Carbon and Sulfur Isotope Methods for Tracing Groundwater Contamination: A Review of Sustainable Utilization in Reclaimed Municipal Landfill Areas
Abstract
:1. Introduction
2. Methods for Detecting Contamination
2.1. Groundwater Contamination
2.2. Gas Pollution
3. Results and Discussion
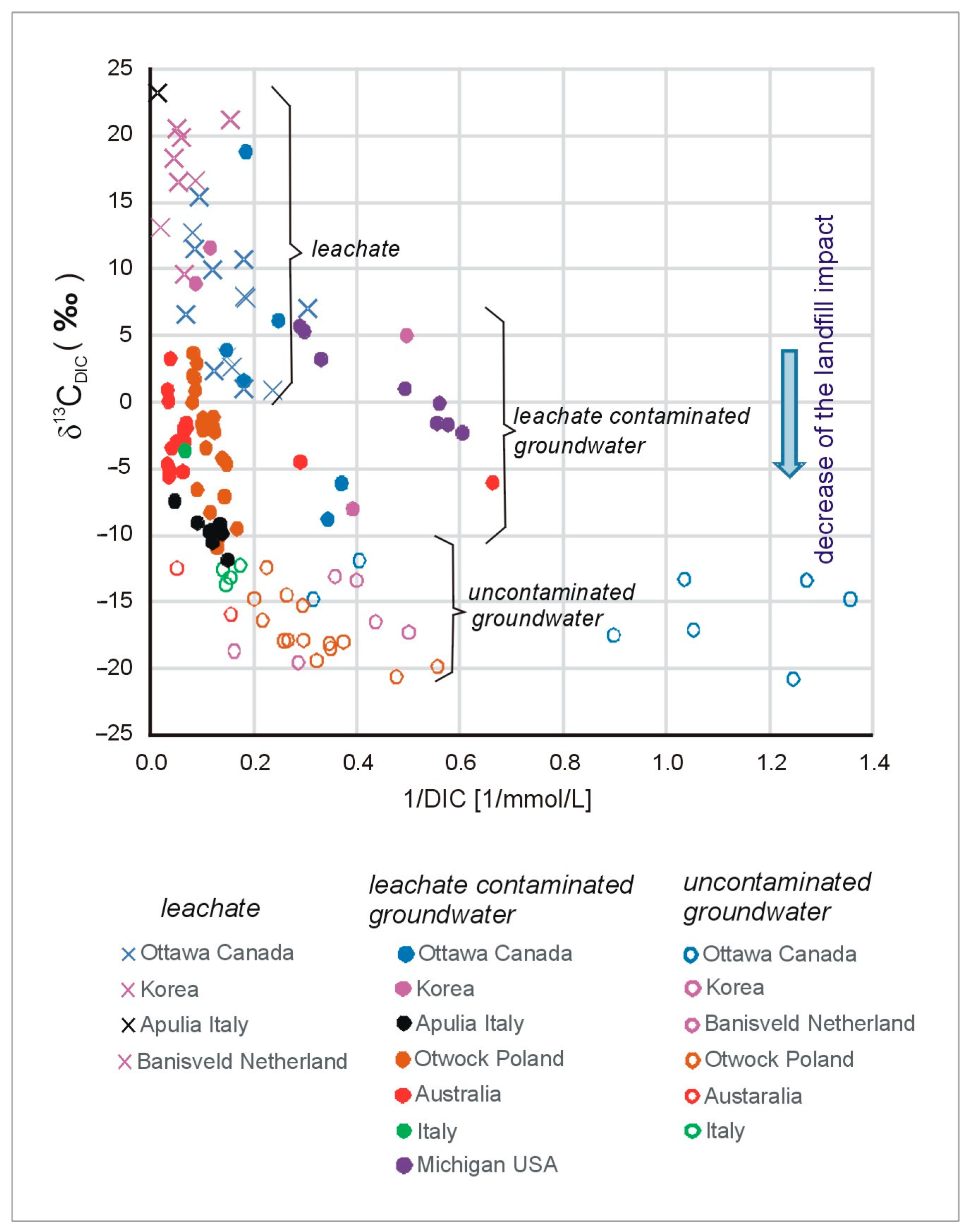
3.1. Carbon and Sulfur Isotope Methods

| Location | δ13CDIC (‰) Uncontaminated Groundwater | δ13CDIC (‰) Contaminated Groundwater | δ13CDIC (‰) Leachate | δ34S (‰) Uncontaminated Groundwater | δ34S (‰) Contaminated Groundwater | δ34S (‰) Leachate | References |
|---|---|---|---|---|---|---|---|
| Europe | |||||||
| Banisveld, The Netherlands | −19.6 | n.a. | from +9.6 to +13.1 | n.a. | from −3.3 to +9.1 | n.a. | [111] |
| Germany | −15.3 | from −18.2 to −10.7 | n.a. | n.a. | from +14 to +36.9 | n.a. | [112] |
| Germany | from −24.2 to −7.4 | from −7.4 to +14.7 | from −5.5 to +25.9 | n.a. | from −3 to +9.1 | n.a. | [64] |
| Germany | from −15 to −12 | n.a. | about +10 | n.a. | n.a. | n.a. | [27] |
| Gajke and Brstje landfills, Slovenia | from −14.9 to −8.2 | n.a. | +6.1 | n.a. | n.a. | n.a. | [113] |
| Apulia, Italy | from −11.88 to −7.44 | n.a. | +23.24 | n.a. | n.a. | n.a. | [114] |
| Italy | about −16 | about −4 | n.a. | n.a. | n.a. | n.a. | [14] |
| Central Italy | from −13.69 to −12.25 | −3.64 | n.a. | n.a. | n.a. | n.a. | [47] |
| Otwock, Poland | from −20.6 to −12.4 | from −10.9 to +3.6 | n.a. | n.a. | n.a. | n.a. | [109] |
| Upper Silesia, Poland | n.a. | n.a. | n.a. | n.a. | from −4.8 to +11.5 | from +10.9 to +25.6 | [115] |
| Moscow, Russia | n.a. | from −10 to +2.7 | n.a. | n.a. | n.a. | n.a. | [116] |
| Asia | |||||||
| Korea | from −18.7 to −14.4 | from +5.0 to +11.6 | from +16.5 to +21.2 | n.a. | n.a. | n.a. | [69] |
| Indonesia | n.a. | n.a. | n.a. | n.a. | from +3.92 to +6.66 | +8.87 | [117] |
| North and South America | |||||||
| Norman, Oklahoma | from −17.8 to −12.5 | from −8.8 to +11.9 | n.a. | −5 | from +8.7 to +50.4 | n.a. | [10,17,94,110] |
| New York | −23.1 | n.a. | from +20.9 to +24.3 | n.a. | n.a. | n.a. | [106] |
| Illinois | n.a. | n.a. | from +16 to +22 | n.a. | n.a. | n.a. | [73] |
| South California | −20.28 | from −17.3 to −13.18 | +2.27 | n.a. | n.a. | n.a. | [61] |
| Kalamazoo, Michigan | from −16.9 to −10.0 | from −2.3 to +5.7 | - | n.a. | n.a. | n.a. | [118] |
| West Lafayette, Indiana | n.a. | n.a. | n.a. | n.a. | from +10 to +17 | n.a. | [119] |
| Trail Road, Ottawa, Canada | −17 | from −6.4 to −1.0 | from +7.0 to +15.4 | n.a. | n.a. | n.a. | [120,121,122] |
| Brazil | from −6.9 to −5.0 | +3.5 | from −1.0 to +18.5 | n.a. | n.a. | n.a. | [46] |
| Australia and Oceania | |||||||
| Sydney, Australia | from −6.05 to −4.48 | from −5.25 to +3.27 | - | n.a. | n.a. | n.a. | [123] |
| Dunedin, New Zealand | n.a. | n.a. | +16.11 ±0.23 | n.a. | n.a. | n.a. | [124,125] |
| Four landfills in New Zealand | n.a. | n.a. | from +2.8 to +15.8 | n.a. | n.a. | n.a. | [62] |
| The most frequent values | from −20 to −10 | from −10 to +5 | from 0 to +20 | −5 | from +5 to +20 even +50 | >+10 | - |
3.2. SWOT Analysis
4. Conclusions
- (1)
- The determination of δ13CDIC and δ34S provides a powerful tool for identifying a zone with natural and leachate-contaminated groundwater. Natural groundwater is characterized by low δ13CDIC and low δ34S values, whereas leachate-contaminated groundwater is characterized by high values. In the study area, these values were as follows: in natural groundwater, the δ13CDIC ranged from −20 to −10‰, and the δ34S values were approximately −5‰, while in the leachate-contaminated groundwater, the δ13CDIC ranged from −10 to + 5‰, and the δ34S ranged from +5 to +20‰.
