Ash Properties and Environmental Impact of Coal and Its Blend with Patent Fuel for Climate Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Fuels
2.2. Ash Samples
2.3. Characterization of Ash Samples
2.4. Leaching Tests in Deionized Water and Acid Rain
3. Results and Discussion
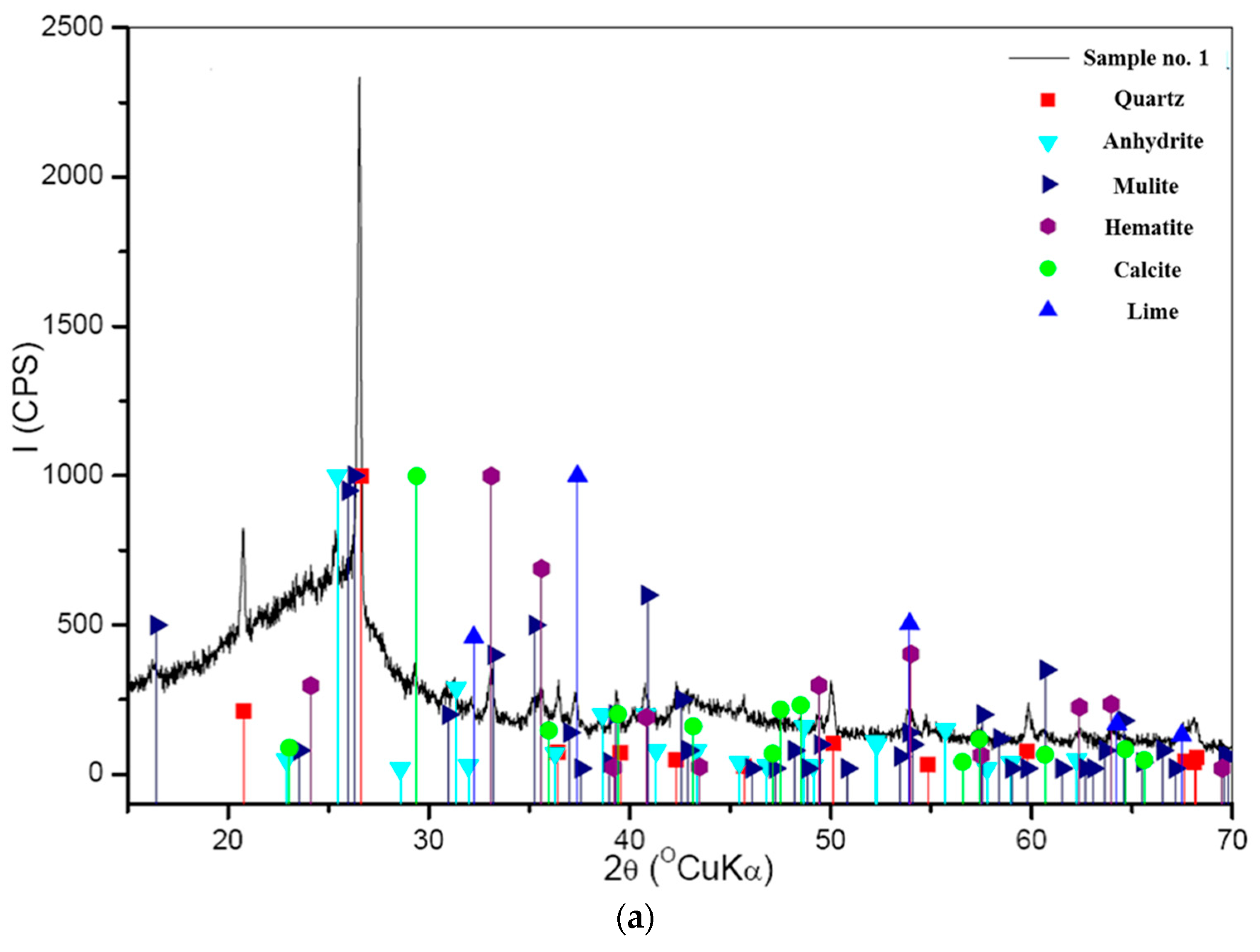
3.1. Chemical and Mineralogical Characterization
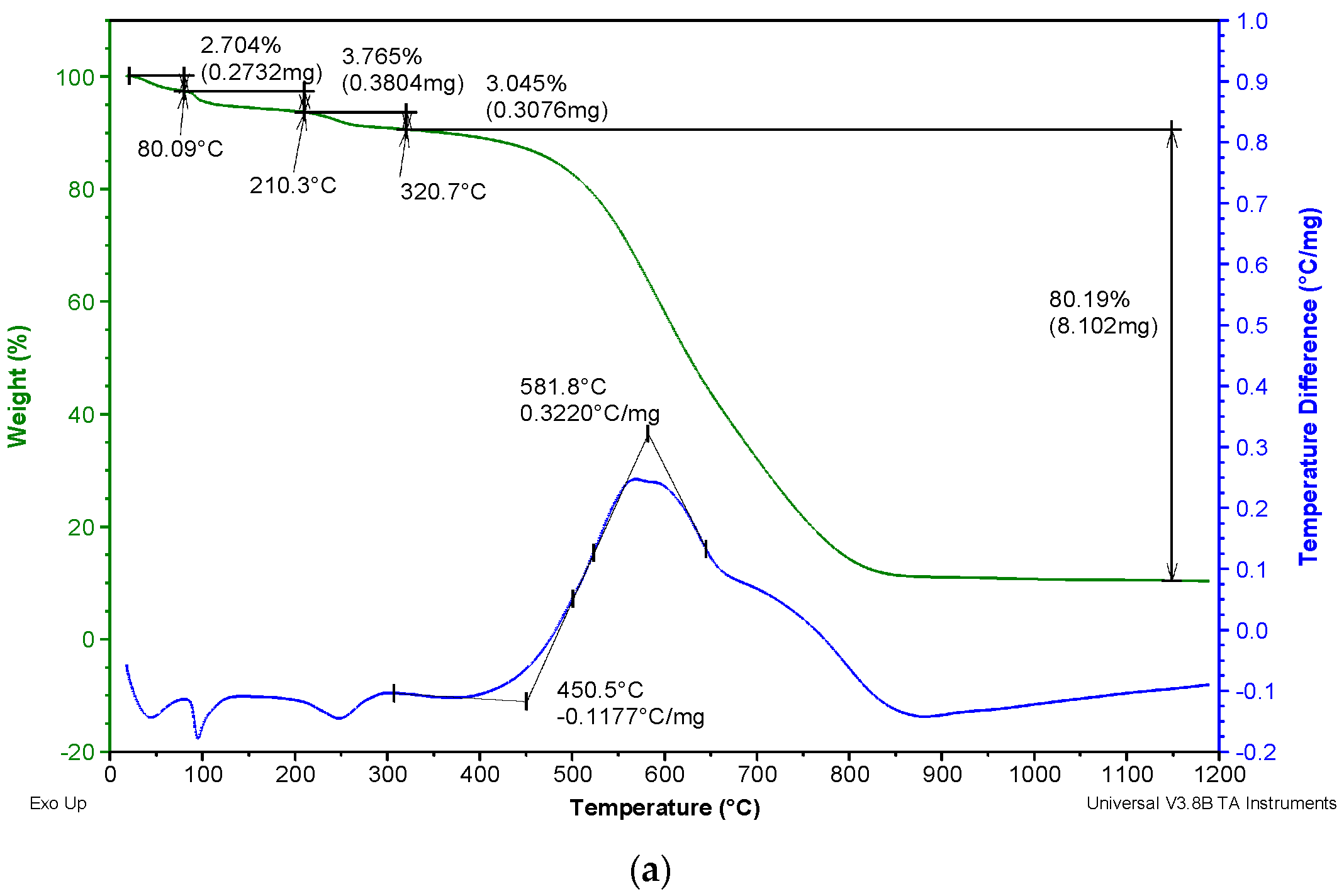
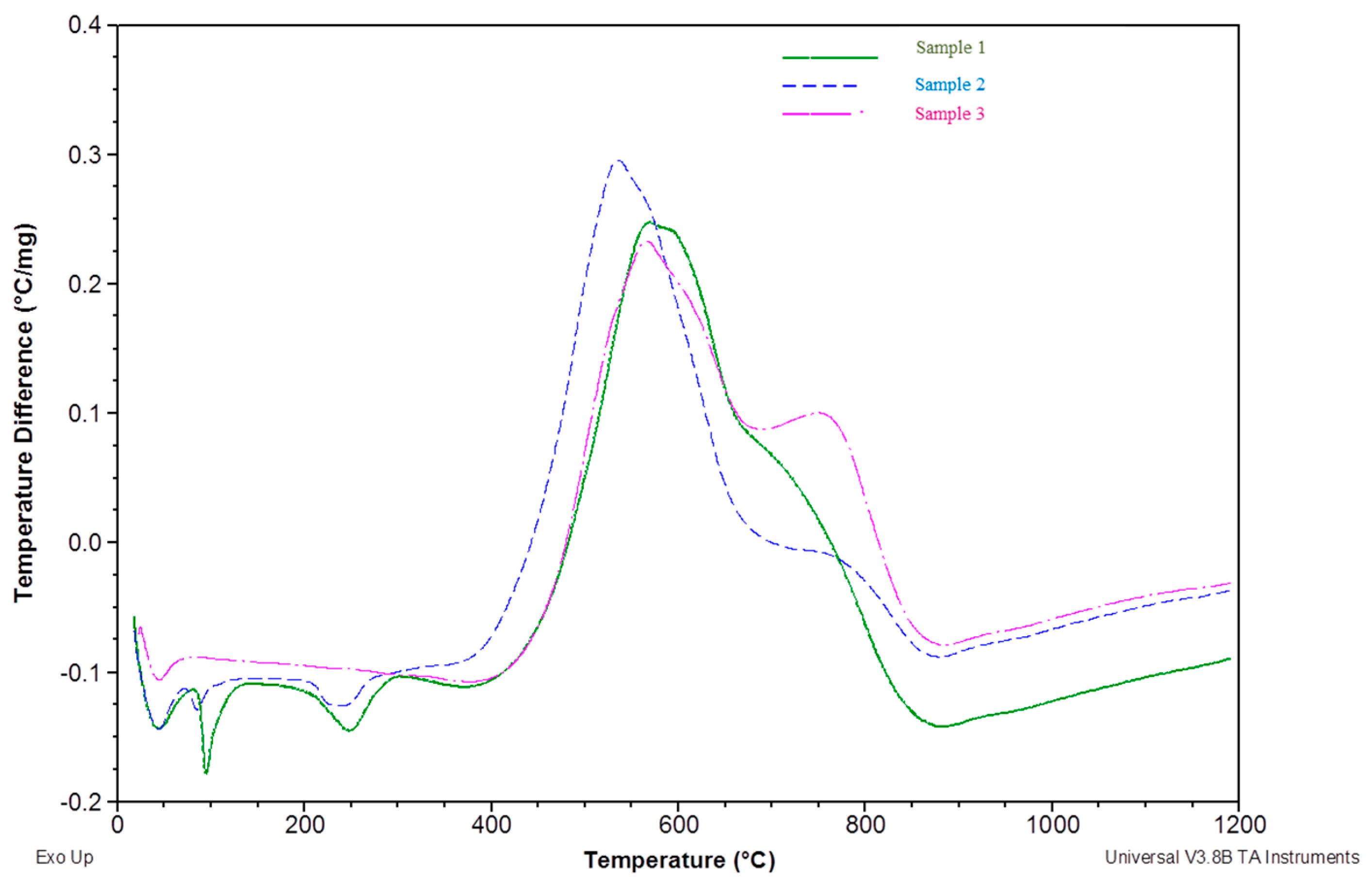
3.2. Thermal Analysis
- A loss of mass from the sample due to the evaporation of absorbed water during the temperature interval from room temperature up to 80 °C;
