 165, 104792. [Google Scholar] [CrossRef]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.V.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 74–81. [Google Scholar] [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation optimization of D-limonene-loaded nanoemulsions as a natural and efficient biopesticide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124746. [Google Scholar] [CrossRef]
- Riyanto; Mulwandari, M.; Asysyafiiyah, L.; Sirajuddin, M.I.; Cahyandaru, N. Direct synthesis of lemongrass (Cymbopogon citratus L.) essential oil-silver nanoparticles (EO-AgNPs) as biopesticides and application for lichen inhibition on stones. Heliyon 2022, 8, e09701. [Google Scholar] [CrossRef]
- Wu, H.Q.; Sun, L.L.; Fang, L.; Wang, Z.Y.; Cao, C.W. Preparation of dry flowable formulations of Clonostachys rosea by spray drying and application for Sclerotinia sclerotiorum control. J. Integr. Agric. 2018, 17, 613–620. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Recent advances in downstream processing and formulations of Bacillus thuringiensis based biopesticides. Process Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Werner, S.J.; DeLiberto, S.T.; Mangan, A.M.; Pettit, S.E.; Ellis, J.W.; Carlson, J.C. Anthraquinone-based repellent for horned larks, great-tailed grackles, American crows and the protection of California’s specialty crops. Crop Prot. 2015, 72, 158–162. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Jeevanandam, J.; Inobeme, A.; Olaniyan, O.T.; Anani, O.A.; Thangadurai, D.; Islam, S. Application of biosurfactant for the production of adjuvant and their synergetic effects when combined with different agro-pesticides. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–277. [Google Scholar]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H.E.; Bagley, W.E.; Derksen, R.C.; Krause, C.R. Adjuvant Effects on Evaporation Time and Wetted Area of Droplets on Waxy Leaves. Trans. ASABE 2010, 53, 13–20. [Google Scholar] [CrossRef]
- Gaskin, R.E.; Murray, R.J.; Krishna, H.; Carpenter, A. Effect of adjuvants on the retention of insecticide spray on cucumber and pea foliage. N. Zeal. Plant Prot. 2000, 53, 355–359. [Google Scholar] [CrossRef]
- Ramsey, R.J.L.; Stephenson, G.R.; Hall, J.C. A review of the effects of humidity, humectants, and surfactant composition on the absorption and efficacy of highly water-soluble herbicides. Pestic. Biochem. Physiol. 2005, 82, 162–175. [Google Scholar] [CrossRef]
- Kudsk, P.; Mathiassen, S.K. Analysis of adjuvant effects and their interactions with variable application parameters. Crop Prot. 2007, 26, 328–334. [Google Scholar] [CrossRef]
- Wang, C.J.; Liu, Z.Q. Foliar uptake of pesticides—Present status and future challenge. Pestic. Biochem. Physiol. 2007, 87, 1–8. [Google Scholar] [CrossRef]
- Stock, D.; Briggs, G. Physicochemical Properties of Adjuvants: Values and Applications. Weed Technol. 2000, 14, 798–806. [Google Scholar] [CrossRef]
- Tu, M.; Hurd, C.; Randall, J.M.; Rice, B. Weed Control Methods Handbook; The Nature Conservancy: Arlington County, VI, USA, 2001; ISBN 5032301221. [Google Scholar]
- Hazen, J.L. Adjuvants: Terminology, Classification, and Chemistry on JSTOR. In Weed Technology; Cambridge University Press: Cambridge, MA, USA, 2000; Volume 14, pp. 773–784. [Google Scholar]
- Yu, Y.; Zhu, H.; Ozkan, H.E.; Derksen, R.C.; Krause, C.R. Evaporation and Deposition Coverage Area of Droplets Containing Insecticides and Spray Additives on Hydrophilic, Hydrophobic, and Crabapple Leaf Surfaces. Trans. ASABE 2009, 52, 39–49. [Google Scholar] [CrossRef]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Schönherr, J.; Baur, P.; Uhlig, B.A. Rates of cuticular penetration of 1-naphthylacetic acid (NAA) as affected by adjuvants, temperature, humidity and water quality. Plant Growth Regul. 2000, 31, 61–74. [Google Scholar] [CrossRef]
