Separation and Recovery of Copper and Nickel in the Leachate of a Waste IC Lead Frame through Synergistic Solvent Extraction Using a Binary Extractant Containing LIX984N and Cyanex302 Followed by Selective Stripping
Abstract
:1. Introduction
2. Materials and Methods
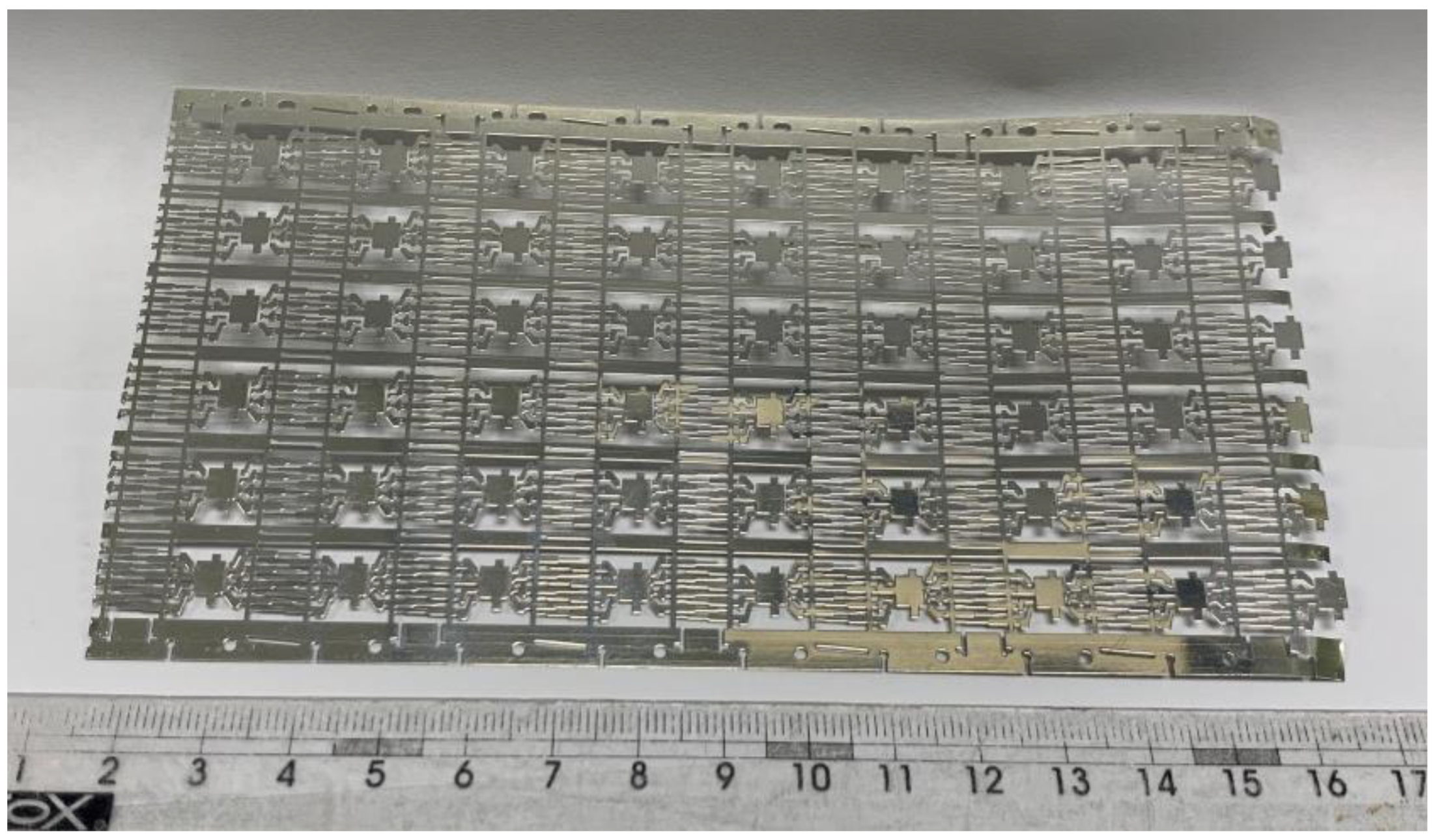
2.1. Materials
2.2. Methods
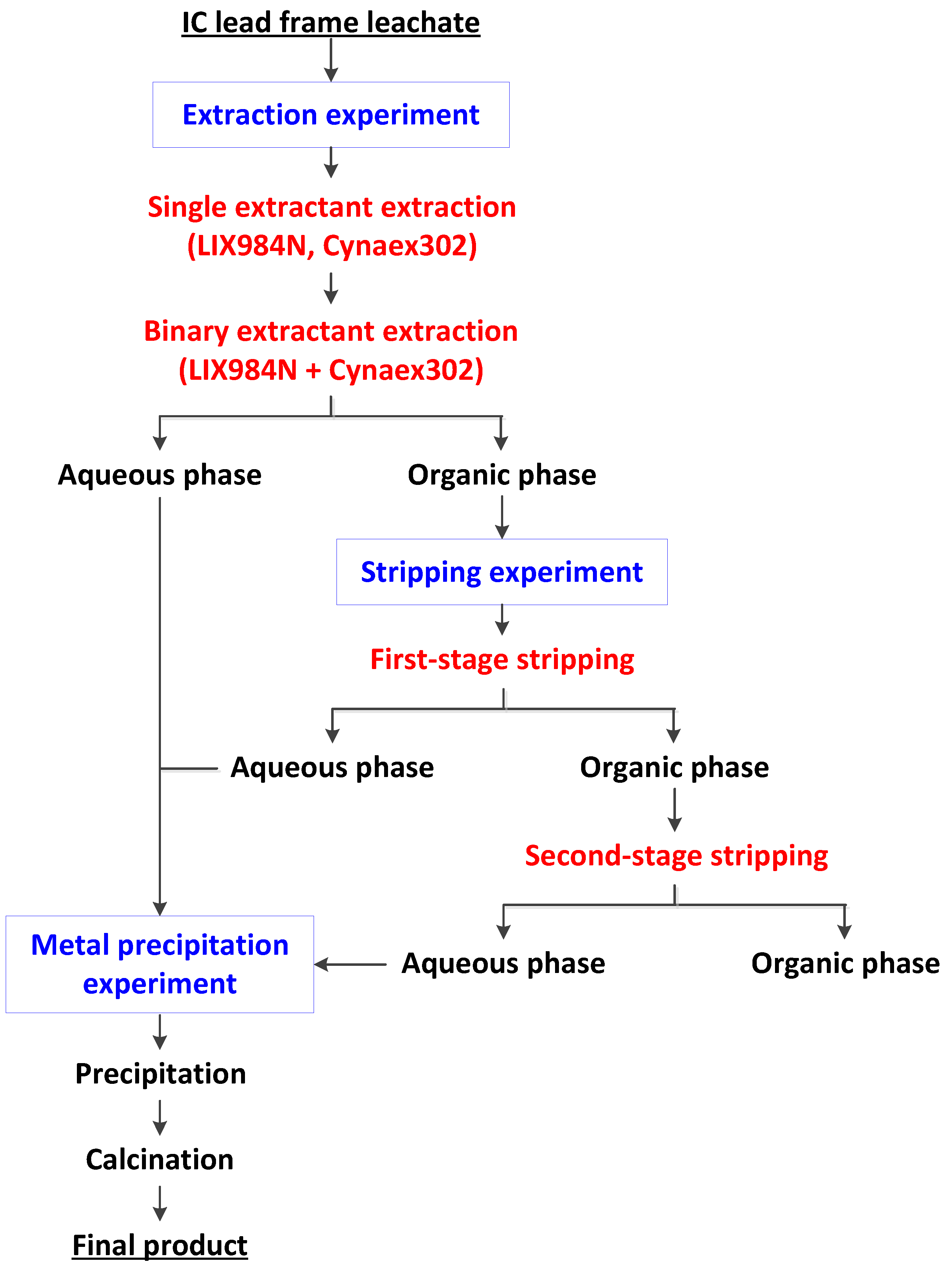
2.2.1. Extraction Experiments
2.2.2. Stripping Experiments
2.2.3. Metal Precipitation Experiments
3. Results and Discussion
3.1. Extraction Experiments
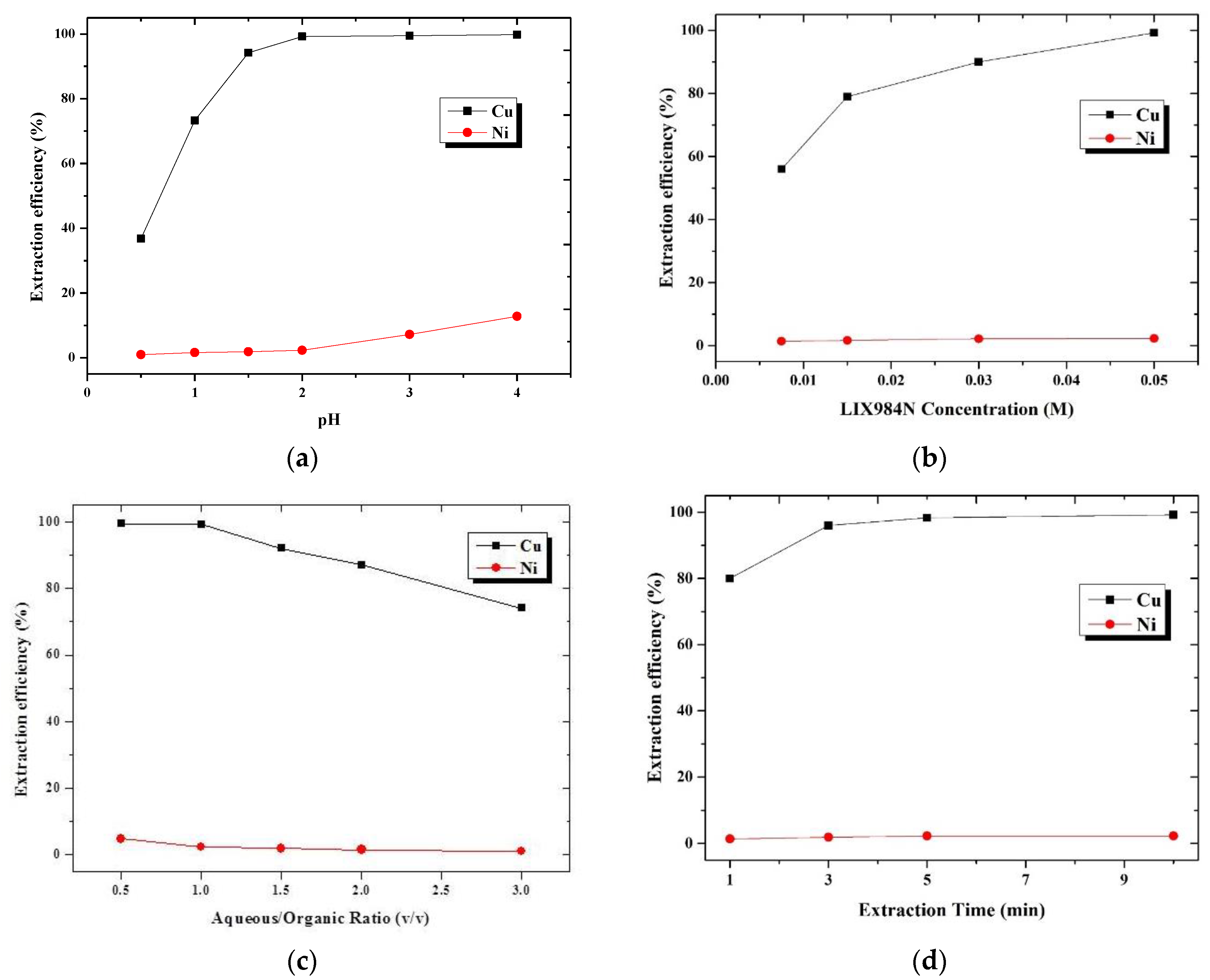
3.1.1. Extraction Using a Single Extractant of LIX984
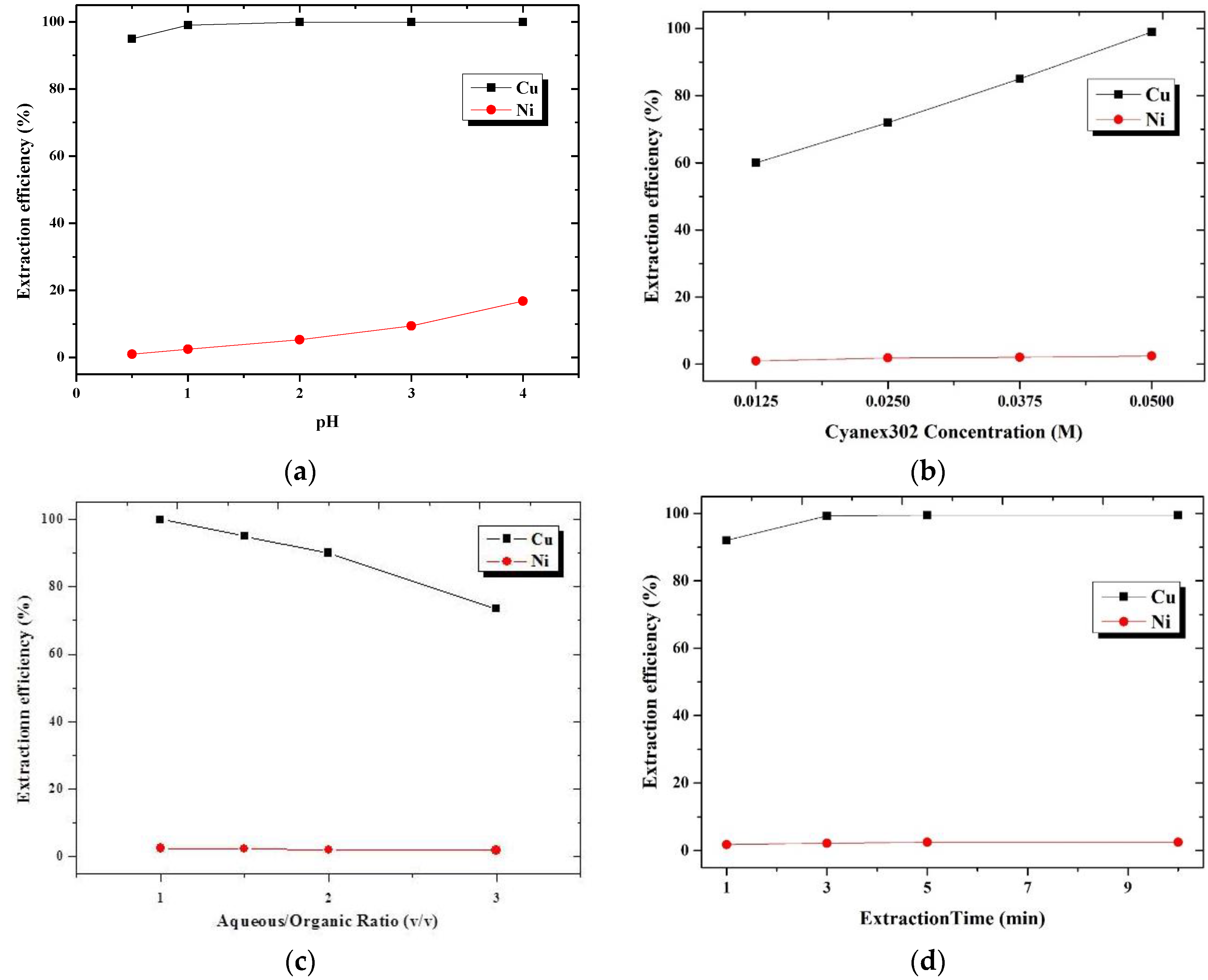
3.1.2. Extraction Using a Single Extractant of Cyanex302
3.1.3. Extraction Using a Binary Extractant Containing LIX984 and Cyanex302
3.1.4. Summarization and Comparison of Solvent Extraction Using Single and Binary Extractant Containing LIX984 and Cyanex302
3.2. Stripping Experiments
3.2.1. Effect of the Type of Stripping Agent
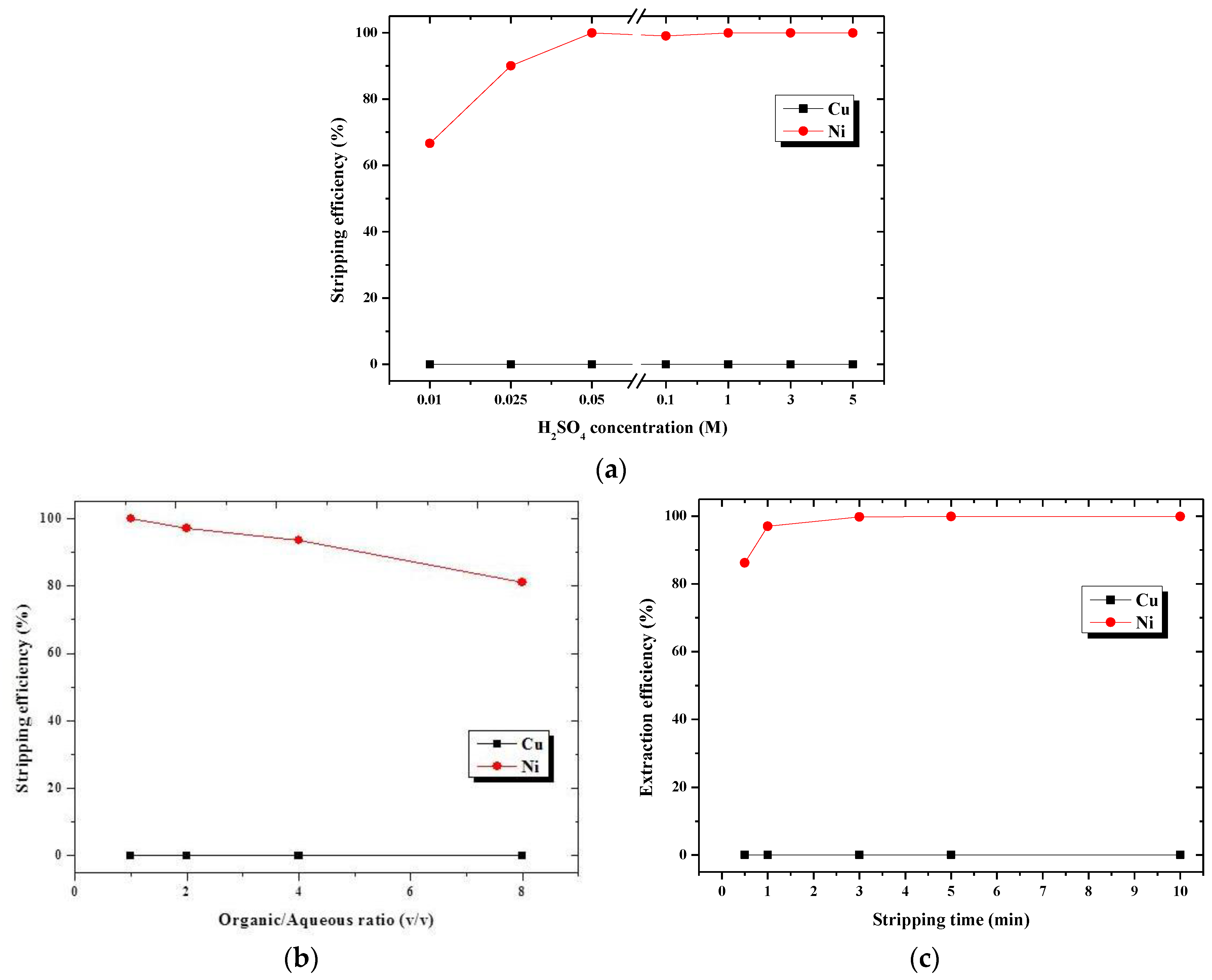
