Bibliometric Analysis of Nanostructured Anodes for Electro-Oxidative Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
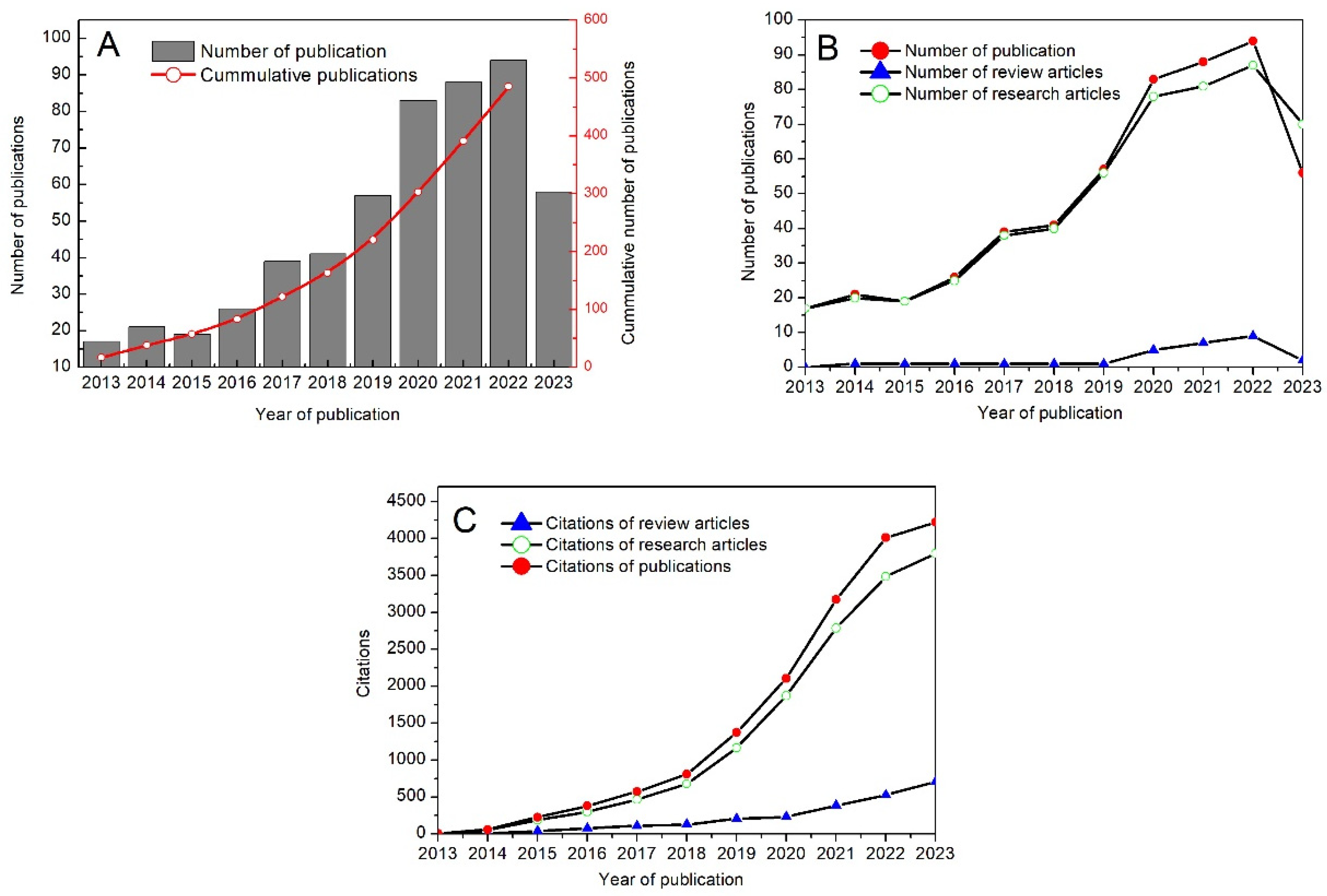
3.1. Publications Trends, Cited and Co-Cited Journals
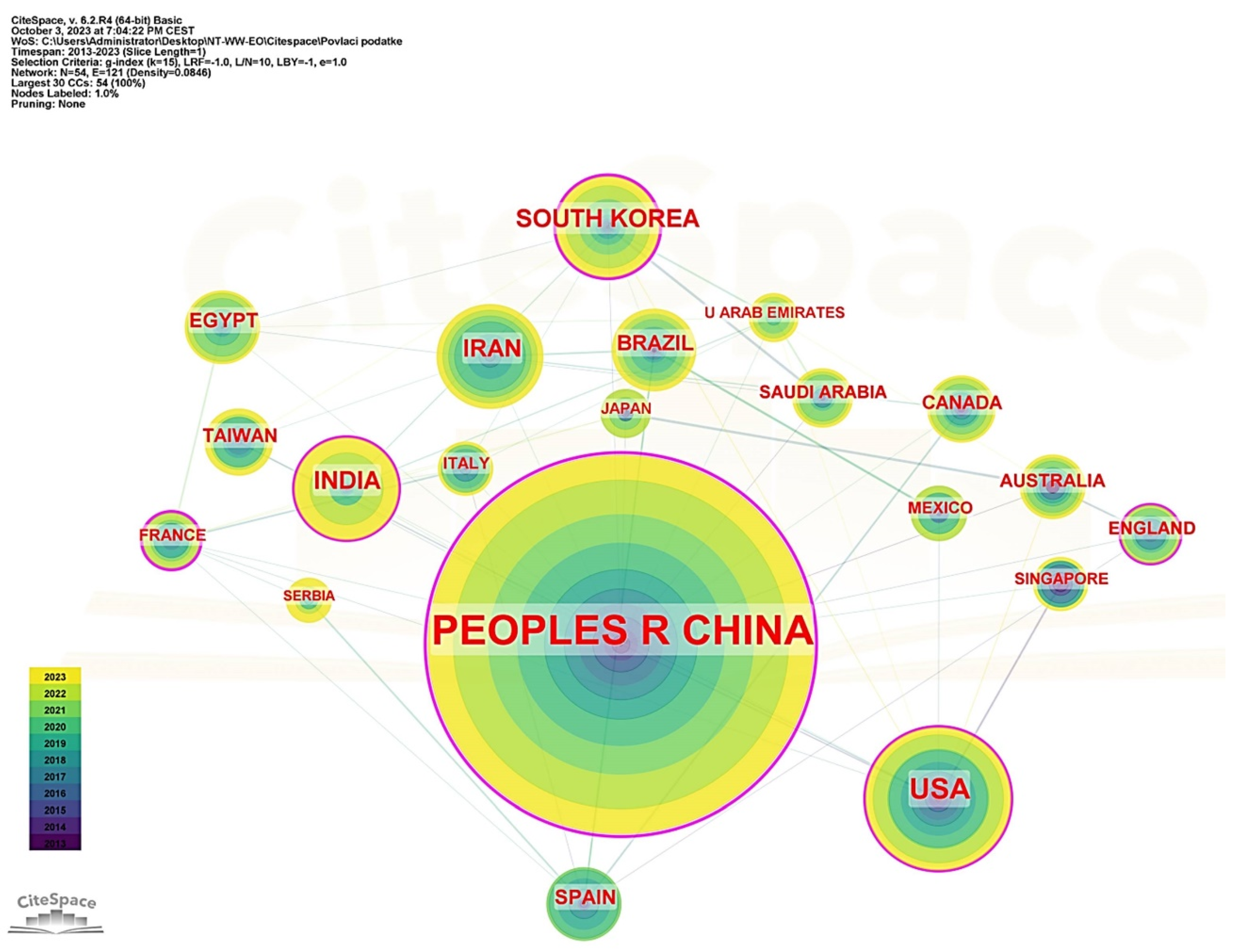
3.2. Countries’ Contribution
3.3. The Contributions of Institutions
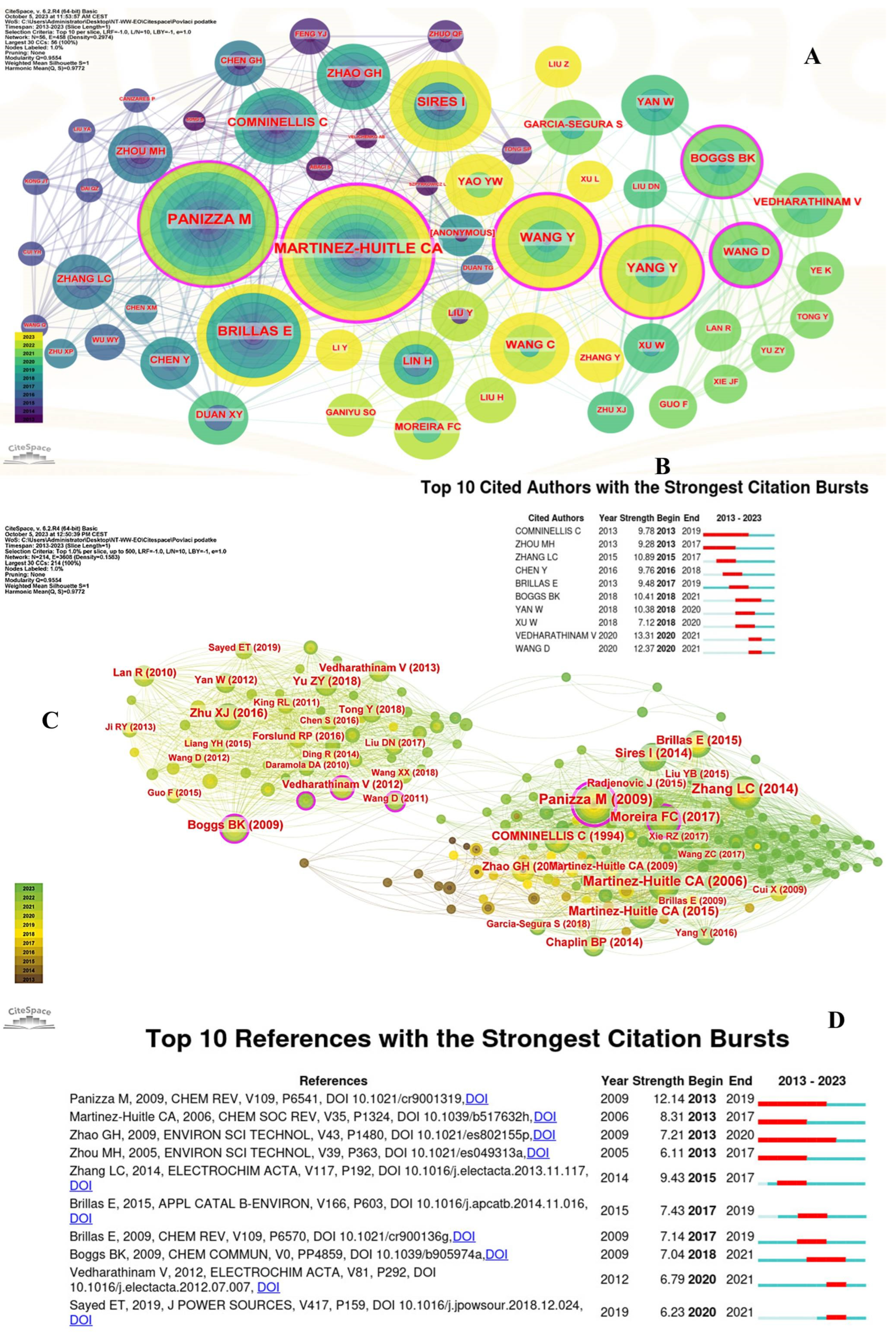
3.4. Contributions of Authors and Reference
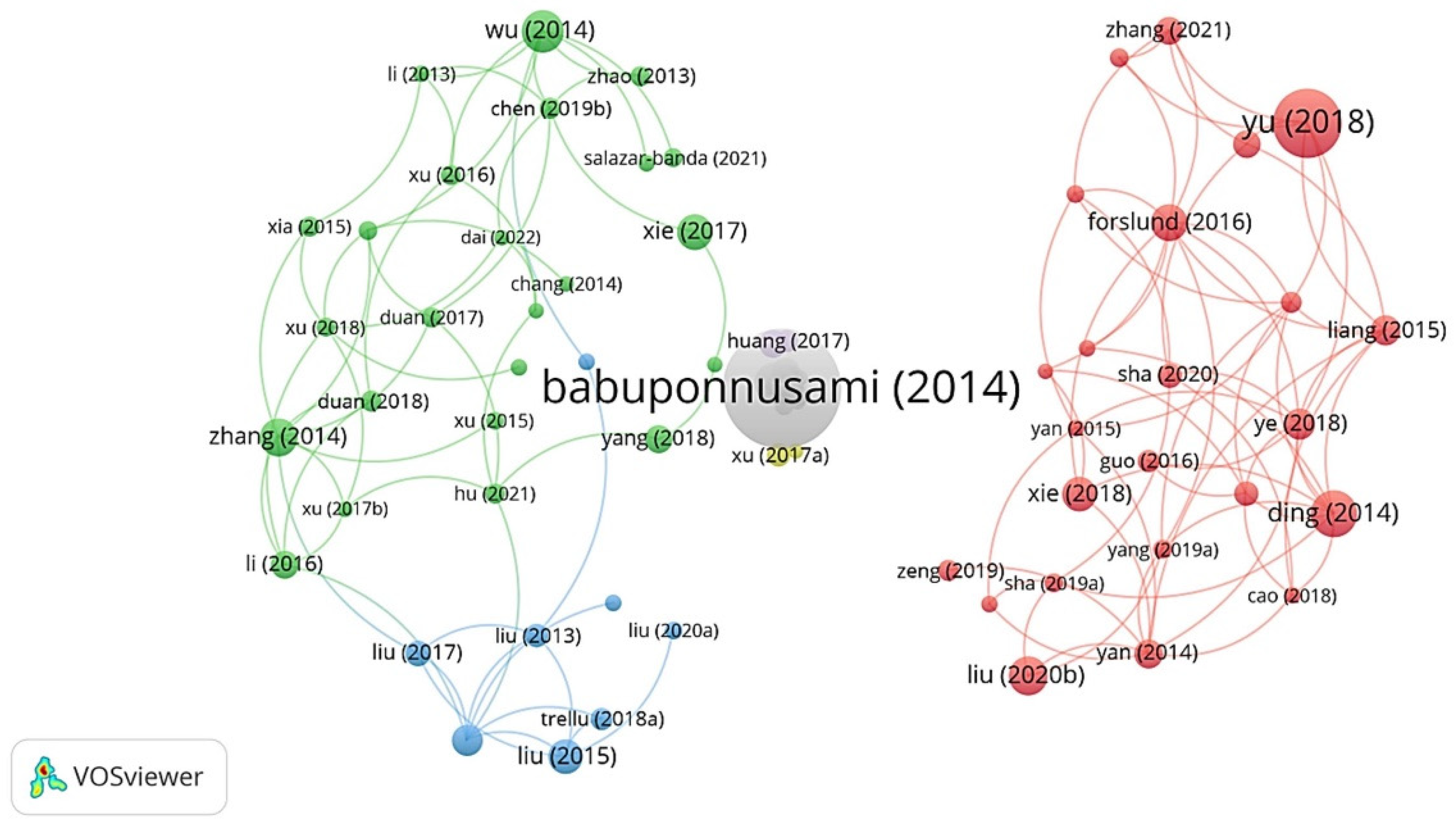
3.4.1. Co-Citation Author and Co-Citation Reference
3.4.2. Co-Citation Reference
3.5. Co Authorship, Cited Author, and Cited Reference
| No. | Document | Title | Journal | Citations | Links |
|---|---|---|---|---|---|
| 1 | Babuponnusami (2014) [59] | A review on Fenton and improvements to the Fenton process for wastewater treatment | Journal of Environmental Chemical Engineering 2014, 2, 557–572 | 1133 | 1 |
| 2 | Yu (2018) [76] | Ni-Mo-O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis | Energy & Environmental Science 2018, 11, 1890–1897 | 491 | 29 |