- Mulqueen, P. Recent advances in agrochemical formulation. Adv. Colloid Interface Sci. 2003, 106, 83–107. [Google Scholar] [CrossRef]
- Blackwell, P.S. Management of water repellency in Australia, and risks associated with preferential flow, pesticide concentration and leaching. J. Hydrol. 2000, 231–232, 384–395. [Google Scholar] [CrossRef]
- Petrović, M.; Barceló, D. Analysis and fate of surfactants in sludge and sludge-amended soils. TrAC Trends Anal. Chem. 2004, 23, 762–771. [Google Scholar] [CrossRef]
- Reddy, S.; Verma, V.; Srivastava, N. (Eds.) Marine Biosurfactants. In Marine Surfactants; CRC Press: Boca Raton, FL, USA, 2022; pp. 255–269. [Google Scholar]
- de Almeida, D.G.; da Silva, M.D.; do Nascimento Barbosa, R.; Silva, D.D.; da Silva, R.O.; de Souza Lima, G.M.; de Gusmão, N.B.; de Queiroz, M.D. Biodegradation of marine fuel MF-380 by microbial consortium isolated from seawater near the petrochemical Suape Port, Brazil. Int. Biodeterior. Biodegrad. 2017, 116, 73–82. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.; Bezerra, K.G.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Paulino, B.N.; Pessôa, M.G.; Mano, M.C.R.; Molina, G.; Neri-Numa, I.A.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. [Google Scholar] [CrossRef]
- Hayes, D.G.; Smith, G.A. Biobased Surfactants: Overview and Industrial State of the Art. Biobased Surfactants Synth. Prop. Appl. 2019, 3–38. [Google Scholar] [CrossRef]
- Fernández Cirelli, A.; Ojeda, C.; Castro, M.J.L.; Salgot, M. Surfactants in sludge-amended agricultural soils: A review. Environ. Chem. Lett. 2008, 6, 135–148. [Google Scholar] [CrossRef]
- Kandasamy, R.; Rajasekaran, M.; Venkatesan, S.K.; Uddin, M. New Trends in the Biomanufacturing of Green Surfactants: Biobased Surfactants and Biosurfactants. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1329, pp. 243–260. [Google Scholar]
- Zahed, M.A.; Mohammad, M.A.; Azari, A.; Mohajeri, L. Biosurfactant, a green and effective solution for bioremediation of petroleum hydrocarbons in the aquatic environment. Discov. Water 2022, 2, 5. [Google Scholar] [CrossRef]
- Silva, M.D.; de Medeiros, A.O.; Sarubbo, L.A. Biosurfactant: A Biodegradable Antimicrobial Substance. In Biodegradable Materials and Their Applications; Inamuddin, T.A., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 471–486. [Google Scholar]
- Santos, D.; Rufino, R.; Luna, J.; Santos, V.; Sarubbo, L. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.-S.; Wong, J.W.C.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Fact. 2021, 20, 120. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial Surfactants: The Next Generation Multifunctional Biomolecules for Applications in the Petroleum Industry and Its Associated Environmental Remediation. Microorganisms 2019, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and characterisation of short chain rhamnolipid production in a previously uninvestigated, non-pathogenic marine pseudomonad. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. [Google Scholar] [CrossRef]
- Rawat, G.; Dhasmana, A.; Kumar, V. Biosurfactants: The next generation biomolecules for diverse applications. Environ. Sustain. 2020, 3, 353–369. [Google Scholar] [CrossRef]
- Wang, H.; Roelants, S.L.K.W.; To, M.H.; Patria, R.D.; Kaur, G.; Lau, N.S.; Lau, C.Y.; Van Bogaert, I.N.A.; Soetaert, W.; Lin, C.S.K. Starmerella bombicola: Recent advances on sophorolipid production and prospects of waste stream utilization. J. Chem. Technol. Biotechnol. 2019, 94, 999–1007. [Google Scholar] [CrossRef]
