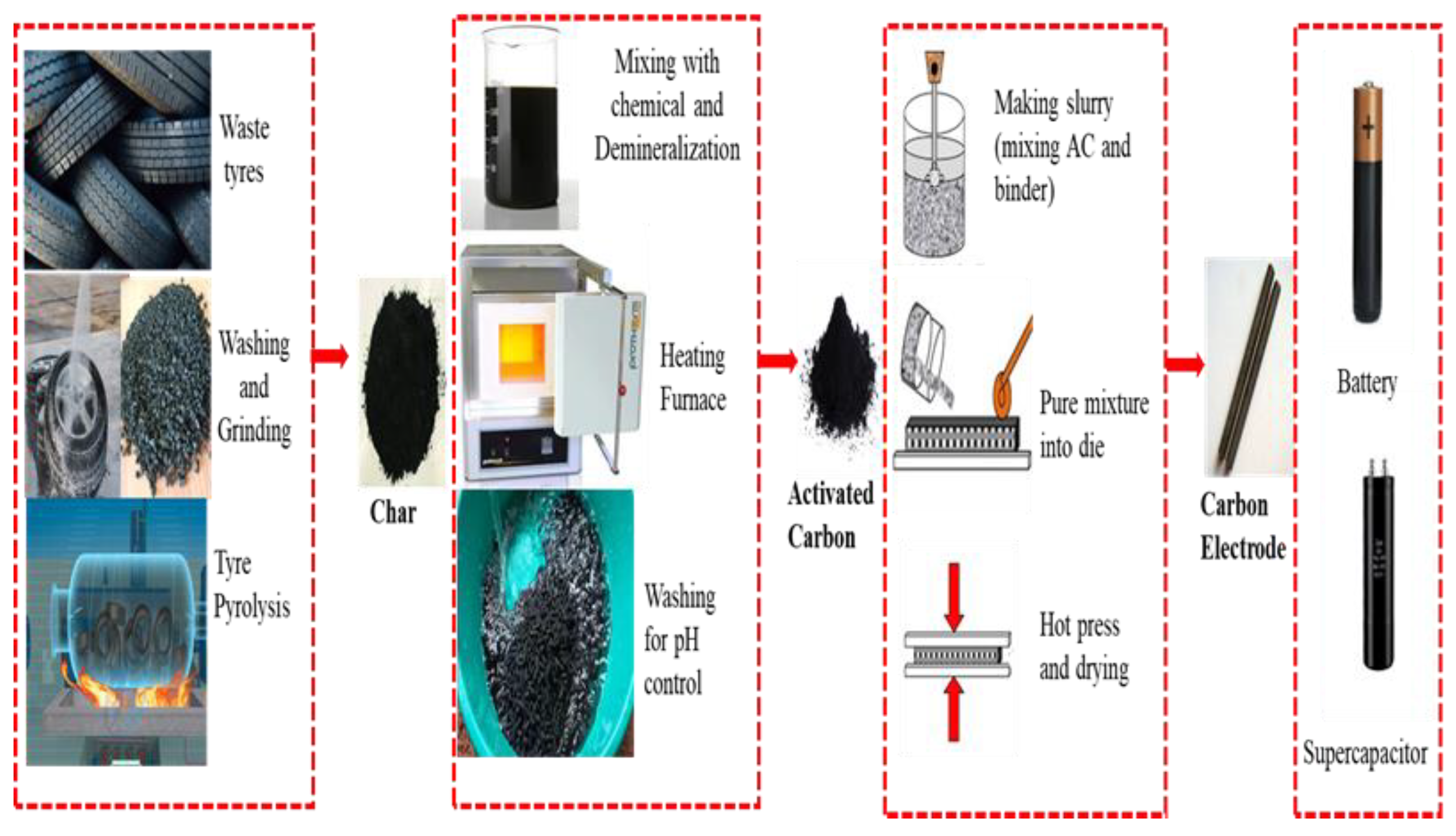
Abstract
Tyre waste is a common form of non-degradable polymer-based solid waste. This solid waste can be effectively managed by converting it into char through the pyrolysis process and then further converting the char into activated carbon (AC) through physical and chemical activation processes. Tyre-derived activated carbon (TDAC) has versatile applications, such as its use as an absorber, catalyst, and electrode material, among others. This study aims to review the electrochemical properties of TDAC. This study employed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta analysis) bibliographic search methodology, with a specific focus on the application of TDAC in a wide variety of energy storage devices, including lithium-ion batteries, sodium-ion batteries, potassium-ion batteries, and supercapacitors. In several experimental studies, TDAC was utilised as an electrode in numerous energy devices due to its high specific capacitance properties. The study found that both activation processes can produce AC with a surface area ranging from 400 to 900 m2/g. However, the study also discovered that the surface morphology of TDAC influenced the electrochemical behaviours of the synthesised electrodes.
1. Introduction
Activated carbon (AC) typically originated from non-renewable sources like wood, coal, and synthetic polymers, incurring high costs and environmental repercussions. To address this, researchers have explored affordable alternatives by conducting experiments with materials like nutshells, plastics, tires, paper, and solid waste. Tsoncheva et al. (2018) activated sunflower seed husk at 500 °C, generating activated carbon (AC) with a maximum surface area of 1511 m2g [1]. Ahmed (2016) subjected rice straw to carbonization at 700 °C, resulting in the production of activated carbon (AC) with a maximum surface area measuring 1154 m2g [2]. A review article emphasized the potential of pyrolysis as a promising method for repurposing waste tyres into valuable products [3]. Pyrolysis, a thermochemical process that decomposes a substance without oxygen, yields three valuable energy products: oil, char, and gas [4,5]. As pyrolytic oil has a high calorimetric value, it can be utilised as a fuel [6]. However, another product of pyrolysis is char, which serves as an excellent precursor for activated carbon (AC). AC is an enhanced version of pyrolytic char due to its high surface area and porous structure. A study was conducted to pyrolyse waste tyres at 600 °C and with a heating rate of 10 °Cmin−1, using a constant inert N2 outflow (200 millilitres per minute), followed by activating with alkali metals [7]. The result found that the activated carbon exhibited a larger surface area compared to pyrolysed char. End-of-life (EOL) tyres can be efficiently transformed into AC, allowing for the extraction of approximately 30–60% black carbon through the thermochemical decomposition via tyre pyrolysis [8]. Numerous studies have been ongoing to investigate the potential of end-of-life (EOL) tyres in generating activated carbon and to compare its efficiency with industrial-scale production.
The production process of activated carbon (AC) can be categorized into two methods: one-step and two-step. In a one-step process, tyres are transformed into activated carbon in a single stage through direct pyrolysis, heating the tyres in the absence of oxygen. This yields volatile compounds, gases, and carbon-rich char, which is subsequently activated using agents like steam or chemicals. Moreover, carbonisation with chemical substances is also considered as a single-step activation. Where the chemical agent is mixed with the shredded tyre and pyrolysed in the reactor. The process involves limited steps, offering simplicity and potential cost reduction. However, drawbacks include limited control over activation, affecting surface area and uniformity. Conversely, a two-step process involves distinct stages for pyrolysis and activation. Following tyre pyrolysis, the resulting char undergoes a dedicated activation step, enhancing control and allowing parameter tailoring. Despite the increased complexity and potential higher operational costs due to extended processing time, the two-step method offers high surface area and porous structure. Carbon activation can be further divided into two types based on the reagent used: physical activation and chemical activation [9]. In numerous experiments, both activation processes were employed to convert EOL tyres into AC. In physical activation, steam and CO2 gas are used to activate the substance. In one study, AC was produced by pyrolysing tyres in a rotary oven [10]. Subsequently, the char was physically activated at 850 °C with 250 mL/min of CO2 for varying durations to achieve a range of burn-off degrees. The surface area of the resulting ACs was 475 and 390 m2/g. In another study, tyre pyrolysic char (TPC) was utilised to produce AC. Pyrolysis was carried out with a rate of heating of 30 °C/min and a temperature of 570 °C [11]. Chemical activation was then conducted using a KOH-TPC weight ratio (W) of 0.5 to 6, resulting in activated carbon with a maximum surface area of 814 m2/g.
Numerous studies investigated the efficacy of TDAC in fulfilling two critical roles: absorption and energy storage [12,13]. As an absorbent material, TDAC exhibits remarkable potential due to its high surface area and porous structure, making it exceptionally efficient in capturing and retaining various substances. TDAC has garnered considerable attention for its role in advanced energy storage applications. Researchers have recognized TDAC as a viable alternative to conventional graphite for producing anodes in lithium-ion batteries and supercapacitors. Additionally, TDAC’s carbonaceous composition ensures good electrical conductivity, a crucial factor in the efficiency of electrode materials. TDAC-based anodes offer the potential for improved energy density, faster charge/discharge rates, and longer cycle life, all of which are highly desirable traits in the field of energy storage technology.
The purpose of this study is to provide a concise synthesis of TDAC research conducted from 2000 to 2023. This study collected experimental data from n = 197 papers to evaluate the AC production processes and reported the relationship between production parameters and TDAC properties. The most noteworthy finding of the article is that chemical activation can produce High quality AC with easy process [14]. TDAC exhibits a more extensive surface structure compared to graphite. This article centres on scrutinizing the electrochemical attributes of TDAC when used as an electrode material and offers a constructive comparative analysis between graphite and TDAC as electrode materials.