| 3 | Ding (2014) [61] | Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation | Nanoscale 2014, 6, 1369–1376 | 268 | 21 |
| 4 | Wu (2014) [77] | Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water | Applied Catalysis A: General 2014, 480, 58–78 | 235 | 23 |
| 5 | Rashid (2021) [60] | A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method | Environmental Science and Pollution Research 2021, 28, 9050–9066 | 224 | 0 |
| 6 | Liu (2020b) [62] | Efficient synergism of NiSe2 Nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis | Applied Catalysis B: Environmental 2020, 276, 119165 | 205 | 6 |
| 7 | Zhang (2014a) [51] | Preparation of Ti/SnO2-Sb electrodes modified by carbon nanotube for anodic oxidation of dye wastewater and combination with nanofiltration | Electrochimica Acta 2014, 117, 192–201 | 199 | 46 |
| 8 | Forslund (2016) [63] | Nanostructured LaNiO3 Perovskite Electrocatalyst for Enhanced Urea Oxidation | ACS Catalysis 2016, 6, 5044–5051 | 187 | 23 |
| 9 | Xie (2017) [78] | Electrochemical oxidation of ofloxacin using a TiO2-based SnO2-Sb/polytetrafluoroethylene resin-PbO2 electrode: Reaction kinetics and mass transfer impact | Applied Catalysis B: Environmental 2017, 203, 515–525 | 179 | 18 |
| 10 | Xie (2018) [64] | Partially Amorphous Nickel-Iron Layered Double Hydroxide Nanosheet Arrays for Robust Bifunctional Electrocatalysis | Journal of Materials Chemistry A 2018, 6, 16121–16129 | 174 | 15 |
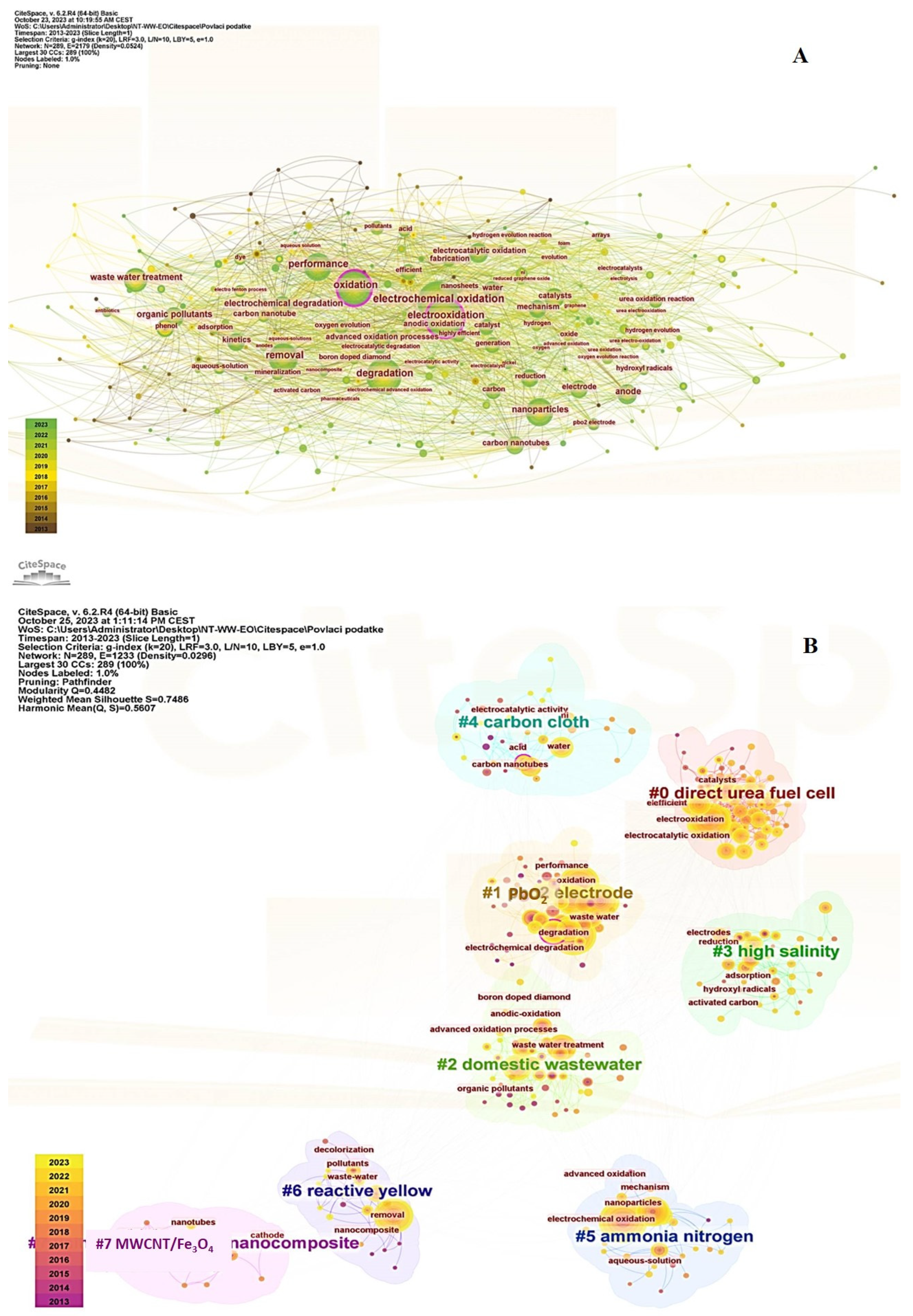
3.6. Keywords
3.7. Retrospection on Bibliometric Results
4. Conclusions
5. Future Directions
- Advanced Nanostructured Materials: researchers may focus on developing novel nanostructured materials with enhanced properties for electro-oxidative wastewater treatment. These materials could offer improved efficiency, durability, and selectivity in pollutant removal.