- Fei, D.; Zhou, G.W.; Yu, Z.Q.; Gang, H.Z.; Liu, J.F.; Yang, S.Z.; Ye, R.Q.; Mu, B.Z. Low-Toxic and Nonirritant Biosurfactant Surfactin and its Performances in Detergent Formulations. J. Surfactants Deterg. 2020, 23, 109–118. [Google Scholar] [CrossRef]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Datta, P.; Tiwari, P.; Pandey, L.M. Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J. Pet. Sci. Eng. 2020, 195, 107612. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Ngueagni, P.T. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar] [CrossRef]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2021, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S. Tapping significance of microbial surfactants as a biopesticide and synthetic pesticide remediator: An ecofriendly approach for maintaining the environmental sustainability. In Insecticides—Advances in Insect Control and Sustainable Pest Management; IntechOpen: London, UK, 2023. [Google Scholar]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Moldes, A.B.; Cruz, J.M. Biodegradability Study of the Biosurfactant Contained in a Crude Extract from Corn Steep Water. J. Surfactants Deterg. 2020, 23, 79–90. [Google Scholar] [CrossRef]
- Silva, E.J.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B Biointerfaces 2018, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arh. Hig. Rada Toksikol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro, P.P.F.; De Almeida, D.G.; De Luna, J.M.; Rufino, R.D.; Dos Santos, V.A.; Sarubbo, L.A. Optimization of biosurfactant production from Candida guiliermondii using a Rotate Central Composed Design. Chem. Eng. Trans. 2015, 43, 1411–1416. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environ. Technol. Innov. 2021, 24, 102090. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Bittencourt Sydney, E.; Bianchi Pedroni Medeiros, A.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Junior Letti, L.A.; Thomaz Soccol, V.; de Melo Pereira, G.V.; et al. Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresour. Technol. 2021, 341, 125795. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef]
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An overview of current research and developments in biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. [Google Scholar] [CrossRef]
- Rostás, M.; Blassmann, K. Insects had it first: Surfactants as a defence against predators. Proc. R. Soc. B Biol. Sci. 2009, 276, 633–638. [Google Scholar] [CrossRef]
- Fukuoka, T.; Yoshida, S.; Nakamura, J.; Koitabashi, M.; Sakai, H.; Abe, M.; Kitamoto, D.; Kitamoto, H. Application of Yeast Glycolipid Biosurfactant, Mannosylerythritol Lipid, as Agrospreaders. J. Oleo Sci. 2015, 64, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Trinchera, A.; Baratella, V. Use of a Non-Ionic Water Surfactant in Lettuce Fertigation for Optimizing Water Use, Improving Nutrient Use Efficiency, and Increasing Crop Quality. Water 2018, 10, 613. [Google Scholar] [CrossRef]
- Abagandura, G.O.; Park, D.; Bridges, W.C. Surfactant and irrigation impacts on soil water content and leachate of soils and greenhouse substrates. Agrosyst. Geosci. Environ. 2021, 4, 1–11. [Google Scholar] [CrossRef]
- Hassan, M.N.; Namood-e-Sahar; Zia-Ul-Husnain Shah, S.; Afghan, S.; Hafeez, F.Y. Suppression of red rot disease by Bacillus sp. based biopesticide formulated in non-sterilized sugarcane filter cake. BioControl 2015, 60, 691–702. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Marecik, R.; Chrzanowski, Ł. Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 2013, 97, 2327–2339. [Google Scholar] [CrossRef]
- Luzuriaga-Loaiza, W.P.; Schellenberger, R.; De Gaetano, Y.; Obounou Akong, F.; Villaume, S.; Crouzet, J.; Haudrechy, A.; Baillieul, F.; Clément, C.; Lins, L.; et al. Synthetic Rhamnolipid Bolaforms trigger an innate immune response in Arabidopsis thaliana. Sci. Rep. 2018, 8, 8534. [Google Scholar] [CrossRef]
