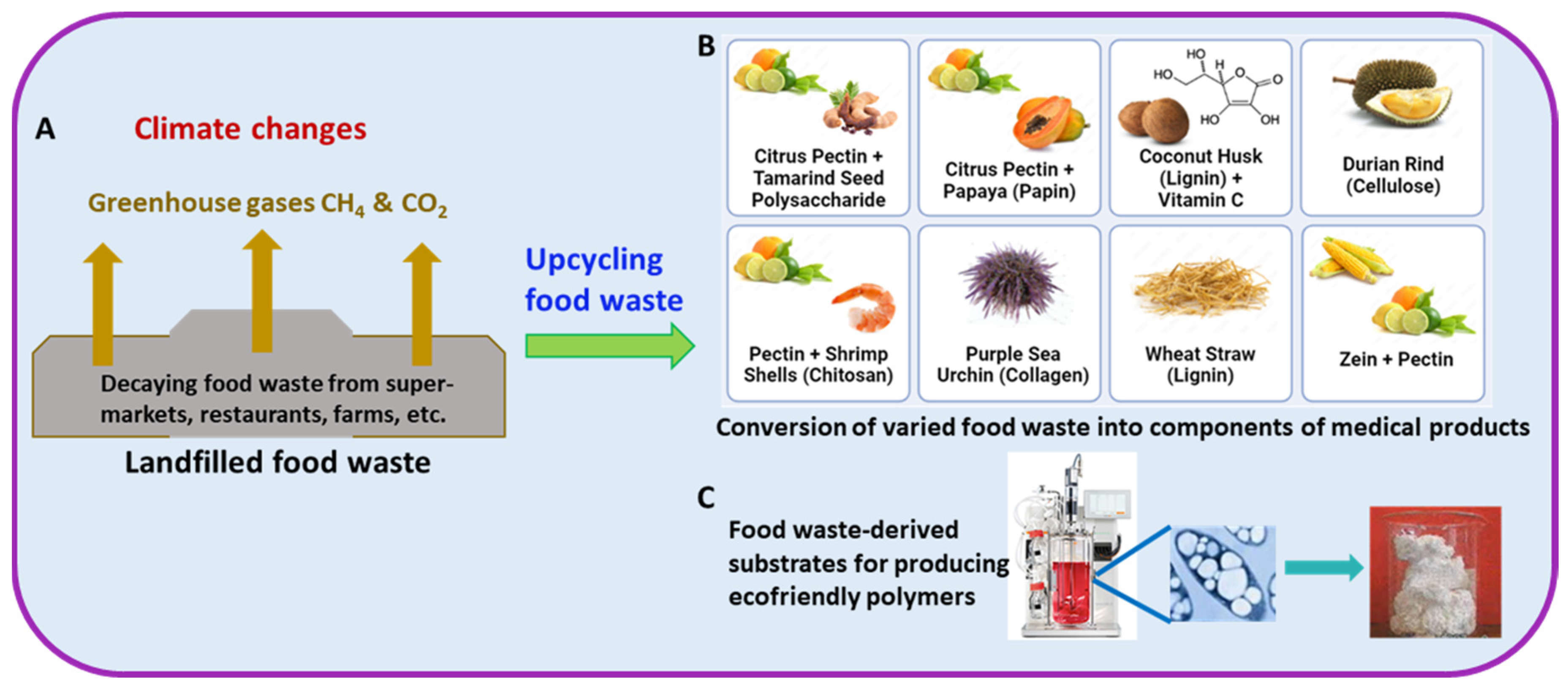
Upcycling Food Waste into Biomaterials Applicable to Medical Products
Abstract
:1. Introduction
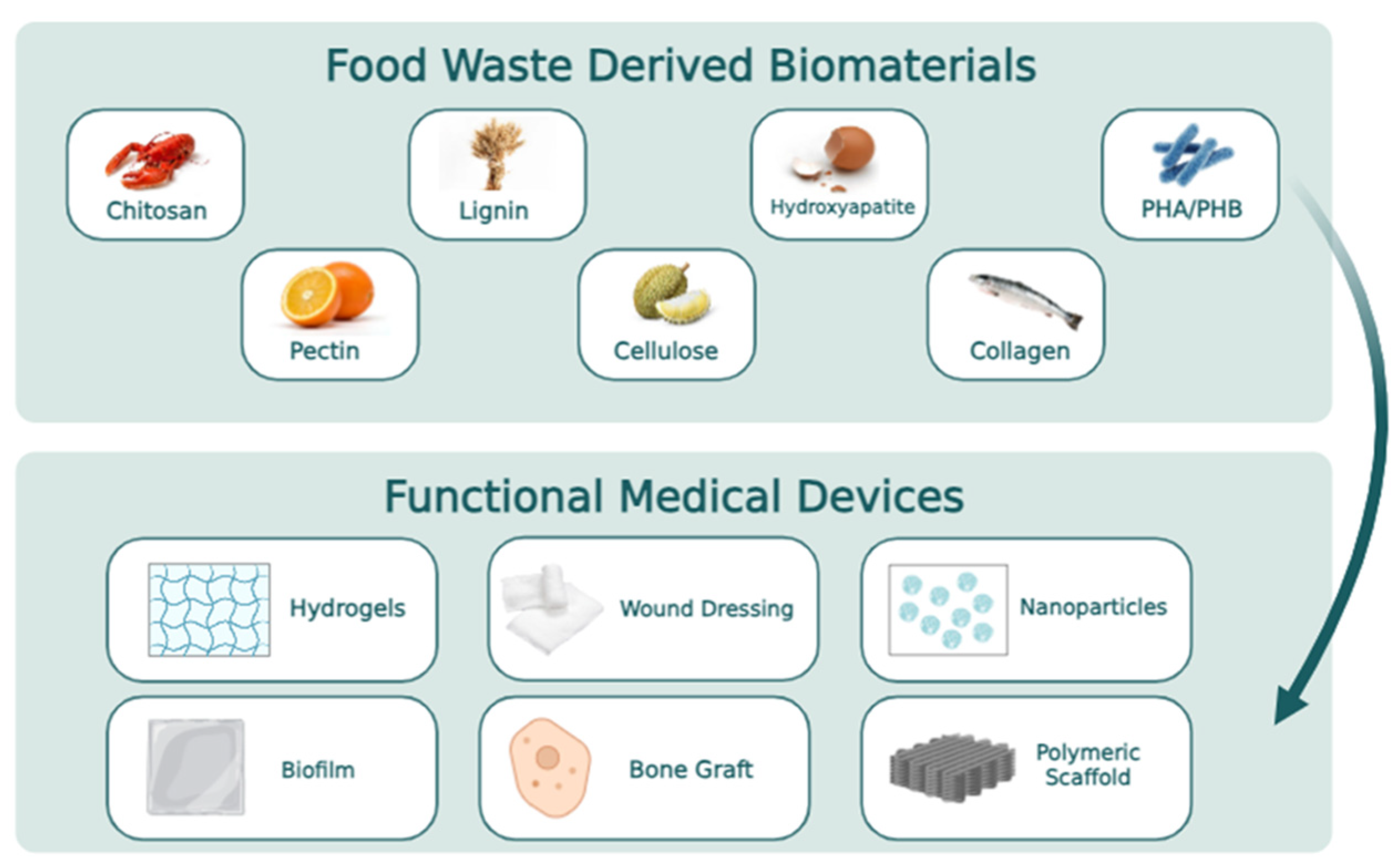
2. Food Waste as a Source for Making Valuable Biomaterials
2.1. Lignin Extraction from Food Waste
2.2. Cellulose Extraction from Food Waste
2.3. Chitosan Extraction from Food Waste
2.4. Pectin Extraction from Food Waste
2.5. Collagen Extraction from Food Waste
2.6. Hydroxyapatite Extraction from Food Waste
3. Conversion of Food Waste into Carbon Sources for the Production of Biodegradable Plastics
3.1. Polyhydroxyalkanoate Production from a Variety of Food Waste Using Natural PHA-Accumulating Microorganisms
| Food Waste | Bacterium | DCW * (g/L) | PHA/PHB ** Content (%) | Reference |
|---|---|---|---|---|
| Spent coffee grounds | Curpriavidus necator | 55.4 | 89.1 | [89] |
| Spent coffee grounds | Cupriavidus necator DSM 428 | 16.7 | 78.4 | [90] |
| Saccharified spent coffee grounds | Burkholderia cepacia CCM 2656 (ATCC 17759) | 4.9 | 54.8 | [104] |
| Rice straw | Bacillus firmus NII 0830 | 1.9 | 89 | [107] |
| Rice husk | B. cepacia USM (JCM 15050) | 7.8 | 50 | [92] |
| Waste cooking oil | Cupriavidus necator H16 | 15.5 | 70 | [94] |
| Used cooking oil | Cupriavidus necator DSM 428 | 10.4 | 37 | [95] |