- Integration of Nanotechnology: further integration of nanotechnology into the electrolytic systems could lead to more efficient and cost-effective wastewater treatment processes. This might involve exploring new methods for fabricating nanostructured anodes and optimizing their performance in real-world applications.
- Multidisciplinary Collaborations: given the international collaboration observed in the bibliometric analysis, future research efforts could involve multidisciplinary collaborations between researchers from different countries and institutions. This collaborative approach can foster innovation and accelerate progress in the field.
- Focus on Urea Electro-oxidation: since the analysis identified urea electro-oxidation as a main research theme, future studies may delve deeper into this area. This could involve investigating the electrochemical mechanisms involved in urea oxidation, optimizing electrode materials for urea removal, and exploring potential applications in various industries, such as agriculture and wastewater treatment.
- Environmental Protection and Sustainable Ecological Engineering: there could be a growing emphasis on developing sustainable and environmentally friendly technologies for electro-oxidative wastewater treatment. Researchers may explore the use of renewable energy sources, such as solar energy, to drive electrochemical processes, as well as the development of eco-friendly electrode materials.
- Data Analysis and Visualization Tools: continued advancements in bibliometric analysis tools, such as VOSviewer and CiteSpace, could enable researchers to gain deeper insights into research trends, collaboration networks, and emerging topics in the field. This could facilitate more informed decision-making and strategic planning for future research endeavors.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Ajala, O.A.; Akinnawo, S.O.; Bamisaye, A.; Adedipe, D.T.; Adesina, M.O.; Okon-Akan, O.A.; Adebusuyi, T.A.; Ojedokun, A.T.; Adegoke, K.A.; Bello, O.S. Adsorptive Removal of Antibiotic Pollutants from Wastewater Using Biomass/Biochar-Based Adsorbents. RSC Adv. 2023, 13, 4678–4712. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.R.; Danish, M.; Alam, M.G.; Majeed, S.; Alanazi, A.M. A Review of Pre- and Post-Surface-Modified Neem (Azadirachta Indica) Biomass Adsorbent: Surface Functionalization Mechanism and Application. Chemosphere 2024, 351, 141180. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zuo, H.; Lv, J.; Wei, S.; Yao, Y.; Liu, Z.; Lin, Q.; Yu, Y.; Yu, W.; Huang, Y. Preparation of Multi-Layered Microcapsule-Shaped Activated Biomass Carbon with Ultrahigh Surface Area from Bamboo Parenchyma Cells for Energy Storage and Cationic Dyes Removal. J. Clean. Prod. 2023, 396, 136517. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A Review of the Photocatalysis Process Used for Wastewater Treatment. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Shi, P.; Qiu, J.; Guo, R.; You, J.; Zhang, H. Hybrid Organic Frameworks: Synthesis Strategies and Applications in Photocatalytic Wastewater Treatment—A Review. Chemosphere 2024, 350, 141143. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.; Dash, P.; Panda, P.K.; Yang, P.-C. A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites. Nanomaterials 2023, 13, 2173. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Gao, X.; Zou, J.; Pang, F. Research on Photocatalytic Wastewater Treatment Reactors: Design, Optimization, and Evaluation Criteria. Catalysts 2023, 13, 974. [Google Scholar] [CrossRef]
- Song, Q.; Chen, X.; Hua, Y.; Chen, S.; Ren, L.; Dai, X. Biological Treatment Processes for Saline Organic Wastewater and Related Inhibition Mechanisms and Facilitation Techniques: A Comprehensive Review. Environ. Res. 2023, 239. [Google Scholar] [CrossRef]
- Ma, Z.; Chang, H.; Liang, Y.; Meng, Y.; Ren, L.; Liang, H. Research Progress and Trends on State-of-the-Art Membrane Technologies in Textile Wastewater Treatment. Sep. Purif. Technol. 2024, 333. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M. Advanced Membrane Technologies for Wastewater Treatment and Recycling. Membranes 2023, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Li, J.; Zeng, P.; Duan, L.; Dong, J.; Ma, Y.; Yang, L. The Application of Membrane Separation Technology in the Pharmaceutical Industry. Membranes 2024, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Sivodia, C.; Sinha, A. Assessment of Graphite Electrode on the Removal of Anticancer Drug Cytarabine via Indirect Electrochemical Oxidation Process: Kinetics & Pathway Study. Chemosphere 2020, 243, 125456. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, A.M.Z.; Martínez-Huitle, C.A.; da Silva, D.R. Application of Electrochemical Technology for Removing Petroleum Hydrocarbons from Produced Water Using a DSA-Type Anode at Different Flow Rates. Fuel 2010, 89, 531–534. [Google Scholar] [CrossRef]
- Santos, I.D.; Dezotti, M.; Dutra, A.J.B. Electrochemical Treatment of Effluents from Petroleum Industry Using a Ti/RuO2 Anode. Chem. Eng. J. 2013, 226, 293–299. [Google Scholar] [CrossRef]