3.2.2. First-Stage Stripping Using H2SO4 as the Stripping Agent
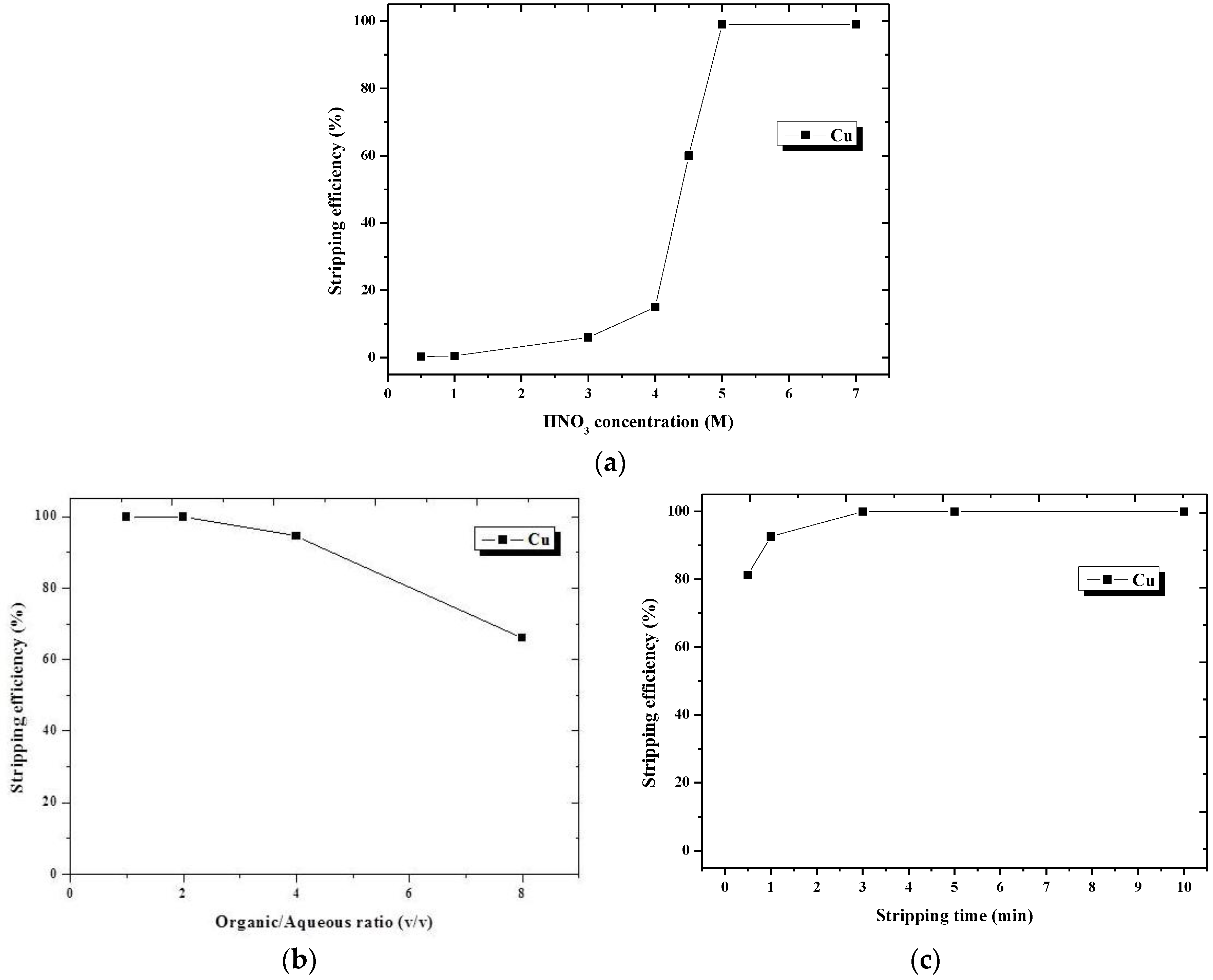
3.2.3. Second-Stage Stripping Using HNO3 as the Stripping Agent
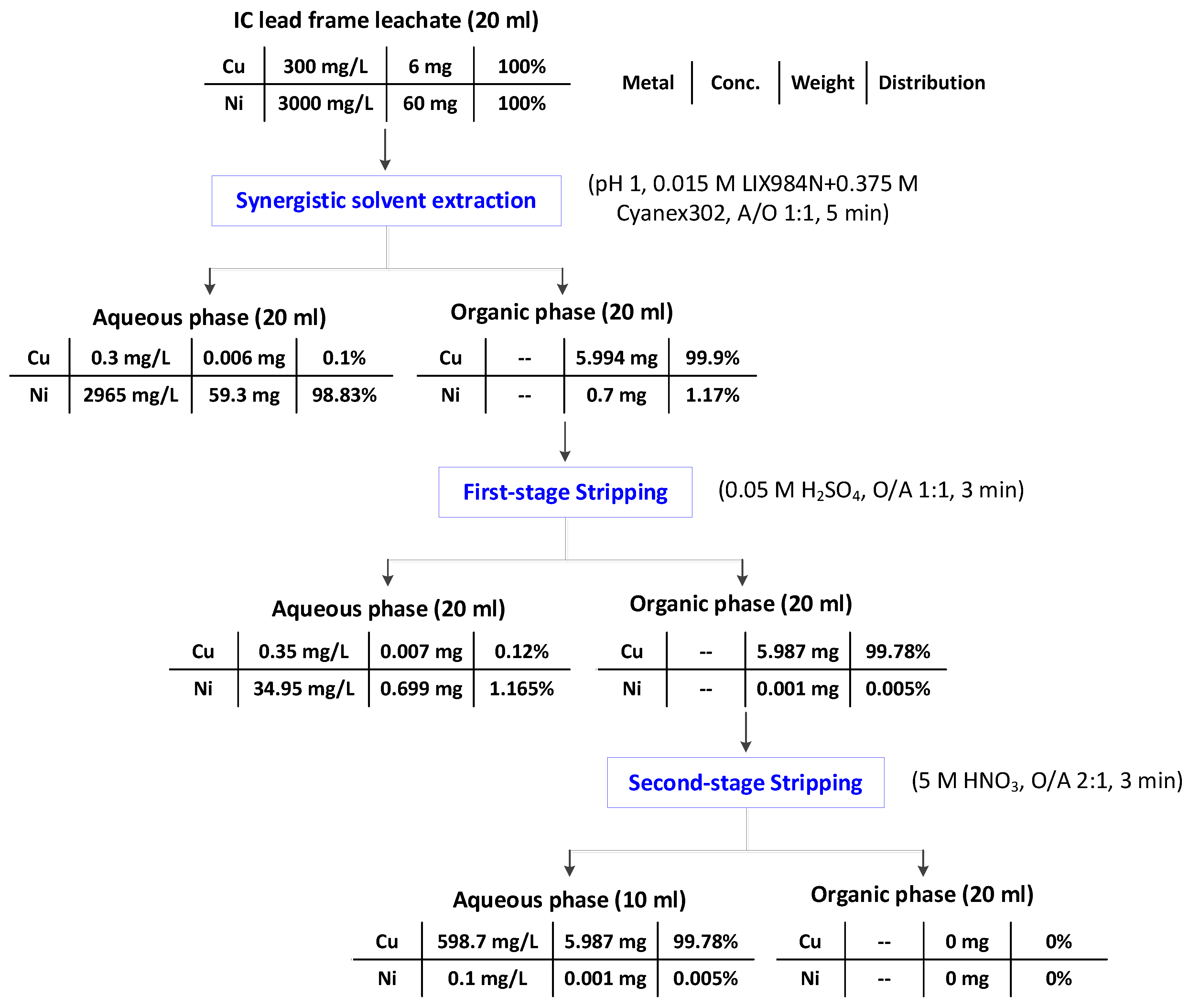
3.3. Mass Balance of Cu and Ni in the Synergistic Solvent Extraction and Selective Stripping Procedure under Optimal Experimental Conditions
3.4. Metal Recovery from Stripping Solution
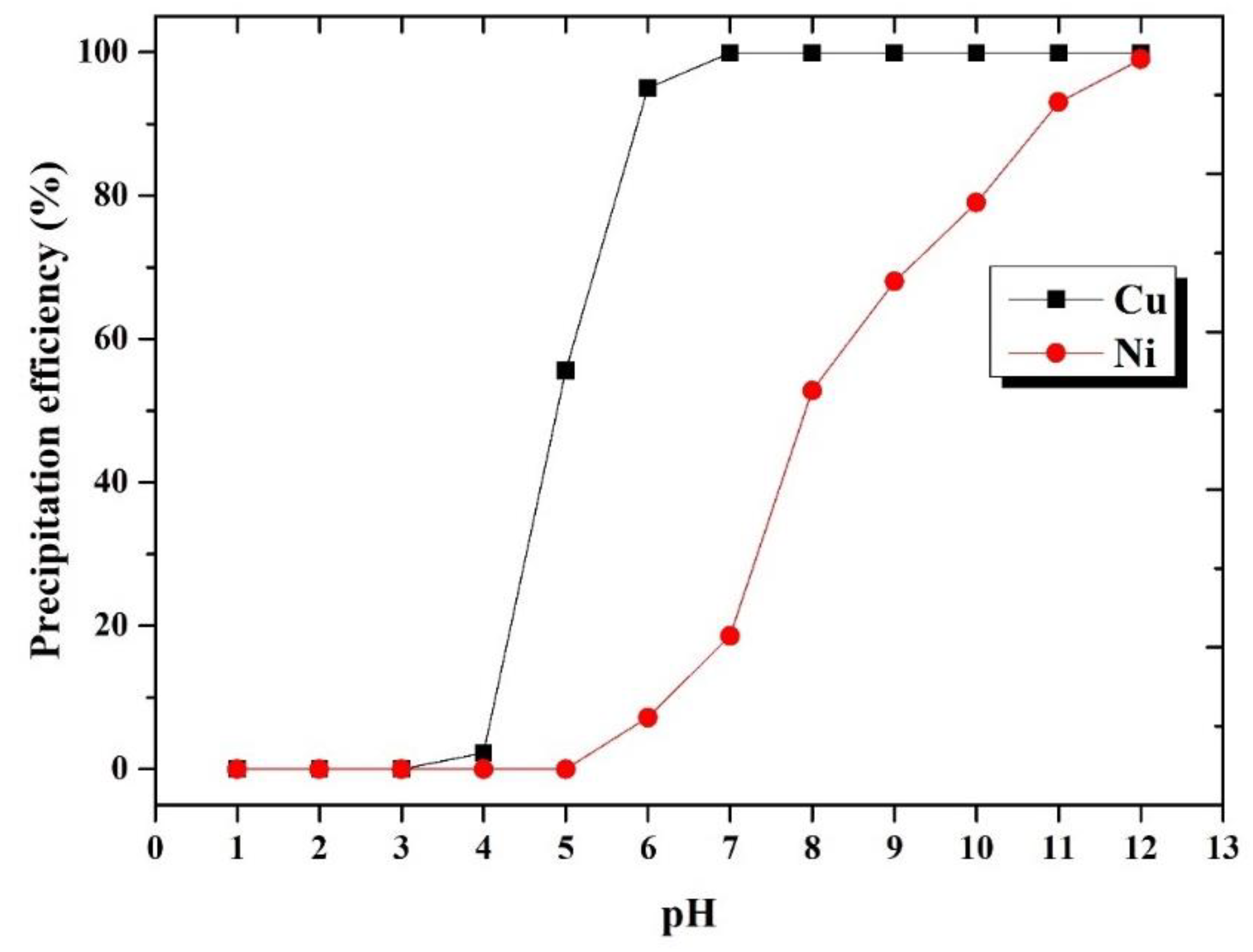
3.4.1. Precipitation pH Value
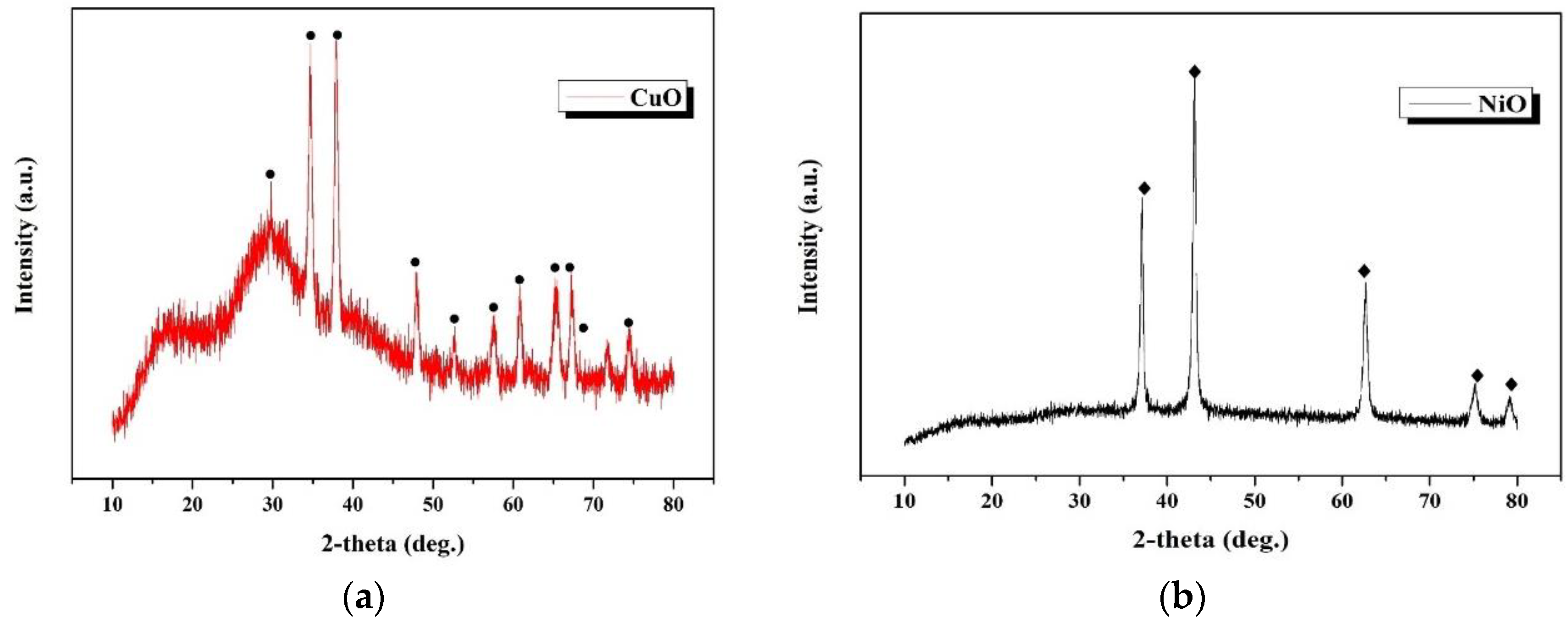
3.4.2. Characterization of Metal Oxide Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, U.K.; Singh, J. Semiconductor Device Physics and Design; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789400797789. [Google Scholar]
- Jackson, K.; Schroter, W. Handbook of Semiconductor Technology; Wiley VCH: Hoboken, NJ, USA, 2000; Volume 2, ISBN 3-527-29835-5. [Google Scholar]
- Han, D. Research status and key technology analysis of integrated circuit lead frame. Acad. J. Eng. Technol. Sci. 2023, 6, 43–49. [Google Scholar]