2. Pyrolysis Process
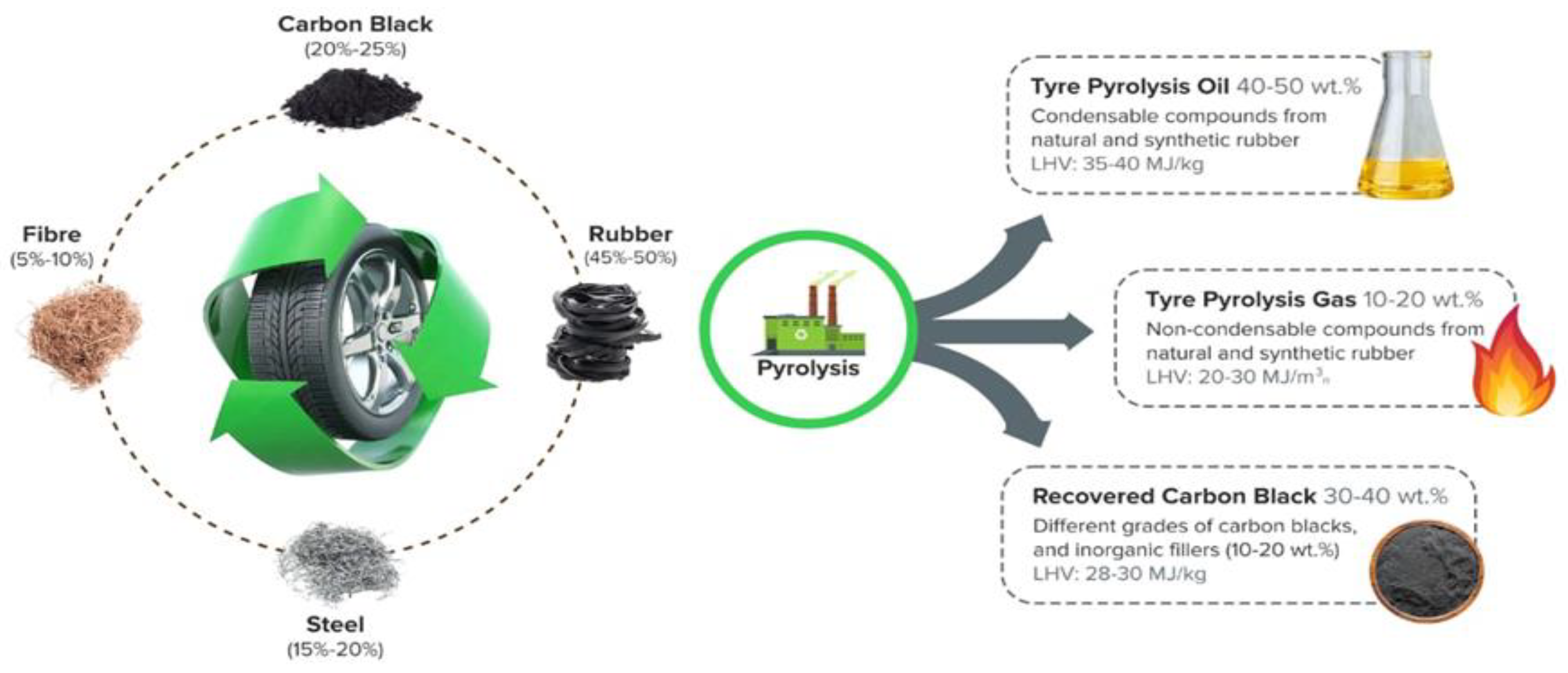
Recently, various thermochemical processes have been employed for the efficient disposal of solid waste. Gasification, pyrolysis, and combustion are common methods for converting solid waste into fuel and thermal energy. All types of solid waste, including plastics, tyres, paper, and wood, can be thermally treated in the absence of oxygen in the pyrolysis process, where the substance decomposes into various forms of energy such as petroleum oil, gas, and carbon. Since it takes approximately 100 years for tyres to naturally degrade, pyrolysis is a promising method for generating energy products from this waste. In general, the primary components of a tyre are a mixture of natural rubber, butadiene rubber, and synthetic rubber [15]. The rubber mixture is vulcanised with various additives (sulphur, zinc) and combined with various infill materials (carbon, steel, and textile fibres) to increase the tyre’s rigidity and elasticity [16]. The pyrolysis process converts rubber and textile fibres into volatile substances that produce crude oil and gases [17]. The carbon material of the tyre is transformed into charcoal, and the steel remains unchanged [18]. In this process, rubber begins to devolatilize at temperatures above 400 °C, and the standard temperature range for tyre pyrolysis is between 400 and 700 °C [19]. Depending on the operational parameters of the pyrolysis process, various chemical reactions occur [20]. Consequently, the proportion and characteristics of pyrolytic yields from tyres depend greatly on the overall pyrolysis process. Reactor type, temperature, heating rate, residence time, gas flow rate, particle size, and catalyst are the primary influencing factors in tire pyrolysis [21,22,23,24,25]. According to previous research on tire pyrolysis, the pyrolytic yields from tyres, including oil, char, and gas, can range from 20% to 70%, 30% to 55%, and 5% to 30%, respectively, under various operating conditions [26,27,28,29,30]. The outcomes of waste tyre treatment through the pyrolysis process are illustrated in Figure 1.
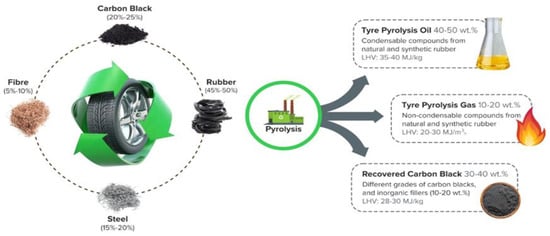
Figure 1.
The outcomes of waste tyre treatment through the pyrolysis process.
According to the mobility of the feedstock, pyrolysis reactors are classified as either stationary feedstock or movable feedstock. In a stationary feedstock reactor, the feedstock remains steady in the reactor, and heat transfer from the reactor wall and inner gas is used only to remove oxygen. Common types of this reactor include fixed-bed and microwave reactors. Taleb et al. (2020) utilised a fixed bed reactor to pyrolyse tyres at temperatures ranging from 300 to 700 °C and with mesh sizes ranging from 18 to 80 [31]. Maximum oil yield was achieved at 500 °C and 80 mesh particle size. In another study, microwave pyrolysis yielded 30 to 44% char and 40 to 65% pyrolytic oil [32,33]. In a movable feedstock reactor, feedstock can be moved by pneumatic or mechanical force. Fluidised bed reactors are frequently used for tyre pyrolysis, in which gas or liquid flows at high velocity from the lower part of the reactor to generate movement in the fine particles. Alvarez et al. (2017) pyrolysed tyres in a fluidised reactor in order to determine the quality of pyrolytic oil [34]. Maximum limonene was discovered at temperatures between 425 and 475 °C. Tyre can be pyrolysed at low pressure and temperature in vacuum reactors. Numerous scientists utilised fluidised and vacuum reactors to optimise pyrolytic oil yield. In a mechanical bed reactor, the feedstock is moved using mechanical apparatus such as an agitator, screw, or shaft. Auger and rotary kiln reactors are common mechanical bed reactors used for pyrolysis of tyres. In this form of reactor, tyres can be pyrolysed at temperatures as high as 1200 °C, which maximises the yield of pyrolytic char. In a study, tyres were pyrolysed using an auger reactor, and the yields of oil, char, and gas were 37%, 55%, and 8%, respectively [33]. Table 1 illustrates the influence of reactor type and operating conditions on the yield percentage of pyrolytic products. Galvagno et al. (2002) pyrolysed tyres in a rotary kiln reactor at 500 °C and obtained 44% char and 45% oil [35]. Similar to reactor conditions, pyrolysis operating conditions have a substantial effect on product yield. Temperature is the most important factor in tyre pyrolysis, and it ranges from 400 to 1000 °C for maximal experimental study. Numerous studies have demonstrated that high temperatures increase gas yield while oil and char yields decrease proportionally. At 550 °C, 45% char was discovered in a pyrolysis experiment [36]. Both heating rate and time have a substantial role in pyrolysis yields. Low heating rate and long residence time maximise char yield, whereas high heating rate and short residence time produce more volatile material and increase liquid yield. Muenpol and Jitkarnka (2016) pyrolysed tyre at 500 °C for 2 h at a heating rate of 5 °C/min [37]. The results found 45% oil, 42% char, and 13% gas. In a distinct investigation, the effect of tyre particle size was examined [38]. They pyrolysed used tyres at 500 to 600 °C for one hour. Maximum oil yield was 34% at 60 mm particle size, and maximum char content was 52% at 150 mm particle size.

Table 1.
Tyre pyrolysis condition and product.