- Da Silva, R.G.; Neto, S.A.; De Andrade, A.R. Electrochemical Degradation of Reactive Dyes at Different DSA® Compositions. J. Braz. Chem. Soc. 2011, 22, 126–133. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Applicability of Electrochemical Methods to Carwash Wastewaters for Reuse. Part 1: Anodic Oxidation with Diamond and Lead Dioxide Anodes. J. Electroanal. Chem. 2010, 638, 28–32. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; De Battisti, A.; Ferro, S.; Reyna, S.; Cerro-López, M.; Quiro, M.A. Removal of the Pesticide Methamidophos from Aqueous Solutions by Electrooxidation Using Pb/PbO2, Ti/SnO2, and Si/BDD Electrodes. Environ. Sci. Technol. 2008, 42, 6929–6935. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Esmilaire, R.; van Hullebusch, E.; Esposito, G.; Oturan, M.A. Sub-Stoichiometric Titanium Oxide (Ti4O7) as a Suitable Ceramic Anode for Electrooxidation of Organic Pollutants: A Case Study of Kinetics, Mineralization and Toxicity Assessment of Amoxicillin. Water Res. 2016, 106, 171–182. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Ning, P.; Xin, P.; Chen, Z.; Wang, Q.; Uvdal, K.; Hu, Z. Nested Hollow Architectures of Nitrogen-Doped Carbon-Decorated Fe, Co, Ni-Based Phosphides for Boosting Water and Urea Electrolysis. Nano Res. 2022, 15, 1916–1925. [Google Scholar] [CrossRef]
- Pourzamani, H.; Mengelizadeh, N.; Hajizadeh, Y.; Mohammadi, H. Electrochemical Degradation of Diclofenac Using Three-Dimensional Electrode Reactor with Multi-Walled Carbon Nanotubes. Environ. Sci. Pollut. Res. 2018, 25, 24746–24763. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarlou, H.; Pedersen, N.L.; Simonsen, M.E.; Muff, J. Nitrogen-Doped Graphene Iron-Based Particle Electrode Outperforms Activated Carbon in Three-Dimensional Electrochemical Water Treatment Systems. Water 2020, 12, 3121. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Tshibangu, J.; Delegan, N.; El Khakani, M.A. Electrochemical Treatment of Domestic Wastewater Using Boron-Doped Diamond and Nanostructured Amorphous Carbon Electrodes. Environ. Sci. Pollut. Res. 2014, 21, 6578–6589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kazim, F.M.; Ma, S.; Qu, K.; Li, M.; Wang, Y.; Hu, H.; Cai, W.; Yang, Z. Nitrogen Dopants in Nickel Nanoparticles Embedded Carbon Nanotubes Promote Overall Urea Oxidation. Appl. Catal. B Environ. 2021, 280, 119436. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, H.; Niu, J.; Zhou, Y.; Wang, C.; Wang, Y. Hydroxyl Multi-Walled Carbon Nanotube-Modified Nanocrystalline PbO2 Anode for Removal of Pyridine from Wastewater. J. Hazard. Mater. 2017, 327, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Gao, S.; Li, X.; Sun, Z.; Hu, X. Preparation of CeO2-ZrO2 and Titanium Dioxide Coated Carbon Nanotube Electrode for Electrochemical Degradation of Ceftazidime from Aqueous Solution. J. Electroanal. Chem. 2019, 841, 10–20. [Google Scholar] [CrossRef]
- Chang, L.; Zhou, Y.; Duan, X.; Liu, W.; Xu, D. Preparation and Characterization of Carbon Nanotube and Bi Co-Doped PbO2 Electrode. J. Taiwan Inst. Chem. Eng. 2014, 45, 1338–1346. [Google Scholar] [CrossRef]
- Sun, Z.; Ni, Y.; Wu, Y.; Yue, W.; Zhang, G.; Bai, J. Electrocatalytic Degradation of Methyl Orange and 4-Nitrophenol on a Ti/TiO2-NTA/La-PbO2 Electrode: Electrode Characterization and Operating Parameters. Environ. Sci. Pollut. Res. 2023, 30, 6262–6274. [Google Scholar] [CrossRef] [PubMed]
- Pourzamani, H.; Hajizadeh, Y.; Mengelizadeh, N. Application of Three-Dimensional Electrofenton Process Using MWCNTs-Fe3O4 Nanocomposite for Removal of Diclofenac. Process Saf. Environ. Prot. 2018, 119, 271–284. [Google Scholar] [CrossRef]
- Savić, B.G.; Stanković, D.M.; Živković, S.M.; Ognjanović, M.R.; Tasić, G.S.; Mihajlović, I.J.; Brdarić, T.P. Electrochemical Oxidation of a Complex Mixture of Phenolic Compounds in the Base Media Using PbO2-GNRs Anodes. Appl. Surf. Sci. 2020, 529, 147120. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Liu, M.; Li, P.; Zhang, Y.; Cao, T.; Li, H.; Lei, Y.; Liu, L.; Li, D. Electrochemical Degradation of Refractory Pollutant Using a Novel Microstructured TiO2 Nanotubes/Sb-Doped SnO2 Electrode. Environ. Sci. Technol. 2009, 43, 1480–1486. [Google Scholar] [CrossRef]
- Yu, S.; Hao, C.; Li, Z.; Zhang, R.; Dang, Y.; Zhu, J.J. Promoting the Electrocatalytic Performance of PbO2 Nanocrystals via Incorporation of Y2O3 Nanoparticles: Degradation Application and Electrocatalytic Mechanism. Electrochim. Acta 2021, 369, 137671. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Cui, H.; Ding, L.; Wei, Z.; Zhai, J. Fabrication of Cerium-Doped Lead Dioxide Anode with Improved Electrocatalytic Activity and Its Application for Removal of Rhodamine B. Chem. Eng. J. 2013, 228, 806–814. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Prasad, K.; Yoo, J.; Kim, J.; Yoo, K. CuO-SnO2 Nanocomposites: Efficient and Cost-Effective Electrocatalysts for Urea Oxidation. Mater. Lett. 2023, 353, 10–13. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.; Zhou, X.; Li, J.; Sun, X.; Shen, J.; Wang, L.; Han, W. Electrochemical Degradation of Pyridine by Ti/SnO2-Sb Tubular Porous Electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, L.; Subramanian, P.; Ji, S.; Kannan, P. Co, Fe-Ions Intercalated Ni(OH)2 Network-like Nanosheet Arrays as Highly Efficient Non-Noble Catalyst for Electro-Oxidation of Urea. Int. J. Hydrogen Energy 2021, 46, 34318–34332. [Google Scholar] [CrossRef]
- Đuričić, T.; Prosen, H.; Kravos, A.; Mićin, S.; Kalčíková, G.; Malinović, B.N. Electrooxidation of Phenol on Boron-Doped Diamond and Mixed-Metal Oxide Anodes: Process Evaluation, Transformation By-Products, and Ecotoxicity. J. Electrochem. Soc. 2023, 170, 023503. [Google Scholar] [CrossRef]
- Audino, F.; Arboleda, J.; Petrovic, M.; Cudinach, R.G.; Pérez, S.S. Pharmaceuticals Removal by Ozone and Electro-Oxidation in Combination with Biological Treatment. Water 2023, 15, 3180. [Google Scholar] [CrossRef]
- Vinayagam, V.; Palani, K.N.; Ganesh, S.; Rajesh, S.; Akula, V.V.; Avoodaiappan, R.; Kushwaha, O.S.; Pugazhendhi, A. Recent Developments on Advanced Oxidation Processes for Degradation of Pollutants from Wastewater with Focus on Antibiotics and Organic Dyes. Environ. Res. 2024, 240, 117500. [Google Scholar] [CrossRef] [PubMed]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Electroplating Wastewater Treatment by Electro-Oxidation Using Synthesized New Electrode: Experimental, Optimization, Kinetics, and Cost Analysis. Process Saf. Environ. Prot. 2024, 183, 735–756. [Google Scholar] [CrossRef]