- Giannopoulou, I.; Panias, D. Copper and nickel recovery from acidic polymetallic aqueous solutions. Miner. Eng. 2007, 20, 753–760. [Google Scholar] [CrossRef]
- Sridhar, V.; Verma, J.K.; Kumar, S.A. Selective separation of copper and nickel by solvent extraction using LIX 984N. Hydrometallurgy 2009, 99, 124–126. [Google Scholar] [CrossRef]
- Li, L.; Zhong, H.; Cao, Z.; Yuan, L. Recovery of Copper(II) and Nickel(II) from Plating Wastewater by Solvent Extraction. Chin. J. Chem. Eng. 2011, 19, 926–930. [Google Scholar] [CrossRef]
- Soeezi, A.; Abdollahi, H.; Shafaei, S.Z.; Rahimi, E. Extraction and stripping of Cu and Ni from synthetic and industrial solutions of Sarcheshmeh Copper Mine containing Cu, Ni, Fe and Zn ions. Trans. Nonferrous Met. Soc. 2020, 30, 518–534. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B. Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy 1995, 37, 129–147. [Google Scholar] [CrossRef]
- Tait, B.K. Cobalt-nickel separation: The extraction of cobalt(II) and nickel(II) by Cyanex 301, Cyanex 302 and Cyanex 272. Hydrometallurgy 1993, 32, 365–372. [Google Scholar] [CrossRef]