- A two-step loss of mass in the temperature interval between 80 and 320 °C due to the evaporation of chemically bonded water (water which occurs in the sample in hydrate form);
- A loss of mass due to coal oxidation and combustion in the temperature interval between 320 and 850 °C.
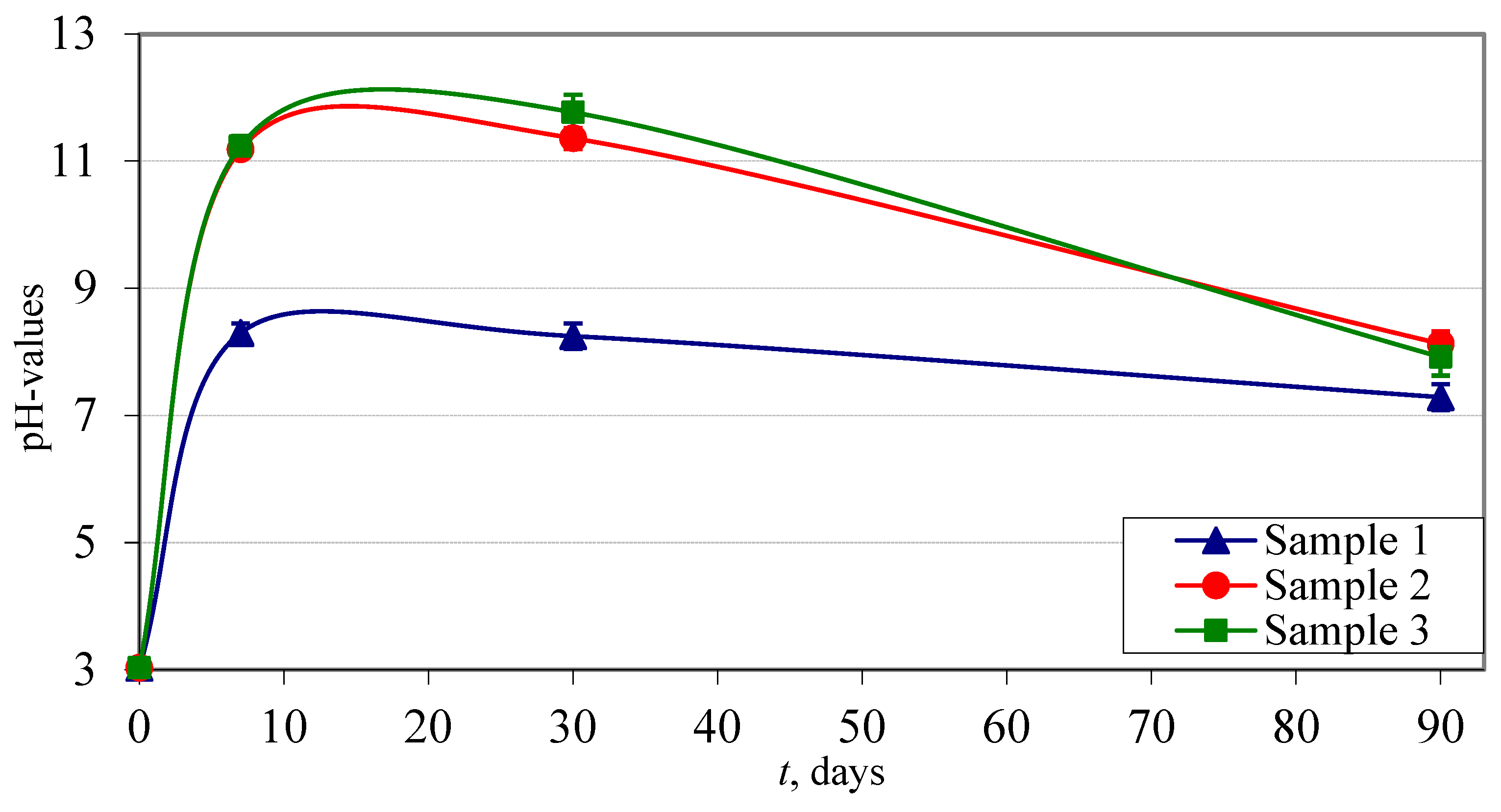
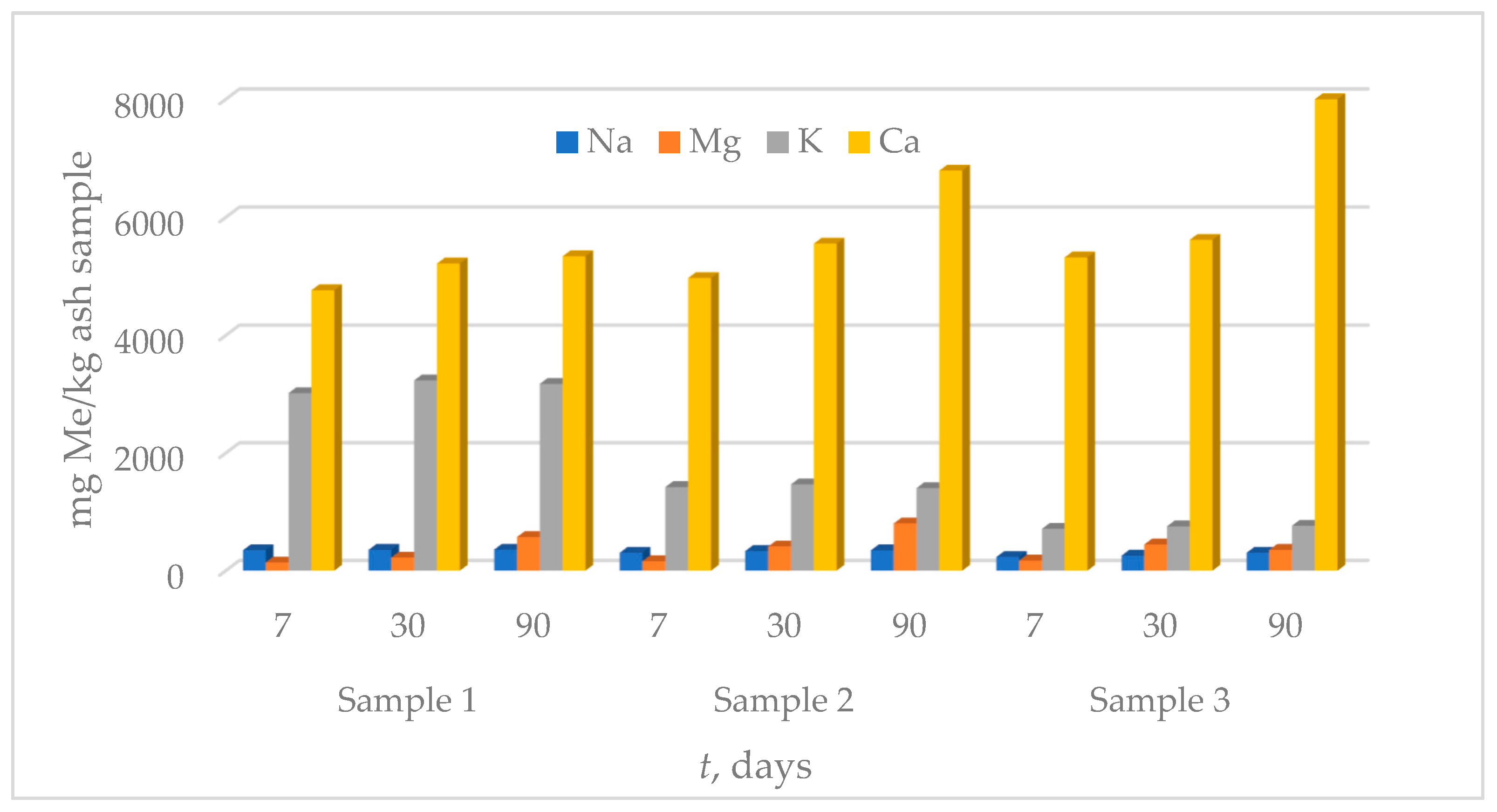
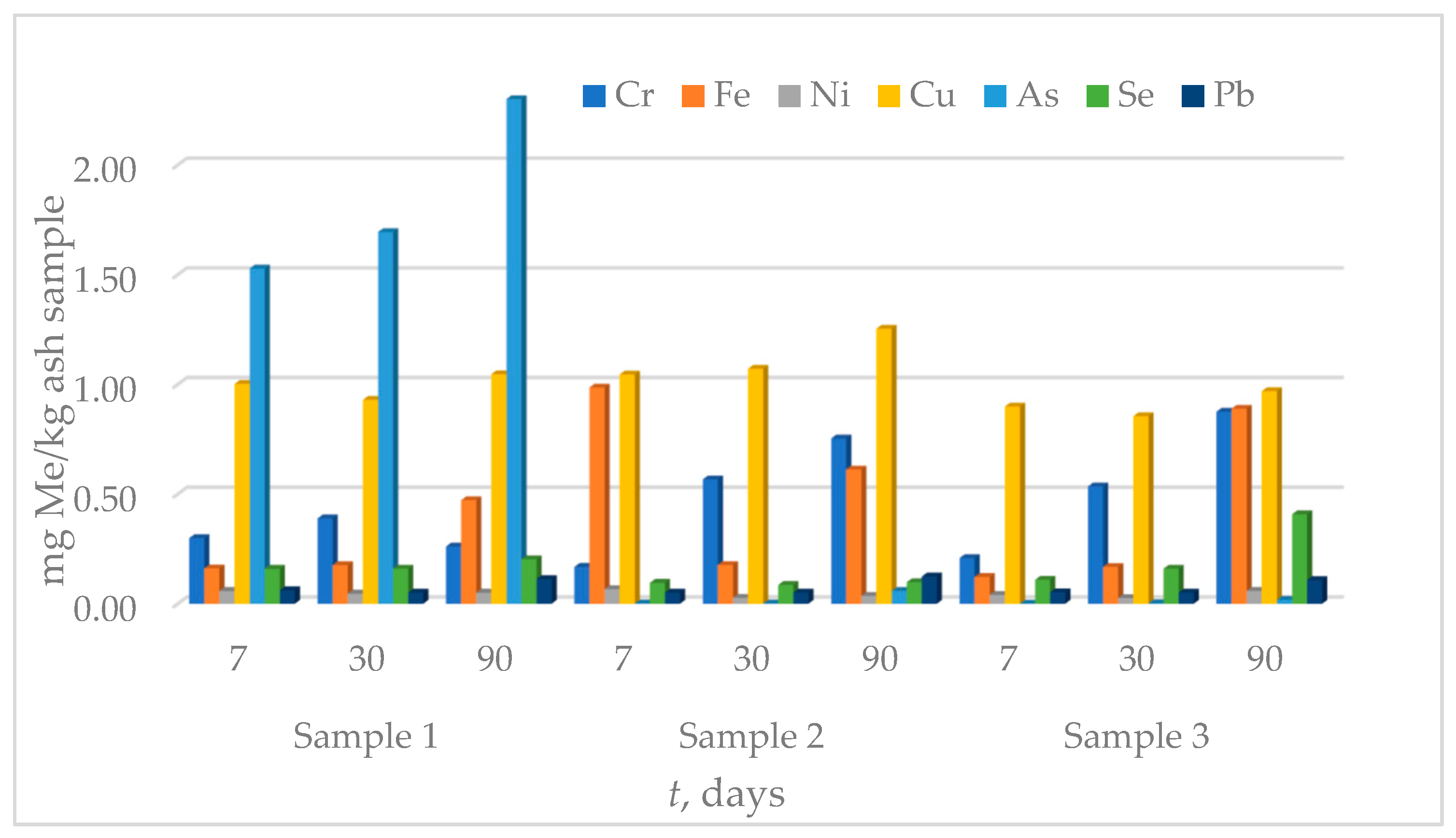
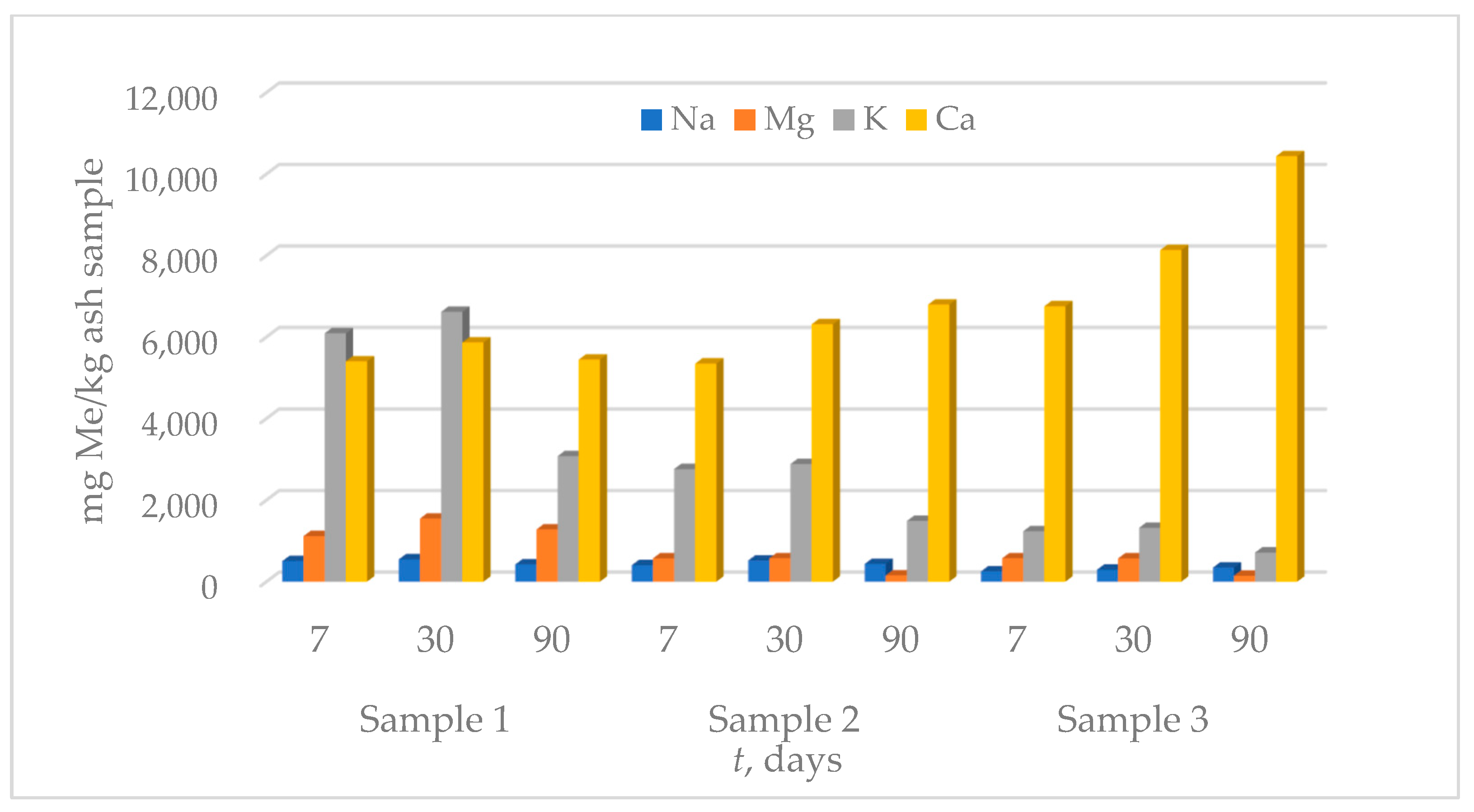
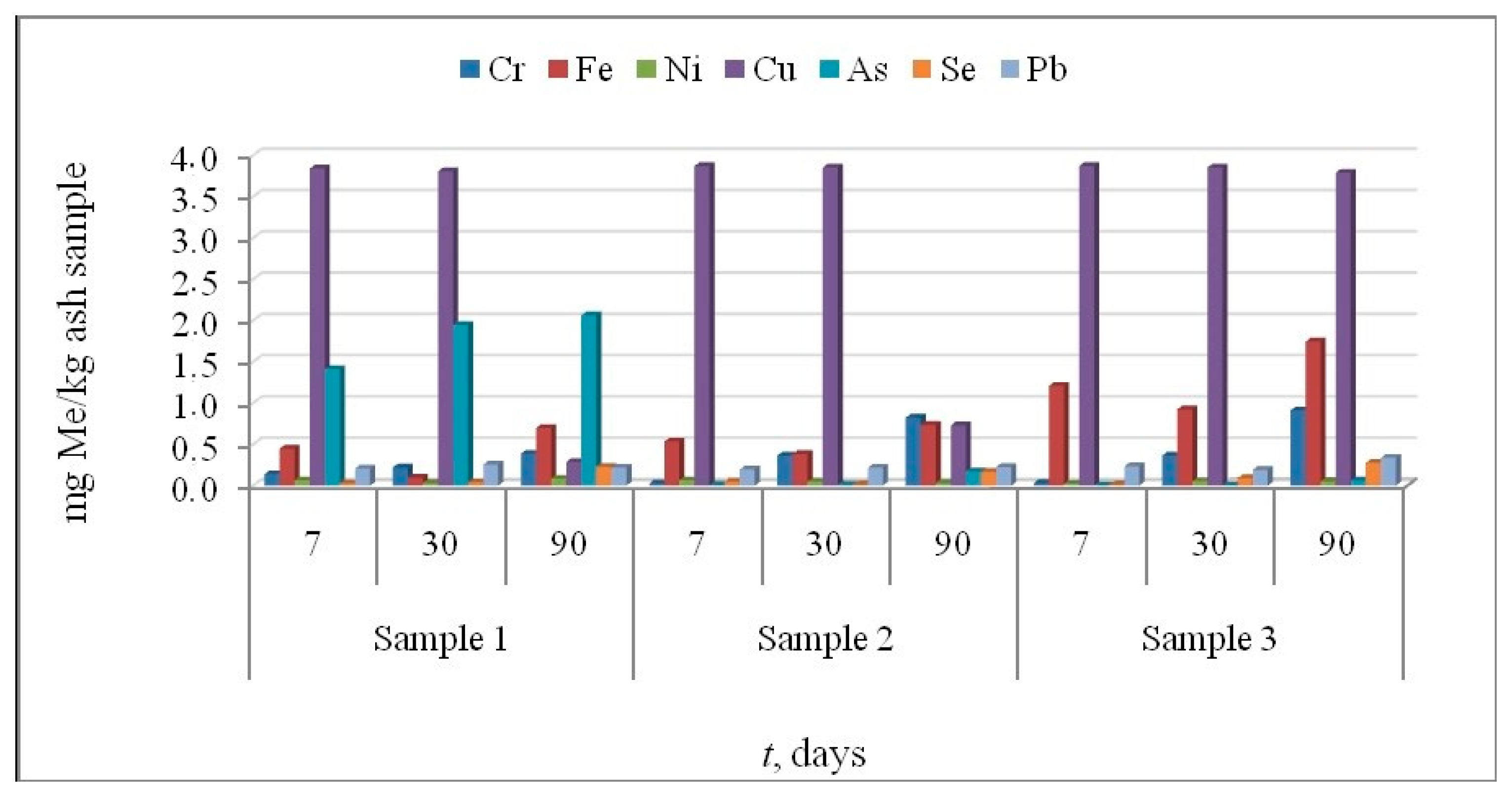
3.3. Leaching Tests
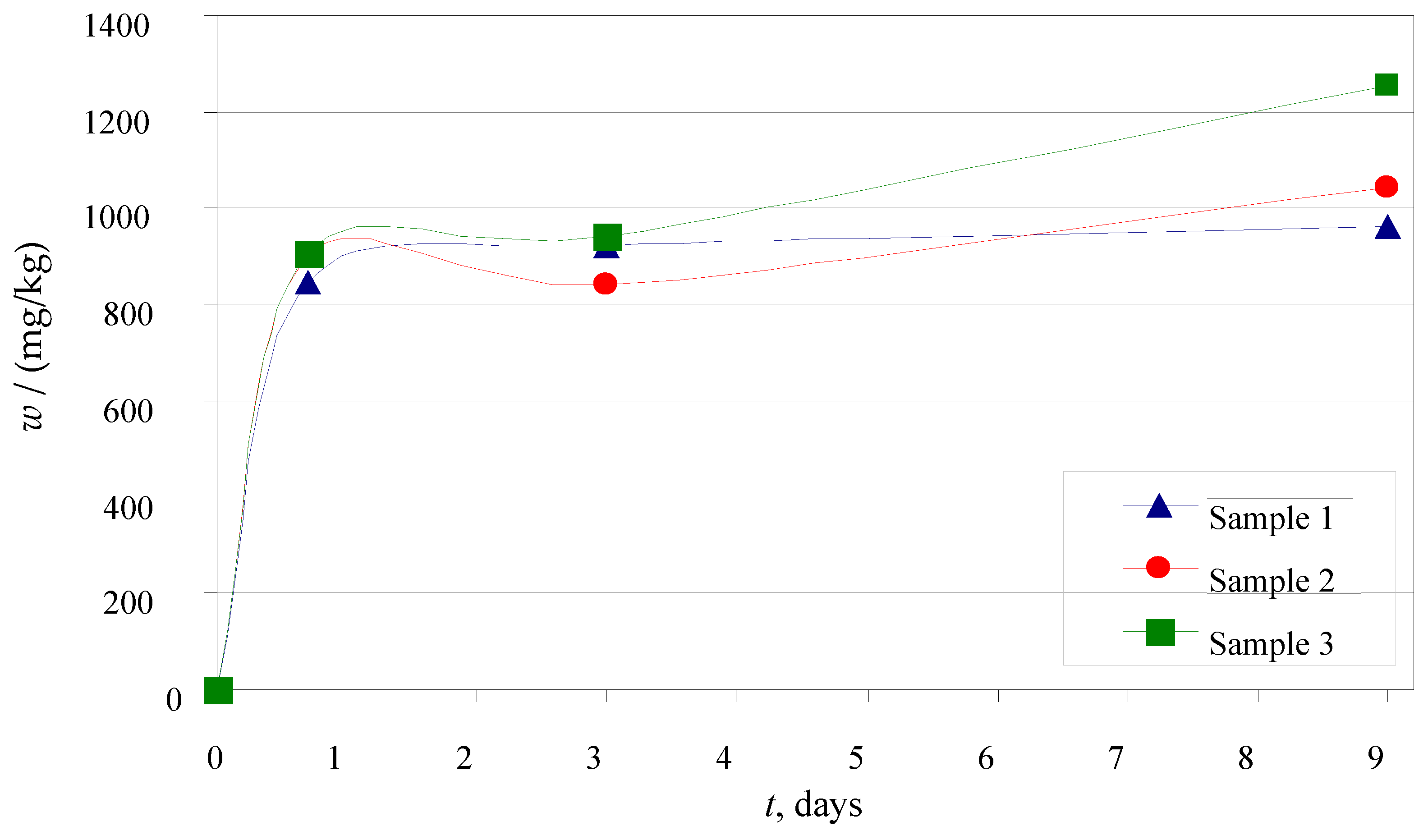
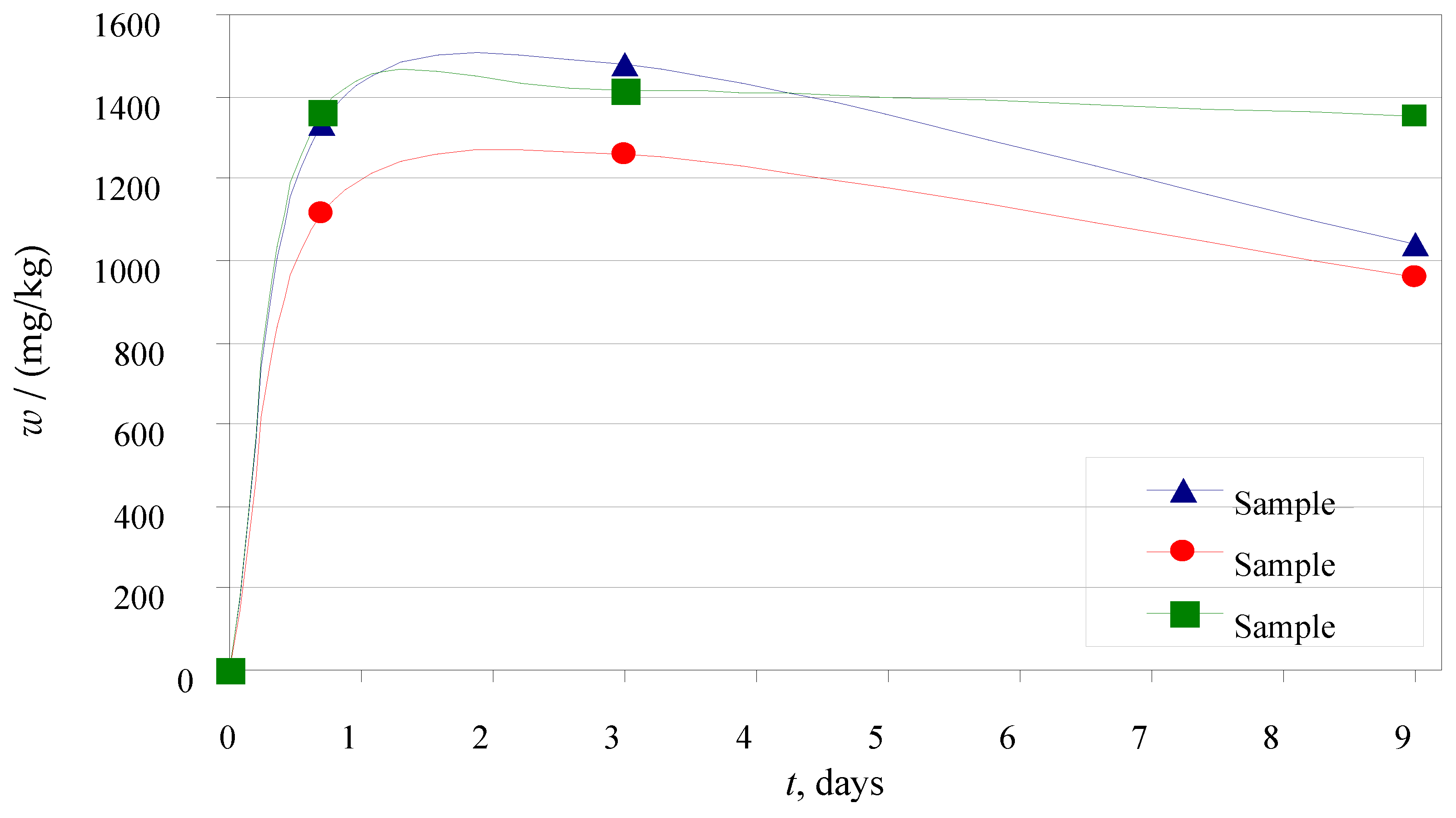
3.3.1. Leaching in Ultra-Pure Water
3.3.2. Leaching in Synthetic Acid Rain
- The concentration of elements in the solid phases;
- The distribution of elements among ash particles;