| Used sunflower oil | Cupriavidus necator DSM 428 | 11.4 | 57 | [95] |
| Waste rapeseed oil with 1% of propanol | Cupriavidus necator H16 | 138 | 75 | [97] |
| Sunflower-derived cooking oil | Burkholderia thailandensis E264 | 12.2 | 61 | [103] |
| Paddy straw mushrooms | Ralstonia eutropha MTCC 1472 | 19.2 | 37.6 | [96] |
| Sugarcane bagasse | Halogeometricum borinquense E3 | 4.15 | 50.4 | [99] |
| Sugarcane bagasse | Burkholderia sp. F24 | 25.04 | 49 | [111] |
| Saccharified waste potato starch | Ralstonia eutropha NCIMB 11599 | 179.0 | 52.5 | [98] |
| Cheese whey | H. mediterranei (ATCC 33500) | 16.01 | 53 | [112] |
| Corn stover | Paracoccus sp. LL1 | 13.41 | 72.4 | [106] |
| Sugarcane molasses | Bacillus megaterium BA-019 | 72.6 | 42 | [109] |
| Liquid bean curd with initial sucrose at 25 g/L | Alcaligenus latus | 3.73 | 66.56 | [110] |
3.2. Polyhydroxyalkanoate Production from Food Waste Using Recombinant Strains
4. Diverse Types of Food Waste-Derived Materials as Essential Components for Fabricating Medical Products
5. Conclusions
Funding
Conflicts of Interest
References
- The Food and Agriculture Organization of the United Nations. 2024. Available online: https://openknowledge.fao.org/home (accessed on 20 May 2024).