- Magdaleno, A.L.; Brillas, E.; Garcia-Segura, S.; dos Santos, A.J. Comparison of Electrochemical Advanced Oxidation Processes for the Treatment of Complex Synthetic Dye Mixtures. Sep. Purif. Technol. 2024, 345, 127295. [Google Scholar] [CrossRef]
- Simić, M.D.; Savić, B.G.; Ognjanović, M.R.; Stanković, D.M.; Relić, D.J.; Aćimović, D.D.; Brdarić, T.P. Degradation of Bisphenol A on SnO2-MWCNT Electrode Using Electrochemical Oxidation. J. Water Process Eng. 2023, 51, 103416. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Mao, G.; Hu, H.; Liu, X.; Crittenden, J.; Huang, N. A Bibliometric Analysis of Industrial Wastewater Treatments from 1998 to 2019. Environ. Pollut. 2021, 275, 115785. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zeng, H. A Bibliometric Analysis of Research Progress in Sulfate-Rich Wastewater Pollution Control Technology. Ecotoxicol. Environ. Saf. 2022, 238, 113626. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, D.; Hamdaoui, O.; Vasseghian, Y.; Momotko, M.; Boczkaj, G.; Kyzas, G.Z.; Wang, C. Bibliometric Analysis and Literature Review of Ultrasound-Assisted Degradation of Organic Pollutants. Sci. Total Environ. 2023, 876, 162551. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, H.; Duan, Z.; Huang, Z.; Wei, K. Research Progress and Trends of Biochar in the Field of Wastewater Treatment by Electrochemical Advanced Oxidation Processes (EAOPs): A Bibliometric Analysis. J. Hazard. Mater. Adv. 2023, 10, 100305. [Google Scholar] [CrossRef]
- Usman, M.; Ho, Y.-S. A Bibliometric Study of the Fenton Oxidation for Soil and Water Remediation. J. Environ. Manag. 2020, 270, 110886. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Cerisola, G. Direct And Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical Oxidation of Organic Pollutants for the Wastewater Treatment: Direct and Indirect Processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; He, J.; Zhang, J. Preparation of Ti/SnO2-Sb Electrodes Modified by Carbon Nanotube for Anodic Oxidation of Dye Wastewater and Combination with Nanofiltration. Electrochim. Acta 2014, 117, 192–201. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea Electrolysis: Direct Hydrogen Production from Urine. Chem. Commun. 2009, 4859. [Google Scholar] [CrossRef] [PubMed]
- Vedharathinam, V.; Botte, G.G. Understanding the Electro-Catalytic Oxidation Mechanism of Urea on Nickel Electrodes in Alkaline Medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Q.; Lei, L.; Ma, C.A.; Wang, D. Long life modified lead dioxide anode for organic wastewater treatment: Electrochemical characteristics and degradation mechanism. Environ. Sci. Technol. 2005, 39, 363–370. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B:Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem.Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Eisa, T.; Mohamed, H.O.; Abdelkareem, M.A.; Allagui, A.; Alawadhi, H.; Chae, K.J. Direct urea fuel cells: Challenges and opportunities. J. Power Sources 2019, 417, 159–175. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, Y.; Liu, H.; Chen, Y. The Role of Nanomaterials and Nanotechnologies in Wastewater Treatment: A Bibliometric Analysis. Nanoscale Res. Lett. 2018, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Facile Synthesis of Mesoporous Spinel NiCo2O4 Nanostructures as Highly Efficient Electrocatalysts for Urea Electro-Oxidation. Nanoscale 2014, 6, 1369–1376. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, H.; Feng, L. Efficient Synergism of NiSe2 Nanoparticle/NiO Nanosheet for Energy-Relevant Water and Urea Electrocatalysis. Appl. Catal. B Environ. 2020, 276, 119165. [Google Scholar] [CrossRef]
- Forslund, R.P.; Mefford, J.T.; Hardin, W.G.; Alexander, C.T.; Johnston, K.P.; Stevenson, K.J. Nanostructured LaNiO3 Perovskite Electrocatalyst for Enhanced Urea Oxidation. ACS Catal. 2016, 6, 5044–5051. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Lei, F.; Peng, X.; Liu, W.; Gao, L.; Hao, P.; Cui, G.; Tang, B. Partially Amorphous Nickel–Iron Layered Double Hydroxide Nanosheet Arrays for Robust Bifunctional Electrocatalysis. J. Mater. Chem. A 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Asiri, A.M.; Sun, X. Enhanced Electrooxidation of Urea Using NiMoO4·xH2O Nanosheet Arrays on Ni Foam as Anode. Electrochim. Acta 2015, 153, 456–460. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Diaz, L.A.; Botte, G.G. Nickel Nanowires as Effective Catalysts for Urea Electro-Oxidation. Electrochim. Acta 2014, 134, 266–271. [Google Scholar] [CrossRef]
- Sha, L.; Yin, J.; Ye, K.; Wang, G.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. The Construction of Self-Supported Thorny Leaf-like Nickel-Cobalt Bimetal Phosphides as Efficient Bifunctional Electrocatalysts for Urea Electrolysis. J. Mater. Chem. A 2019, 7, 9078–9085. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, C.; Liu, W.; Zhao, X.; Chang, L. Fabrication of a Novel PbO2 Electrode with a Graphene Nanosheet Interlayer for Electrochemical Oxidation of 2-Chlorophenol. Electrochim. Acta 2017, 240, 424–436. [Google Scholar] [CrossRef]