- Padhan, E.; Sarangi, K.; Subbaiah, T. Recovery of manganese and nickel from polymetallic manganese nodule using commercial extractants. Int. J. Miner. Process. 2014, 126, 55–61. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep. Purif. Technol. 2015, 144, 197–205. [Google Scholar] [CrossRef]
- Shi, T.; Zou, S.; Liu, J.; Zuo, L.; Lv, C.; Jia, S.; Qiu, R.L. Separation and recovery of nickel and copper from multi-metal electroplating sludge by co-extracting and selective stripping. J. Civ. Environ. Eng. 2011, 1, 103. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Suzuki, T.M.; Inoue, K. The synergistic extraction of nickel and cobalt with a mixture of di(2-ethylhexyl) phosphoric acid and 5-dodecylsalicylaldoxime. Hydrometallurgy 2001, 61, 223–227. [Google Scholar] [CrossRef]
- Fouad, E.A. Separation of copper from aqueous sulfate solutions by mixtures of Cyanex 301 and LIX® 984N. J. Hazard. Mater. 2009, 166, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.N.R.; Othman, N. Solvent extraction of nickel ions from electroless nickel plating wastewater using synergistic green binary mixture of D2EHPA-octanol system. J. Environ. Chem. Eng. 2018, 6, 1814–1820. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, L.; Huang, S.; Xu, Z.; Li, Y.; Wang, W. Synergistic solvent extraction of nickel by 2-hydroxy-5-nonylacetophenone oxime mixed with neodecanoic acid and bis(2-ethylhexyl) phosphoric acid: Stoichiometry and structure investigation. Miner. Eng. 2019, 132, 284–292. [Google Scholar] [CrossRef]
- Qi, D. Hydrometallurgy of Rare Earths: Separation and Extraction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 187–389. ISBN 978-0-12-813920-2. [Google Scholar]
- Devi, N.B.; Nayak, B. Liquid-liquid extraction and separation of copper(II) and nickel(II) using LIX®984N. J. S. Afr. Inst. Min. Metall. 2014, 114, 937–943. [Google Scholar]