- (2)
- The decomposition of organic compounds in the landfill results in the formation of carbon compounds (in the forms of CH4 and CO2) and sulfur compounds (in the forms of H2S, CH3SH, (CH3)2S, CS2, and (CH3)2S2) that undergo numerous transformations during migration; moreover, their concentrations outside the landfill may be quite low. However, even low concentrations can have an impact on people’s health during long stays in such places.
- (3)
- The use of combined carbon and sulfur isotope methods leads to a better under-standing of the geochemical processes occurring in a landfill and its surroundings, is a good method for diagnosing the activity of the landfill, and facilitates the concept of developing this site without unnecessary risk and financial losses.
- (4)
- The relationship between the measured δ13CDIC and δ34S is appropriate for detecting groundwater contamination within the vicinity of old municipal landfills and facilitates decision-making with respect to the management of these sites.
- (5)
- The δ13CDIC methods of dissolved inorganic carbon and the δ34S method of dissolved sulfates (which is carried out much less frequently) are recommended for the identification of groundwater contamination around reclaimed municipal waste landfills and to assess the safety of the development of these sites after reclamation. It is suggested that sulfur isotope tests be used more frequently in the case of old landfills.
- (6)
- The evaluation of groundwater quality near landfills (with SWOT analysis) is an essential part of land management. SWOT analyses may provide guidance for decision-makers (municipalities, planners, developers, etc.) regarding the development of spaces around reclaimed landfills. The results of carbon and sulfur isotope research extended with SWOT analyses can be used to justify investments in the development of spatial planning strategies and schemes at the local and regional levels.
Funding
Conflicts of Interest
References
- Malek, W.; Mortazavi, R.; Cialani, C.; Nordström, J. How have waste management policies impacted the flow of municipal waste? An empirical analysis of 14 European countries. Waste Manag. 2023, 164, 84–93. [Google Scholar] [CrossRef]
- Available online: https://www.eea.europa.eu/data-and-maps/daviz/municipal-waste-recycled-and-composted-3#tab-chart_3 (accessed on 16 February 2024).
- Available online: https://www.eea.europa.eu/data-and-maps/daviz/municipal-waste-landfill-rates-in#tab-chart_1 (accessed on 16 February 2024).
- Abarca-Guerrero, L.; Lobo-Ugalde, S.; Méndez-Carpio, N.; Rodríguez-Leandro, R.; Rudin-Vega, V. Zero Waste Systems: Barriers and measures to recycling of construction and demolition waste. Sustainability 2022, 14, 15265. [Google Scholar] [CrossRef]
- EU. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste. OJ L 182, 16.07.1999; pp. 1–19. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31999L0031 (accessed on 7 March 2024).
- EU. Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. OJ L 150, 14.6.2018; pp. 100–108, 32018L0850. Available online: https://eur-lex.europa.eu/eli/dir/2018/850/oj (accessed on 7 March 2024).