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; Ujang, Z.B. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. 2024. Available online: https://www.epa.gov/ (accessed on 20 May 2024).
- Awogbemi, O.; Von Kallon, D.V. Valorization of agricultural wastes for biofuel applications. Heliyon 2022, 8, e11117. [Google Scholar] [CrossRef] [PubMed]
- Jarunglumlert, T.; Bampenrat, A.; Sukkathanyawat, H.; Prommuak, C. Enhanced energy recovery from food waste by co-production of bioethanol and biomethane process. Fermentation 2021, 7, 265. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-food wastes for bioplastics: European prospective on possible applications in their second life for a circular economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef] [PubMed]
- Jazi, M.E.; Narayanan, G.; Aghabozorgi, F.; Farajidizaji, B.; Aghaei, A.; Kamyabi, M.A.; Navarathna, C.M.; Mlsna, T.E. Structure, chemistry and physicochemistry of lignin for material functionalization. SN Appl. Sci. 2019, 1, 1–19. [Google Scholar]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-based polyurethane: Recent advances and future perspectives. Macromol. Rapid Commun. 2021, 42, 2000492. [Google Scholar] [CrossRef]
- Park, J.; Hwang, H.; Kim, J.Y.; Choi, J.W. Applicability of lignin polymers for automobile brake pads as binder and filler materials and their performance characteristics. Environ. Technol. 2020, 41, 488–497. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Assessment of Osteogenic Differentiation Potential of Cytocompatible Rice Husk-Derived Lignin/Silica Nanohybrids for Bone Tissue Engineering. Silicon 2023, 15, 7235–7245. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.-K.; Kadam, A.; Kumar, G.; Jeon, B.-H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, J.J.; Lin, Q.; Jiang, L.; Zhang, D.; Li, Z.; Ma, B.; Zhang, C.; Li, L.; Kai, D. Lignin-incorporated nanogel serving as an antioxidant biomaterial for wound healing. ACS Appl. Bio Mater. 2020, 4, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Jaganathan, G.; Manivannan, K.; Lakshmanan, S.; Sithique, M.A. Fabrication and characterization of Artocarpus heterophyllus waste derived lignin added chitosan biocomposites for wound dressing application. Sustain. Chem. Pharm. 2018, 10, 27–32. [Google Scholar] [CrossRef]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z. A paper-making transformation: From cellulose-based superwetting paper to biomimetic multifunctional inorganic paper. J. Mater. Chem. A 2020, 8, 20238–20259. [Google Scholar] [CrossRef]
- Wüstenberg, T. Cellulose and Cellulose Derivatives in the Food Industry: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Liu, T.; Du, G.; Wu, Y.; Liu, C.; Yang, H.; Ni, K.; Yin, C.; Ran, X.; Gao, W.; Yang, L. Activated wood surface and functionalized cellulose co-building strong chemical wood bonding performance. Carbohydr. Polym. 2023, 305, 120573. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.; Prates, A.; Evtuguin, A. Production of rayon fibres from cellulosic pulps: State of the art and current developments. Carbohydr. Polym. 2021, 273, 118466. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Life cycle assessment of a new technology to extract, functionalize and orient cellulose nanofibers from food waste. ACS Sustain. Chem. Eng. 2015, 3, 1047–1055. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.; Ng, K.R.; Chen, W.N. Food waste Durian rind-derived cellulose organohydrogels: Toward anti-freezing and antimicrobial wound dressing. ACS Sustain. Chem. Eng. 2021, 9, 1304–1312. [Google Scholar] [CrossRef]
- Sommano, S.R.; Jantrawut, P.; Sangta, J.; Chanabodeechalermrung, B.; Sunanta, P.; Bakshani, C.; Willats, W. Utilization of coffee pulp for the production of sustainable cellulosic composite and plant-based hydrogel as a potential human wound dressing. Food Struct. 2023, 37, 100347. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent advances in chitosan-based applications—A review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Pottathara, Y.B.; Tiyyagura, H.R.; Ahmad, Z.; Thomas, S. Chitin and chitosan composites for wearable electronics and energy storage devices. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–88. [Google Scholar]