- Xiao, B.; Jiang, F.; Yin, S.; Zhang, L.; Peng, J.; Ju, S.; Wang, S. Fast separation of Cu2+ and Ni2+ in sulfate solution by Lix984N extraction using a microchannel chip. Green Process. Synth. 2018, 7, 207–216. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction, Principles and Applications to Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 1984; Part I; ISBN 978-0-12-813920-2. [Google Scholar]
- Jun, M.; Srivastava, R.R.; Jeong, J.; Lee, J.C.; Kim, M.S. Simple recycling of copper by the synergistic exploitation of industrial wastes: A step towards sustainability. Green Chem. 2016, 18, 3823. [Google Scholar] [CrossRef]
- Chen, W.S.; Chen, Y.J.; Lee, C.H.; Cheng, Y.J.; Chen, Y.A.; Liu, F.W.; Chueh, Y.L. Recovery of valuable materials from the waste crystalline-silicon photovoltaic cell and ribbon. Processes 2021, 9, 712. [Google Scholar] [CrossRef]

| Extractant | LIX984N | Cyanex302 | LIX984N + Cyanex302 |
|---|---|---|---|
| Initial extraction pH value | 2 | 1 | 1 |
| Extractant concentration | 0.05 M | 0.05 M | 0.015 M + 0.0375 M |
| Extraction A/O ratio | 1 (v/v) | 1 (v/v) | 1 (v/v) |
| Extraction time | 5 min | 5 min | 5 min |