- Nguyen, B.V.G.; Nagakubo, T.; Toyofuku, M.; Nomura, N.; Utada, A.S. Synergy between Sophorolipid Biosurfactant and SDS Increases the Efficiency of P. aeruginosa Bio film. Disruption 2020, 36, 6411–6420. [Google Scholar] [CrossRef]
- Karamchandani, B.M.; Pawar, A.A.; Pawar, S.S.; Syed, S.; Mone, N.S.; Dalvi, S.G.; Rahman, P.K.S.M.; Banat, I.M.; Satpute, S.K. Biosurfactants’ multifarious functional potential for sustainable agricultural practices. Front. Bioeng. Biotechnol. 2022, 10, 1047279. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhong, H.; Zeng, G.; Liang, Y.; Chen, M.; Wu, Z.; Zhou, Y.; Yu, M.; Shao, B. Recent advances in the environmental applications of biosurfactant saponins: A review. J. Environ. Chem. Eng. 2017, 5, 6030–6038. [Google Scholar] [CrossRef]
- Sales da Silva, I.G.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil Bioremediation: Overview of Technologies and Trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Rocha, R.B.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015, 31, 707–723. [Google Scholar] [CrossRef]
- Selva Filho, A.A.P.; Converti, A.; Soares da Silva, R.d.C.F.; Sarubbo, L.A. biosurfactants as multifunctional remediation agents of environmental pollutants generated by the petroleum industry. Energies 2023, 16, 1209. [Google Scholar] [CrossRef]
- Mata-Sandoval, J.C.; Karns, J.; Torrents, A. Influence of Rhamnolipids and Triton X-100 on the Biodegradation of Three Pesticides in Aqueous Phase and Soil Slurries. J. Agric. Food Chem. 2001, 49, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Odukkathil, G.; Vasudevan, N. Enhanced biodegradation of endosulfan and its major metabolite endosulfate by a biosurfactant producing bacterium. J. Environ. Sci. Health. B 2013, 48, 462–469. [Google Scholar] [CrossRef]
- Wattanaphon, H.T.; Kerdsin, A.; Thammacharoen, C.; Sangvanich, P.; Vangnai, A.S. A biosurfactant from Burkholderia cenocepacia BSP3 and its enhancement of pesticide solubilization. J. Appl. Microbiol. 2008, 105, 416–423. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental Applications of Biosurfactants: Recent Advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Wang, S. Remediation of a heavy metal-contaminated soil by a rhamnolipid foam. Eng. Geol. 2006, 85, 75–81. [Google Scholar] [CrossRef]
- Colores, G.M.; Macur, R.E.; Ward, D.M.; Inskeep, W.P. Molecular Analysis of Surfactant-Driven Microbial Population Shifts in Hydrocarbon-Contaminated Soil. Appl. Environ. Microbiol. 2000, 66, 2959. [Google Scholar] [CrossRef]
- Reid, R.; Hayes, J. Mechanisms and control of nutrient uptake in plants. Int. Rev. Cytol. 2003, 229, 73–114. [Google Scholar] [CrossRef]
- Mitra, G.N. Regulation of Nutrient Uptake by Plants; Springer: New Delhi, India, 2015; ISBN 978-81-322-2333-7. [Google Scholar]
- Adhikari, S.; Anuragi, H.; Chandra, K.; Tarte, S.H.; Dhaka, S.R.; Jatav, H.S.; Hingonia, K. Molecular basis of plant nutrient use efficiency—Concepts and challenges for its improvement. Sustain. Plant Nutr. 2023, 107–151. [Google Scholar] [CrossRef]
- Osman, K.T. Plant Nutrients and Soil Fertility Management. In Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 129–159. [Google Scholar]
- Dietel, K.; Beator, B.; Budiharjo, A.; Fan, B.; Borriss, R. Bacterial Traits Involved in colonization of Arabidopsis thaliana roots by Bacillus amyloliquefaciens FZB42. Plant Pathol. J. 2013, 29, 59–66. [Google Scholar] [CrossRef]
- Le Mire, G.; Siah, A.; Brisset, M.-N.; Gaucher, M.; Deleu, M.; Jijakli, M. Surfactin Protects Wheat against Zymoseptoria tritici and Activates Both Salicylic Acid- and Jasmonic Acid-Dependent Defense Responses. Agriculture 2018, 8, 11. [Google Scholar] [CrossRef]