- The possibility of the incorporation of elements into secondary solids.
4. Conclusions
- (1)
- Patent fuel can absorb sulphur from flue gases during combustion, with 0.85 mas.% of sulphur in the ash sample after the combustion of pure steam coal (Sample 1) compared to 1.44 mas.% in the ash sample (Sample 3) after the combustion of 0.44 kg steam coal with ten ampoules of patent fuel.
- (2)
- The addition of patent fuel led to lower concentrations of trace elements in the ash, specifically for the elements Ba, Cr, Ga, Li, Mn, V, and Zn. For Ba, Ga, and Zn, the reduction in these elements in the ash produced by patent fuel–coal combinations compared to the ash produced by pure coal combustion was up to 70%.
- (3)
- Qualitative XRD diffraction identified that the main mineral phase in ash samples is quartz (SiO2). Other minor mineral components were mulite (3Al2O3 × 2SiO2), calcite (CaCO3), lime (CaO), anhydrite (CaSO4), and hematite (Fe2O3). Ash samples after the combustion of mixtures of coal and patent fuel also had cristobalite (SiO2), portlandite (Ca(OH)2), and rutile (TiO2).
- (4)
- Adding patent fuel to coal results in an earlier start of decomposition, stronger combustion, and faster oxidation, resulting in the release of more energy in the same temperature range.
- (5)
- The leaching tests revealed that most of the elements, particularly Co, Ag, Cd, Ce, Tl, Pb, and Bi, were found to leach more easily in acid rain compared to water. Co showed the biggest difference, with thirteen times better leaching in acid rain than in water, followed by Ag and Cd with seven times better leaching in acid rain.
- (6)
- The leaching characteristics of the measured elements were found to be dependent on the pH value.
- (7)
- The coal ash leachates contained higher levels of environmental pollutants, including As, Cd, Co, Mn, Mo, Sb, and U, than the patent fuel leachates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. Coal 2023 Analysis and Forecast to 2026. Available online: https://www.iea.org/reports/coal-2023 (accessed on 3 March 2024).