- Valachová, K.; Šoltés, L. Versatile use of chitosan and hyaluronan in medicine. Molecules 2021, 26, 1195. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment, and wound healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Borić, M.; Puliyalil, H.; Novak, U.; Likozar, B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018, 20, 1199–1204. [Google Scholar] [CrossRef]
- Theapsak, S.; Watthanaphanit, A.; Rujiravanit, R. Preparation of chitosan-coated polyethylene packaging films by DBD plasma treatment. ACS Appl. Mater. Interfaces 2012, 4, 2474–2482. [Google Scholar] [CrossRef]
- Chakravarty, J.; Yang, C.-L.; Palmer, J.; Brigham, C.J. Chitin extraction from lobster shell waste using microbial culture-based methods. Appl. Food Biotechnol. 2018, 5, 141–154. [Google Scholar]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Arafat, A.; Samad, S.A.; Masum, S.M.; Moniruzzaman, M. Preparation and characterization of chitosan from shrimp shell waste. Int. J. Sci. Eng. Res. 2015, 6, 538–541. [Google Scholar]
- Ghorbani, M.; Roshangar, L.; Rad, J.S. Development of reinforced chitosan/pectin scaffold by using the cellulose nanocrystals as nanofillers: An injectable hydrogel for tissue engineering. Eur. Polym. J. 2020, 130, 109697. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Kedir, W.M.; Deresa, E.M.; Diriba, T.F. Pharmaceutical and drug delivery applications of pectin and its modified nanocomposites. Heliyon 2022, 8, e10654. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Shivamathi, C.S.; Gunaseelan, S.; Soosai, M.R.; Vignesh, N.S.; Varalakshmi, P.; Kumar, R.S.; Karthikumar, S.; Kumar, R.V.; Baskar, R.; Rigby, S.P. Process optimization and characterization of pectin derived from underexploited pineapple peel biowaste as a value-added product. Food Hydrocoll. 2022, 123, 107141. [Google Scholar] [CrossRef]
- Yu, N.; Wang, X.; Ning, F.; Jiang, C.; Li, Y.; Peng, H.; Xiong, H. Development of antibacterial pectin from Akebia trifoliata var. australis waste for accelerated wound healing. Carbohydr. Polym. 2019, 217, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Petkowicz, C.; Vriesmann, L.; Williams, P. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Demir, D.; Ceylan, S.; Göktürk, D.; Bölgen, N. Extraction of pectin from albedo of lemon peels for preparation of tissue engineering scaffolds. Polym. Bull. 2021, 78, 2211–2226. [Google Scholar] [CrossRef]
- Saurabh, V.; Vathsala, V.; Yadav, S.K.; Sharma, N.; Varghese, E.; Saini, V.; Singh, S.P.; Dutta, A.; Kaur, C. Extraction and characterization of ultrasound assisted extraction: Improved functional quality of pectin from jackfruit (Artocarpus heterophyllus Lam.) peel waste. J. Food Meas. Charact. 2023, 17, 6503–6521. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chen, H.-H.; Chang, S.-H.; Ni, T.-S. Pectin-chitosan-PVA nanofibrous scaffold made by electrospinning and its potential use as a skin tissue scaffold. J. Biomater. Sci. Polym. Ed. 2013, 24, 470–484. [Google Scholar] [CrossRef]
- Méndez, P.A.; López, B.L. Polyelectrolyte nanoparticles of amphiphilic chitosan/pectin from banana peel as potential carrier system of hydrophobic molecules. Polymers 2020, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing green methods for pectin extraction from waste orange peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Freitas, C.; Sousa, R.; Dias, M.; Coimbra, J. Extraction of pectin from passion fruit peel. Food Eng. Rev. 2020, 12, 460–472. [Google Scholar] [CrossRef]
- Govindaraj, D.; Rajan, M.; Hatamleh, A.A.; Munusamy, M.A. From waste to high-value product: Jackfruit peel derived pectin/apatite bionanocomposites for bone healing applications. Int. J. Biol. Macromol. 2018, 106, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Harshith, N.; Yogesh, P.S.; Bibin, K.N.K.; Rabin, R.; Anusiya, G.; Ganesh, R.J. Synthesis of bio-nanofiber from pectin/polyvinyl alcohol for therapeutic application. J. Appl. Biol. Biotechnol. 2023, 11, 101–105. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Millan-Linares, M.C.; la Paz, S.M.-D.; Martin, M.E. Pectins and olive pectins: From biotechnology to human health. Biology 2021, 10, 860. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The integrin–collagen connection–a glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Ye, K.; Jin, S. Proteomic analysis of decellularized pancreatic matrix identifies collagen V as a critical regulator for islet organogenesis from human pluripotent stem cells. Biomaterials 2020, 233, 119673. [Google Scholar] [CrossRef]