- Sobrinho, H.B.S.; Luna, J.M.; Rufino, R.D.; Porto, A.L.F.; Sarubbo, L.A. Assessment of toxicity of a biosurfactant from Candida sphaerica UCP 0995 cultivated with industrial residues in a bioreactor. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; de Campos-Takaki, G.M. Evaluation Antimicrobial and Antiadhesive Properties of the Biosurfactant Lunasan Produced by Candida sphaerica UCP 0995. Curr. Microbiol. 2011, 62, 1527–1534. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surf. B Biointerfaces 2011, 84, 1–5. [Google Scholar] [CrossRef]
- Kim, P.I.; Bai, H.; Bai, D.; Chae, H.; Chung, S.; Kim, Y.; Park, R.; Chi, Y.-T. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 2004, 97, 942–949. [Google Scholar] [CrossRef]
- Boyette, C.D.; Lynn Walker, H.; Abbas, H.K. Biological Control of Kudzu (Pueraria lobata) with an Isolate of Myrothecium verrucaria. Biocontrol Sci. Technol. 2010, 12, 75–82. [Google Scholar] [CrossRef]
- Rodriguez, S.B.; Mahoney, N.E. Inhibition of Aflatoxin Production by Surfactants. Appl. Environ. Microbiol. 1994, 60, 106–110. [Google Scholar] [CrossRef]
- Krzyzanowska, D.M.; Potrykus, M.; Golanowska, M.; Polonis, K.A.; Gwizdek-Wisniewska, E.; Lojkowska, E.; Jafra, S. Rhizosphere bacteria as potential biocontrol agents against soft rot caused by various Pectobacterium and Dickeya spp. strains. J. Plant Pathol. 2012, 94, 367–378. [Google Scholar]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid Biosurfactants as New Players in Animal and Plant Defense against Microbes. Int. J. Mol. Sci. 2010, 11, 5095. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.C.; Lee, S.; Kim, J.C.; Yun, M.Y.; Kim, I.S. Insecticidal Activity of Rhamnolipid Isolated from Pseudomonas sp. EP-3 against Green Peach Aphid (Myzus persicae). J. Agric. Food Chem. 2011, 59, 934–938. [Google Scholar] [CrossRef]
- Kruijt, M.; Tran, H.; Raaijmakers, J.M. Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267. J. Appl. Microbiol. 2009, 107, 546–556. [Google Scholar] [CrossRef]
- Velho, R.V.; Medina, L.F.C.; Segalin, J.; Brandelli, A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. 2011, 56, 297–303. [Google Scholar] [CrossRef]
- Haddad, N.I.A.; Wang, J.; Mu, B. Isolation and characterization of a biosurfactant producing strain, Brevibacilis brevis HOB1. J. Ind. Microbiol. Biotechnol. 2008, 35, 1597–1604. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Sørensen, J. Production of Cyclic Lipopeptides by Pseudomonas fluorescens Strains in Bulk Soil and in the Sugar Beet Rhizosphere. Appl. Environ. Microbiol. 2003, 69, 861–868. [Google Scholar] [CrossRef]
- Kim, P.L.; Ryu, J.; Kim, Y.H.; Chi, Y.-T. Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010, 20, 138–145. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and eco-friendly material for sustainable agriculture and environmental safety—A review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- De Giani, A.; Zampolli, J.; Di Gennaro, P. Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: Different perspectives on their properties, challenges, and potential applications. Front. Microbiol. 2021, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.G.; Zeng, G.M.; Yuan, X.Z. The stimulatory effects of surfactants on composting of waste rich in cellulose. World J. Microb. Biot. 2006, 22, 1121–1127. [Google Scholar] [CrossRef]
- Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F. Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from Lactobacillu rhamnosus with its physicochemical and functional properties. Antibiotics 2021, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Yaraguppi, D.A.; Bagewadi, Z.K.; Patil, N.R.; Mantri, N. Iturin: A promising cyclic lipopeptide with diverse applications. Biomolecules 2023, 13, 1515. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Navarro, K.; López-Gutiérrez, T.; García-Fajardo, V.; Gómez-Cornelio, S.; Zarza, E.; De la Rosa-García, S.; Chan-Bacab, M. Broad-spectrum antifungal, biosurfactants and bioemulsifier activity of Bacillus subtilis subsp. spizizenii—A potential biocontrol and bioremediation agent in agriculture. Plants 2023, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Jumpathong, W.; Intra, B.; Euanorasetr, J.; Wanapaisan, P. Biosurfactant-producing Bacillus velezensis PW192 as an anti-fungal biocontrol agent against Colletotrichum gloeosporioides and Colletotrichum musae. Microorganisms 2022, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- López-Prieto, A.; Xanel Vecino, X.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M. A multifunctional biosurfactant extract obtained from corn steep water as bactericide for agrifood industry. Foods 2019, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Stahlman, P.W.; Currie, R.S.; El-Hamid, M.A. Nitrogen Carrier and Surfactant Increase Foliar Herbicide Injury in Winter Wheat (Triticum aestivum). Weed Technol. 1997, 11, 7–12. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B Biointerfaces 2013, 102, 202–209. [Google Scholar] [CrossRef]
- Silva, S.N.R.L.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf. B Biointerfaces 2010, 79, 174–183. [Google Scholar] [CrossRef]
- Luiz, V.; Lovaglio, R.B.; Tozzi, H.H.; Contiero, J. Rhamnolipids: A New Application in Seeds Development. J. Med. Biol. Sci. Res. 2015, 1, 100–106. [Google Scholar]
- Santos, A.P.P.; Silva, M.D.S.; Costa, E.V.L.; Rufino, R.D.; Santos, V.A.; Ramos, C.S.; Sarubbo, L.A.; Porto, A.L.F. Production and characterization of a biosurfactant produced by Streptomyces sp. DPUA 1559 isolated from lichens of the Amazon region. Brazilian J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Matosinhos, R.D.; Cesca, K.; Carciofi, B.A.M.; de Oliveira, D.; de Andrade, C.J. The Biosurfactants Mannosylerythritol Lipids (MELs) as stimulant on the germination of Lactuca sativa L. Agriculture 2023, 13, 1646. [Google Scholar] [CrossRef]
- Buttrós, V.H.; Araújo, N.A.F.; D’Ávila, V.d.A.; Pereira, M.M.A.; Melo, D.d.S.; Pasqual, M.; Dória, J. A Little Helper: Beneficial bacteria with growth-promoting mechanisms can reduce asian soybean rust severity in a cell-free formulation. Agronomy 2022, 12, 2635. [Google Scholar] [CrossRef]
- Mishra, I.; Fatima, T.; Egamberdieva, D.; Arora, N.K. Novel bioformulations developed from Pseudomonas putida BSP9 and its bio-surfactant for growth promotion of Brassica juncea (L.). Plants 2020, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.H.; Zinjarde, S.S.; Venugopalan, V.P.; McLean, R.J.C.; Weber, M.M.; Rahman, P.K.S.M. Quorum sensing: Implications on rhamnolipid biosurfactant production. Biotechnol. Genet. Eng. Rev. 2010, 27, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants. Minireview. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef]
- Chopra, A.; Bobate, S.; Rahi, P.; Banpurkar, A.; Mazumder, P.B.; Satpute, S. Pseudomonas aeruginosa RTE4: A tea rhizobacterium with potential for plant growth promotion and biosurfactant production. Front. Bioeng. Biotechnol. 2020, 861, 861. [Google Scholar] [CrossRef]
- Khare, E.; Arora, N.K. Biosurfactant based formulation of Pseudomonas guariconensis LE3 with multifarious plant growth promoting traits controls charcoal rot disease in Helianthus annus. World J. Microbiol. Biotechnol. 2021, 37, 55. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.K.; Kant, C.; Verma, H.; Kumar, D.; Singh, P.P.; Modi, A.; Droby, S.; Kesawat, M.S.; Alavilli, H.; et al. Microbial biosurfactant: A new frontier for sustainable agriculture and pharmaceutical industries. Antioxidants 2021, 10, 1472. [Google Scholar] [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. Prog. Biol. Control 2020, 21, 275–293. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd Allah, E.F. Exploring the human microbiome: The potential future role of next generation sequencing in disease diagnosis and treatment. Front. Immunol. 2019, 9, 2868. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef]