- Uyanik, S.; Dogerlioglu Isiksungur, O. How Realistic Are Coal Phase-Out Timeline Targets for Turkey? Sustainability 2024, 16, 1649. [Google Scholar] [CrossRef]
- Chicherin, S.; Zhuikov, A.; Kuznetsov, P. The Return of Coal-Fired Combined Heat and Power Plants: Feasibility and Environmental Assessment in the Case of Conversion to Another Fuel or Modernizing an Exhaust System. Sustainability 2024, 16, 1974. [Google Scholar] [CrossRef]
- Alhassan, A.; Ozturk, I.; Fahmi AL-Zyoud, M.; Bekun, F.V. Coal consumption-environmental sustainability nexus in developed and developing major coal-consuming economies. Heliyon 2024, 10, E25619. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 5 March 2024).
- European Commission. In focus: Towards a Just and Clean Energy Transition. Available online: https://commission.europa.eu/news/focus-towards-just-and-clean-energy-transition-2020-10-01_en (accessed on 5 March 2024).
- Nakamura, T.; Maruyama, A.; Masuda, S.; Lloyd, S. The Impact of Russia’s Invasion of Ukraine on Germany’s Energy Choice Attitudes among Residents in German States with Nuclear Power Plants in Commission or Decommissioned. Sustainability 2024, 16, 1999. [Google Scholar] [CrossRef]
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Žuromskaitė-Nagaj, B.; Grebski, W.W. The Influence of the Global Energy Crisis on Energy Efficiency: A Comprehensive Analysis. Energies 2024, 17, 947. [Google Scholar] [CrossRef]
- Shu, L.; Wang, H.; He, X. Physicochemical Properties and Planting Performance of Artificial Soil Developed from Multiple Coal-Based Solid Waste Materials. Sustainability 2024, 16, 1955. [Google Scholar] [CrossRef]
- Pandey, S.P.; Yu, H.; Lau, C.; Ng, K. New Coal Char-Based Building Products: Manufacturing, Engineering Performance, and Techno-Economic Analysis for the USA Market. Sustainability 2024, 16, 1854. [Google Scholar] [CrossRef]
- Goodarzi, F. Mineralogy, elemental composition and modes of occurrence of elements in Canadian feed-coals. Fuel 2002, 81, 1199–1213. [Google Scholar] [CrossRef]
- Srinivasa Reddy, M.; Basha, S.; Joshi, H.V.; Jha, B. Evaluation of the emission characteristics of trace metals from coal and fuel oil fired power plants and their fate during combustion. J. Hazard. Mater. 2005, 123, 242–249. [Google Scholar] [CrossRef]
- Vejahati, F.; Xu, Z.; Gupta, R. Trace elements in coal: Associations with coal and minerals and their behavior during coal utilization—A review. Fuel 2010, 89, 904–911. [Google Scholar] [CrossRef]
- Nelson, P.F.; Shah, P.; Strezov, V.; Halliburton, B.; Carras, J.N. Environmental impacts of coal combustion: A risk approach to assessment of emissions. Fuel 2010, 89, 810–816. [Google Scholar] [CrossRef]
- Van Heek, K.H. Progress of coal science in the 20th century. Fuel 2000, 79, 1–26. [Google Scholar] [CrossRef]
- Ma, L.; Fan, J.; Guo, R.-Z.; Zhang, P.-Y.; Li, C.-H. Characteristics of fires in coal mine roadways and comparative analysis of control effectiveness between longitudinal ventilation and cross-section sealing. Case Stud. Therm. Eng. 2024, 53, 103878. [Google Scholar] [CrossRef]
- Deng, J.; Qu, G.; Ren, S.; Wang, C.; Su, H.; Yuan, Y.; Duan, X.; Yang, N.; Wang, J. Effect of water soaking and air drying on the thermal effect and heat transfer characteristics of coal oxidation at the low-temperature oxidation stage. Energy 2024, 288, 129705. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Analysis of fly ash heavy metal content and disposal in three thermal power plants in India. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Armesto, L.; Merino, J.L. Characterization of some coal combustion solid residues. Fuel 1999, 78, 613–618. [Google Scholar] [CrossRef]
- Goodarzi, F. Morphology and chemistry of fine particles emitted from a Canadian coal- fired power plant. Fuel 2006, 85, 273–280. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Ye, T.; Guo, Y.; Gao, X. A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure. J. Hazard. Mater. 2006, 134, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Nathan, Y.; Dvorachek, M.; Pelly, I.; Mimran, U. Characterization of coal fly ash from Israel. Fuel 1999, 78, 205–213. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G. Heavy metal characterization of CFB- derived coal fly ash. Fuel Process. Technol. 2011, 92, 441–446. [Google Scholar] [CrossRef]
- Medina, A.; Gamero, P.; Querol, X.; Moreno, N.; Leon, B.; Almanza, M.; Vargas, G.; Izquierdo, M.; Font, O. Fly ash from a Mexican mineral coal I: Mineralogical and chemical characterization. J. Hazard. Mater. 2010, 181, 82–90. [Google Scholar] [CrossRef]
- Lewandowski, J.; Kalkreuth, W. Chemical and petrographical characterization of feed coal, fly ash and bottom ash from the FigueiraPower Plant, Paraná, Brazil. Int. J. Coal Geol. 2009, 77, 269–281. [Google Scholar] [CrossRef]
- Querol, X.; Umana, J.C.; Alustuey, A.; Bertrana, C.; Lopez-Soler, A.; Plana, F. Physicochemical Characterization of Spanish Fly Ashes. Energ. Source Part A 2010, 21, 883–898. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Methods for Characterization of Composition of Fly Ashes from Coal- Fired Power Stations: A Critical Overview. Energ. Fuel 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Marrero, J.; Polla, G.; Rebagliati, R.J.; Pla, R.; Gomez, D.; Smichowski, P. Characterization and determination of 25 elements in fly ashes collected in a thermal power plant in Argentina using different instrumental techniques. Spectrochim. Acta 2007, 62, 101–108. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Tascon, J.M.D. Methods for Characterization of Inorganic and Mineral Matter in Coal: A Critical Overview. Energy Fuels 2003, 17, 271–281. [Google Scholar] [CrossRef]
- Koukouzas, N.; Hämäläinen, J.; Papanikolaou, D.; Tourunen, A.; Jäntti, T. Mineralogical and elemental composition of fly ash from pilot scale fluidised bed combustion of lignite, bituminous coal, wood chips and their blends. Fuel 2007, 86, 2186–2193. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behaviour of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2011, 94, 54–66. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Andrés, J.M.; Stanton, K.; Towler, K.; Nugteren, H.; Janssen- Jurkovicova, M.; Jones, R. Physico- chemical characteristics of European pulverized coal combustion fly ashes. Fuel 2005, 84, 1351–1363. [Google Scholar] [CrossRef]