- Freeman, S.; Ramos, R.; Chando, P.A.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs. Acta Biomater 2019, 95, 152–164. [Google Scholar] [CrossRef]
- Huang, H.; Karanth, S.S.; Guan, Y.; Freeman, S.; Soron, R.; Godovich, D.S.; Guan, J.; Ye, K.; Jin, S. Oxygenated Scaffolds for Pancreatic Endocrine Differentiation from Induced Pluripotent Stem Cells. Adv. Healthc. Mater. 2024, 13, 2302275. [Google Scholar] [CrossRef]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen as an implantable material in medicine and dentistry. J. Oral Implantol. 2002, 28, 220–225. [Google Scholar] [CrossRef]
- Ihanamäki, T.; Pelliniemi, L.J.; Vuorio, E. Collagens and collagen-related matrix components in the human and mouse eye. Prog. Retin. Eye Res. 2004, 23, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.W.; Yeo, D.C.L.; Tan, V.; Singh, S.; Choudhury, D.; Naing, M.W. Additive biomanufacturing with collagen inks. Bioengineering 2020, 7, 66. [Google Scholar] [CrossRef]
- Huang, N.F.; Zaitseva, T.S.; Paukshto, M.V. Biomedical Applications of Collagen. Bioengineering 2023, 10, 90. [Google Scholar] [CrossRef]
- Milan, E.P.; Bertolo, M.R.; Martins, V.C.; Sobrero, C.E.; Plepis, A.M.; Fuhrmann-Lieker, T.; Horn, M.M. Effects of mangosteen peel phenolic compounds on tilapia skin collagen-based mineralized scaffold properties. ACS Omega 2022, 7, 34022–34033. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Characterization of acid-and pepsin-soluble collagen extracted from the skin of purple-spotted bigeye snapper. Gels 2022, 8, 665. [Google Scholar] [CrossRef]
- Carolo, A.; Melotti, L.; Zivelonghi, G.; Sacchetto, R.; Akyürek, E.E.; Martinello, T.; Venerando, A.; Iacopetti, I.; Sugni, M.; Martinelli, G. Mutable collagenous tissue isolated from echinoderms leads to the production of a dermal template that is biocompatible and effective for wound healing in rats. Mar. Drugs 2023, 21, 506. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Shih, M.-K.; Hsieh, S.-L.; Hsieh, C.-W.; Liu, T.T.; Chen, M.-H.; Huang, Y.-W.; Hou, C.-Y. Structural characteristics of collagen from cuttlefish skin waste extracted at optimized conditions. Int. J. Food Prop. 2022, 25, 2211–2222. [Google Scholar] [CrossRef]
- Abbas, A.A.; Shakir, K.A.; Walsh, M.K. Functional properties of collagen extracted from catfish (Silurus triostegus) waste. Foods 2022, 11, 633. [Google Scholar] [CrossRef]
- Martins, E.; Fernandes, R.; Alves, A.L.; Sousa, R.O.; Reis, R.L.; Silva, T.H. Skin byproducts of reinhardtius hippoglossoides (Greenland halibut) as ecosustainable source of marine collagen. Appl. Sci. 2022, 12, 11282. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen derived from fish industry waste: Progresses and challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the retention of tissue niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Bee, S.-L.; Hamid, Z.A. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Borciani, G.; Fischetti, T.; Ciapetti, G.; Montesissa, M.; Baldini, N.; Graziani, G. Marine biological waste as a source of hydroxyapatite for bone tissue engineering applications. Ceram. Int. 2023, 49, 1572–1584. [Google Scholar] [CrossRef]
- Teoh, M.; Ng, C.; Lee, S.K.; Ramesh, S.; Ting, C.; Chuah, Y.; Lim, I.Y.; Tan, C.; Sutharsini, U. Densification behaviors of hydroxyapatite/pectin bio-ceramics. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Boudreau, S.; Hrapovic, S.; Liu, Y.; Leung, A.C.; Lam, E.; Kerton, F.M. Isolation of hydroxyapatite from Atlantic salmon processing waste using a protease and lipase mixture. RSC Sustain. 2023, 1, 1554–1564. [Google Scholar] [CrossRef]
- Arslan, Y.E.; Arslan, T.S.; Derkus, B.; Emregul, E.; Emregul, K.C. Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductive biocomposite scaffolds for bone tissue engineering: From waste to regenerative medicine products. Colloids Surf. B Biointerfaces 2017, 154, 160–170. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a valuable biomolecule from seafood industry waste in the design of green food packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef]