- Datta, S.; Rajnish, K.N.; Samuel, M.S.; Pugazlendhi, A.; Selvarajan, E. Metagenomic applications in microbial diversity, bioremediation, pollution monitoring, enzyme and drug discovery. A review. Environ. Chem. Lett. 2020, 18, 1229–1241. [Google Scholar] [CrossRef]
- Shikha, S.; Shankar, S. Microbial metagenomics. In Advances in Animal Genomics; Mondal, S., Singh, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–122. [Google Scholar] [CrossRef]
- Singh, S.; Singh, H.; Rout, B.; Tripathi, R.B.M.; Chopra, C.; Chopra, R.S. The new science of metagenomics: Revealing the secrets of microbial physiology. In Metagenomics: Techniques, Applications, Challenges and Opportunities; Chopra, R.S., Chopra, C., Sharma, N.R., Eds.; Springer: Singapore, 2020; pp. 3–22. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Raj, A.; Kumar, A.; Dames, J.F. Tapping the role of microbial biosurfactants in pesticide remediation: An eco-friendly approach for environmental sustainability. Front. Microbiol. 2021, 12, 791723. [Google Scholar] [CrossRef]
- Tsuge, K.; Ohata, Y.; Shoda, M. Gene yerP, involved in surfactin self resistance in Bacillus subtilis. Antimicrob. Agents Chemother. 2001, 45, 3566–3573. [Google Scholar] [CrossRef]
- Nishio, E.; Ichiki, Y.; Tamura, H.; Morita, S.; Watanabe, K.; Yoshikawa, H. Isolation of Bacterial Strains that Produce the Endocrine Disruptor, Octylphenol Diethoxylates, in Paddy Fields. Biosci. Biotechnol. Biochem. 2002, 66, 1792–1798. [Google Scholar] [CrossRef]
- Manga, E.B.; Celik, P.A.; Cabuk, A.; Banat, I.M. Biosurfactants: Opportunities for the development of a sustainable future. Curr. Opin. Colloid Interface Sci. 2021, 56, 101514. [Google Scholar] [CrossRef]
- Purvis, B.; Mao, Y.; Robinson, D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019, 14, 681–695. [Google Scholar] [CrossRef]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K.; Dayarathne, H.N.; Jamal, A.; Aftab, M.N.; Mainali, B.; Choi, Y. Current advances in the classification, production, properties and applications of microbial biosurfactants—A critical review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef] [PubMed]
- Rebello, S.; Anoopkumar, A.N.; Sindhu, R.; Binod, P.; Pandey, A.; Aneesh, E.M. Comparative life-cycle analysis of synthetic detergents and biosurfactants—An overview. Refin. Biomass Residues Sustain. Energy Bioprod. 2020, 511–521. [Google Scholar] [CrossRef]
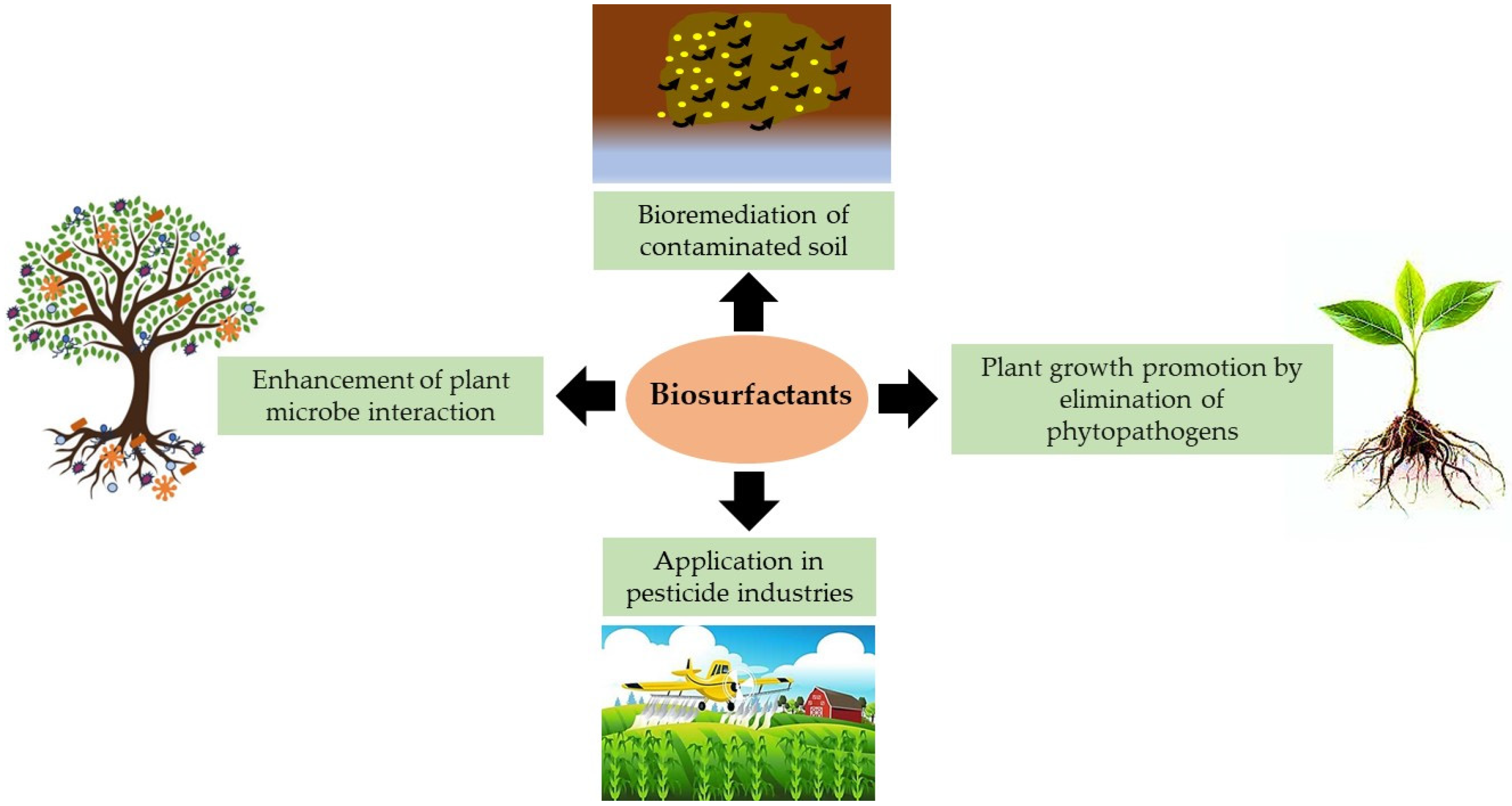
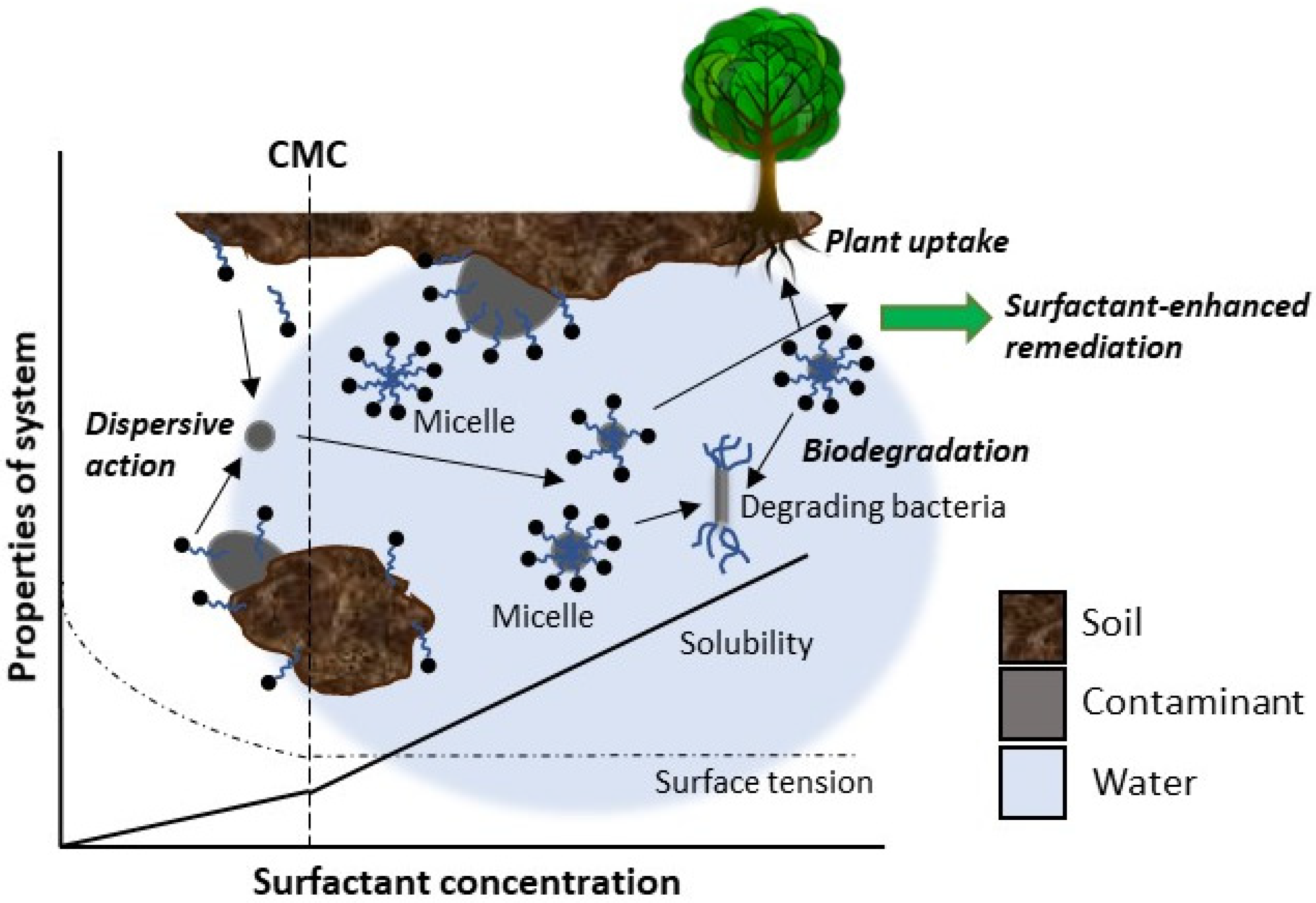

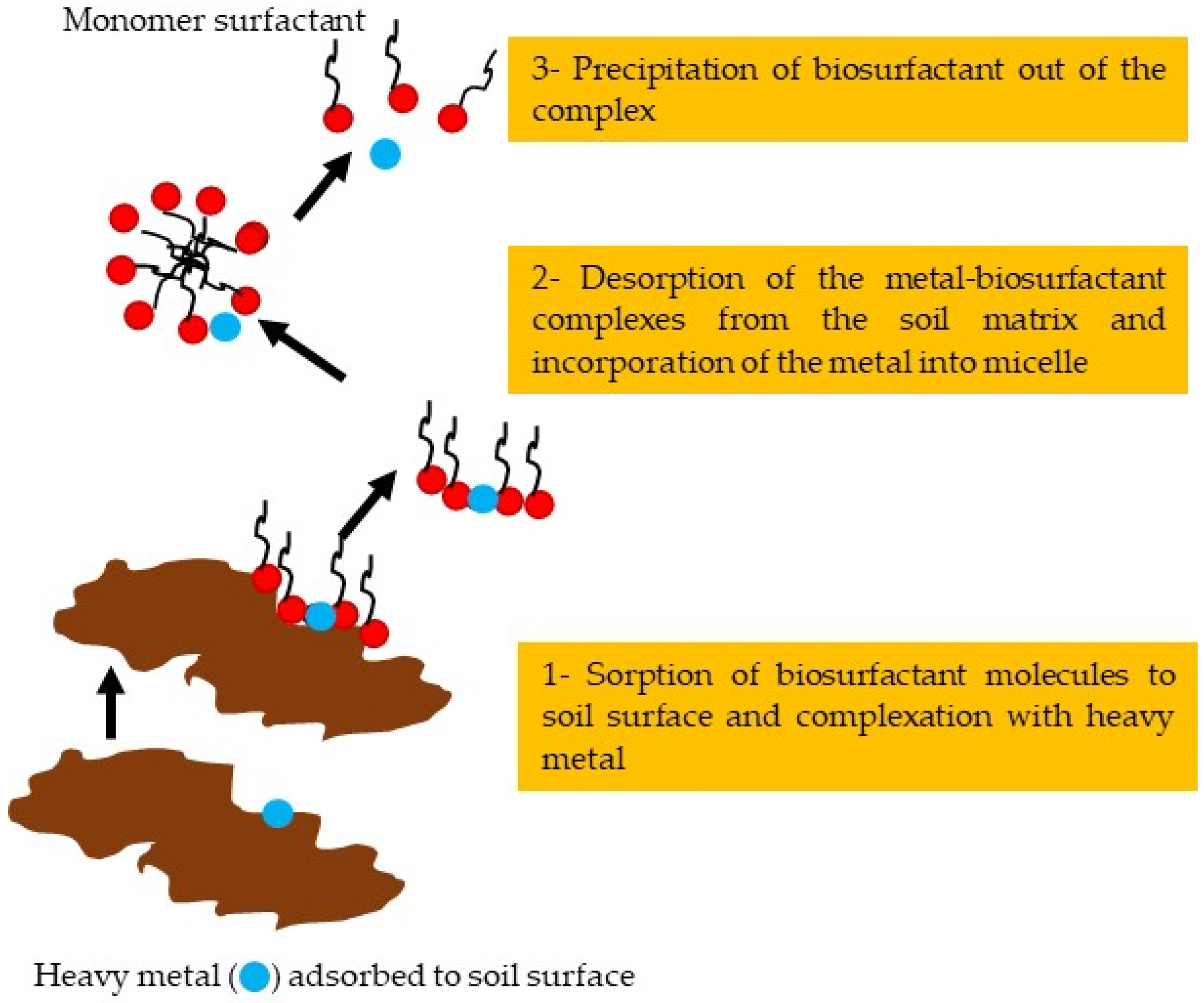
| Trends in Biosurfactant Application in Agriculture | Other Promising Applications of Biosurfactants in Agriculture |
|---|---|
| Development of more effective and affordable biopesticides and biofertilizers [96]. | Utilization of biosurfactants in irrigation systems [97,98]. |
| Biocontrol of plant pathogens [99]. | Use of biosurfactants to enhance biofuel production [100]. |
| Stimulation of plant growth [101]. | Removal of biofilms in irrigation systems [102]. |
| Stabilization of pesticide and fertilizer emulsions [103]. | Application of biosurfactants for remediation of contaminated soils [104]. |
| Enhancement of herbicide and foliar nutrient absorption [96]. |
| Product | Specifications | Country | Patent ID/Year |
|---|---|---|---|
| Biopesticide | Biopesticide compositions and/or biopesticide formulations obtained from Eucalyptus species. The addition of rhamnolipid biosurfactant was cited in the composition of one of the formulations. | Australia | WO2011/013133A3/2011 |
| Biocontrol agent | Application of microorganisms as biological control agents, more specifically, the Serratia plymuthica strain A30, BCCM Deposit Nº. LMG P-26170, which is capable of degrading acyl-homoserine lactones and producing biosurfactants. | The Netherlands | EP2663659B1/2013 |