- Sangam, H.P.; Rowe, R.K. Migration of dilute aqueous organic pollutants through HDPE geomembranes. Geotext. Geomembr. 2001, 19, 329–357. [Google Scholar] [CrossRef]
- Cerar, S.; Serianz, L.; Koren, K.; Prestor, J.; Mali, N. Synoptic Risk Assessment of Groundwater Contamination from Landfills. Energies 2022, 15, 5150. [Google Scholar] [CrossRef]
- Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste (Consolidated text). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01999L0031-20180704 (accessed on 7 March 2024).
- Cozzarelli, I.M.; Böhlke, J.K.; Masoner, J.; Breit, G.N.; Lorah, M.M.; Tuttle, M.L.W.; Jaeschke, J.B. Biogeochemical evolution of a landfill leachate plume, Norman, Oklahoma. Ground Water. 2011, 49, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, H.; Tong, L.; Wang, Y.; Chen, R.; Liu, S.; Zhao, L.; Li, Z.; Cai, L. Relationships between microbial communities and groundwater chemistry in two pristine confined groundwater aquifers in central China. Hydrol. Processes. 2019, 23, 1993–2005. [Google Scholar] [CrossRef]
- Porowska, D. Review of research methods for assessing the activity of a municipal landfill based on the landfill gas analysis. Period. Polytech. Chem. Eng. 2021, 65, 167–176. [Google Scholar] [CrossRef]
- Guo, Y.; Li, P.; He, X.; Wang, L. Groundwater quality in and around a landfill in Northwest China: Characteristic pollutant identification, health risk assessment, and controlling factor analysis. Expo. Health 2022, 14, 885–901. [Google Scholar] [CrossRef]
- Stevenazzi, S.; Del Gaudio, E.; Ruggiero, D.; D’Aniso, C.; Patelli, A.M.; Ducci, D. Geochemical and isotopic evidence for investigating the impacts of landfills on groundwater: A case study in the Campania Region (Southern Italy). Sustainability 2023, 15, 15822. [Google Scholar] [CrossRef]
- Folino, A.; Gentili, E.; Komilis, D.; Calabrò, P.S. A 35-year monitoring of an Italian landfill: Effect of recirculation of reverse osmosis concentrate on leachate characteristics. Sci. Total Environ. 2024, 915, 170234. [Google Scholar] [CrossRef]
- Kamal, A.; Makhatova, A.; Yergali, B.; Baidullayeva, A.; Satayeva, A.; Kim, J.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Biological Treatment, Advanced Oxidation and Membrane Separation for Landfill Leachate Treatment: A Review. Sustainability 2022, 14, 14427. [Google Scholar] [CrossRef]
- Scholl, M.A.; Cozzarelli, I.M.; Christenson, S.C. Recharge processes drive sulfate reduction in an alluvial aquifer contaminated with landfill leachate. J. Contam. Hydrol. 2006, 104, 4–35. [Google Scholar] [CrossRef] [PubMed]
- Masoner, J.R.; Cozzarelli, I.M. Spatial and temporal migration of a landfill leachate plume in alluvium. Water Air Soil. Pollut. 2015, 226, 18. [Google Scholar] [CrossRef]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, J.B.; Baun, A.; Albrechtsen, H.-J.; Heron, G. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
- Randazzo, A.; Venturi, S.; Tassi, F. Soil processes modify the composition of volatile organic compounds (VOCs) from CO2- and CH4-dominated geogenic and landfill gases: A comprehensive study. Sci. Total Environ. 2024, 923, 171483. [Google Scholar] [CrossRef] [PubMed]
- Lar, K.; Złotkowska, R. Skutki zdrowotne zamieszkiwania w sąsiedztwie składowisk odpadów. Medycyna Środowiskowa 2013, 16, 71–78. (In Polish) [Google Scholar]
- Pivato, A.; Savino, M.; Peres, F.; Lavagnolo, M.C. Landscape requalification of landfills: An open issue between legal and technical aspects. UPLanD J. Urban. Plan. Landsc. Environ. Des. 2019, 4, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Koda, E.; Osiński, P.; Podlasek, A.; Markiewicz, A.; Winkler, J.; Vaverková, M.D. Geoenvironmental approaches in an old municipal waste landfill reclamation process: Expectations vs reality. Soils Found. 2023, 63, 101273. [Google Scholar] [CrossRef]
- First, M.A.; Viles, F.J.; Levin, S. Control of toxic and explosive hazards in buildings erected on landfills. Public Health Rep. 1966, 1, 419–428. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc1919733/ (accessed on 7 March 2024). [CrossRef]
- Emberton, J.R.; Parker, A. The problems associated with building on landfill sites. Waste Manag. Res. 1987, 5, 473–482. [Google Scholar] [CrossRef]
- Singh, R.K.; Datta, M.; Nema, A.K. Review of groundwater contamination hazard rating systems for old landfills. Waste Manag. Res. 2010, 28, 97–108. [Google Scholar] [CrossRef]
- Wimmer, B.; Hrad, M.; Huber-Humer, M.; Watzinger, A.; Wyhlidal, S.; Reichenauer, T.G. Stable isotope signatures for characterising the biological stability of landfilled municipal solid waste. Waste Manag. 2013, 33, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.A.; Lokshina, L.Y. Carbon and hydrogen dynamic isotope equations are used to describe the dominant processes of waste biodegradation: Effect of aeration in methanogenic phase of the landfill. Waste Manag. 2023, 166, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.D.; Silva, S.M.; Fernandes, F.; Teixeira, R.S.; Celligoi, A.; Dall’Antônia, L.H. Geophysical technique and groundwater monitoring to detect leachate contamination in the surrounding area of a landfill—Londrina (PR—Brazil). J. Environ. Manag. 2012, 113, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sunmonu, L.A.; Olafisoye, E.R.; Adagunodo, T.A.; Ojoawo, I.A.; Oladejo, O.P. Integrated Geophysical Survey In A Refuse Dumpsite of Aarada, Ogbomoso, Southwestern Nigeria. J. Appl. Phys. 2012, 2, 11–20. [Google Scholar] [CrossRef]