| Extraction efficiency for Cu | 99.2% | 99.5% | 99.8% |
| Extraction efficiency for Ni | 2.3% | 2.5% | 1.17% |
| Distribution ratio of Cu (DCu) | 149 | 559 | 999 |
| Distribution ratio of Ni (DNi) | 0.024 | 0.027 | 0.012 |
| Separation factor of Cn and Ni (βCu/Ni) | 6208.3 | 22,185.2 | 83,250 |
| Stripping Efficiency | HCl | HNO3 | H2SO4 |
|---|---|---|---|
| Ni | 98.5% | 98.2% | 99.9% |
| Cu | 48.2% | 99.9% | 0.01% |
| First-stage stripping | |
| [H2SO4] | 0.05 M |
| Stripping O/A ratio | 1:1 |
| Stripping time | 3 min |
| Stripping efficiency for Ni | 99.9% |
| Stripping efficiency for Cu | 0.12% |
| Second-stage stripping | |
| [HNO3] | 5 M |
| Stripping O/A ratio | 2:1 |
| Stripping time | 3 min |
| Stripping efficiency for Cu | 99.9% |
| Content | NiO | CuO |
|---|---|---|
| NiO | 99.74% | 0.05% |
| CuO | 0.075% | 99.82% |
| NaO | 0.18% | 0.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-P.; Lin, J.-Y.; Chen, Y.-J.; Tseng, B.-C.; Hsu, C.