3. Carbon Activation
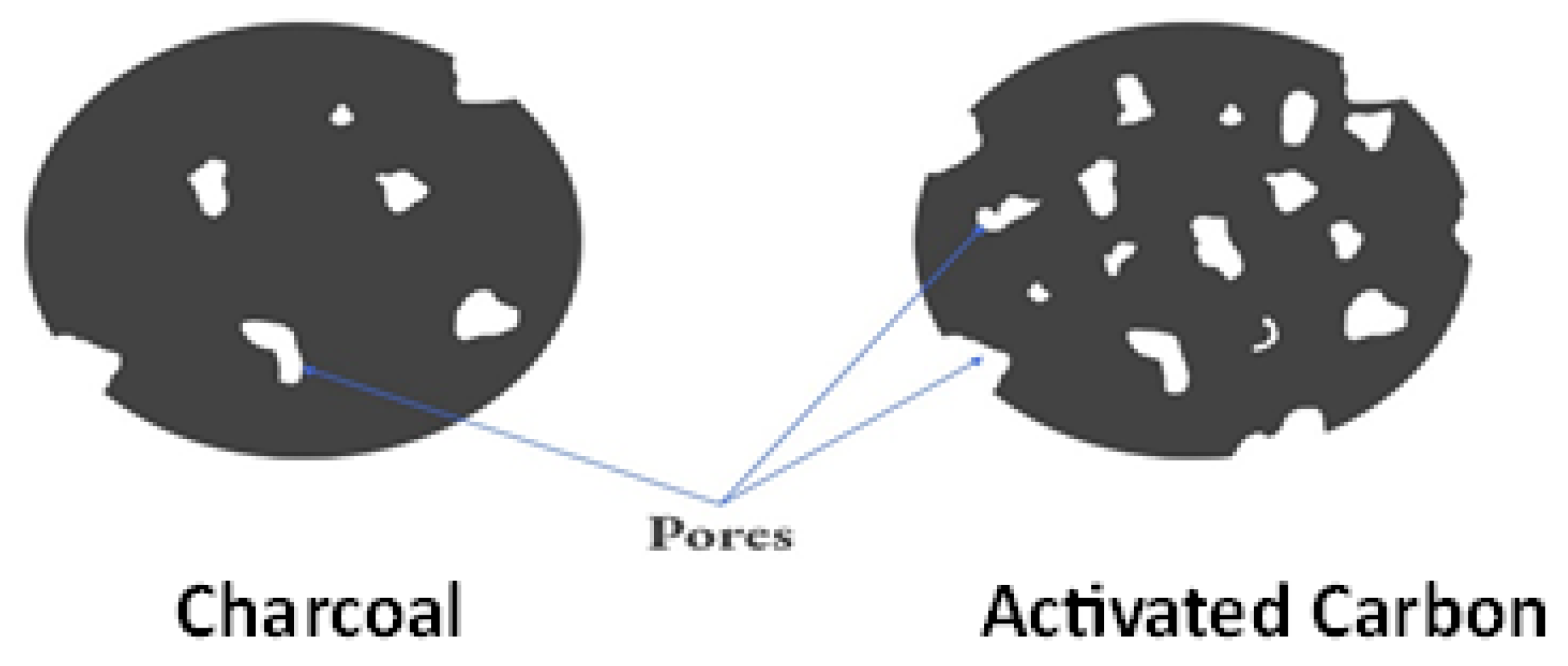
Activated carbon and charcoal are both carbon-rich substances, but they differ in their properties and applications. Activated carbon is a highly porous material with an extensive surface area, created through specific activation processes [61]. Charcoal, on the other hand, is a more general term for carbonized organic matter, often used for cooking and heating. While charcoal has limited adsorption capabilities compared to activated carbon, it is commonly employed for grilling and as a fuel source due to its ability to generate high heat [62]. As it can absorb various heavy metal ions, chemicals, dyes, and other contaminants from the aquatic phase, it has a significant applicability in water treatment [63,64]. In addition, it is utilised in the electronic industry to manufacture electrodes for many energy storage devices. It is also used to retain and purify a variety of gases, including H2, SO2, N2, CO2, and others. The contrast between the 2D atomic structures of AC and charcoal is visually depicted in Figure 2
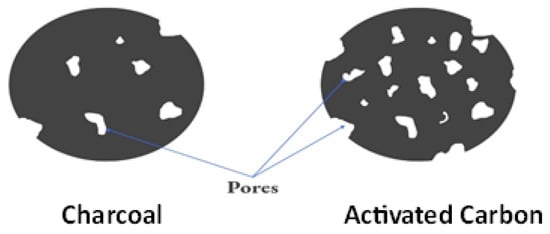
Figure 2.
Two-dimensional atomic structures of AC and charcoal.
Numerous studies have produced AC carbon from EOL tyre char and examined its performance for a variety of applications, whereas chemical activation and physical activation are the two types of activation processes typically used to produce tyre-derived activated carbon (TDAC). In both instances, substances are heated at high temperatures (500 to 1200 °C) for a period, but the activating agent is distinct [65,66]. In physical activation, gaseous substances such as steam and CO2 are used to expand the surface area, whereas in chemical activation, different chemicals such as KOH, H2O2, K2CO3, and NaCO3 are employed [67]. The process is divided into two types based on the number of activation steps required to produce TDAC: one-step and two-step processes. Shredded tyres are combined with an activator in a one-step procedure [68]. Then, the substance is heated for a specific period of time. In contrast, tyre shreds are converted into charcoal in a two-step procedure. The charcoal is then transformed into TDAC in the presence of chemicals. Several experimental studies have concluded that the crystal and surface structure of the TDAC is influenced by a variety of factors, including activation type, temperature, time, gas flow rate, and chemical mixing ratio, among others. In general, AC with a larger surface area and a highly porous structure with varied pore sizes is preferred for both absorption and electrical applications. Belgacem et al. (2014) combined tyre shreds with KOH and activated them at 650 °C for two hours [69]. They determined the surface area of TDAC to be 558 m2/g and applied it to the removal of uranium ion in the aquatic phase. A. Nieto-Márquez (2017) also applied a single-step activation with KOH to produce TDAC [70]. The SSA was 265 m2/g and used to remove Pd2+, Cr3+, and Cd2+. In a study, waste tyres were pyrolysed at 800 °C for one hour [7]. The produced pyrolytic char was then chemically activated, and the TDAC’s surface area was found to be 630 m2/g. Numerous studies have confirmed the capability of TDAC to produce anodes for lithium-ion batteries with an outstanding crystal structure, which will aid in reducing battery costs and enhancing performance.
4. Bibliographical Search and Methods
A scoping review was conducted with a focus on consolidating and synthesizing the existing knowledge concerning the production of activated carbon from end-of-life (EOL) tyres. To ensure rigorous methodology, the scoping review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) approach for conducting a bibliographic search. Different keywords were employed to search for relevant journal and conference papers, as well as project reports related to this study, with the aim of enhancing the comprehensiveness of our search. The objective of this study was to gather data from the maximum number of publications available between 2000 and 2023. The researchers of this study employed Scopus, Google Scholar, and ResearchGate databases to search for relevant articles. The most popular search terms included ‘Tyre to AC conversion’, ‘AC from tyre for energy storage’, ‘Absorbent from tyre-derived black carbon’, ‘Porous structure of tyre-derived AC’, ‘Chemical activation of pyrolyzed tyre char’, and ‘Steam and CO2 activation of pyrolyzed tyre char.’ The selection of the most relevant papers was based on the inclusion and exclusion criteria provided below for paper selection.
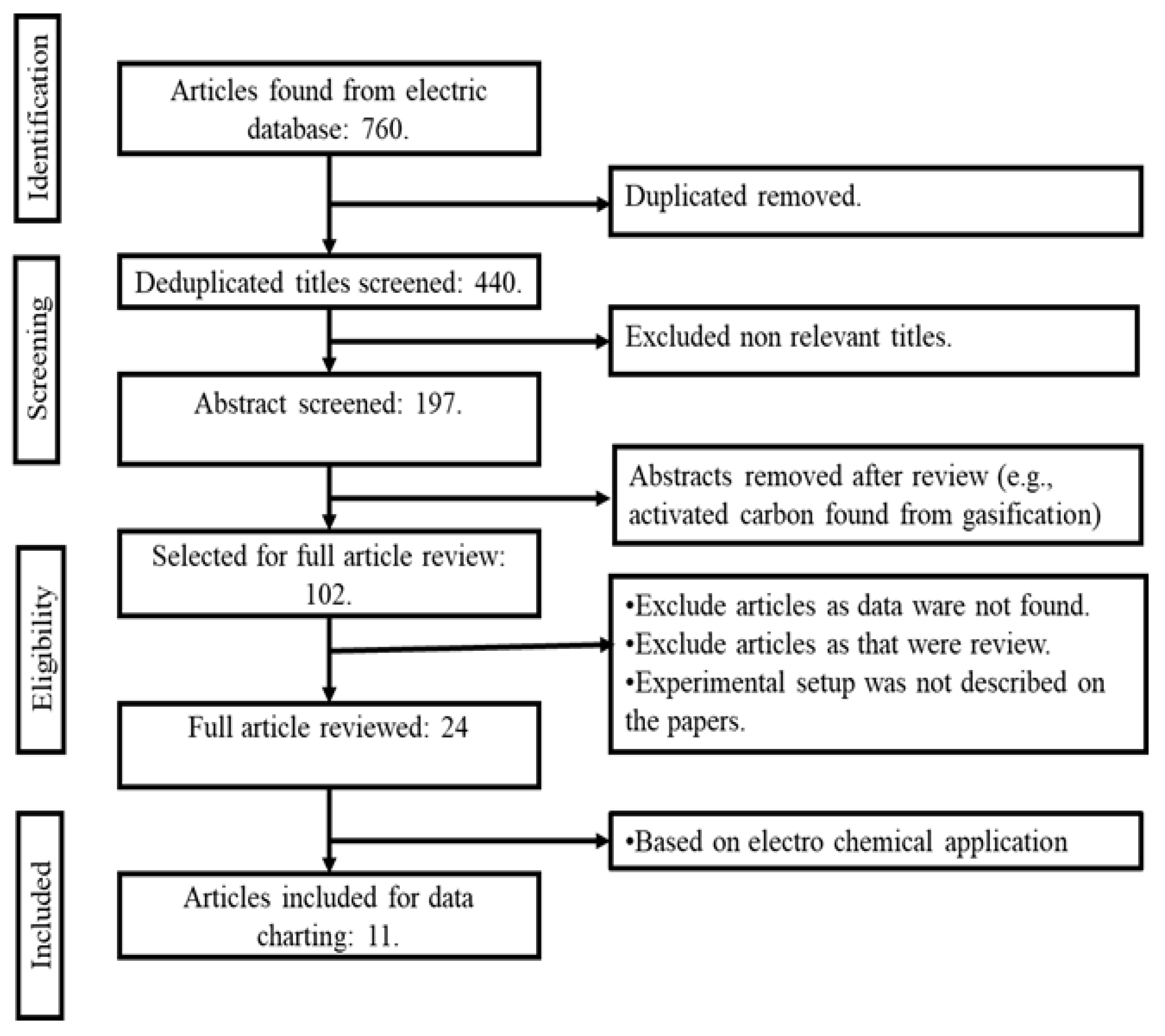
In this review, the inclusion criteria comprised the following conditions: (i) the production of AC from EOL tyres, (ii) the tyre-derived char being transformed into AC through a pyrolysis method, and (iii) the selection of only experimental research was selected for additional evaluation. Articles were omitted according to the following exclusion criteria: (i) works published before the year 2000, (ii) simulations and computer-based modelling research, (iii) AC produced through thermochemical gasification process, and (iv) if the papers were not peer-reviewed. Figure 3 depicts a flowchart of the PRISMA method used in this study. A total of n = 760 articles were found from the initial search. The duplicated items were then removed. After de-duplication, n = 440 articles were identified from the databases. Following another round of removal of irrelevant articles, n = 197 articles remained, relevant to the thermochemical conversion of EOL tyres into AC (encompassing both pyrolysis and gasification processes). Then, the articles pertaining to gasification (thermochemical conversion) were then excluded, leaving n = 102 papers for a comprehensive review of the pyrolysis process. Finally, inclusion and exclusion criteria were applied to all n = 102 papers, resulting in the selection of n = 11 full-length papers for review. This review focuses on the electrochemical application of TDAC.
