Interspecific Differences in the Effects of Calcium and Phosphorus Coprecipitation Induced by Submerged Plants on the Water-to-Phosphorus Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Sediments, and Their Preculture
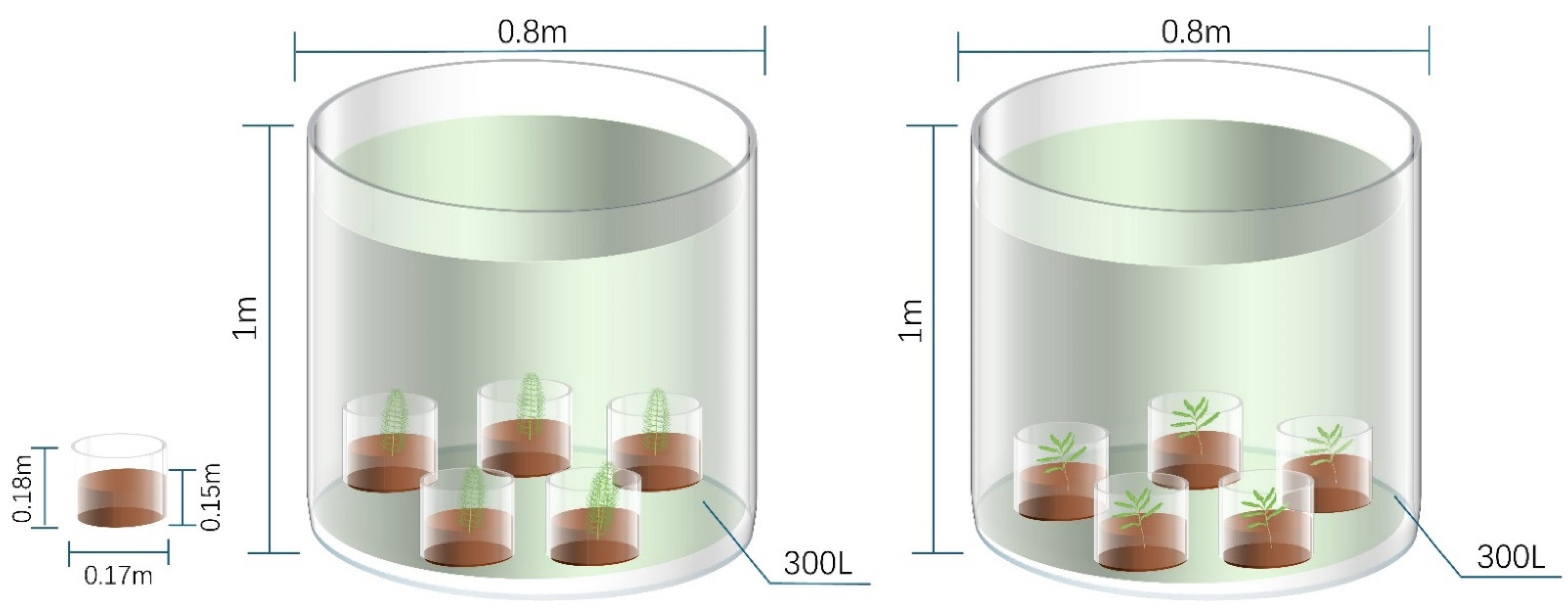
2.2. Experimental Design
2.3. Experimental Measurements