- De Carlo, L.; Perri, M.T.; Caputo, M.C.; Deiana, R.; Vurro, M.; Cassiani, G. Characterization of a dismissed landfill via electrical resistivity tomography and mise-à-la-masse metod. J. Appl. Geophys. 2013, 98, 1–10. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Nawawi, M.; Saad, R.; Abu-Rizaiza, A.S.; Yusoff, M.S.; Khalil, A.E.; Ishola, K.S. Characterization of active and closed landfill sites using 2D resistivity/IP imaging: Case studies in Penang, Malaysia. Environ. Earth Sci. 2016, 75, 347. Available online: https://link.springer.com/article/10.1007/s12665-015-5003-5 (accessed on 7 March 2024). [CrossRef]
- Yin, K.; Tong, H.; Giannis, A.; Wang, J.Y.; Chang, V.W.C. Multiple geophysical surveys for old landfill monitoring in Singapore. Env. Monit. Assess. 2017, 189, 20. [Google Scholar] [CrossRef]
- Giang, N.V.; Kochanek, K.; Vu, N.T.; Duan, N.B. Landfill leachate assessment by hydrological and geophysical data: Case study NamSon, Hanoi, Vietnam. J. Mater. Cycles Waste Manag. 2018, 20, 1648–1662. [Google Scholar] [CrossRef]
- Moreira, C.A.; Helene, L.P.I.; Nogara, P.; Ilha, L.M. Analysis of leaks from geomembrane in a sanitary landfill through models of electrical resistivity tomography in South Brazil. Environ. Earth Sci. 2018, 77, 7. [Google Scholar] [CrossRef]
- Islami, N.; Irianti, M.; Fakhruddin, F.; Azhar, A.; Nor, M. Application of geoelectrical resistivity method for the assessment of shallow aquifer quality in landfill areas. Environ. Monit. Assess. 2020, 192, 606. [Google Scholar] [CrossRef]
- Helene, L.P.I.; Moreira, C.A. Analysis of Leachate Generation Dynamics in a Closed Municipal Solid Waste Landfill by Means of Geophysical Data (DC Resistivity and SelfPotential Methods). Pure Appl. Geophys. 2021, 178, 1355–1367. [Google Scholar] [CrossRef]
- Akiang, F.B.; Emujakporue, G.O.; Nwosu, L.I. Leachate delineation and aquifer vulnerability assessment using geo-electric imaging in a major dumpsite around Calabar Flank, Southern Nigeria. Environ. Monit. Assess. 2023, 195, 123. [Google Scholar] [CrossRef] [PubMed]
- Juarez, M.B.; Mondelli, G.; Giacheti, H.L. An overview of in situ testing and geophysical methods to investigate municipal solid waste landfills. Env. Sci. Pollut. Res. 2023, 30, 24779–24789. [Google Scholar] [CrossRef] [PubMed]
- Morsy, E.A. Geo-Environmental Evaluation of the Kaakia Landfill, Southwest Makkah, Saudi Arabia. Sustainability 2023, 15, 500. [Google Scholar] [CrossRef]
- Porowska, D. Wykorzystanie metod geofizycznych w badaniu składowisk odpadów komunalnych. In Najnowsze Trendy w Gospodarce Odpadami Komunalnymi i Przemysłowymi; Wydawnictwo Naukowe TYGIEL sp. z o.o.: Lublin, Poland, 2023; pp. 68–82. ISBN 978-83-67881-00-5. Available online: https://bc.wydawnictwo-tygiel.pl/publikacja/AAF9ED89-E308-0458-E19D-1F90AA7D3865 (accessed on 7 March 2024).
- Li, J.; He, Z.; Wu, X.; Zhang, Z. Mise-a-la-masse-based induced-polarization method for heavy-metal pollution leakage monitoring. Methodol. Model. Results. Geophys. 2022, 87, EN57–EN67. [Google Scholar] [CrossRef]
- Holm, J.V.; Ruegge, K.; Bjerg, P.L.; Christensen, T.H. Occurrence and distribution of pharmaceutical organic compounds in the groundwater downgradient of a landfill (Grindsted, Denmark). Environ. Sci. Technol. 1995, 29, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.S.; Sucgang, R.J.; Almoneda, R.V.; Mendoza, N.D.S.; David, C.P.C. Environmental isotopes and major ions for tracing leachate contamination from a municipal landfill in Metro Manila, Philippines. J. Environ. Radioact. 2012, 110, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Porowska, D. Assessment of groundwater contamination around reclaimed municipal landfill—Otwock area, Poland. J. Ecol. Eng. 2014, 15, 69–81. [Google Scholar] [CrossRef]
- de Medeiros Engelmann, P.; dos Santos, V.H.J.M.; Barbieri, C.B.; Augustin, A.H.; Ketzer, J.M.M.; Rodrigues, L.F. Environmental monitoring of a landfill area through the application of carbon stable isotopes, chemical parameters and multivariate analysis. Waste Manag. 2018, 76, 591–605. [Google Scholar] [CrossRef]
- Preziosi, E.; Frollini, E.; Zoppini, A.; Ghergo, S.; Melita, M.; Parrone, D.; Rossi, D.; Amalfitano, S. Disentangling natural and anthropogenic impacts on groundwater by hydrogeochemical, isotopic and microbiological data: Hints from a municipal solid waste landfill. Waste Manag. 2019, 84, 245–255. [Google Scholar] [CrossRef]
- Fattahzadeh, M.; Hoshyari, E.; Parang, S.; Fereidoni, H.; Khoshbakht, R.; Rajmjooe, J.; Charkhestani, A. Assessment of heavy metal concentration and their source in the groundwa-ter near the landfill site: Case study (Shiraz landfill). J. Mater. Environ. Sci. 2021, 12, 1430–1443. Available online: http://www.jmaterenvironsci.com (accessed on 9 March 2024).