-H.; Kou, M.; Zhou, H.; Sreearunothai, P. Separation and Recovery of Copper and Nickel in the Leachate of a Waste IC Lead Frame through Synergistic Solvent Extraction Using a Binary Extractant Containing LIX984N and Cyanex302 Followed by Selective Stripping. Sustainability 2024, 16, 77. https://doi.org/10.3390/su16010077
Wang L-P, Lin J-Y, Chen Y-J, Tseng B-C, Hsu C-H, Kou M, Zhou H, Sreearunothai P. Separation and Recovery of Copper and Nickel in the Leachate of a Waste IC Lead Frame through Synergistic Solvent Extraction Using a Binary Extractant Containing LIX984N and Cyanex302 Followed by Selective Stripping. Sustainability. 2024; 16(1):77. https://doi.org/10.3390/su16010077
Chicago/Turabian StyleWang, Li-Pang, Jia-Yan Lin, Yan-Jhang Chen, Bu-Ching Tseng, Ching-Hsiang Hsu, Mingyin Kou, Heng Zhou, and Paiboon Sreearunothai. 2024. "Separation and Recovery of Copper and Nickel in the Leachate of a Waste IC Lead Frame through Synergistic Solvent Extraction Using a Binary Extractant Containing LIX984N and Cyanex302 Followed by Selective Stripping" Sustainability 16, no. 1: 77. https://doi.org/10.3390/su16010077