- Dai, J.; Feng, H.; Shi, K.; Ma, X.; Yan, Y.; Ye, L.; Xia, Y. Electrochemical Degradation of Antibiotic Enoxacin Using a Novel PbO2 Electrode with a Graphene Nanoplatelets Inter-Layer: Characteristics, Efficiency and Mechanism. Chemosphere 2022, 307, 135833. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, Z.; Zhou, M.; Zhou, L.; Xi, B. Efficient Degradation of P-Nitrophenol by Electro-Oxidation on Fe Doped Ti/TiO2 Nanotube/PbO2 Anode. Sep. Purif. Technol. 2014, 128, 67–71. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Z.; Wang, F.; Hong, P.; Wang, C.; Ouyang, X.; Zhu, C.; Wei, Y.; Hun, Y.; Fang, W. Fabrication of Cerium Doped Ti/NanoTiO2/PbO2 Electrode with Improved Electrocatalytic Activity and Its Application in Organic Degradation. Electrochim. Acta 2016, 201, 240–250. [Google Scholar] [CrossRef]
- Yang, C.; Shang, S.; Li, X. Fabrication of Sulfur-Doped TiO2 Nanotube Array as a Conductive Interlayer of PbO2 Anode for Efficient Electrochemical Oxidation of Organic Pollutants. Sep. Purif. Technol. 2021, 258, 118035. [Google Scholar] [CrossRef]
- Gao, G.; Pan, M.; Vecitis, C.D. Effect of the Oxidation Approach on Carbon Nanotube Surface Functional Groups and Electrooxidative Filtration Performance. J. Mater. Chem. A 2015, 3, 7575–7582. [Google Scholar] [CrossRef]
- Xu, W.; Lan, R.; Du, D.; Humphreys, J.; Walker, M.; Wu, Z.; Wang, H.; Tao, S. Directly Growing Hierarchical Nickel-Copper Hydroxide Nanowires on Carbon Fibre Cloth for Efficient Electrooxidation of Ammonia. Appl. Catal. B Environ. 2017, 218, 470–479. [Google Scholar] [CrossRef]
- Luo, M.; Yuan, S.; Tong, M.; Liao, P.; Xie, W.; Xu, X. An Integrated Catalyst of Pd Supported on Magnetic Fe3O4 Nanoparticles: Simultaneous Production of H2O2 and Fe2+ for Efficient Electro-Fenton Degradation of Organic Contaminants. Water Res. 2014, 48, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Lang, C.C.; Gao, M.R.; Chen, Y.; Fu, Q.Q.; Duan, Y.; Yu, S.H. Ni-Mo-O Nanorod-Derived Composite Catalysts for Efficient Alkaline Water-to-Hydrogen Conversion: Via Urea Electrolysis. Energy Environ. Sci. 2018, 11, 1890–1897. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.H.; Lim, T.T. Recent Development of Mixed Metal Oxide Anodes for Electrochemical Oxidation of Organic Pollutants in Water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Xie, R.; Meng, X.; Sun, P.; Niu, J.; Jiang, W.; Bottomley, L.; Li, D.; Chen, Y.; Crittenden, J. Electrochemical Oxidation of Ofloxacin Using a TiO2-Based SnO2-Sb/Polytetrafluoroethylene Resin-PbO2 Electrode: Reaction Kinetics and Mass Transfer Impact. Appl. Catal. B Environ. 2017, 203, 515–525. [Google Scholar] [CrossRef]
- Cheng, Y.; Liao, F.; Dong, H.; Wei, H.; Geng, H.; Shao, M. Engineering CoN/Ni(OH)2 Heterostructures with Improved Intrinsic Interfacial Charge Transfer toward Simultaneous Hydrogen Generation and Urea-Rich Wastewater Purification. J. Power Sources 2020, 480, 229151. [Google Scholar] [CrossRef]
- Yan, X.; Hu, Q.-T.; Wang, G.; Zhang, W.-D.; Liu, J.; Li, T.; Gu, Z.-G. NiCo Layered Double Hydroxide/Hydroxide Nanosheet Heterostructures for Highly Efficient Electro-Oxidation of Urea. Int. J. Hydrogen Energy 2020, 45, 19206–19213. [Google Scholar] [CrossRef]
- Wei, D.; Tang, W.; Ma, N.; Wang, Y. NiCo Bimetal Organic Frames Derived Well-Matched Electrocatalyst Pair for Highly Efficient Overall Urea Solution Electrolysis. J. Alloys Compd. 2021, 874, 159945. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, G.; Yin, S.; Yu, C.; Chen, W.; Yu, T.; Luo, L.; Xia, Y.; Tsiakaras, P. Design and Synthesis of Highly Performing Bifunctional Ni-NiO-MoNi Hybrid Catalysts for Enhanced Urea Oxidation and Hydrogen Evolution Reactions. ACS Sustain. Chem. Eng. 2020, 8, 7174–7181. [Google Scholar] [CrossRef]
- He, M.; Hu, S.; Feng, C.; Wu, H.; Liu, H.; Mei, H. Interlaced Rosette-like MoS2/Ni3S2/NiFe-LDH Grown on Nickel Foam: A Bifunctional Electrocatalyst for Hydrogen Production by Urea-Assisted Electrolysis. Int. J. Hydrogen Energy 2020, 45, 23–35. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G. Reduced Graphene Oxide Supported Nickel Tungstate Nano-Composite Electrocatalyst for Anodic Urea Oxidation Reaction in Direct Urea Fuel Cell. Int. J. Hydrogen Energy 2020, 45, 33500–33511. [Google Scholar] [CrossRef]
- Gopi, S.; Al-Mohaimeed, A.M.; Elshikh, M.S.; Yun, K. Facile Fabrication of Bifunctional SnO–NiO Heteromixture for Efficient Electrocatalytic Urea and Water Oxidation in Urea-Rich Waste Water. Environ. Res. 2021, 201, 111589. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Luo, L.; Zhang, H.; Chen, W.; Gao, Z.; Yin, S.; Tsiakaras, P. Novel Bifunctional V2O3 Nanosheets Coupled with N-Doped-Carbon Encapsulated Ni Heterostructure for Enhanced Electrocatalytic Oxidation of Urea-Rich Wastewater. ACS Appl. Mater. Interfaces 2020, 12, 38061–38069. [Google Scholar] [CrossRef]
- El Aggadi, S.; Kerroum, Y.; El Hourch, A. Elaboration and Characterization of Fe/C-Doped Lead Dioxide-Modified Anodes for Electrocatalytic Degradation of Reactive Yellow 14. J. Appl. Electrochem. 2023, 53, 109–119. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, K.; Han, W.; Wang, L.; Sun, X.; Li, J. Electrochemical Degradation of Tricyclazole in Aqueous Solution Using Ti/SnO2-Sb/PbO2 Anode. J. Electroanal. Chem. 2013, 705, 68–74. [Google Scholar] [CrossRef]
- Wen-wu, L.; Xiu-ping, W.; Xue-yan, T.; Chang-yong, W. Treatment of Pretreated Coking Wastewater by Flocculation, Alkali out, Air Stripping, and Three-Dimensional Electrocatalytic Oxidation with Parallel Plate Electrodes. Environ. Sci. Pollut. Res. 2014, 21, 11457–11468. [Google Scholar] [CrossRef]
- Sharan, S.; Khare, P.; Shankar, R.; Tyagi, A.; Khare, A. Development of 3D Network of Zn-Oxide Nanorods Assisted with PbO2/Pb Electrode for Electrochemical Oxidation of Methylene Blue in Aqueous Phase. J. Taiwan Inst. Chem. Eng. 2023, 144, 104739. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a Hydrogen Carrier: A Perspective on Its Potential for Safe, Sustainable and Long-Term Energy Supply. Energy Environ. Sci. 2011, 4, 1216. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea Removal from Aqueous Solutions—A Review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef]