2.4. Data Analyses
3. Results
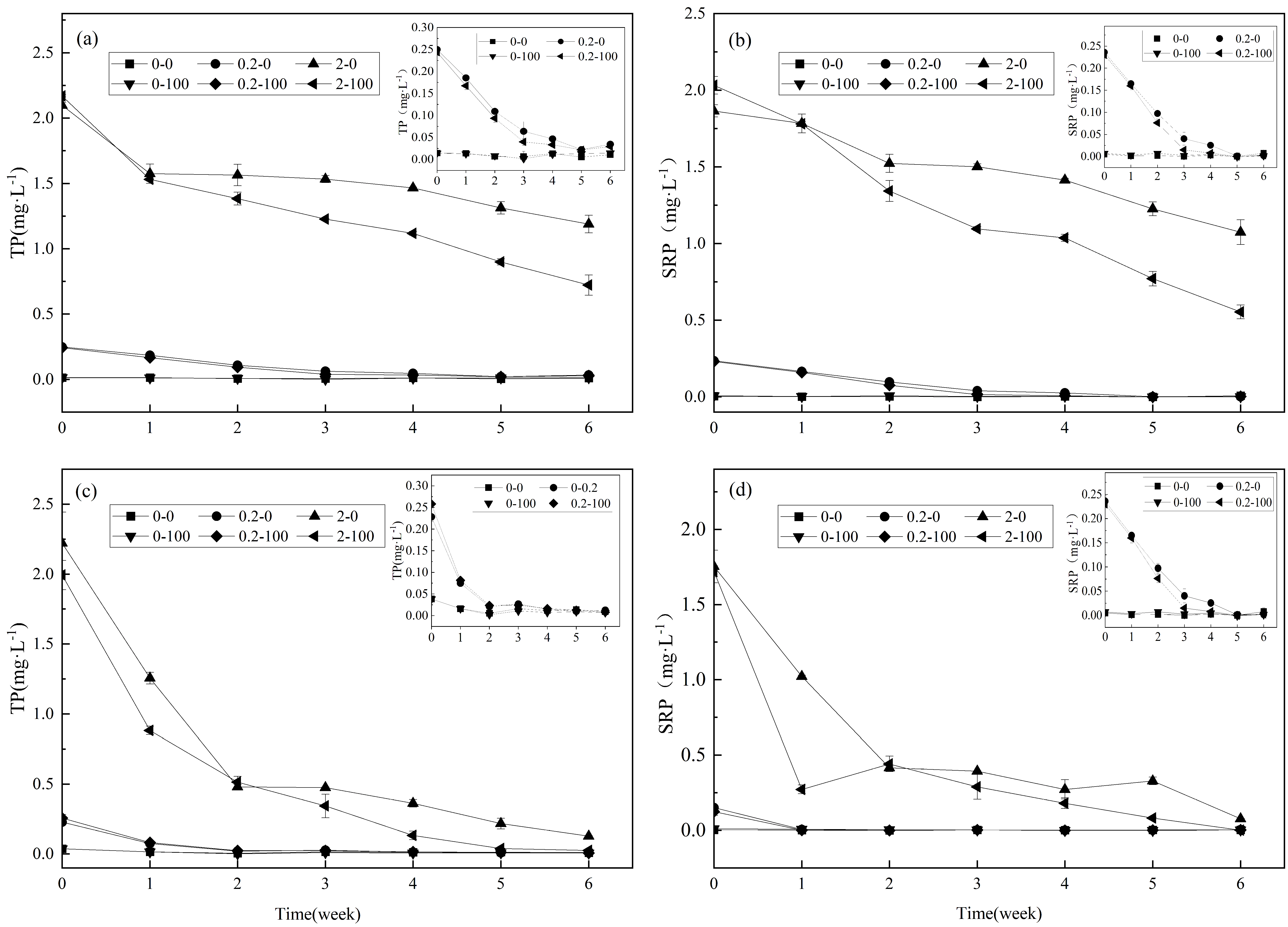
3.1. Variation in TP and SRP in the Overlying Water
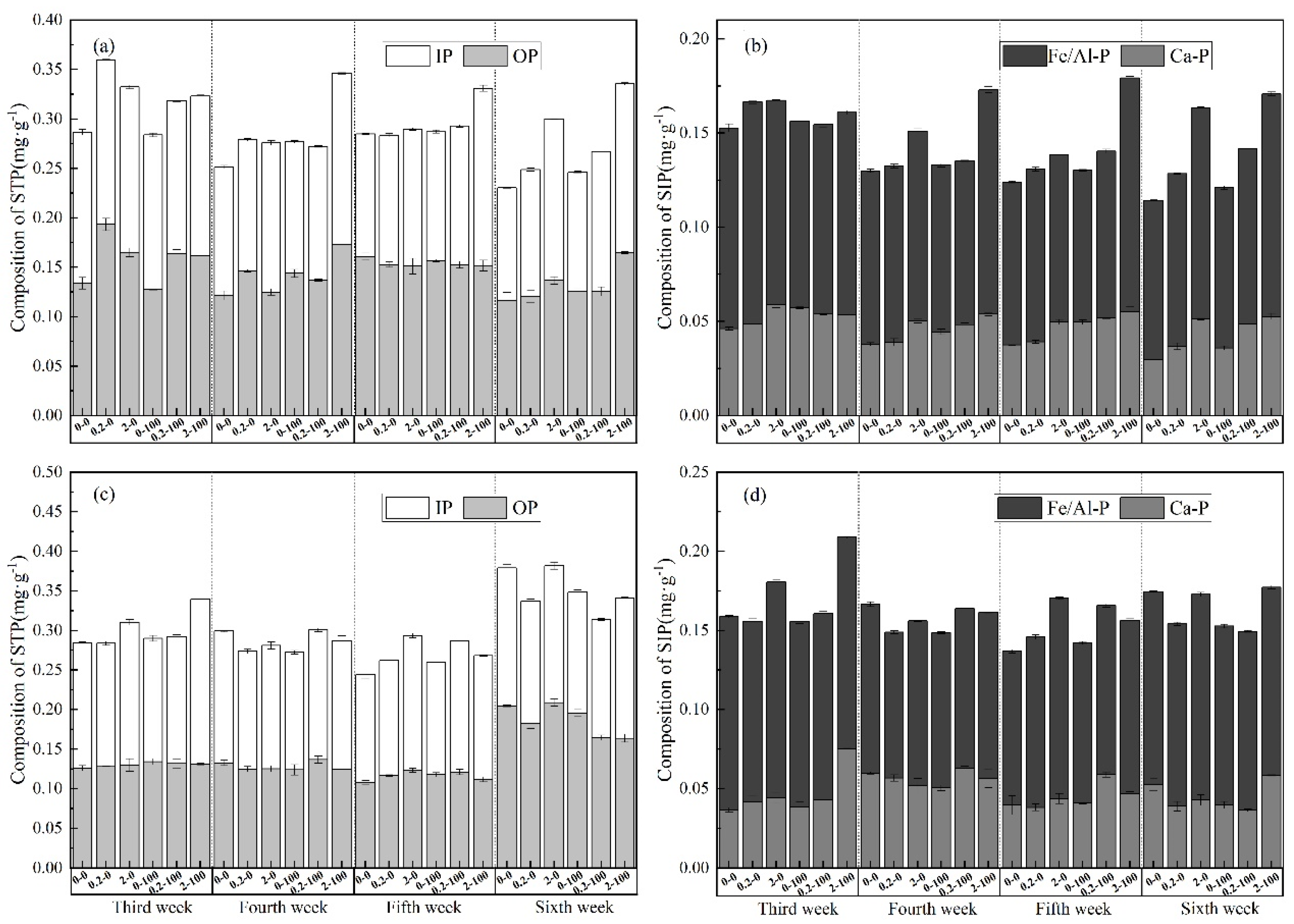
3.2. Changes in Ash Phosphorus Composition
3.3. Changes in the Phosphorus Concentration in the Sediments
4. Discussion
4.1. Variations in TP and SRP in the Overlying Water
4.2. Composition and Variation in Ash Phosphorus
4.3. Composition and Variation in Phosphorus in Sediments
5. Conclusions
- (1)
- During the experiment, the decrease in TP and SRP in overlying water with C. demersum was higher than that with P. crispus. And, this reduction increased after the addition of calcium ions.