- Liu, X.; Wang, Y. Identification and Assessment of Groundwater and Soil Contamination from an Informal Landfill Site. Sustainability 2022, 14, 16948. [Google Scholar] [CrossRef]
- Lalik, M.; Dąbrowska, D. Groundwater Chemical Status Assessment in the Area of the Waste Landfill in Chorzów—Southern Poland. Sustainability 2024, 16, 763. [Google Scholar] [CrossRef]
- Baun, A.; Reitzel, L.A.; Ledin, A.; Christensen, T.C.; Bjerg, P.L. Natural attenuation of xenobiotic organic compounds in a landfill leachate plume (Vejen, Denmark). J. Contam. Hydrol. 2003, 65, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, F.; Hassanvand, M.S.; Nodehi, R.N.; Amini, H.; Rastkari, N.; Aghaei, M.; Yunesian, M.; Yaghmaeian, K. The concentration of BTEX compounds and health risk assessment in municipal solid waste facilities and urban areas. Environ. Res. 2020, 191, 110068. [Google Scholar] [CrossRef] [PubMed]
- Guadaño, J.; Gómez, J.; Fernández, J.; Lorenzo, D.; Domínguez, C.M.; Cotillas, S.; García-Cervilla, R.; Santos, A. Remediation of the Alluvial Aquifer of the Sardas Landfill (Sabiñánigo, Huesca) by Surfactant Application. Sustainability 2022, 14, 16576. [Google Scholar] [CrossRef]
- Salikova, N.S.; Rodrigo-Ilarri, J.; Rodrigo-Clavero, M.-E.; Urazbayeva, S.E.; Askarova, A.Z.; Magzhanov, K.M. Environmental Assessment of Microplastic Pollution Induced by Solid Waste Landfills in the Akmola Region (North Kazakhstan). Water 2023, 15, 2889. [Google Scholar] [CrossRef]
- Li, J.; Sha, H.; Liu, W.; Yuan, Y.; Zhu, G.; Meng, F.; Xi, B.; Tan, W. Transport of per-/polyfluoroalkyl substances from leachate to groundwater as affected by dissolved organic matter in landfills. Environ. Res. 2024, 247, 118230. [Google Scholar] [CrossRef] [PubMed]
- Porowska, D. Ocena agresywności wód podziemnych w rejonie zrekultywowanego składowiska odpadów komunalnych w Otwocku. Geol. Rev. 2015, 63, 1011–1014. (In Polish) [Google Scholar]
- Abunama, T.; Moodley, T.; Abualqumboz, M.; Kumari, S.; Bux, F. Variability of leachate quality and polluting potentials in light of leachate pollution index (LPI)—A global perspective. Chemosphere 2021, 282, 131119. [Google Scholar] [CrossRef]
- Bisht, T.S.; Kumar, D.; Alappat, B.J. Revised leachate pollution index (r-LPI): A tool to quantify the contamination potential of landfill leachate. Process Saf. Env. Prot. 2022, 168, 1142–1154. [Google Scholar] [CrossRef]
- Teja, D.R.; Kumar, P.S.S.; Jariwala, N. Application of multi-criteria decision-making techniques to develop modify-leachate pollution index. Env. Sci. Pollut. Res. 2023, 30, 41172–41186. [Google Scholar] [CrossRef] [PubMed]
- Sidle, W.C. Environmental isotopes for resolution of hydrology problems. Environ. Monit. Assess. 1998, 52, 389–410. [Google Scholar] [CrossRef]
- Kerfoot, H.B.; Baker, J.A.; Burt, D.M. The use of isotopes to identify landfill gas effects on groundwater. J. Environ. Monit. 2003, 5, 896. [Google Scholar] [CrossRef] [PubMed]
- North, J.C.; Frew, R.D.; Van Hale, R. Can stable isotopes be used to monitor landfill leachate impact on surface waters? J. Geochem. Explor. 2006, 88, 49–53. [Google Scholar] [CrossRef]
- Knöller, K.; Vogt, C.; Feisthauer, S.; Weise, S.M.; Weiss, H.; Richnow, H.-H. Sulfur Cycling and Biodegradation in Contaminated Aquifers: Insights from Stable Isotope Investigations. Environ. Sci. Technol. 2008, 42, 7807–7812 . [Google Scholar] [CrossRef] [PubMed]
- Haarstad, K.; Mæhlum, T. Tracing solid waste leachate in groundwater using δ13C from dissolved inorganic carbon. Isot. Environ. Health Stud. 2013, 9, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Porowska, D. Identification of groundwater contamination zone around a reclaimed landfill using carbon isotopes. Water Sci. Technol. 2017, 75, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Porowska, D. Determination of the origin of dissolved inorganic carbon in groundwater around a reclaimed landfill in Otwock using stable carbon isotopes. Waste Manag. 2015, 39, 216–225. [Google Scholar] [CrossRef]
- Porowska, D. Precipitation method for determination of carbon and oxygen isotopes to detect groundwater contamination near a municipal landfill. Period. Polytech. Chem. Eng. 2022, 66, 565–575. [Google Scholar] [CrossRef]