- Senadheera, T.R.; Dave, D.; Shahidi, F. Sea cucumber derived type I collagen: A comprehensive review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Babaremu, K.; John, M.; Mfoh, U.; Akinlabi, E.; Okokpujie, I. Behavioral characteristics of magnesium as a biomaterial for surface engineering application. J. Bio-Tribo-Corros. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Kabilan, S.; Ayyasamy, M.; Jayavel, S.; Paramasamy, G. Pseudomonas sp. as a source of medium chain length polyhydroxyalkanoates for controlled drug delivery: Perspective. Int. J. Microbiol. 2012, 2012, 317828. [Google Scholar] [CrossRef] [PubMed]
- Bonartsev, A.; Bonartseva, G.; Reshetov, I.; Kirpichnikov, M.; Shaitan, K. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly (3-hydroxybutyrate). Acta Naturae (англoязычная версия) 2019, 11, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of C/N ratio on polyhydroxyalkanoates (PHA) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Heng, K.S.; Hatti-Kaul, R.; Adam, F.; Fukui, T.; Sudesh, K. Conversion of rice husks to polyhydroxyalkanoates (PHA) via a three-step process: Optimized alkaline pretreatment, enzymatic hydrolysis, and biosynthesis by Burkholderia cepacia USM (JCM 15050). J. Chem. Technol. Biotechnol. 2017, 92, 100–108. [Google Scholar] [CrossRef]
- Hassan, M.A.; Shirai, Y.; Umeki, H.; Yamazumi, H.; Jin, S.; Yamamoto, S.; Karim, M.I.A.; Nakanishi, K.; Hashimoto, K. Acetic acid separation from anaerobically treated palm oil mill effluent by ion exchange resins for the production of polyhydroxyalkanoate by Alcaligenes eutrophus. Biosci. Biotechnol. Biochem. 1997, 61, 1465–1468. [Google Scholar] [CrossRef]
- Kamilah, H.; Al-Gheethi, A.; Yang, T.A.; Sudesh, K. The use of palm oil-based waste cooking oil to enhance the production of polyhydroxybutyrate [P (3HB)] by Cupriavidus necator H16 strain. Arab. J. Sci. Eng. 2018, 43, 3453–3463. [Google Scholar] [CrossRef]
- Martino, L.; Cruz, M.V.; Scoma, A.; Freitas, F.; Bertin, L.; Scandola, M.; Reis, M.A. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. Int. J. Biol. Macromol. 2014, 71, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, M.; Aravind, J.; Kanmani, P. Production of polyhydroxyalkanoates from Ralstonia eutropha using paddy straw as cheap substrate. Int. J. Environ. Sci. Technol. 2013, 10, 47–54. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Jin, B.; Zepf, F.T. Production of poly (3-hydroxybutyrate) from waste potato starch. Biosci. Biotechnol. Biochem. 2008, 72, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.; Van Keulen, F.; Ferreira, B.S.; Telo, J.P.; da Fonseca, M.M.R. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Burkholderia sacchari using wheat straw hydrolysates and gamma-butyrolactone. Int. J. Biol. Macromol. 2014, 71, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.; Sciarria, T.P.; Reis, M.; Scaglia, B.; Adani, F. Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 2016, 218, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Follonier, S.; Goyder, M.S.; Silvestri, A.-C.; Crelier, S.; Kalman, F.; Riesen, R.; Zinn, M. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 42–52. [Google Scholar] [CrossRef]
- Kourmentza, C.; Costa, J.; Azevedo, Z.; Servin, C.; Grandfils, C.; De Freitas, V.; Reis, M. Burkholderia thailandensis as a microbial cell factory for the bioconversion of used cooking oil to polyhydroxyalkanoates and rhamnolipids. Bioresour. Technol. 2018, 247, 829–837. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Petrik, S.; Oborna, J.; Prikryl, R.; Marova, I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem. 2014, 49, 1409–1414. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Vlysidis, A.; Papanikolaou, S.; Kookos, I.K.; Martínez, B.M.; Rondán, M.C.E.; Koutinas, A.A. Downstream separation of poly (hydroxyalkanoates) using crude enzyme consortia produced via solid state fermentation integrated in a biorefinery concept. Food Bioprod. Process. 2016, 100, 323–334. [Google Scholar] [CrossRef]
- Sawant, S.S.; Salunke, B.K.; Kim, B.S. Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp. LL1. Bioresour. Technol. 2015, 194, 247–255. [Google Scholar] [CrossRef]
- Sindhu, R.; Silviya, N.; Binod, P.; Pandey, A. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 2013, 78, 67–72. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar]