- (2)
- Compared with C. demersum, P. crispus enriched more phosphorus during the experiment because of its higher abilities to induce carbonate and phosphorus coprecipitation.
- (3)
- When exogenous phosphorus and calcium are added, two submerged species show differentiated responses and effects on sediment phosphorus fractions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, T.; Wang, C.; Wang, P.; Qian, J.; Wang, X. How physiological and physical processes contribute to the phenology of cyanobacterial blooms in large shallow lakes: A new Euler-Lagrangian coupled model. Water Res. 2018, 140, 34–43. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.A.; Jarvie, H.P. Delivery and cycling of phosphorus in rivers: A review. Sci. Total Environ. 2008, 400, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Ni, Z.; Wang, S. Characteristics of bioavailable phosphorus in sediment and potential environmental risks in Poyang Lake: The largest freshwater lake in China. Ecol. Indic. 2020, 115, 106409. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Huang, X.-L. Relative importance of solid-phase phosphorus and iron on the sorption behavior of sediments. Environ. Sci. Technol. 2007, 41, 2789–2795. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Song, H.; An, J.; Dong, B.; Wu, Y.; Wang, Y.; Li, B.; Liu, Q.; Yu, W. Influence of Potamogeton crispus harvesting on phosphorus composition of Lake Yimeng. Sci. Rep. 2022, 12, 17616. [Google Scholar] [CrossRef]
- Dai, Y.; Jia, C.; Liang, W.; Hu, S.; Wu, Z. Effects of the submerged macrophyte Ceratophyllum demersum L. on restoration of a eutrophic waterbody and its optimal coverage. Ecol. Eng. 2012, 40, 113–116. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Zhang, Y.; Liu, B.; Zhou, Q.; Zeng, L.; He, F.; Wu, Z. Synergistic removal effect of P in sediment of all fractions by combining the modified bentonite granules and submerged macrophyte. Sci. Total Environ. 2018, 626, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Chao, C.; Yu, H.; Yu, D.; Liu, C. Submerged macrophytes successfully restored a subtropical aquacultural lake by controlling its internal phosphorus loading. Environ. Pollut. 2021, 268, 115949. [Google Scholar] [CrossRef]
- Siong, K.; Asaeda, T. Does calcite encrustation in Chara provide a phosphorus nutrient sink? J. Environ. Qual. 2006, 35, 490–494. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, Z.; Zhang, J.; Zhang, W.; Mba, F.O. Phosphorus removal from water of eutrophic Lake Donghu by five submerged macrophytes. Desalination 2009, 242, 193–204. [Google Scholar] [CrossRef]
- Kufel, L.; Biardzka, E.; Strzałek, M. Calcium carbonate incrustation and phosphorus fractions in five charophyte species. Aquat. Bot. 2013, 109, 54–57. [Google Scholar] [CrossRef]
- Kufel, L.; Strzałek, M.; Biardzka, E. Site-and species-specific contribution of charophytes to calcium and phosphorus cycling in lakes. Hydrobiologia 2016, 767, 185–195. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.Y. Effect of calcium addition on phosphorus enrichment capacity of two submerged plants (Potamogeton crispus L. and Ceratophyllum demersum L.) in water bodies. J. Lake Sci. 2020, 32, 406–416. [Google Scholar] [CrossRef]