- Nisi, B.; Raco, B.; Dotsika, E. Groundwater Contamination Studies by Environmental Isotopes: A review. In Environment, Energy and Climate Change I: Environmental Chemistry of Pollutants and Wastes; Jimenez, E., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Lee, K.S.; Ko, K.S.; Kim, E.Y. Application of stable isotopes and dissolved ions for monitoring landfill leachate contamination. Environ. Geochem. Health 2020, 42, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Sankoh, A.A.; Derkyi, N.S.A.; Frazer-Williams, R.A.D.; Laar, C.; Kamara, I. A Review on the Application of Isotopic Techniques to Trace Groundwater Pollution Sources within Developing Countries. Water 2022, 14, 35. [Google Scholar] [CrossRef]
- Sabarathinam, C.; Al-Rashidi, A.; Alsabti, B.; Samayamanthula, D.R.; Kumar, U.S. A Review of the Publications on Carbon Isotopes in Groundwater and Rainwater. Water 2023, 15, 3392. [Google Scholar] [CrossRef]
- Bhagwat, A.; Ojha, C.S.P.; Kumar, S.; Kumar, B. Use of environmental isotopes in leachate studies through multiple isotopic analysis—A review. Environ. Technol. Rev. 2024, 13, 214–234. [Google Scholar] [CrossRef]
- Hackley, K.C.; Liu, C.L.; Coleman, D.D. Environmental isotope characteristics of landfill leachates and gases. Ground Water 1996, 34, 827–836. [Google Scholar] [CrossRef]
- Robinson, H.D.; Gronow, J.R. Tritium levels in leachates and condensates from domestic waste in landfill sites. Water Environ. J. 1996, 10, 391–398. [Google Scholar] [CrossRef]
- Vilomet, J.D.; Angeletti, B.; Moustier, S.; Ambrosi, J.P.; Wiesner, M.; Bottero, J.Y.; Snidaro, L.C.H. Application of strontium isotopes for tracing landfill leachate plumes in groundwater. Environ. Sci. Technol. 2001, 35, 4675–4679. [Google Scholar] [CrossRef] [PubMed]
- Park, S.D.; Kim, J.G.; Kim, W.H.; Kim, H.S. Distribution of tritium in the leachates and methane gas condensates from municipal waste landfills in Korea. Water Environ. J. 2005, 19, 91–99. [Google Scholar] [CrossRef]
- Nigro, A.; Sappa, G.; Barbieri, M. Application of Boron and Tritium Isotopes for Tracing Landfill Contamination in Groundwater. J. Geochem. Explor. 2017, 172, 101–108. [Google Scholar] [CrossRef]
- Raco, B.; Battaglini, R. Tritium as a tool to assess leachate contamination: An example from Conversano landfill (Southern Italy). J. Geochem. Explor. 2022, 235, 106939. [Google Scholar] [CrossRef]
- Tazioli, A.; Fronzi, D.; Mammoliti, E. Tritium as a Tracer of Leachate Contamination in Groundwater: A Brief Review of Tritium Anomalies Method. Hydrology 2022, 9, 75. [Google Scholar] [CrossRef]
- Christophersen, M.; Kjeldsen, P. Lateral gas transport in soil adjacent to an old landfill: Factors governing gas migration. Waste Manag. Res. 2001, 19, 144–159. [Google Scholar] [CrossRef]
- Nastev, M.; Therrien, R.; Lefebvre, R.; Gelinas, P. Gas production and migration in landfills and geological materials. J. Contam. Hydrol. 2001, 52, 187–211. [Google Scholar] [CrossRef]
- Kim, K.-H. Emissions of reduced sulfur compounds (RSC) as a landfill gas (LFG): A comparative study of young and old landfill facilities. Atmos. Environ. 2006, 40, 6567–6578. [Google Scholar] [CrossRef]
- Jin, Z.; Ci, M.; Yang, W.; Shen, D.; Hu, L.; Fang, C.; Long, Y. Sulfate reduction behavior in the leachate saturated zone of landfill sites. Sci. Total Environ. 2020, 730, 138946. [Google Scholar] [CrossRef]
- Börjesson, G.; Chanton, J.; Svensson, B.H. Methane Oxidation in Swedish Landfill Quantified with the stable carbon isotope technique in combination with an optical method for emitted methane. Environ. Sci. Technol. 2007, 41, 6684–6690. [Google Scholar] [CrossRef]
- Abichou, T.; Chanton, J.; Powelson, D.; Fleiger, J.; Escoriaza, S.; Lei, Y.; Stern, J. Methane flux and oxidation at two types of intermediate landfill covers. Waste Manag. 2006, 26, 1305–1312. [Google Scholar] [CrossRef]
- Scheutz, C.; Kjeldsen, P. Guidelines for landfill gas emission monitoring using the tracer gas dispersion method. Waste Manag. 2019, 85, 351–360. [Google Scholar] [CrossRef] [PubMed]
- EPA. Guidance on the management of landfill gas, doc. LFTGN03, Environment Agency, Bristol. 2002. Available online: https://assets.publishing.service.gov.uk/media/5a7e090b40f0b62305b80615/LFTGN03.pdf (accessed on 7 March 2024).