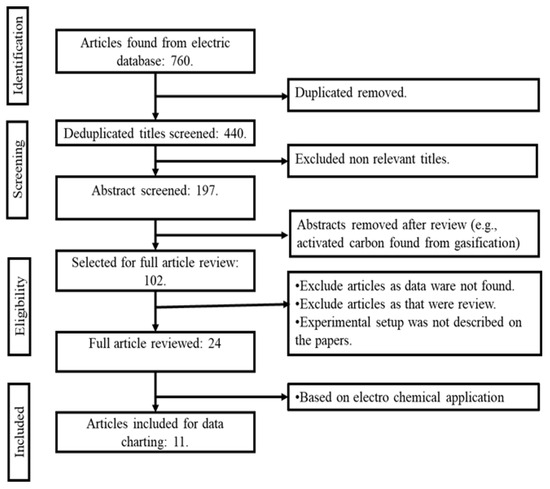
Figure 3.
Flow diagram of the study procedure.
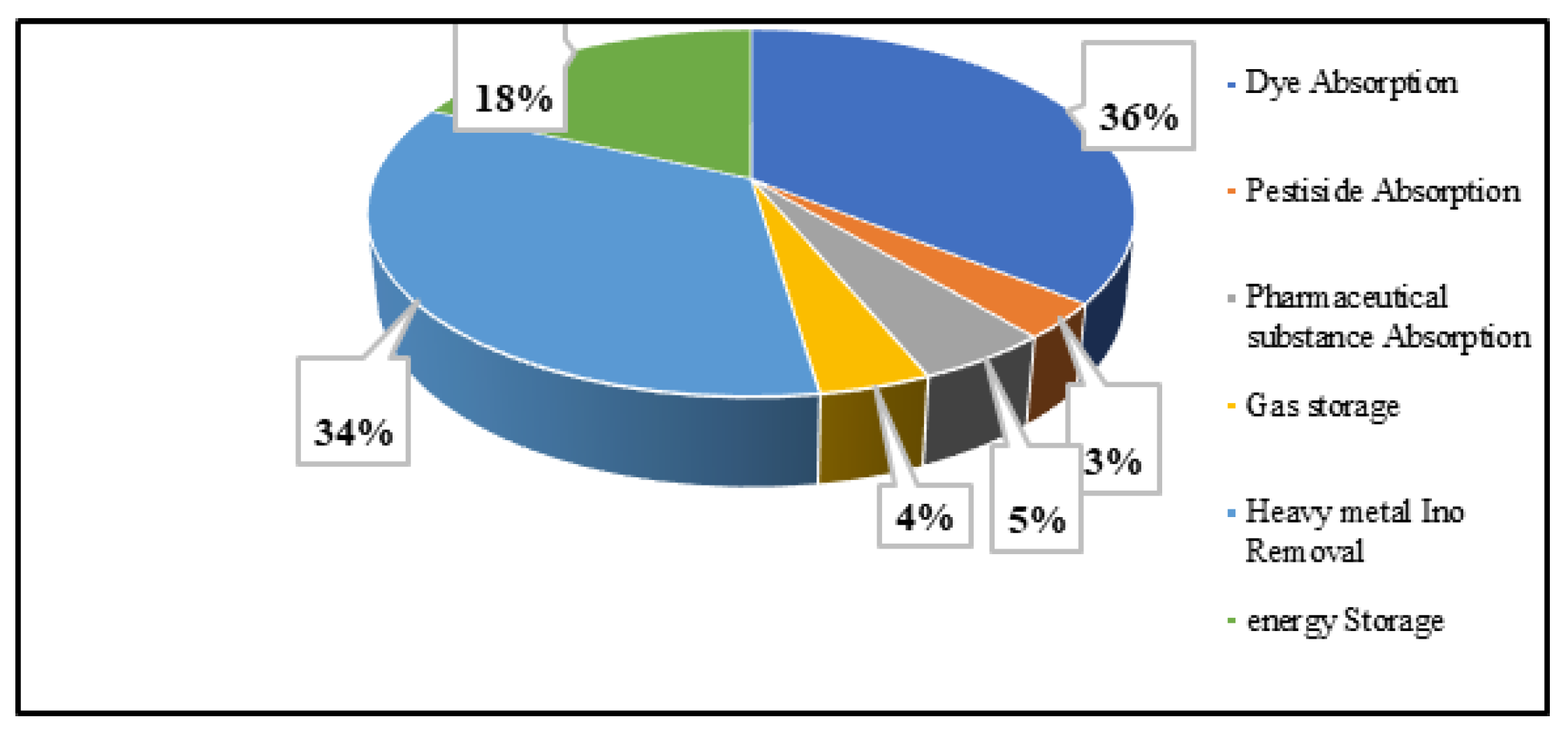
Each article investigates the absorption and electrochemical use of TDAC. Among the collected n = 102 publications, n = 23 articles concentrated on the utilization of AC in energy storage devices, while the remaining publications highlighted the exceptional absorbent qualities of TDAC. The majority of these studies demonstrated the remarkable absorption capacity of TDAC, effectively removing pollutants from both water and air, making it a valuable resource for water and air purification. Figure 4 depicts the percentage of the removal of various substances via TDAC. Notably, a significant portion of the research, about 36%, focused on the removal of dyes, while 34% concentrated on eliminating heavy metal ions from liquid phases. Despite only 18% of research being dedicated to the electrical application of TDAC, researchers emphasized its considerable potential in developing low-cost effective energy storage devices.
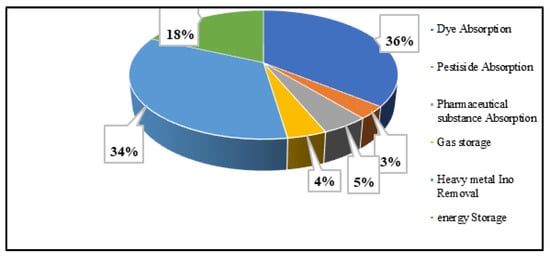
Figure 4.
Categorise papers according to the application of TDAC.
Activated carbon, which is derived from waste tyres, has a high surface area and good porosity, making it an outstanding material for energy storage applications. In supercapacitors, tyre-derived carbon electrode allows them to charge and discharge rapidly. The surface construction makes it an ideal material for applications where high-power density and rapid charging and discharging are required. Moreover, the high porosity of activated carbon allows for a greater amount of electrolyte to be stored, which increases the energy density of the battery. Additionally, the porosity of the material can also reduce the stress on the battery during charging and discharging cycles, leading to a longer cycle life. Although a small number of experimental research has been done on the electrochemical properties of TDAC, all the research concluded that it can be potential source of electrode material for batteries and capacitors.
5. Findings on Electrochemical Applications of TDAC
Activated carbon (AC) is a common electrode material, which is used commercially for supercapacitors. Waste materials are widely used to make AC, which can reduce the electrode cost in energy devices [71]. In this study, 11 papers were intensely studied to uncover the future opportunities of TDAC as electrode material. Various researchers used various ways to convert waste tyres into AC and examined their electrochemical properties. In some of the experiments, the researcher produced AC directly from waste crumb tyres, whereas some researchers first produced char through pyrolysis and then converted char into AC. The operation conditions highly impact the electrochemical behaviour of TDAC. This study discusses tyre-derived activated carbon (TDAC) applications as electrodes in different energy storage devices. The discussion is divided into two parts; the first part explains the potentiality of TDAC as electrode material and the second part presents the electrochemical properties of TDAC. Table 2 and Figure 5 present entire processes used in literature to convert waste tyres into electrode material.

Table 2.
Conversion of the waste tire into electrode for energy storage device.
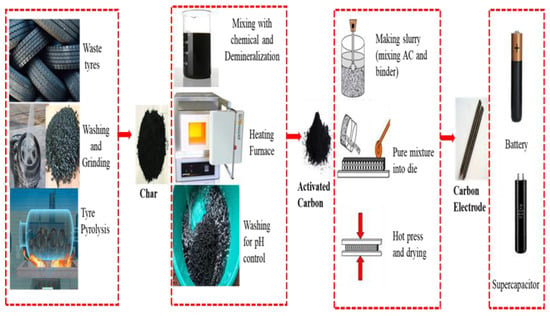
Figure 5.
Waste tyre conversion into energy device.
5.1. TDAC as Electrode Material
Graphite is a material commonly used as an anode in lithium-ion batteries on account of its unique properties that make it suitable for electrochemical application. Firstly, graphite has a layered structure that allows lithium ions to intercalate (insert) and de-intercalate (remove) easily during charging and discharging. This means that graphite anodes can accommodate a large number of lithium ions, allowing for a high energy density and longer battery life. Secondly, graphite is relatively inexpensive and readily available, making it a cost-effective choice for battery manufacturers. Graphite is also used as an anode in supercapacitors for its high specific capacitance of 372 mAhg−1 with good cyclic stability and lower hysteresis [73]. Due to its large specific surface area (SSA), superior electrical conductivity, and broad working temperature range, as electrode materials for supercapacitors, carbon materials of various forms have been extensively studied. Due to their large SSA (up to 3000 m2 g−1), extended operating temperature, and affordable manufacturing procedure, porous activated carbons (AC) are now the most popular electrode materials used in battery, capacitor and supercapacitor [82]. Currently, TDAC is also used as anode material in many experiments for its surface and crystal structure. Much research found that the electrochemical performance of TDAC is compatible with graphite. In both aqueous and organic electrolytes, TDAC yields capacitances of 200 Fg−1 and 100 Fg−1, respectively [83,84,85,86,87]. Due to having a very high power density >500 W kg−1, a long lifespan (>106 cycles), and a quick charging-discharging efficiency (0.3–60 s), TDAC has potential to make anodes for supercapacitors (SC) [88,89,90]. Much research has found that the performance of graphite is highly dependent on its crystal structure and surface morphology. Many experimental analyses justified that the crystal structure and surface structure of TDAC is more developed than graphite as an electrode material [87]. Table 3 presents a comparison between the electrochemical behaviours and structure of various electrode material as well as TDAC. Comparing tyre-derived activated carbon (TDAC) and traditional graphite anodes, TDAC offers potential durability with its high surface area, enabling efficient charge cycles. However, its specific durability depends on the activation process and material impurities. Traditional graphite anodes are known for stability but may degrade under high charging rates. Regarding safety, TDAC, if properly processed, can be considered safe, utilising recycled tire material. Graphite anodes are generally safe, but concerns arise from the environmental impact of graphite production.