- Danen-Louwerse, H.J.; Lijklema, L.; Coenraats, M. Coprecipitation of phosphate with calcium carbonate in Lake Veluwe. Water Res. 1995, 29, 1781–1785. [Google Scholar] [CrossRef]
- House, W.; Casey, H.; Donaldson, L.; Smith, S. Factors affecting the coprecipitation of inorganic phosphate with calcite in hardwaters—I Laboratory studies. Water Res. 1986, 20, 917–922. [Google Scholar] [CrossRef]
- Dittrich, M.; Koschel, R. Interactions between calcite precipitation (natural and artificial) and phosphorus cycle in the hardwater lake. Hydrobiologia 2002, 469, 49–57. [Google Scholar] [CrossRef]
- Wang, H.; Ni, L.; Yu, D. Significance of HCO3-alkalinity in calcification and utilization of dissolved inorganic carbon in Chara vulgaris. Aquat. Biol. 2017, 26, 169–178. [Google Scholar] [CrossRef][Green Version]
- Sand-Jensen, K.; Martinsen, K.T.; Jakobsen, A.L.; Sø, J.S.; Madsen-Østerbye, M.; Kjaer, J.E.; Kristensen, E.; Kragh, T. Large pools and fluxes of carbon, calcium and phosphorus in dense charophyte stands in ponds. Sci. Total Environ. 2021, 765, 142792. [Google Scholar] [CrossRef]
- Li, W.; Zhong, J.; Liu, J.; Dai, T.; Curtis, J. Allocation of phosphorus (P) into biomass and calcite encrustation in Chara at high and low P availability. Aquat. Sci. 2023, 85, 36. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Wu, X.; An, J.; Wu, Y.; Wang, Y.; Li, B.; Liu, Q.; Dong, B. Relationship between the coprecipitation of phosphorus-on-calcite by submerged macrophytes and the phosphorus cycle in water. J. Environ. Manag. 2022, 314, 115110. [Google Scholar] [CrossRef] [PubMed]
- Shilla, D.; Dativa, J. Biomass dynamics of charophyte-dominated submerged macrophyte communities in Myall Lake, NSW, Australia. Chem. Ecol. 2008, 24, 367–377. [Google Scholar] [CrossRef]
- Liu, G.; Guo, W.; Yuan, S.; Zhu, H.; Yang, T.; Zhou, Y.; Zhu, D. Occurrence and characterization of CaCO3- coprecipitation on the leaf surface of Potamogeton crispus in water. Environ. Sci. Pollut. Res. 2016, 23, 23308–23315. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yu, D. Turion production and nutrient reserves in Potamogeton crispus are influenced by sediment nutrient level. Aquat. Biol. 2011, 14, 21–28. [Google Scholar] [CrossRef][Green Version]
- Dai, Y.; Tang, H.; Chang, J.; Wu, Z.; Liang, W. What’s better, Ceratophyllum demersum L. or Myriophyllum verticillatum L., individual or combined? Ecol. Eng. 2014, 70, 397–401. [Google Scholar] [CrossRef]
- Jin, H.; Chen, K.; Wang, H.Y. Distinguished responses of curled pondweed (Potamogeton crispus L.) and hornwort (Ceratophyllum demersum L.) to NH4-N stress under elevated HCO3− conditions. Appl Ecol. Environ. Res. 2022, 20, 4737–4748. [Google Scholar] [CrossRef]
- GB11893-89; Water Quality—Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1989.