- Kulpreecha, S.; Boonruangthavorn, A.; Meksiriporn, B.; Thongchul, N. Inexpensive fed-batch cultivation for high poly (3-hydroxybutyrate) production by a new isolate of Bacillus megaterium. J. Biosci. Bioeng. 2009, 107, 240–245. [Google Scholar] [CrossRef]
- Kumalaningsih, S.; Hidayat, N.; Aini, N. Optimization of polyhydroxyalkanoates (PHA) production from liquid bean curd waste by Alcaligenes latus bacteria. J. Agric. Food Technol. 2011, 1, 63–67. [Google Scholar]
- Lopes, M.S.G.; Gomez, J.G.C.; Taciro, M.K.; Mendonça, T.T.; Silva, L.F. Polyhydroxyalkanoate biosynthesis and simultaneous remotion of organic inhibitors from sugarcane bagasse hydrolysate by Burkholderia sp. J. Ind. Microbiol. Biotechnol. 2014, 41, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Pais, J.; Serafim, L.S.; Freitas, F.; Reis, M.A. Conversion of cheese whey into poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. New Biotechnol. 2016, 33, 224–230. [Google Scholar] [CrossRef]
- Patel, S.K.; Singh, M.; Kumar, P.; Purohit, H.J.; Kalia, V.C. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy 2012, 36, 218–225. [Google Scholar] [CrossRef]
- Hong, K.; Leung, Y.C.; Kwok, S.Y.; Law, K.H.; Lo, W.H.; Chua, H.; Yu, P.H.F. Construction of recombinant Escherichia coli strains for polyhydroxybutyrate production using soy waste as nutrient. Appl. Biochem. Biotechnol. 2000, 84, 381–390. [Google Scholar] [CrossRef]
- Law, K.-H.; Chan, P.-L.; Lau, W.-S.; Cheng, Y.-C.; Leung, Y.-C.; Lo, W.-H.; Lawford, H.; Yu, H.-F. Construction of recombinant Escherichia coli strains for production of poly-(3-hydroxybutyrate-co-3-hydroxyvalerate). In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; Springer: Berlin/Heidelberg, Germany, 2004; pp. 361–372. [Google Scholar]
- Lee, S.; Middelberg, A.; Lee, Y. Poly (3-hydroxybutyrate) production from whey using recombinant Escherichia coli. Biotechnol. Lett. 1997, 19, 1033–1035. [Google Scholar] [CrossRef]
- Pais, J.; Farinha, I.; Freitas, F.; Serafim, L.S.; Martínez, V.; Martínez, J.C.; Arévalo-Rodríguez, M.; Prieto, M.A.; Reis, M.A. Improvement on the yield of polyhydroxyalkanotes production from cheese whey by a recombinant Escherichia coli strain using the proton suicide methodology. Enzym. Microb. Technol. 2014, 55, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Park, S.J.; Lee, S.Y. Production of poly (3-hydroxybutyrate) by fed-batch culture of recombinant Escherichia coli with a highly concentrated whey solution. Appl. Environ. Microbiol. 2000, 66, 3624–3627. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Kim, J.-H.; Kim, M.-S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.-J.; Jeon, J.-M.; Kim, S.-H.; Ahn, J. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2018, 41, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.G.; Antonio, R.V. Polyhydroxyalkanoates production by recombinant Escherichia coli harboring the structural genes of the polyhydroxyalkanoate synthases of Ralstonia eutropha and Pseudomonas aeruginosa using low cost substrate. J. Appl. Sci. 2006, 6, 1745–1750. [Google Scholar] [CrossRef]
- Pandit, A.P.; Koyate, K.R.; Kedar, A.S.; Mute, V.M. Spongy wound dressing of pectin/carboxymethyl tamarind seed polysaccharide loaded with moxifloxacin beads for effective wound heal. Int. J. Biol. Macromol. 2019, 140, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Jáuregui, K.M.G.; Cabrera, J.C.C.; Ceniceros, E.P.S.; Hernández, J.L.M.; Ilyina, A. A new formulated stable papin-pectin aerosol spray for skin wound healing. Biotechnol. Bioprocess Eng. 2009, 14, 450–456. [Google Scholar] [CrossRef]
- Fiorentini, F.; Suarato, G.; Summa, M.; Miele, D.; Sandri, G.; Bertorelli, R.; Athanassiou, A. Plant-Based, Hydrogel-like Microfibers as an Antioxidant Platform for Skin Burn Healing. ACS Appl. Bio Mater. 2023, 6, 3103–3116. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Kocaaga, B.; Kurkcuoglu, O.; Tatlier, M.; Batirel, S.; Guner, F.S. Low-methoxyl pectin–zeolite hydrogels controlling drug release promote in vitro wound healing. J. Appl. Polym. Sci. 2019, 136, 47640. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, A.; Afzal, Z.; Umer, M.F.; Khan, J.; Khan, G.M. Formulation development and characterization of cefazolin nanoparticles-loaded crosslinked films of sodium alginate and pectin as wound dressings. Int. J. Biol. Macromol. 