Table 3.
Comparison between graphite and TDAC as electrode material.
5.1.1. Crystal Structure of TDAC
The crystal structure of graphite can affect battery efficiency through its impact on ion interaction, electrical conductivity, and structural stability. Generally, ion interaction depends on the spacing between the graphite layers, which is influenced by the crystal structure. The interlayer spacing can affect the ability of ion diffusion in and out of the graphite, which can impact the battery’s charging and discharging rates. The interlayer spacing of graphite is the distance between the two layers, while basal distance spacing is the sum of interlayer distance and the thickness of a single layer [91]. Graphite’s interlayer distance typically measures 0.334 nm, although depending on the synthesis technique, this number can change. Zhang et al. (2022) found that TDAC had an interlayer spacing d002 of 0.35 nm, which was larger than that of graphite [72]. The report concludes that TDAC can provide more ion interaction and specific capacitance at different current density. The lattice fringes are mainly a layer of graphite having higher random orientation and it represents the presence of sp2 hybridized carbon atoms in graphite. Veldevi et al. (2022) found interlayer spacing of 0.349 nm for TDAC (at 1600 °C calcination temperature), which was greater than regular graphite crystals [73]. Li et al. (2016) showed that the higher temperature pyrolysed carbon had more crystalline phases than lower temperature carbon having interlayer spacing of 0.4 nm [81]. In Raman Spectroscopy, the G peak is caused by the in-plane lengthening of sp2 carbon atoms. The D band is an irregular band resulting from structural defects, edge effects, and asymmetrical sp2 carbon bonds. The intensity ratio of the D- and G- Raman peaks (ID/IG) is frequently used to characterize carbon films to calculate the size and number of sp2 clusters that are present in the carbon structures [92,93]. The standard ID/IG for graphite is 0.04 [94]. Some experiments reported that the ID/IG ratio of TDAC is 1.42 [72]. The amount of graphitization or number of flaws within carbon materials is often shown by the intensity ratio of ID to IG, and a greater ID/IG ratio denotes more defects. In a report, the ID/IG ration of TDAC was recorded as 0.56 [73]. In a study, ID/IG ratio of 0.99 was obtained when tyre was pyrolysed at a temperature of 1600 °C [81]. During charging and discharging, the graphite experiences expansion and contraction due to the intercalation and deintercalation of ions. If the crystal structure is not stable, this can lead to cracking and degradation of the graphite electrode, which can reduce battery efficiency. Researchers continue to investigate ways to optimize the crystal structure of graphite and other materials to improve the performance of energy storge devices. Numerous experiments justify that TDAC has a high potentiality as anode material for various device, for example Li-ion batteries, K-ion batteries, Na-ion batteries, and others.
5.1.2. Surface Morphology
The surface structure of activated carbon has a significant impact on anode performance in batteries. The pore size distribution, specific surface area, and surface chemistry are all important parameters that should be carefully optimized for specific battery applications. One important surface structure parameter is the specific surface area (SSA), which represents the total surface area per unit mass of the material. Generally, a higher SSA corresponds to a higher electrochemical performance, as it provides more sites for electrochemical reactions to occur. However, excessively high SSA can also lead to a decrease in structural stability and increased internal resistance, which can negatively impact battery performance [14]. However, the larger specific surface area and hierarchical porous carbon increase the surface’s accessibility and use for ion storage, and they offered a large number of ion channels for quicker ion-transmission throughout the electro-chemical reaction [95]. The surface area of TDAC is varied from 200–1000 m2g−1 [72]. Veldevi et al. (2022) observed the maximum surface area of 369 m2g−1 where AC was prepared by varying temperature [73]. Han et al. (2014) found the BET surface area of nitric acid concentrated AC was 915 m2g−1 [79]. The BET surface area of PMC 800 (phosphorus-doped mesoporous carbon) was 241.5 m2 g−1 obtained by Gong et al. [80].
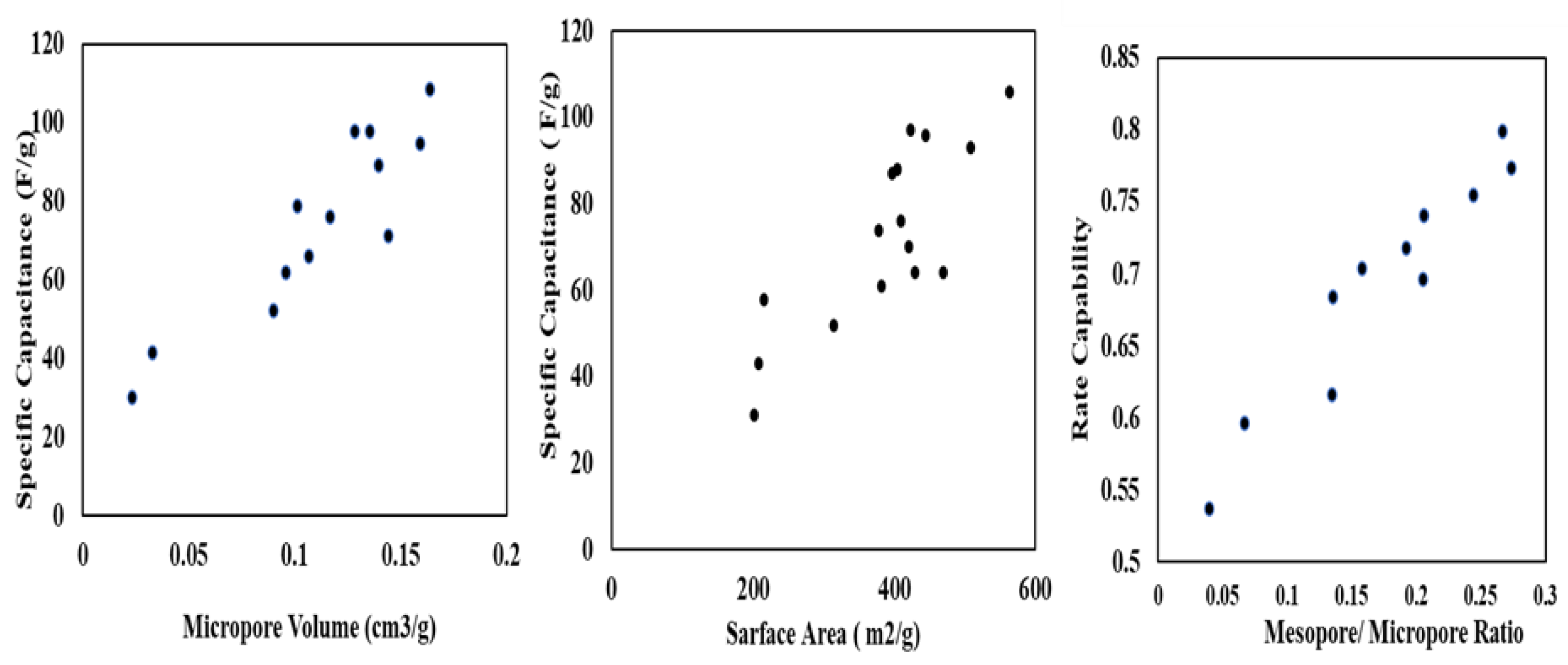
Another important surface structure parameter is the pore-size distribution (PSD), which affects the accessibility of the ions of electrolyte to the active sites in the material. The pore size distribution of tyre-derived activated carbon (TDAC) is influenced by factors such as the carbonization process, activation conditions, and the type of activating agent used. The choice of temperature, time, and activating agents during production determines the formation of micropores, mesopores, and macropores in TDAC. Additionally, the characteristics of the initial tire material and the presence of impurities can impact pore size distribution. Optimizing these parameters is crucial for constructing the pore structure of TDAC, influencing its suitability for specific applications like energy storage devices. A narrow PSD with a high proportion of micropores (<2 nm) can increase the surface area accessible to electrolyte ions and enhance capacity but can also lead to diffusion. On the other hand, a broader PSD with a higher proportion of mesopores (2–50 nm) can improve cycling stability and rate capability but may lead to lower overall capacity. Micropores provide a good amount of ion-absorption active sites, mesopores help ion-accessibility, and the distance of ion-diffusion between electrode and electrolyte interface is shortened by macropores [96]. Also, the mesoporous structure can be advantageous to increase the conductivity of ions [80]. Micropores (those smaller than 2 nm in diameter) are known to have a crucial adsorption function in the formation of the electrical double layer. Meso-/micro-porosity and large pore volume are often necessary properties for charge storage since they assure the pores’ accessibility to electrolyte ions [97,98]. Zhang et al. (2022) showed that activated carbon with more micropores and regular mesopores had 408 Fg−1 charge storage capacity at 0.25 Ag−1 in 6 M KOH retaining 97% of their capacitance after 10,000 cycles [72]. Hou et al. (2022) depicts that pore size of 1.4 nm for TDAC is more conductive to the infiltration of the electrolyte [14]. They also conclude that higher pore volume ensures more adsorption of electrolyte. The presence of micropores in carbon particles allows more penetration of electrolytes during electrochemical cycling [76]. Figure 6 shows the relationship between the physical construction of TDAC and its electrochemical performance. At a micropore volume of 0.1 cm3/g, the specific capacitance reaches 50 F/g, and it significantly rises with increasing volume. This increase is also directly proportional to the surface area and pore ratio.
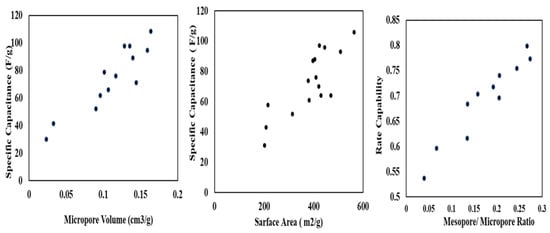
Figure 6.
Specific capacitance of TDAC electrode versus surface morphology [52,76].