- Ruban, V.; López-Sánchez, J.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment. Environ. Monit. 1999, 1, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ruban, V.; López-Sánchez, J.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Development of a harmonised phosphorus extraction procedure and certification of a sediment reference material. J. Environ. Monit. 2001, 3, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; He, J.; Liu, Y.; Jiao, L.; Zhao, H.; Cheng, Y. The adsorption-release behavior of sediment phosphorus in a typical “grass-algae” coexisting lake and its influence mechanism during the transition sensitive period. Chemosphere 2022, 307, 135903. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, M.; Yin, P.; Li, J. Characterization of internal phosphorus loading in the sediment of a large eutrophic lake (Lake Taihu, China). Water Res. 2022, 225, 119125. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Song, H.; An, J.; Wu, Y.; Wang, Y.; Li, B.; Liu, Q.; Dong, B.; Yu, W. Effect of environmental factors on phosphorus transformation during the growth of submerged macrophytes. SN Appl. Sci. 2023, 5, 111. [Google Scholar] [CrossRef]
- Rao, Q.; Su, H.; Ruan, L.; Xia, W.; Deng, X.; Wang, L.; Xu, P.; Shen, H.; Chen, J.; Xie, P. Phosphorus enrichment affects trait network topologies and the growth of submerged macrophytes. Environ. Pollut. 2022, 292, 118331. [Google Scholar] [CrossRef]
- Lombardo, P.; Cooke, G.D. Ceratophyllum demersum–phosphorus interactions in nutrient enriched aquaria. Hydrobiologia 2003, 497, 79–90. [Google Scholar] [CrossRef]
- Wetzel, R.G. Marl encrustation on hydrophytes in several Michigan lakes. Oikos 1960, 11, 223–236. [Google Scholar] [CrossRef]
- Scheffer, M. Multiplicity of stable states in freshwater systems. Hydrobiologia 1994, 200, 475–486. [Google Scholar] [CrossRef]
- Drizo, A.; Comeau, Y.; Forget, C.; Chapuis, R.P. Phosphorus saturation potential: A parameter for estimating the longevity of constructed wetland systems. Environ. Sci. Technol. 2002, 36, 4642–4648. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Abramovich, D.; Siegrist, H.; Gujer, W. Kinetics of biologically induced phosphorus precipitation in waste-water treatment. Water Res. 1999, 33, 484–493. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Pang, Y.; Zhao, H.; Zhou, X.; Wu, F. Phosphorus fractions and phosphate sorption characteristics in relation to the sediment compositions of shallow lakes in the middle and lower reaches of Yangtze River region, China. J. Colloid Interface Sci. 2005, 289, 339–346. [Google Scholar] [CrossRef]
- Han, C.; Ren, J.; Wang, Z.; Yang, S.; Ke, F.; Xu, D.; Xie, X. Characterization of phosphorus availability in response to radial oxygen losses in the rhizosphere of Vallisneria spiralis. Chemosphere 2018, 208, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, S.; Luo, J. Transfer kinetics of phosphorus (P) in macrophyte rhizosphere and phytoremoval performance for lake sediments using DGT technique. J. Hazard. Mater. 2018, 350, 189–200. [Google Scholar] [CrossRef]
- Yuan, H.; Cai, Y.; Yang, Z.; Li, Q.; Liu, E.; Yin, H. Phosphorus removal from sediments by Potamogeton crispus: New high-resolution in-situ evidence for rhizosphere assimilation and oxidization-induced retention. J. Environ. Sci. 2021, 109, 181–192. [Google Scholar] [CrossRef]
- Horppila, J.; Nurminen, L. Effects of different macrophyte growth forms on sediment and P resuspension in a shallow lake. Hydrobiologia 2005, 545, 167–175. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, G.; Zou, Y.; Ding, Z.; Tang, Y.; Wang, R.; Liu, Z.; Zhou, Q.; Wu, Z.; Zhang, Y. Combined remediation mechanism of bentonite and submerged plants on lake sediments by DGT technique. Chemosphere 2022, 298, 134236. [Google Scholar] [CrossRef]