- Ćosić, D. Vodikovo gorivo kao ekološki doprinos funkcioniranju postojećih termoelektrana na ugljen. In Proceedings of the 18th Forum: Day of Energy in Croatia, HED, Zagreb, Croatia, 18 November 2009. [Google Scholar]
- Ćosić, D.; Rakin, P. Vodonično gorivo kao ekološki doprinos funkcionisanju postojećih termoelektrana na ugalj. In Proceedings of the Zbornik Radova Hrvati u BIH Danas, Banja Luka, Bosnia and Herzegovina, 6 March 2011. [Google Scholar]
- Dobrović, S.; Švaić, S.; Ljubas, D.; Juretić, H.; Rodić, A. Report on Applicability of Patented Fuel Hydrogen-Industrial; Faculty of Mechanical Engineering and Naval Architecture, University of Zagreb: Zagreb, Croatia, 2011. [Google Scholar]
- ASTM D 4326-21; Standard Test Method for Major and Minor Elements in Coal and Coke Ash by X-ray Fluorescence. American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- Trkmić, M.; Ćurković, L.; Ašperger, D. Chemical analysis of solid residue from liquid and solid fuel combustion: Method development and validation. Materialwiss. Werkst. 2012, 43, 503–510. [Google Scholar] [CrossRef]
- Iwashita, A.; Nakajima, T.; Takanashi, H.; Ohki, H.; Fujita, Y.; Yamashita, T. Determination of trace elements in coal and coal fly ash by joint- use of ICP-AES and atomic absorption spectrometry. Talanta 2007, 71, 251–257. [Google Scholar] [CrossRef]
- Wang, J.; Nakazato, T.; Sakanishi, K.; Yamada, O.; Tao, H.; Saito, I. Single-step microwave digestion with HNO3 alone for determination of trace elements in coal by ICP spectrometry. Talanta 2006, 68, 1584–1590. [Google Scholar] [CrossRef]
- Wang, J.; Nakazato, T.; Sakanishi, K.; Yamada, O.; Tao, H.; Saito, I. Microwave digestion with HNO3/H2O2 mixture at high temperatures for determination of trace elements in coal by ICP-OES and ICP-MS. Anal. Chim. Acta 2004, 514, 115–124. [Google Scholar] [CrossRef]
- Iwashita, A.; Nakaijima, T.; Takanashi, H.; Ohki, A.; Fujita, Y.; Yamashita, T. Effects of pretreatment conditions on the determination of major and trace elements in coal fly ash using ICP-AES. Fuel 2006, 85, 257–263. [Google Scholar] [CrossRef]
- Sandroni, V.; Smith, C.M.M. Microwave digestion of sludge, soil and sediment samples for metal analysis by inductively coupled plasma-atomic emission spectrometry. Anal. Chim. Acta 2002, 468, 335–344. [Google Scholar] [CrossRef]
- Das, A.K.; Chakraborty, R.; Guardia, M.; Cerera, M.L.; Goswami, D. ICP-MS multielement determination in fly ash after microwave-assisted digestion of samples. Talanta 2001, 54, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Iwashita, A.; Nakajima, T.; Yamashita, H.; Takanashi, H.; Ohki, A. Effect of HF addition on the microwave-assisted acid-digestion for the determination of metals in coal by inductively coupled plasma- atomic emission spectrometry. Talanta 2005, 66, 58–64. [Google Scholar] [CrossRef]
- Font, O.; Moreno, N.; Querol, X.; Izquierdo, M.; Alvarez, E.; Diez, S.; Elvira, J.; Antenucci, D.; Nugteren, H.; Plana, F.; et al. X-ray powder diffraction- based method for the determination of the glass content and mineralogy of coal (co)-combustion fly ashes. Fuel 2010, 89, 2971–2976. [Google Scholar] [CrossRef]
- Kim, A.G.; Hesbach, P. Comparison of fly ash leaching method. Fuel 2009, 88, 926–937. [Google Scholar] [CrossRef]
- Dutta, B.K.; Khanra, S.; Mallick, D. Leaching of elements from coal fly ash: Assessment of its potential for use in filling abandoned coal mines. Fuel 2009, 88, 1314–1323. [Google Scholar] [CrossRef]
- Raman, N.; Sathiyanarayanan, D. An USEPA approach of heavy metal analysis in solid waste by multiple extraction procedure using synthetic acid rain as extraction fluid. Rasayan J. Chem. 2010, 3, 539–544. [Google Scholar] [CrossRef]
- USEPA Method 1320 Multipleextraction procedure, Publication SW-846: Test methods for Evaluating Solid Waste, Physical/Chemical Methods, Washington, DC, USA. 1986. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/1320.pdf (accessed on 3 March 2024).
- Hesbach, P.A.; Kim, A.G.; Abel, A.S.P.; Lamey, S.C. Serial batch procedure for characterization of coal fly ash. Environ. Monit. Asses. 2010, 168, 523–545. [Google Scholar] [CrossRef]
- Jegadeesan, G.; Al-Abed, S.R.; Pinto, P. Influence of trace metal distribution on its leachability from coal fly ash. Fuel 2008, 87, 1887–1893. [Google Scholar] [CrossRef]
- Gitari, W.M.; Fatoba, O.O.; Petrik, L.F.; Vadapalli, V.R.K. Leaching characteristics of selected South African fly ashes: Effect of pH on the release of major and trace species. J. Environ. Sci. Health Part A 2009, 44, 206–220. [Google Scholar] [CrossRef]
- Palumbo, A.V.; Tarver, J.R.; Fagan, L.A.; McNeilly, M.S.; Ruter, R.; Fisher, L.S.; Amonette, J.E. Comparing metal leaching and toxicity from high pH, low pH, and high ammonia fly as. Fuel 2007, 86, 1623–1630. [Google Scholar] [CrossRef]
- Pathan, S.M.; Aylmore, L.A.G.; Colmer, T.D. Properties of Several Fly Ash Materials in Relation to Use as Soil Amendments. J. Environ. Qual. 2003, 32, 687–693. [Google Scholar] [CrossRef]
- Jankowski, J.; Ward, C.R.; French, D.; Groves, S. Mobility of trace elements from selected Australian fly ashes and its potential impact on aquatic ecosystems. Fuel 2006, 85, 243–256. [Google Scholar] [CrossRef]



| Parameters | Test Methods | Results |
|---|---|---|
| Surface moisture | ASTM D 3302 | 2.96% |
| Inherent moisture | ASTM D 3173 | 3.25% |
| Total moisture | ASTM D 3302 | 6.21% |
| Ash content | ISO 1171 | 10.04% |
| Volatile matter | ISO 562 | 34.06% |
| C-fix | by calculation | 49.68% |
| Total sulphur | ASTM D 4239 | 1.43% |
| C content | ASTM D 5373 | 70.00% |
| H content | ASTM D 5373 | 5.18% |
| N content | ASTM D 5373 | 1.42% |
| O content | by calculation | 5.72% |
| Gross calorific value | ISO 1928 | 27.97 MJ/kg |
| Net calorific value | ISO 1928 | 26.69 MJ/kg |
| Emission factor | EC 2018/2066 | 96.17 tCO2/TJ |
| No. | Sample Description | Masss (Sample)/g | Day of Experiment |
|---|---|---|---|
| 1. | Bottom ash after hard coal combustion | 500 | 1st day of combustion |
| 2. | Bottom ash Mixture: packages with a ratio of 0.44 kg hard coal + 10 ampoules | 500 | |
| 3. | Bottom ash Mixture: packages with a ratio of 0.54 kg hard coal + 8 ampoules | 500 | |
| 4. | Bottom ash from combustion chamber, after cleaning the combustion remains from the previous day. | 500 | 2nd day of combustion |