5.2. Electrochemical Performance of TDAC Electrode
Electrochemical properties of TDAC can be examined by investigating the specific capacitance, capacitance retention, cyclic voltammetry (CV) curve, galvanostatic charge-discharge (GCD) curve, Nyquist plot, cycling performance. The equation used to obtain the specific capacitance from CV curve is C = Q/V_m, where C is the specific capacitance (F g−1), m is the mass of the active components (g), V is the potential window (V), Q is the average charge during the charging and discharging operation (C). Specific capacitance of activated carbon is influenced by its mesoporosity and specific surface area. The specific capacitance will increase with increasing mesoporosity and specific surface area [99]. In an experiment, specific capacitance was found to be 408 Fg−1 at 0.25 A g−1 for TDAC electrode in 6 M KOH electrolyte [72]. Generally, the specific capacitance of TDAC electrode decreases with the increasing density of current because of the insufficient OH_ ions move into pores through diffusion [100]. Hou et al. (2022) observed 192 F g−1 specific capacitance for TDAC electrode at 0.5 A g−1 [14]. Many experiments proved that the specific capacitance of tyre-derived electrode was only 3% less after 1000 cycles, which shows that the newly made anode is good to use in supercapacitors [75]. Table 4 depicts the electrochemical performance of TDAC in various energy devices.

Table 4.
Electrochemical performance of TDAC as electrode material.
In addition, gravimetric capacitance is used to calculate the capacitance retention percentage (%R). The initial-to-final cycle ratio (during the CV process) based on specific capacitance is known as the R% [101]. High capacitance retention after a given large number of cycles indicates the excellent quality energy storing device [102,103]. Zhang et al. (2022) found capacitance retention of up to 97% for tyre driven electrode after 10,000 charge/discharge cycles [72]. In same way, Hou et al. (2022) showed 106% capacitance retention, at 2 A g−1, after 10,000 cycles in two-electrode systems [14].
A method for measuring the current that forms in an electrochemical cell when the voltage exceeds what the Nernst equation predicts is called cyclic voltammetry (CV). The working electrode’s potential is cycled during CV, and then the resulting current is measured. Thus, this process characterised as the electro-chemical behaviour of the electrode [14]. Zhang et al. (2022) characterized electrochemical properties of TDAC using CV curve in 6 M KOH at various scanning rates (5 to 200 mV s−1) in the voltage range of −0.8 to 0.0 V) [72]. In this study, the larger current density in CV curve indicates the excellent capacitive behaviour for a three-electrode system. A report showed that when the scanning rate was increased from 2 to 100 mVs−1 the CV curve’s characteristic rectangular shape could continue to be kept up for good AC with excellent rate capability [76]. CV curve was observed for tyre derived electrode in 1 M HNO3 electrolyte with a scanning rate of 10 to 50 mVs−1 and found that energy efficiency was 70% after 1000 cycles having capacity 50 mAg−1 [36]. Han et al. (2014) found more current density from CV curve at the same scanning rate for nitric acid concentrated TDAC electrode, indicating more capacitance with the increase in oxygen content in electrode materials [79]. The scanning rates in cyclic voltammetry (CV) significantly impact the electrochemical performance of tyre-derived activated carbon (TDAC). Higher scanning rates may lead to reduced capacitance due to limited ion diffusion within the material, affecting charge storage. Conversely, lower scanning rates enhance ion accessibility, promoting higher capacitance. The relationship between scanning rates and energy efficiency in TDAC involves a trade-off, where slower rates offer increased capacitance but may sacrifice operational speed. Optimization of scanning rates is crucial to balance capacitance and energy efficiency in TDAC for effective utilization in electrochemical applications like supercapacitors.
The GCD curve uses a fixed potential (similar to that used in CV) and current to monitor the passing of time (both the charging and discharging times). For the purpose of calculating specific capacitance values, CV and GCD are both helpful [75]. One may compute the values of the energy and power densities using the GCD data. Zhang et al. (2012) observed the potentiality of tyre-derived electrode after exploring GCD curve with larger charge- discharge time and excellent specific capacitance [100]. The almost symmetric charge-discharge patterns over the potential windows shown in the GCD curves of the AC sample at various current densities from 0.25 to 5 A g−1, demonstrating the electrode’s double layer mechanism. In this experiment, the specific capacitance was 408 Fg−1, 266 Fg−1, 229 Fg−1, and 171 Fg−1 at 0.25 A g−1, 0.5 A g−1, 1 A g−1, and 5 A g−1, respectively [72]. Hou et al. (2022) investigated GCD curve at 1 A g−1 current density [14]. The study found the highest specific capacitance of 185 F g−1. In a report, sulfonated tyre rubber carbon showed the first discharge capacity of 545 mA h g−1 and reversible charge capacity of 387 mA h g−1, which leads to irreversible capacity around 158 mA h g−1 [74].
A parametric representation of a frequency response is known as a Nyquist plot. In the Nyquist plot, the real axis (Z’) is represented by a semicircle curve with two intersecting points. The values of solution resistance (Rs) are determined from the first intercept point, which is closest to the origin, and the value of total resistance (Rs + Rct), which is determined from the second intercept point farthest from the origin [104,105]. Shang et al. (2020) observed a Nyquist curve of TDAC and found steep and linear at lower frequency, which was the same as the ideal capacitive behaviour [106]. In another research, CV and GCD curve was investigated at different scanning rate (10 to 200 mV−1) and current density (0.5 to 50 Ag−1) that displayed a quasi-rectangular shape for CV curve and quasi triangular shape for GCD curve without any obvious voltage drop that confirmed lower internal resistance and excellent capacitive behaviour with 73% rate capability at 50 A g−1 in a three-electrode system [14]. Results show that Rs and Rct were 1.156 and 0.3325 Ω for tyre-derived electrode.
Cycling performance of an electrode is described by the specific capacitance and capacitance retention or charge discharge capacity and columbic efficiency. The electrode performance of tire-derived activated carbon (TDAC) is intricately linked to specific capacitance and capacitance retention, influencing energy density, power density, and cycle stability in electrochemical applications. Specific capacitance, representing the charge storage capacity per unit mass, directly impacts energy density. Higher specific capacitance in TDAC translates to increased energy storage capability, enhancing the overall energy density of supercapacitors. Simultaneously, high specific capacitance contributes to improved power density by facilitating rapid charge and discharge rates. Capacitance retention, reflecting the ability to maintain capacitance over repeated cycles, is crucial for cycle stability. TDAC with robust capacitance retention ensures prolonged and reliable performance, mitigating capacity loss during extended use. Thus, the intricate balance between specific capacitance and capacitance retention in TDAC electrodes is essential for optimizing energy density, power density, and cycle stability, collectively influencing the effectiveness and longevity of energy storage devices [81]. An experimental study obtained capacitance retention of 97% for TDAC electrode at 5 Ag−1 current density after 10,000 cycles [72]. The article reported that when the current density was increased from 0.5 to 20 A g−1, the TDAC electrode consistently maintained a gravimetric capacitance of 113 F g−1, with a remarkable capacitance retention of 84% [14]. A study observed the capacity remains of 155 mAh g−1 for tyre-derived electrode after 200 cycles—that is 80.7% capacity retention [77]. In another experimental study, 140 Fg−1 specific capacitance was obtained after 1000 cycles and the retention rate was 72% for a tyre-derived electrode [79].
6. Conclusions
This study demonstrates a viable pathway for recovering high-value-added AC from waste-tyre rubber for use as an electrode material. The TDAC anode exhibits superior rate capability in K-ion, Na-ion, and Li-ion batteries. Moreover, it provides a high specific capacitance and current density, even after 500 cycles in supercapacitors. The study reveals that the specific capacitance of the tyre-derived electrodes typically falls within a range of 50 to 200 F/g, with the rate capacitance typically ranging from 10 to 100 F/g. Moreover, the power density of these tyre-derived electrodes commonly ranges from 1000 to 10,000 W/kg and, on occasion, surpasses these values, with variations influenced by electrode design and material properties. Given the cost effectiveness and abundant availability of the raw material for TDAC, it holds the potential to reduce the overall production costs of batteries and superconductors. The crystal structure and surface morphology of TDAC enable efficient ion absorption and storage in the anode, essential for enhancing energy storage capacitances.
This research demonstrates the promise of employing tyre-derived carbon as an electrode material, presenting an ecofriendly approach to recycling waste tyres. Moreover, owing to its extensive surface area and porosity, TDAC finds application in water treatment and various gas refining processes, further emphasizing its potential in sustainable and versatile recycling practice. Furthermore, it is crucial to optimize processing techniques for obtaining tire-derived carbon materials, refining methods to convert these materials into electrode structures, and prioritizing scalability and cost-effectiveness.