- Mortimer, C.H. The exchange of dissolved substances between mud and water in lakes. J. Ecol. 1941, 29, 280–329. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.; Wang, C.; Hou, J.; Miao, L.; Yuan, Y.; Wang, T.; Liu, C. Assessment of mobilization of labile phosphorus and iron across sediment-water interface in a shallow lake (Hongze) based on in situ high-resolution measurement. Environ. Pollut. 2016, 219, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Cheng, H.; Tysklind, M.; Xie, J.; Lu, L.; Yang, S. Occurrence of water phosphorus at the water-sediment interface of a freshwater shallow lake: Indications of lake chemistry. Ecol. Indic. 2017, 81, 443–452. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Y.; Wang, D.; Li, Y.Y.; Gong, M.; Zhang, C. In situ, high-resolution evidence for iron-coupled mobilization of phosphorus in sediments. Sci. Rep. 2016, 6, 24341. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, T.; Tian, S.; Wang, L.; Holm, P.E.; Hansen, H.C.B. High-resolution imaging of labile phosphorus and its relationship with iron redox state in lake sediments. Environ. Pollut. 2016, 219, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Tai, Z.; Li, Q.; Liu, E. In-situ, high-resolution evidence from water-sediment interface for significant role of iron bound phosphorus in eutrophic lake. Sci. Total Environ. 2020, 706, 136040. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Lemoine, D.G.; Mermillod-Blondin, F.; Barrat-Segretain, M.-H.; Massé, C.; Malet, E. The ability of aquatic macrophytes to increase root porosity and radial oxygen loss determines their resistance to sediment anoxia. Aquat. Ecol. 2012, 46, 191–200. [Google Scholar] [CrossRef]
- House, W.A. Inhibition of calcite crystal growth by inorganic phosphate. J. Colloid Interface Sci. 1987, 119, 505–511. [Google Scholar] [CrossRef]
- House, W. The prediction of phosphate coprecipitation with calcite in freshwaters. Water Res. 1990, 24, 1017–1023. [Google Scholar] [CrossRef]
- Dove, P.M.; Hochella, M.F., Jr. Calcite precipitation mechanisms and inhibition by orthophosphate: In situ observations by Scanning Force Microscopy. Geochim. Cosmochim. Acta 1993, 57, 705–714. [Google Scholar] [CrossRef]
- Neal, C. The potential for phosphorus pollution remediation by calcite precipitation in UK freshwaters. Hydrol. Earth Syst. Sci. 2001, 5, 119–131. [Google Scholar] [CrossRef]
- Ding, S.; Han, C.; Wang, Y.; Yao, L.; Wang, Y.; Xu, D.; Sun, Q.; Williams, P.N.; Zhang, C. In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res. 2015, 74, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Wang, S.; Cai, J.; Li, H.; Jenkins, A.; Maberly, S.C.; May, L. The potential role of sediment organic phosphorus in algal growth in a low nutrient lake. Environ. Pollut. 2019, 255, 113235. [Google Scholar] [CrossRef]
- Worsfold, P.J.; Monbet, P.; Tappin, A.D.; Fitzsimons, M.F.; Stiles, D.A.; McKelvie, I.D. Characterisation and quantification of organic phosphorus and organic nitrogen components in aquatic systems: A review. Anal. Chim. Acta. 2008, 624, 37–58. [Google Scholar] [CrossRef]