2019, 124, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Koshy, J.; Sangeetha, D. Hemigraphis Alternata Leaf Extract Incorporated Agar/Pectin-Based Bio-Engineered Wound Dressing Materials for Effective Skin Cancer Wound Care Therapy. Polymers 2022, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Kamel, N.A.; El-messieh, S.L.A.; Saleh, N.M. Chitosan/banana peel powder nanocomposites for wound dressing application: Preparation and characterization. Mater. Sci. Eng. C 2017, 72, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, M.; Ahn, D.U.; Huang, X. Preparation and characterization of novel eggshell membrane-chitosan blend films for potential wound-care dressing: From waste to medicinal products. Int. J. Biol. Macromol. 2019, 123, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kiadeh, S.Z.H.; Ghaee, A.; Farokhi, M.; Nourmohammadi, J.; Bahi, A.; Ko, F.K. Electrospun pectin/modified copper-based metal–organic framework (MOF) nanofibers as a drug delivery system. Int. J. Biol. Macromol. 2021, 173, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jin, S.; Ye, K. Development of islet organoids from H9 human embryonic stem cells in biomimetic 3D scaffolds. Stem. Cells Dev. 2017, 26, 394–404. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.; Lee, J.-K. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef]
- Najah, M.; Aisyah, R.; Adzila, S.; Haq, R. Mechanical properties of calcium phosphate reinforced polyhydroxyalkanoate (PHA) biocomposite. J. Thermoplast. Compos. Mater. 2023, 36, 3294–3311. [Google Scholar] [CrossRef]
- Injorhor, P.; Trongsatitkul, T.; Wittayakun, J.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Biodegradable Polylactic Acid-Polyhydroxyalkanoate-Based Nanocomposites with Bio-Hydroxyapatite: Preparation and Characterization. Polymers 2023, 15, 1261. [Google Scholar] [CrossRef]
- Phuegyod, S.; Pramual, S.; Wattanavichean, N.; Assawajaruwan, S.; Amornsakchai, T.; Sukho, P.; Svasti, J.; Surarit, R.; Niamsiri, N. Microbial Poly (hydroxybutyrate-co-hydroxyvalerate) Scaffold for Periodontal Tissue Engineering. Polymers 2023, 15, 855. [Google Scholar] [CrossRef] [PubMed]
- Ghadirian, S.; Karbasi, S.; Kharazi, A.Z.; Setayeshmehr, M. Evaluation of the effects of halloysite nanotubes on physical, mechanical, and biological properties of polyhydroxy butyrate electrospun scaffold for cartilage tissue engineering applications. J. Polym. Environ. 2024, 32, 1170–1187. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.; Kim, I. Smart l-borneol-loaded hierarchical hollow polymer nanospheres with antipollution and antibacterial capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.G.; Song, W.; Kim, B.J. Rational design of hyper-crosslinked polymers for biomedical applications. J. Polym. Sci. 2024, 62, 1517–1535. [Google Scholar] [CrossRef]

| Bacterium | Inserted Genes | Food Waste | DCW * (g/L) | PHA/PHB ** Content (%) | Reference |
|---|---|---|---|---|---|
| E. coli | phb operon | Soy waste | 3.03 | 27.83 | [114] |
| E. coli | phaC, phaA, and phaB | Malt waste | 10.27 | 43 | [115] |
| E. coli | Alcaligenes eutrophus polyhydroxyalkanoate (PHA) biosynthesis genes | Whey | 6.42 | 81 | [116] |
| E. coli | C. necator phbC, phbA and phbB genes | Cheese Whey | 36 | 21.73 | [117] |
| E. coli | A. latus PHA biosynthesis genes | Whey | 6.6 | 76 | [118] |
| E. coli | Amp, phaC1 gene from P. aeruginosa and PHA operon from R. eutropha | Corn starch and soybean Oil | 0.92 | 5.9 | [120] |
| Cupriavidus necator | Overexpressing phaJ and PHA synthetase (phaC2) with deletion of phaB1, phaB2, and phaB3 | Coffee waste oil | 0.89 | 69 | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahabeer, G.; Jin, S. Upcycling Food Waste into Biomaterials Applicable to Medical Products. Sustainability 2024, 16, 4473. https://doi.org/10.3390/su16114473
Mahabeer G, Jin S. Upcycling Food Waste into Biomaterials Applicable to Medical Products. Sustainability. 2024; 16(11):4473. https://doi.org/10.3390/su16114473
Chicago/Turabian StyleMahabeer, Genna, and Sha Jin. 2024. "Upcycling Food Waste into Biomaterials Applicable to Medical Products" Sustainability 16, no. 11: 4473. https://doi.org/10.3390/su16114473