- Spokas, K.A.; Bogner, J.; Corcoran, M. Modeling landfill CH4 emissions: CALMIM international field validation, using CALMIM to simulate management strategies, current and future climate scenarios. Elem. Sci. Anth 2021, 9, 50. [Google Scholar] [CrossRef]
- Kendall, C.; McDonnell, J.J. (Eds.) Isotope Tracers in Catchment Hydrology; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Krouse, H.R.; Mayer, B. Sulfur and oxygen isotopes in sulfate. In Environmental Tracers in Subsurface Hydrology; Cook, P., Herczeg, A.L., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2000; pp. 195–231. [Google Scholar]
- De Groot, P.A. (Ed.) Handbook of Stable Isotope Analytical Techniques; Elsevier: Amsterdam, The Netherlands, 2004; Volume I, pp. 1–1258. [Google Scholar]
- De Groot, P.A. (Ed.) Handbook of Stable Isotope Analytical Techniques; Elsevier: Amsterdam, The Netherlands, 2009; Volume II, pp. 1–1398. [Google Scholar]
- Clark, I. Groundwater Geochemistry and Isotopes; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2015. [Google Scholar]
- Grossman, E.L.; Cifuentes, L.A.; Cozzarelli, I.M. Anaerobic methane oxidation in a landfill leachate plume. Environ. Sci. Technol. 2002, 36, 2436–2442. [Google Scholar] [CrossRef]
- Van Breukelen, B.M.; Prommer, H. Beyond the Rayleigh equation: Reactive transport modeling of isotope fractionation effects to improve quantification of biodegradation. Environ. Sci. Technol. 2008, 42, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.D.; Amos, R.T.; Blowes, D.W. 34S/32S fractionation during sulfate reduction in groundwater treatment systems: Reactive transport modeling. Environ. Sci. Technol. 2011, 45, 2863–2870. [Google Scholar] [CrossRef]
- Sim, M.S.; Ogata, H.; Lubitz, W.; Adkins, J.F.; Sessions, A.L.; Orphan, V.J.; McGlynn, S.E. Role of APS reductase in biogeochemical sulfur isotope fractionation. Nat. Commun. 2019, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Ci, M.; Yang, W.; Jin, H.; Hu, L.; Fang, C.; Shen, D.; Long, Y. Evolution of sulfate reduction behavior in leachate saturated zones in landfills. Waste Manag. 2022, 141, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Bakkaloglu, S.; Lowry, D.; Fisher, R.E.; France, J.L.; Nisbet, E.G. Carbon isotopic characterisation and oxidation of UK landfill methane emissions by atmospheric measurements. Waste Manag. 2021, 132, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Porowski, A.; Porowska, D.; Halas, S. Identification of Sulfate Sources and Biogeochemical Processes in an Aquifer Affected by Peatland: Insights from Monitoring the Isotopic Composition of Groundwater Sulfate in Kampinos National Park, Poland. Water 2019, 11, 1388. [Google Scholar] [CrossRef]
- Grossman, E.L. Stable carbon isotopes as indicators of microbial activity in aquifers. In Manual of Environmental Microbiology; Hurst, C.I., Ed.; American Society for Microbiology: Washington, DC, USA, 1997; pp. 565–576. [Google Scholar]
- Cook, P.G.; Herczeg, A.L. Environmental Tracers in Subsurface Hydrology; Kluwer: Boston, MA, USA, 2000; 529p. [Google Scholar]
- Butler, T.W. Isotope geochemistry of drainage from an acid mine impaired watershed, Oakland, California. App Geochem. 2007, 22, 1416–1426. [Google Scholar] [CrossRef]
- Wachniew, P.; Różański, K. Carbon budget of a mid–latitude, groundwater–controlled lake: Isotopic evidence for the importance of dissolved inorganic carbon recycling. Geochim. Et Cosmochim. Acta 1997, 61, 2453–2465. [Google Scholar] [CrossRef]
- Cerling, T.E. The stable isotopic composition of modern soil carbonate and its relationship to climate. Earth Planet. Sc. Lett. 1984, 71, 229–240. [Google Scholar] [CrossRef]
- Walsh, D.C.; LaFleur, R.G.; Bopp, R.F. Stable carbon isotopes in dissolved inorganic carbon of landfill leachate. Ground Water Manag. 1993, 16, 153–167. [Google Scholar]
- Manning, D. Calcite precipitation in landfills: An essential product of waste stabilization. Mineral. Mag. 2001, 65, 603–610. [Google Scholar] [CrossRef]
- Deines, P. The isotopic composition of reduced organic carbon. In Handbook of Environmental Isotope Geochemistry; Fritz, P., Fontes, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1980; Volume 1, pp. 329–406. [Google Scholar]
- Porowska, D. Pochodzenie węgla nieorganicznego w wodach podziemnych strefy hipergenezy w warunkach naturalnych i przekształconych antropogenicznie na przykładzie poligonów Pożary i Otwock; University of Warsaw: Warsaw, Poland, 2016. (In Polish) [Google Scholar]
- Breit, G.N.; Tuttle, M.L.W.; Cozzarelli, I.M.; Christenson, S.C.; Jaeschke, J.B.; Fey, D.L.; Berry, C.J. Results of the chemical and isotopic analyses of sediment and ground water from alluvium of the Canadian River near a closed municipal landfill, Norman, Ok-lahoma. 2008, U.S. Dept. of the Interior, U.S. Geological Survey.; U.S. Geological Survey Toxic Substances Hydrology Program. Available online: https://d1wqtxts1xzle7.cloudfront.net/69991715/OF08-1134_508-libre.pdf?1632150729=&response-content-disposition=inline%3B+filename%3DResults_of_the_Chemical_and_Isotopic_Ana.pdf&Expires=1716609839&Signature=NA2neyEmCO1rMhN2~oJa0td9GL3QDYK3mBycDTHob~FStIXwSwjl5zjmMYcaEywdmNBBZFRKkbsybe5zddES44CeE3oZSbQcoVMAaLuZ~1U4tmG3QxEqin0DaRfsyVlEu5-AzjSXU1M3WkKO3Z~RkBwQb32tGuLtWS~PHRGylMdjUtKsho5fFjHW~-DAGovacPdsn5Erc3lIoBniOfKPXT9cdBsjraCXVgjM106lD7XnaQu~uUOnalbeF4xgqN9AkXu6p5jKWoW7vs3rTY8dcYnnUw8uMTu73DA5aSz6IEljvIif4rdYXXTJqUIa3sx69joqd~lJv5EiPNfCk3ITtA__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 8 March 2024).