Author Contributions
Conceptualization, N.H.Z., M.I.J.; Data collection and curation, N.H.Z., M.I.J. and A.S.M.S.; Formal analysis, M.I.J., A.S.M.S. and M.G.R.; Writing original draft, M.I.J. and R.H.; Writing—review and editing, M.G.R., M.I.J. and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank CQUniversity, Australia for the financial support provided to Nusrat Hossain Zerin through elevate scholarship. The authors also acknowledge the organisations for their financial support to this study through 2021 FAPEx Grant for project “Technology development to process Municipal Solid Waste (MSW) as a biochar for producing activated carbon” and Advance Queensland Industry Research Fellowship project “Cheaper greener Queensland: Optimising renewable fuel production from mixed waste” (project no: RSH/6017).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsoncheva, T.; Mileva, A.; Tsyntsarski, B.; Paneva, D.; Spassova, I.; Kovacheva, D.; Velinov, N.; Karashanova, D.; Georgieva, B.; Petrov, N. Activated carbon from Bulgarian peach stones as a support of catalysts for methanol decomposition. Biomass Bioenergy 2018, 109, 135–146. [Google Scholar] [CrossRef]
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Hossain, F.M.; Rasul, M.G.; Chowdhury, A.A. A review on the thermochemical recycling of waste tyres to oil for automobile engine application. Energies 2021, 14, 3837. [Google Scholar] [CrossRef]
- Hasan, M.; Rasul, M.; Jahirul, M.; Khan, M. Characterization of pyrolysis oil produced from organic and plastic wastes using an auger reactor. Energy Convers. Manag. 2023, 278, 116723. [Google Scholar] [CrossRef]
- Faisal, F.; Rasul, M.; Jahirul, M.; Schaller, D. Pyrolytic conversion of waste plastics to energy products: A review on yields, properties, and production costs. Sci. Total Environ. 2023, 861, 160721. [Google Scholar] [CrossRef] [PubMed]
- Mustayen, A.; Rasul, M.; Wang, X.; Hazrat, M.; Jahirul, M.; Negnevitsky, M. Plastic-made diesel (PMD) from pyrolysis via vacuum distillation process-A waste recycling fuel to diesel engine performance and emissions improvement. J. Energy Inst. 2023, 107, 101198. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Production of activated carbons from waste tyres for low temperature NOx control. Waste Manag. 2016, 49, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Guo, J.; Sun, Y.; Liu, X.; Pan, J. Pyrolytic carbon black-derived porous carbon with spherical skeleton as recovered and enduring electrode material for supercapacitor. J. Energy Storage 2021, 44, 103372. [Google Scholar] [CrossRef]
- Özbaşa, E.E.; Balçıkb, B.; Ozcana, H. Preparation of activated carbon from waste tires, and its use for dye removal. Desalination Water Treat. 2019, 172, 78–85. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C. Texture and surface chemistry of activated carbons obtained from tyre wastes. Fuel Process. Technol. 2015, 134, 275–283. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; De Yuso, A.M.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Arias, A.M.; Moreno-Piraján, J.C.; Giraldo, L. Adsorption of Triton X-100 in aqueous solution on activated carbon obtained from waste tires for wastewater decontamination. Adsorption 2020, 26, 303–316. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Zhai, T.; Wang, H.; Sun, Q.; Li, H. Direct conversion of waste tires into three-dimensional graphene. Energy Storage Mater. 2019, 23, 499–507. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Xie, Z.; Kang, Y.; Tang, Z.; Dai, Y.; Lei, Y.; Chen, J.; Liang, F. Activated carbon prepared from waste tire pyrolysis carbon black via CO2/KOH activation used as supercapacitor electrode. Sci. China Technol. Sci. 2022, 65, 2337–2347. [Google Scholar] [CrossRef]
- Rowhani, A.; Rainey, T.J. Scrap tyre management pathways and their use as a fuel—A review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Shulman, V. Tyre Recycling; iSmithers Rapra Publishing: Shawbury, UK, 2004; Volume 15. [Google Scholar]
- Mushunje, K.; Otieno, M.; Ballim, Y. A review of waste tyre rubber as an alternative concrete consituent material. MATEC Web Conf. 2018, 199, 11003. [Google Scholar] [CrossRef]
- Jusli, E.; Nor, H.M.; Jaya, R.P.; Haron, Z.; Mohamed, A. A Review of Double Layer Rubberized Concrete Paving Blocks. J. Eng. Res. Technol. 2016, 2. [Google Scholar]
- Aziz, M.A.; Al-Khulaidi, R.A.; Rashid, M.; Islam, M.; Rashid, M. Design and fabrication of a fixed-bed batch type pyrolysis reactor for pilot scale pyrolytic oil production in Bangladesh. IOP Conf. Ser. Mater. Sci. Eng. 2017, 184, 012056. [Google Scholar] [CrossRef]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–29. [Google Scholar] [CrossRef]
- Kordoghli, S.; Khiari, B.; Paraschiv, M.; Zagrouba, F.; Tazerout, M. Impact of different catalysis supported by oyster shells on the pyrolysis of tyre wastes in a single and a double fixed bed reactor. Waste Manag. 2017, 67, 288–297. [Google Scholar] [CrossRef]
- Oyedun, A.; Lam, K.-L.; Fittkau, M.; Hui, C.-W. Optimisation of particle size in waste tyre pyrolysis. Fuel 2012, 95, 417–424. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Czajka, K.; Krzyżyńska, R.; Jouhara, H. Waste tyre pyrolysis–Impact of the process and its products on the environment. Therm. Sci. Eng. Prog. 2020, 20, 100690. [Google Scholar] [CrossRef]
- Mkhize, N.M.; Danon, B.; Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M.; van der Gryp, P.; Görgens, J. Influence of reactor and condensation system design on tyre pyrolysis products yields. J. Anal. Appl. Pyrolysis 2019, 143, 104683. [Google Scholar] [CrossRef]
- Quek, A.; Balasubramanian, R. An algorithm for the kinetics of tire pyrolysis under different heating rates. J. Hazard. Mater. 2009, 166, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Jones, S.B.; Valkenburt, C.; Walton, C.W.; Elliott, D.C.; Holladay, J.E.; Stevens, D.J.; Kinchin, C.; Czernik, S. Production of Gasoline and Diesel from Biomass via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2009.
- Lira, C.S.; Berruti, F.M.; Palmisano, P.; Berruti, F.; Briens, C.; Pécora, A.A. Fast pyrolysis of Amazon tucumã (Astrocaryum aculeatum) seeds in a bubbling fluidized bed reactor. J. Anal. Appl. Pyrolysis 2013, 99, 23–31. [Google Scholar] [CrossRef]
- Iribarren, D.; Peters, J.F.; Dufour, J. Life cycle assessment of transportation fuels from biomass pyrolysis. Fuel 2012, 97, 812–821. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M. Biomass pyrolysis for liquid fuels and chemicals: A review. NIScPR Online Period. Repos. 2007, 66, 797–804. [Google Scholar]
- Taleb, D.A.; Abd Hamid, H.; Deris, R.R.R.; Zulkifli, M.; Khalil, N.A.; Yahaya, A.N.A. Insights into pyrolysis of waste tire in fixed bed reactor: Thermal behavior. Mater. Today Proc. 2020, 31, 178–186. [Google Scholar] [CrossRef]
- Undri, A.; Meini, S.; Rosi, L.; Frediani, M.; Frediani, P. Microwave pyrolysis of polymeric materials: Waste tires treatment and characterization of the value-added products. J. Anal. Appl. Pyrolysis 2013, 103, 149–158. [Google Scholar] [CrossRef]
- Lozhechnik, A.; Savchin, V. Pyrolysis of rubber in a screw reactor. J. Eng. Phys. Thermophys. 2016, 89, 1482–1486. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Mkhize, N.; Danon, B.; Van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Evaluation of the properties of tyre pyrolysis oils obtained in a conical spouted bed reactor. Energy 2017, 128, 463–474. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Sadhukhan, A.K.; Gupta, P. Pyrolysis of three different categories of automotive tyre wastes: Product yield analysis and characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Muenpol, S.; Jitkarnka, S. Effects of Fe supported on zeolites on structures of hydrocarbon compounds and petrochemicals in waste tire-derived pyrolysis oils. J. Anal. Appl. Pyrolysis 2016, 117, 147–156. [Google Scholar] [CrossRef]
- Ye, W.; Xu, X.; Zhan, M.; Huang, Q.; Li, X.; Jiao, W.; Yin, Y. Formation behavior of PAHs during pyrolysis of waste tires. J. Hazard. Mater. 2022, 435, 128997. [Google Scholar] [CrossRef]
- Choi, G.-G.; Jung, S.-H.; Oh, S.-J.; Kim, J.-S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- de Marco Rodriguez, I.; Laresgoiti, M.; Cabrero, M.; Torres, A.; Chomon, M.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- Ucar, S.; Karagoz, S.; Ozkan, A.R.; Yanik, J. Evaluation of two different scrap tires as hydrocarbon source by pyrolysis. Fuel 2005, 84, 1884–1892. [Google Scholar] [CrossRef]
- Ahoor, A.H.; Zandi-Atashbar, N. Fuel production based on catalytic pyrolysis of waste tires as an optimized model. Energy Convers. Manag. 2014, 87, 653–669. [Google Scholar] [CrossRef]
- Boxiong, S.; Chunfei, W.; Cai, L.; Binbin, G.; Rui, W. Pyrolysis of waste tyres: The influence of USY catalyst/tyre ratio on products. J. Anal. Appl. Pyrolysis 2007, 78, 243–249. [Google Scholar] [CrossRef]
- Witpathomwong, C.; Longloilert, R.; Wongkasemjit, S.; Jitkarnka, S. Improving light olefins and light oil production using Ru/MCM-48 in catalytic pyrolysis of waste tire. Energy Procedia 2011, 9, 245–251. [Google Scholar] [CrossRef]
- Dũng, N.A.; Klaewkla, R.; Wongkasemjit, S.; Jitkarnka, S. Light olefins and light oil production from catalytic pyrolysis of waste tire. J. Anal. Appl. Pyrolysis 2009, 86, 281–286. [Google Scholar] [CrossRef]
- Díez, C.; Sánchez, M.; Haxaire, P.; Martínez, O.; Moran, A. Pyrolysis of tyres: A comparison of the results from a fixed-bed laboratory reactor and a pilot plant (rotatory reactor). J. Anal. Appl. Pyrolysis 2005, 74, 254–258. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Mohamed, T.J.; Hussain, A.A.; Abas, F.O. Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J. Anal. Appl. Pyrolysis 2004, 72, 165–170. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C.; Alvarez, R. Pyrolysis of blends of coal and tyre wastes in a fixed bed reactor and a rotary oven. Fuel 2013, 113, 817–825. [Google Scholar] [CrossRef]
- Williams, P.T.; Brindle, A.J. Aromatic chemicals from the catalytic pyrolysis of scrap tyres. J. Anal. Appl. Pyrolysis 2003, 67, 143–164. [Google Scholar] [CrossRef]