| 5. | Bottom ash Mixture: packages with a ratio of 0.54 kg hard coal + 8 ampoules | 500 | |
| 6. | Bottom ash Mixture: packages with a ratio of 0.44 kg hard coal + 10 ampoules | 500 | |
| 7. | Samples from the filter: 3 quartz filters with ash and soot from the flue gases—after hard coal combustion. | 0.06 | |
| 8. | Samples from the filter: 2 quartz filters with ash and soot from the flue gases—after combustion of 0.54 kg hard coal + 8 ampoules. | 0.02 | |
| 9. | Samples from the filter: 1 quartz filter with ash and soot from the flue gases—after combustion of 0.44 kg hard coal + 10 ampoules. | 0.06 |
| Samples | Description |
|---|---|
| Sample 1 | Ash sample after combustion of pure steam coal |
| Sample 2 | Ash sample after combustion block of 0.54 kg steam coal + 8 ampoules of patent fuel |
| Sample 3 | Ash sample after combustion block of 0.44 kg steam coal + 10 ampoules of patent fuel |
| Element | DLs | SLRS-5 | SRM 1643e | ||||
|---|---|---|---|---|---|---|---|
| Certified (Mean ± SD) | Found (Mean ± SD) | % D | Certified (Mean ± SD) | Found (Mean ± SD) | % D | ||
| Li | 0.0116 | - | 0.28 ± 0.02 | - | 17.00 ± 1.70 | 17.22 ± 0.23 | 101.3 |
| Be | 0.0009 | 0.005 2 | 0.003 ± 0.001 | 61.9 | 13.64 ± 0.16 | 8.91 ± 0.11 | 65.3 |
| Na 1 | 1.8102 | 5.38 ± 0.10 | 4.75 ± 0.02 | 88.4 | 20.23 ± 0.25 | 20.01 ± 0.82 | 98.9 |
| Mg 1 | 0.6240 | 2.54 ± 0.16 | 2.28 ± 0.14 | 89.7 | 7.84 ± 0.09 | 7.42 ± 0.15 | 94.7 |
| Al | 0.1774 | 49.50 ± 5.00 | 50.12 ± 0.52 | 101.3 | 138.33 ± 8.40 | 151.69 ± 0.60 | 109.7 |
| K 1 | 6.5270 | 0.84 ± 0.04 | 0.77 ± 0.01 | 91.6 | 1.98 ± 0.03 | 2.01 ± 0.03 | 101.1 |
| Ca 1 | 4.6519 | 10.50 ± 0.40 | 9.66 ± 0.04 | 92.0 | 31.50 ± 1.10 | 32.77 ± 0.28 | 104.0 |
| V | 0.0007 | 0.32 ± 0.03 | 0.30 ± 0.01 | 95.0 | 36.93 ± 0.57 | 35.73 ± 0.27 | 96.8 |
| Cr | 0.0052 | 0.21 ± 0.02 | 0.22 ± 0.02 | 104.0 | 19.90 ± 0.23 | 21.21 ± 0.16 | 106.6 |
| Mn | 0.0053 | 4.33 ± 0.18 | 4.17 ± 0.05 | 96.3 | 38.02 ± 0.44 | 39.27 ± 0.29 | 103.3 |
| Fe | 0.2430 | 91.20 ± 5.80 | 93.71 ± 0.74 | 102.7 | 95.70 ± 1.40 | 98.44 ± 5.57 | 102.9 |
| Co | 0.0006 | 0.05 2 | 0.05 ± 0.07 | 102.1 | 26.40 ± 0.32 | 27.04 ± 0.10 | 102.4 |
| Ni | 0.0138 | 0.48 ± 0.06 | 0.50 ± 0.02 | 104.9 | 60.89 ± 0.67 | 62.93 ± 0.74 | 103.4 |
| Cu | 0.0974 | 17.40 ± 1.30 | 16.74 ± 0.06 | 96.2 | 22.20 ± 0.31 | 16.20 ± 0.14 | 73.0 |
| Zn | 0.9186 | 0.85 ± 0.09 | 0.86 ± 0.54 | 101.6 | 76.50 ± 2.10 | 93.22 ± 10.87 | 121.9 |
| As | 0.0016 | 0.41 ± 0.04 | 0.40 ± 0.01 | 97.3 | 58.98 ± 0.70 | 61.48 ± 1.36 | 104.2 |
| Se | 0.0029 | - | 0.04 ± 0.00 | - | 11.68 ± 0.13 | 11.32 ± 0.46 | 96.9 |
| Rb | 0.0010 | - | 1.19 ± 0.01 | - | 13.80 ± 0.17 | 13.80 ± 0.13 | 100.0 |
| Sr | 0.0071 | 53.60 ± 1.30 | 52.92 ± 0.51 | 98.7 | 315.20 ± 3.50 | 331.66 ± 2.50 | 105.2 |
| Mo | 0.0278 | 0.50 2 | 0.38 ± 0.04 | 75.6 | 118.50 ± 1.30 | 119.75 ± 1.04 | 101.1 |
| Ag | 0.0007 | 0.01 ± 0.00 | - | 1.04 ± 0.07 | 0.78 ± 0.15 | 75.2 | |
| Cd | 0.0002 | 0.006 ± 0.001 | 0.007 ± 0.000 | 123.3 | 6.41 ± 0.07 | 6.39 ± 0.06 | 99.7 |
| Sb | 0.0011 | 0.30 2 | 0.30 ± 0.01 | 100.1 | 56.88 ± 0.60 | 58.27 ± 0.63 | 102.4 |
| Ba | 0.0400 | 14.00 ± 0.50 | 13.87 ± 0.12 | 99.0 | 531.00 ± 5.60 | 554.59 ± 5.67 | 104.4 |
| Ce | 0.0005 | - | 0.31 ± 0.02 | - | - | 0.76 ± 0.02 | - |
| Tl | 0.0062 | - | 0.06 ± 0.00 | - | 7.26 ± 0.09 | 7.12 ± 0.30 | 98.1 |
| Pb | 0.0030 | 0.08 ± 0.01 | 0.09 ± 0.01 | 116.6 | 19.15 ± 0.20 | 18.09 ± 0.69 | 94.5 |
| Bi | 0.0033 | - | 0.03 ± 0.00 | - | 13.75 ± 0.15 | 12.10 ± 0.51 | 88.0 |
| U | 0.0008 | 0.10 2 | 0.12 ± 0.03 | 116.2 | - | 0.001 ± 0.000 | - |
| Sample | wt.(S)/% | wt.(C)/% | wt.(H)/% | wt.(N)/% | Gross Calorific Value/MJ/kg |
|---|---|---|---|---|---|
| Sample 1 | 0.85 | 59.80 | 0.48 | 1.04 | 20.60 |
| Sample 2 | 1.37 | 63.07 | 0.48 | 1.17 | 21.66 |
| Sample 3 | 1.44 | 64.80 | 0.31 | 1.06 | 21.47 |
| Sample | TiO2 | Fe2O3 | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 1.28 | 6.45 | 0.7 | 1.8 | 18.21 | 54.62 | 1.42 | 7.4 | 1.88 | 5.94 |
| Sample 2 | 1.16 | 6.09 | 1.32 | 1.89 | 15.1 | 48.42 | 1.28 | 8.15 | 1.84 | 14.49 |
| Sample 3 | 1.50 | 4.6 | 1.2 | 1.92 | 15.91 | 44.09 | 1.24 | 8.01 | 1.64 | 19.62 |
| Element | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| X ± SD | X ± SD | X ± SD | |
| As | <QL | <QL | <QL |
| Ba | 1053.34 ± 30.47 | 321.78 ± 11.34 | 312.76 ± 6.73 |
| Cd | 7.87 ± 0.19 | 7.63 ± 0.59 | 7.57 ± 0.10 |
| Co | 21.50 ± 0.41 | 17.22 ± 0.87 | 20.19 ± 0.65 |
| Cr | 242.88 ± 2.39 | 127.12 ± 6.84 | 113.62 ± 2.92 |
| Cu | 76.28 ± 1.06 | 55.48 ± 3.60 | 47.55 ± 1.29 |
| Ga | 343.81 ± 0.96 | 126.23 ± 6.48 | 162.39 ± 1.66 |
| Li | 110.78 ± 7.11 | 70.59 ± 3.73 | 75.10 ± 0.15 |
| Mn | 320.67 ± 2.47 | 114.81 ± 6.16 | 97.04 ± 1.52 |
| Ni | 154.69 ± 1.72 | 134.34 ± 5.70 | 117.36 ± 3.25 |
| Pb | 48.58 ± 3.31 | 35.46 ± 2.23 | 41.80 ± 7.70 |
| Se | 3.29 ± 1.16 | 3.05 ± 1.16 | 3.30 ± 0.47 |
| Sr | 402.52 ± 9.19 | 341.96 ± 13.85 | 408.10 ± 3.68 |
| Tl | <QL | <QL | <QL |
| V | 420.19 ± 2.90 | 207.14 ± 9.39 | 277.96 ± 3.61 |
| Zn | 93.51 ± 1.08 | 27.98 ± 2.03 | 27.23 ± 1.13 |
| Leachate | Sample | Conductivity, µS/cm |
|---|---|---|
| Ultra-pure water | 1 | 370.1 |
| 2 | 394.30 | |
| 3 | 441.33 | |
| Acid rain | 1 | 325.50 |
| 2 | 398.67 | |
| 3 | 411.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trkmić, M.; Ćurković, L.; Ašperger, D.; Ujević Bošnjak, M.; Vinković Vrček, I.; Matečić Mušanić, S. Ash Properties and Environmental Impact of Coal and Its Blend with Patent Fuel for Climate Sustainability. Sustainability 2024, 16, 4413. https://doi.org/10.3390/su16114413
Trkmić M, Ćurković L, Ašperger D, Ujević Bošnjak M, Vinković Vrček I, Matečić Mušanić S. Ash Properties and Environmental Impact of Coal and Its Blend with Patent Fuel for Climate Sustainability. Sustainability. 2024; 16(11):4413. https://doi.org/10.3390/su16114413
Chicago/Turabian StyleTrkmić, Marija, Lidija Ćurković, Danijela Ašperger, Magdalena Ujević Bošnjak, Ivana Vinković Vrček, and Sanja Matečić Mušanić. 2024. "Ash Properties and Environmental Impact of Coal and Its Blend with Patent Fuel for Climate Sustainability" Sustainability 16, no. 11: 4413. https://doi.org/10.3390/su16114413
APA StyleTrkmić, M., Ćurković, L., Ašperger, D., Ujević Bošnjak, M., Vinković Vrček, I., & Matečić Mušanić, S. (2024). Ash Properties and Environmental Impact of Coal and Its Blend with Patent Fuel for Climate Sustainability. Sustainability, 16(11), 4413. https://doi.org/10.3390/su16114413