| Biopesticides | The invention relates to methods for pest (nematodes) control with a microbial rhamnolipid biosurfactant, implying providing the microbial biosurfactant to pests in such an amount that pests are controlled. | United States | EP1750738B1/2007 |
| Insecticide | Obtaining an insecticide that contains biosurfactant in its formulation. Preferably, the biosurfactant is a glycolipid, a glycoside, or their derivatives. | France | EP3122186B1/2017 |
| Additive | A method of producing surfactin, a lipopeptide produced by Bacillus subtilis, and its application in aquafeeds to reduce the occurrence of mold contamination. | Taiwan | EP3039968B1/2016 |
| Additive | A rhamnolipid is implemented to replace a chemical surfactant to be used as the additive of the pesticide, the fertilizer, and the feed additive to ensure significant effects. | China | CN103070167B/2010 |
| Biofertilizers, biostimulants, bio dispersants, and other applications | Formulations comprising microbes and/or their growth by-products to be used to improve fertility, salinity, water retention, and other soil characteristics, as well as to control pests and stimulate plant growth. In some of them, growth by-products are biosurfactants. | United States | WO2021030385A1/2020 |
| Bioremediators of soil | The invention reveals a type of method in which the surfactant repairs the soil contaminated with organochlorine pesticides, removing more than 85% of the pesticides and making the soil reach the environmental safety standard. The operation is simple, economical, and efficient and can be applied on a large scale in the repair of soils contaminated with organic pollutants. | China | CN104923558B/2015 |
| Enhancers of fertility and health of soil, pesticides, plant immune modulators, and/or plant growth stimulants | Microbe-based formulations for restoring soil health and controlling pests. They can comprise one or more biosurfactants (glycolipids and/or lipopeptides). | United States | WO2021030385A1/2021 |
| Fruit preservative | The invention belongs to the technical field of food preservation and relates to a sophorolipid fruit preservative and a method for prolonging the preservation life of fruits. Using microbiological fermentation technology, a sophorolipid was obtained, which was used in the preparation of a solution (3 mg/mL) sprayed evenly on the fruits to prevent fruit corrosion, maintain freshness, and extend the shelf life of fruits at room temperature. | China | CN101886047B/2010 |
| Biofertilizers, biostimulants | Use of sophorolipids to increase the yield of crops. | Germany | DE102014209346A1/2014 |
| Biopesticide | Sophorolipid agricultural antibiotic and its application to control fungal diseases of crops. | China | CN104178537A/2014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.d.G.C.; Medeiros, A.O.; Converti, A.; Almeida, F.C.G.; Sarubbo, L.A. Biosurfactants: Promising Biomolecules for Agricultural Applications. Sustainability 2024, 16, 449. https://doi.org/10.3390/su16010449
Silva MdGC, Medeiros AO, Converti A, Almeida FCG, Sarubbo LA. Biosurfactants: Promising Biomolecules for Agricultural Applications. Sustainability. 2024; 16(1):449. https://doi.org/10.3390/su16010449
Chicago/Turabian StyleSilva, Maria da Glória C., Anderson O. Medeiros, Attilio Converti, Fabiola Carolina G. Almeida, and Leonie A. Sarubbo. 2024. "Biosurfactants: Promising Biomolecules for Agricultural Applications" Sustainability 16, no. 1: 449. https://doi.org/10.3390/su16010449
APA StyleSilva, M. d. G. C., Medeiros, A. O., Converti, A., Almeida, F. C. G., & Sarubbo, L. A. (2024). Biosurfactants: Promising Biomolecules for Agricultural Applications. Sustainability, 16(1), 449. https://doi.org/10.3390/su16010449