- Akkouche, N.; Balistrou, M.; Loubar, K.; Awad, S.; Tazerout, M. Heating rate effects on pyrolytic vapors from scrap truck tires. J. Anal. Appl. Pyrolysis 2017, 123, 419–429. [Google Scholar] [CrossRef]
- Islam, M.R.; Haniu, H.; Beg, M.R.A. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel 2008, 87, 3112–3122. [Google Scholar]
- Raj, R.E.; Kennedy, Z.R.; Pillai, B. Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers. Manag. 2013, 67, 145–151. [Google Scholar]
- Li, S.-Q.; Yao, Q.; Chi, Y.; Yan, J.-H.; Cen, K.-F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Antoniou, N.; Zabaniotou, A. Experimental proof of concept for a sustainable End of Life Tyres pyrolysis with energy and porous materials production. J. Clean. Prod. 2015, 101, 323–336. [Google Scholar] [CrossRef]
- Li, W.; Huang, C.; Li, D.; Huo, P.; Wang, M.; Han, L.; Chen, G.; Li, H.; Li, X.; Wang, Y. Derived oil production by catalytic pyrolysis of scrap tires. Chin. J. Catal. 2016, 37, 526–532. [Google Scholar] [CrossRef]
- Martínez, J.D.; Murillo, R.; García, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C. Pyrolysis of synthetic tire rubber in a fluidised-bed reactor to yield 1, 3-butadiene, styrene and carbon black. J. Anal. Appl. Pyrolysis 2001, 58, 803–811. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Mkhize, N.; Danon, B.; Van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Waste truck-tyre processing by flash pyrolysis in a conical spouted bed reactor. Energy Convers. Manag. 2017, 142, 523–532. [Google Scholar] [CrossRef]
- Labaki, M.; Jeguirim, M. Thermochemical conversion of waste tyres—A review. Environ. Sci. Pollut. Res. 2017, 24, 9962–9992. [Google Scholar] [CrossRef]
- Suuberg, E.M.; Aarna, I. Porosity development in carbons derived from scrap automobile tires. Carbon 2007, 45, 1719–1726. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Adv. Colloid Interface Sci. 2014, 211, 93–101. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Atanes, E.; Morena, J.; Fernández-Martínez, F.; Valverde, J.L. Upgrading waste tires by chemical activation for the capture of SO2. Fuel Process. Technol. 2016, 144, 274–281. [Google Scholar] [CrossRef]
- Alexandre-Franco, M.; Fernández-González, C.; Alfaro-Domínguez, M.; Gómez-Serrano, V. Adsorption of cadmium on carbonaceous adsorbents developed from used tire rubber. J. Environ. Manag. 2011, 92, 2193–2200. [Google Scholar] [CrossRef]
- Sirimuangjinda, A.; Hemra, K.; Atong, D.; Pechyen, C. Comparison on pore development of activated carbon produced from scrap tire by potassium hydroxide and sodium hydroxide for active packaging materials. Key Eng. Mater. 2013, 545, 129–133. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, J.; Sun, C.; He, W.; Huang, J.; Li, G. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 2020, 742, 140235. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilization. J. Anal. Appl. Pyrolysis 2003, 70, 711–722. [Google Scholar] [CrossRef]
- Belgacem, A.; Rebiai, R.; Hadoun, H.; Khemaissia, S.; Belmedani, M. The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ. Sci. Pollut. Res. 2014, 21, 684–694. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Pinedo-Flores, A.; Picasso, G.; Atanes, E.; Kou, R.S. Selective adsorption of Pb2+, Cr3+ and Cd2+ mixtures on activated carbons prepared from waste tires. J. Environ. Chem. Eng. 2017, 5, 1060–1067. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Z.; Zhang, Y.; Xu, Y.; Liu, H.; Wu, J.; Li, P. Activated Carbon Tailored by Potassium Hydroxide from Waste Tires as a Supercapacitor Electrode. ECS J. Solid State Sci. Technol. 2022, 11, 061004. [Google Scholar] [CrossRef]
- Veldevi, T.; Raghu, S.; Kalaivani, R.; Shanmugharaj, A. Waste tire derived carbon as potential anode for lithium-ion batteries. Chemosphere 2022, 288, 132438. [Google Scholar] [CrossRef]
- Naskar, A.K.; Bi, Z.; Li, Y.; Akato, S.K.; Saha, D.; Chi, M.; Bridges, C.A.; Paranthaman, M.P. Tailored recovery of carbons from waste tires for enhanced performance as anodes in lithium-ion batteries. Rsc Adv. 2014, 4, 38213–38221. [Google Scholar] [CrossRef]
- Zhao, P.; Han, Y.; Dong, X.; Zhang, C.; Liu, S. Application of activated carbons derived from scrap tires as electrode materials for supercapacitors. ECS J. Solid State Sci. Technol. 2015, 4, M35. [Google Scholar] [CrossRef]
- Zhi, M.; Yang, F.; Meng, F.; Li, M.; Manivannan, A.; Wu, N. Effects of pore structure on performance of an activated-carbon supercapacitor electrode recycled from scrap waste tires. ACS Sustain. Chem. Eng. 2014, 2, 1592–1598. [Google Scholar] [CrossRef]
- Li, Y.; Adams, R.A.; Arora, A.; Pol, V.G.; Levine, A.M.; Lee, R.J.; Akato, K.; Naskar, A.K.; Paranthaman, M.P. Sustainable potassium-ion battery anodes derived from waste-tire rubber. J. Electrochem. Soc. 2017, 164, A1234. [Google Scholar] [CrossRef]
- Bello, A.; Momodu, D.Y.; Madito, M.; Makgopa, K.; Rambau, K.M.; Dangbegnon, J.K.; Musyoka, N.M.; Manyala, N. Influence of K3Fe (CN) 6 on the electrochemical performance of carbon derived from waste tyres by K2CO3 activation. Mater. Chem. Phys. 2018, 209, 262–270. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, P.-P.; Dong, X.-T.; Zhang, C.; Liu, S.-X. Improvement in electrochemical capacitance of activated carbon from scrap tires by nitric acid treatment. Front. Mater. Sci. 2014, 8, 391–398. [Google Scholar] [CrossRef]
- Gong, H.; Wang, D.; Jiang, Y.; Wang, L.; Zhang, K.; Qian, Y. Phosphorus-doped mesoporous carbon derived from waste tires as anode for K-ion batteries. Mater. Lett. 2021, 285, 128983. [Google Scholar] [CrossRef]
- Li, Y.; Paranthaman, M.P.; Akato, K.; Naskar, A.K.; Levine, A.M.; Lee, R.J.; Kim, S.-O.; Zhang, J.; Dai, S.; Manthiram, A. Tire-derived carbon composite anodes for sodium-ion batteries. J. Power Source 2016, 316, 232–238. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Source 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Yeh, M.H.; Lin, L.Y.; Li, T.J.; Leu, Y.A.; Chen, G.L.; Tien, T.C.; Hsieh, C.Y.; Lo, S.C.; Huang, S.J.; Chiang, W.H. Synthesis of boron–doped multi–walled carbon nanotubes by an ammonia–assisted substitution reaction for applying in supercapacitors. Energy Procedia 2014, 61, 1764–1767. [Google Scholar] [CrossRef]
- Liao, M.D.; Peng, C.; Hou, S.P.; Chen, J.; Zeng, X.G.; Wang, H.-L.; Lin, J.-H. Large-Scale Synthesis of Nitrogen-Doped Activated Carbon Fibers with High Specific Surface Area for High-Performance Supercapacitors. Energy Technol. 2020, 8, 1901477. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Vellacheri, R.; Lei, Y. Recent advances in designing and fabricating self-supported nanoelectrodes for supercapacitors. Adv. Sci. 2017, 4, 1700188. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, C.; Ma, C.; Li, G.; Wang, Y.; Zhang, K.; Li, F.; Liu, C.; Cheng, H.M.; Du, Y. Nitrogen-superdoped 3D graphene networks for high-performance supercapacitors. Adv. Mater. 2017, 29, 1701677. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K. Montmorillonite supported metal nanoparticles: An update on syntheses and applications. Rsc Adv. 2013, 3, 13583–13593. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe vera derived activated high-surface-area carbon for flexible and high-energy supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef]
- Liang, Q.; Ye, L.; Huang, Z.-H.; Xu, Q.; Bai, Y.; Kang, F.; Yang, Q.-H. A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 2014, 6, 13831–13837. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, O.-K.; Lee, J.H.; Ku, B.-C. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, L.; Zhao, H.; Liang, F.; Chang, S.; Li, L.; Zhang, Y.; Lin, Z.; Kröger, J.; Lei, Y. Nanoelectrode design from microminiaturized honeycomb monolith with ultrathin and stiff nanoscaffold for high-energy micro-supercapacitors. Nat. Commun. 2020, 11, 299. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Lei, Y. Review on nanoarchitectured current collectors for pseudocapacitors. Small Methods 2019, 3, 1800341. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Charge storage mechanism in nanoporous carbons and its consequence for electrical double layer capacitors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3457–3467. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Li, X.-H.; Wang, Z.-X.; Guo, H.-J.; Peng, W.-J. Effect of activated carbon and electrolyte on properties of supercapacitor. Trans. Nonferrous Met. Soc. China 2007, 17, 1328–1333. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Jiang, L.; Wu, H.; Wu, C.; Su, J. Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Source 2012, 216, 290–296. [Google Scholar] [CrossRef]
- Boota, M.; Paranthaman, M.P.; Naskar, A.K.; Li, Y.; Akato, K.; Gogotsi, Y. Waste tire derived carbon–polymer composite paper as pseudocapacitive electrode with long cycle life. ChemSusChem 2015, 8, 3576–3581. [Google Scholar] [CrossRef]
- Hsiao, C.; Lee, C.; Tai, N. High retention supercapacitors using carbon nanomaterials/iron oxide/nickel-iron layered double hydroxides as electrodes. J. Energy Storage 2022, 46, 103805. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, R.; Liu, Q.; Ou, K.; Li, D.-S.; Fang, J.; Fu, Q.; Sun, Y. A flexible supercapacitor with high capacitance retention at an ultra-low temperature of −65.0 °C. Electrochim. Acta 2022, 424, 140644. [Google Scholar] [CrossRef]
- Wang, K.-P.; Teng, H. Structural feature and double-layer capacitive performance of porous carbon powder derived from polyacrylonitrile-based carbon fiber. J. Electrochem. Soc. 2007, 154, A993. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Lu, J.; Zhan, C.; Lv, R.; Liang, Q.; Huang, Z.-H.; Shen, W.; Kang, F. Synthesis of activated carbon nanospheres with hierarchical porous structure for high volumetric performance supercapacitors. Electrochim. Acta 2015, 182, 908–916. [Google Scholar] [CrossRef]
- Shang, T.; Xu, Y.; Li, P.; Han, J.; Wu, Z.; Tao, Y.; Yang, Q.-H. A bio-derived sheet-like porous carbon with thin-layer pore walls for ultrahigh-power supercapacitors. Nano Energy 2020, 70, 104531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).