- Joshi, S.R.; Kukkadapu, R.K.; Burdige, D.J.; Bowden, M.E.; Sparks, D.L.; Jaisi, D.P. Organic matter remineralization predominates phosphorus cycling in the mid-bay sediments in the Chesapeake Bay. Environ. Sci. Technol. 2015, 49, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, H.; Chen, J.; Liao, P.; Chen, Q.; Yang, Y.; Liu, Y. Organic phosphorus mineralization dominates the release of internal phosphorus in a macrophyte-dominated eutrophication lake. Environ. Sci. 2022, 9, 747. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, X.; Yao, Y.; Li, L.; Wu, F. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef]

| Plant Species | Parameters | Percentage of Explained Variance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | P | Time (T) | Ca2+ × P | Ca2+ × T | P × T | Ca2+ × P × T | Error | ||
| P. crispus | TAP | 1.16 *** | 67.54 *** | 5.17 *** | 3.39 *** | 7.31 *** | 6.42 *** | 9.00 *** | 0.01 |
| H2O-P | 1.12 *** | 30.73 *** | 39.91 *** | 4.09 *** | 5.21 *** | 5.91 *** | 12.94*** | 0.09 | |
| NaOH-P | 0.28 *** | 55.36 *** | 13.92 *** | 4.97 *** | 6.19 *** | 7.51 *** | 11.60 *** | 0.17 | |
| HCl-P | 3.21 *** | 36.10 *** | 48.31 *** | 0.93 *** | 4.48 *** | 3.97 *** | 2.91 *** | 0.09 | |
| C. demersum | TAP | 3.56 *** | 45.16 *** | 34.11 *** | 1.13 *** | 4.48 *** | 7.54 *** | 4.01 *** | 0.01 |
| H2O-P | 0.30 *** | 54.29 *** | 24.95 *** | 0.30 *** | 10.97 *** | 3.81 *** | 5.37 *** | 0.01 | |
| NaOH-P | 2.27 *** | 1.79 *** | 80.07 *** | 2.84 *** | 2.60 *** | 2.22 *** | 8.20 *** | 0.01 | |
| HCl-P | 1.01 *** | 67.87 *** | 9.96 *** | 1.53 *** | 3.67 *** | 6.94 *** | 8.93 *** | 0.09 | |
| Plant Species | Parameters | Percentage of Explained Variance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | P | Time (T) | Ca2+ × P | Ca2+ × T | P × T | Ca2+ × P × T | Error | ||
| P. crispus | STP | 10.89 *** | 56.03 *** | 14.01 *** | 4.67 * | 1.56 ** | 7.78 *** | 4.67 * | 0.39 |
| OP | 1.73 * | 15.15 *** | 28.72 * | 7.96 ** | 11.12 *** | 26.90 ** | 5.18 * | 13.2 | |
| IP | 5 ** | 49.77 *** | 20.32 *** | 3.97 * | 5.47 ns | 11.85 * | 1.4 ns | 0.47 | |
| SCa-P | 16.57 *** | 27.91 *** | 24.82 *** | 7.3 *** | 25 * | 10.41 ** | 1.8 ns | 8.69 | |
| Fe/Al-P | 0.3 *** | 43.55 *** | 15.53 *** | 12.88 *** | 6.31 ns | 14.18 ** | 6.82 *** | 0.44 | |
| C. demersum | STP | 0.13 *** | 5.36 ** | 71.98 *** | 1.4 * | 5.86 ** | 9.8 *** | 4.12 ** | 1.37 |
| OP | 0.44 *** | 0.39 ns | 87.32 *** | 1.06 * | 4.13 * | 3.49 ** | 1.86 * | 1.32 | |
| IP | 0.37 * | 32.14 * | 18.54 *** | 7.17 *** | 4.4 * | 23.08 *** | 11.89 ** | 2.41 | |
| SCa-P | 6.14 *** | 9.84 ns | 22.7 *** | 13.58 ** | 5.72 ns | 23.10 * | 13.70 * | 5.23 | |
| Fe/Al-P | 2.01 *** | 22.44 *** | 58.19 *** | 3.21 * | 1.67 *** | 7.62 *** | 4.28 *** | 0.57 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, R.; Chen, Q.; Chen, K.; Hu, R. Interspecific Differences in the Effects of Calcium and Phosphorus Coprecipitation Induced by Submerged Plants on the Water-to-Phosphorus Cycle. Sustainability 2024, 16, 4200. https://doi.org/10.3390/su16104200
Wang H, Zhang R, Chen Q, Chen K, Hu R. Interspecific Differences in the Effects of Calcium and Phosphorus Coprecipitation Induced by Submerged Plants on the Water-to-Phosphorus Cycle. Sustainability. 2024; 16(10):4200. https://doi.org/10.3390/su16104200
Chicago/Turabian StyleWang, Heyun, Runlong Zhang, Qi Chen, Kuang Chen, and Rui Hu. 2024. "Interspecific Differences in the Effects of Calcium and Phosphorus Coprecipitation Induced by Submerged Plants on the Water-to-Phosphorus Cycle" Sustainability 16, no. 10: 4200. https://doi.org/10.3390/su16104200
APA StyleWang, H., Zhang, R., Chen, Q., Chen, K., & Hu, R. (2024). Interspecific Differences in the Effects of Calcium and Phosphorus Coprecipitation Induced by Submerged Plants on the Water-to-Phosphorus Cycle. Sustainability, 16(10), 4200. https://doi.org/10.3390/su16104200