- Aćimović, D.; Vasić Anićijević, D. Electrooxidative Removal of Organophosphates—A Combined Experimental and Theoretical Approach. In Organophosphates: Detection, Exposure and Occurrence. Volume 1: Impact on Health and the Natural Environment; Lazarević-Pašti, T., Ed.; Nova Science Publishers: New York, NY, USA, 2022; Volume 1, pp. 215–250. ISBN 9781685076528. [Google Scholar]
- Han, Q.; Wang, M.; Sun, F.; Yu, B.; Dong, Z.; Li, P.; Luo, J.; Li, M.; Jin, X.; Dai, Z. Effectiveness and Degradation Pathways of Bisphenol A (BPA) Initiated by Hydroxyl Radicals and Sulfate Radicals in Water: Initial Reaction Sites Based on DFT Prediction. Environ. Res. 2023, 216. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Goel, S. Electrocoagulation and Electrooxidation Technologies for Pesticide Removal from Water or Wastewater: A Review. Chemosphere 2022, 302, 134709. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Reddy, N.; Zhu, Z.; Zheng, J.; Wang, W.; Liu, B.; Hu, C. MOFFeCo/B-CN Composites Achieve Efficient Degradation of Antibiotics in a Non-Homogeneous Concurrent Photocatalytic-Persulfate Activation System. Sci. Total Environ. 2023, 858, 159795. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Xu, F.; Chen, J.; Hao, Y.; Guo, Y.; Zhu, C.; Luo, S.; Jiang, B. Electrogenerated Oxychlorides Induced Overlooked Negative Effects on Electro-Oxidation Wastewater Treatment in Terms of over-Evaluated COD Removal Efficiency and Biotoxicity. J. Hazard. Mater. 2023, 456, 131667. [Google Scholar] [CrossRef] [PubMed]
- Ječmenica Dučić, M.; Krstić, A.; Zdolšek, N.; Aćimović, D.; Savić, B.; Brdarić, T.; Vasić Anićijević, D. Low-Cost Graphene-Based Composite Electrodes for Electrochemical Oxidation of Phenolic Dyes. Crystals 2023, 13, 125. [Google Scholar] [CrossRef]

| 10 Most Cited Journals | 10 Most Co-Cited Journals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Journal | Documents | Citations Counts | IF (2022) | h Index | WOS Category | Journal | Co-Citations Counts | IF (2022) | h Index | WOS Category |
| 1 | Electrochimica Acta | 43 | 1999 | 6.6 | 263 | Electrochemistry (8/30) | Electrochimica acta | 2354 | 6.6 | 263 | Chemical Engineering (miscellaneous) (Q1); Electrochemistry (Q1) |
| 2 | Journal of Environmental Chemical Engineering | 12 | 1263 | 7.7 | 107 | Engineering, Chemical (16/141) Engineering, Environmental (12/55 | Chemical Engineering Journal | 1572 | 15.1 | 280 | Engineering, Chemical (5/141) Engineering, Environmental (3/55) |
| 3 | Chemical Engineering Journal | 26 | 1036 | 15.1 | 280 | Engineering, Chemical (5/141) Engineering, Environmental (3/55) | Environmental Science and Technology | 1210 | 11.4 | 456 | Engineering, Environmental (7/55) Environmental Sciences (19/274) |
| 4 | Chemosphere | 36 | 1017 | 8.8 | 288 | Environmental Sciences (30/274) | Journal of Hazardous Materials | 1195 | 13.6 | 329 | Engineering, Environmental (4/55), Environmental Sciences (10/274) |
| 5 | Applied Catalysis. B: Environmental | 12 | 886 | 22.1 | 301 | Chemistry, Physical (6/161), Engineering, Chemical (3/141), Engineering, Environmental (1/55) | Applied Catalysis. B: Environmental | 1172 | 22.1 | 301 | Chemistry, Physical (6/161), Engineering, Chemical (3/141), Engineering, Environmental (1/55) |
| 6 | Separation and Purification Technology | 26 | 504 | 8.6 | 191 | Engineering, Chemical (12/141) | Chemosphere | 1096 | 8.8 | 288 | Environmental Sciences (30/274) |
| 7 | Journal of Electroanalytical Chemistry | 17 | 477 | 4.5 | 167 | Chemistry, Analytical (18/86), Electrochemistry (12/30) | Water Research | 1000 | 12.8 | 354 | Engineering, Environmental (6/55) Environmental Sciences (13/274) Water Resources (1/103) |
| 8 | Journal of Materials Chemistry A | 5 | 428 | 11.9 | 270 | Chemistry, Physical (24/161), Energy & Fuels (11/117), Materials Science, Multidisciplinary (32/342) | Separation and Purification Technology | 719 | 8.6 | 191 | Engineering, Chemical (12/141) |
| 9 | Journal of Hazardous Materials | 16 | 376 | 13.6 | 329 | Engineering, Environmental (4/55), Environmental Sciences (10/274) | Journal of Electroanalytical Chemistry | 634 | 4.5 | 167 | Chemistry, Analytical (18/86), Electrochemistry (12/30) |
| 10 | Journal of Power Sources | 8 | 367 | 9.2 | 339 | Chemistry, Physical (36/161) Electrochemistry (4/30) Energy & Fuels (21/117) Materials Science, Multidisciplinary (59/342) | Journal of Materials Chemistry A | 503 | 11.9 | 270 | Chemistry, Physical (24/161), Energy & Fuels (11/117), Materials Science, Multidisciplinary (32/342) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brdarić, T.P.; Aćimović, D.D.; Savić Rosić, B.G.; Simić, M.D.; Stojanović, K.D.; Vranješ, Z.M.; Vasić Anićijević, D. Bibliometric Analysis of Nanostructured Anodes for Electro-Oxidative Wastewater Treatment. Sustainability 2024, 16, 3982. https://doi.org/10.3390/su16103982
Brdarić TP, Aćimović DD, Savić Rosić BG, Simić MD, Stojanović KD, Vranješ ZM, Vasić Anićijević D. Bibliometric Analysis of Nanostructured Anodes for Electro-Oxidative Wastewater Treatment. Sustainability. 2024; 16(10):3982. https://doi.org/10.3390/su16103982
Chicago/Turabian StyleBrdarić, Tanja P., Danka D. Aćimović, Branislava G. Savić Rosić, Marija D. Simić, Katarina D. Stojanović, Zdravko M. Vranješ, and Dragana Vasić Anićijević. 2024. "Bibliometric Analysis of Nanostructured Anodes for Electro-Oxidative Wastewater Treatment" Sustainability 16, no. 10: 3982. https://doi.org/10.3390/su16103982