- Van Breukelen, B.M. Natural Attenuation of Landfill Leachate: A Combined Biogeochemical Processes Analysis and Microbial ecology Approach. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 2003. [Google Scholar]
- Asmussen, G.; Strauch, G. Sulfate Reduction in a Lake and the Groundwater of a Former Lignite Mining Area Studied by Stable Sulfur and Carbon Isotopes. Water Air Soil. Pollut. 1998, 108, 271–284. [Google Scholar] [CrossRef]
- Cerar, S.; Serianz, L.; Vreča, P.; Štrok, M.; Kanduč, T. Impact assessment of the Gajke and Brstje landfills on groundwater status using stable and radioactive isotopes. Geologija 2023, 66, 285–299. [Google Scholar] [CrossRef]
- Beneduce, L.; Piergiacomo, F.; Limoni, P.P.; Zuffianò, L.E.; Polemio, M. Microbial, chemical, and isotopic monitoring integrated approach to assess potential leachate contamination of groundwater in a karstic aquifer (Apulia, Italy). Env. Monit. Assess. 2024, 196, 312. [Google Scholar] [CrossRef] [PubMed]
- Jakóbczyk-Karpierz, S.; Ślósarczyk, K. Isotopic signature of anthropogenic sources of groundwater contamination with sulfate and its application to groundwater in a heavily urbanized and industrialized area (Upper Silesia, Poland). J. Hydrol. 2022, 612, 128255. [Google Scholar] [CrossRef]
- Nozhevnikova, A.; Lifshitz, A.B.; Lebedev, V.S.; Zavarzin, G.A. Emission of Methane into the Atmosphere from Landfills in the Former USSR. Chemosphere 1993, 26, 401–417. [Google Scholar] [CrossRef]
- Pujiindiyati, E.R.; Sidauruk, P. Study ofleachate contamination in Bantar Gebang landfill toits shallow groundwater using natural isotopetracers of 18O, 2H and 3H. At. Indones. 2015, 41, 31–39. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Krishnamurthy, R.V. Dissolved Inorganic Carbon (DIC) in Natural Waters for Isotopic Analysis. In Handbook of Stable Isotope Analytical Techniques; De Groot, P.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume I, pp. 203–228. [Google Scholar]
- Fritz, S.J.; Bryan, J.D.; Harvey, F.E.; Leap, D.I. A geochemical and isotopic approach to delineate landfill leachates in a RCRA study. Ground Water 1994, 32, 743–750. [Google Scholar] [CrossRef]
- Mohammadzadeh, H.; Clark, I.; Marschner, M.; St-Jean, G. Compound Specific Isotopic Analysis (CSIA) of landfill leachate DOC components. Chem. Geol. 2005, 218, 3–13. [Google Scholar] [CrossRef]
- Mohammadzadeh, H.; Clark, I.; Aravena, R.; Bourbonnais, A.; Middlestead, P. Isotopic analysis of ammonium (δ15N), nitrate (δ18O & δ15N) and dissolved carbon (δ13C) in landfill leachate plume. Environ. Sci. Technol. Proc. II 2006, 2, 145–150. [Google Scholar]
- Mohammadzadeh, H.; Clark, I. Application of 13C isotope and carbon geochemistry to identify impact from landfill on surrounding groundwater. In Proceedings of the 8th International Congress on Civil Engineering, Shiraz, Iran, 11–13 May 2009; Shiraz University: Shiraz, Iran. [Google Scholar]
- Jorstad, L.B. Analysis of Variation in Inorganic Contaminant Concentration and Distribution in a Landfill Leachate Plume. Ph.D. Thesis, Astrolabe Park, Sydney, Australia, 2006. [Google Scholar] [CrossRef]
- North, J.C.; Frew, R.D.; Peake, B.M. The use of carbon and nitrogen isotope ratios to identify landfill leachate contamination: Green Island Landfill, Dunedin, New Zealand. Environ. Int. 2004, 30, 631–637. [Google Scholar] [CrossRef] [PubMed]
- North, J.C.; Frew, R.D. Isotopic Characterization of Leachate from Seven New Zealand Landfills. In Landfill Research Focus; Lehmann, E., Ed.; NOVA Publishing: Hauppauge, NY, USA, 2008; pp. 199–261. [Google Scholar]
- Hornibrook, E.R.C.; Longstaffe, F.J.; Fyfe, W.S. Evolution of stable carbon–isotope compositions for methane and carbon dioxide in freshwater wetlands and other anaerobic environments. Geochim. Et Cosmochim. Acta 2000, 64, 1013–1027. [Google Scholar] [CrossRef]
- Zimnoch, M.; Florkowski, T.; Nęcki, J.M.; Neubert, R.E.M. Diurnal variability of δ13C and δ18O of atmospheric CO2 in the urban atmosphere of Krakow, Poland. Isot. Environ. Healt. S. 2004, 40, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Parlov, J.; Kovač, Z.; Nakić, Z.; Barešić, J. Using Water Stable Isotopes for Identifying Groundwater Recharge Sources of the Unconfined Alluvial Zagreb Aquifer (Croatia). Water 2019, 11, 2177. [Google Scholar] [CrossRef]
- Lee, E.S.; Krothe, N.C. A four–component mixing model for water in a karst terrain in south–central Indiana, USA. Using solute concentration and stable isotopes as tracers. Chem. Geol. 2001, 179, 129–143. [Google Scholar] [CrossRef]
- Vitoria, L.; Otero, N.; Soler, A.; Canals, A. Fertilizer characterization: Isotopic data (N, S, O, C, and Sr). Environ. Sci. Technol. 2004, 38, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, M.; Cao, M.; Huang, X.; Zhang, Z.; Zhang, L. Sources and behaviors of dissolved sulfate in the Jinan karst spring catchment in northern China identified by using environmental stable isotopes and a Bayesian isotope-mixing model. Appl. Geochem. 2021, 134, 105109. [Google Scholar] [CrossRef]
- Wong, C.T.; Leung, M.K.; Wong, M.K.; Tang, W.C. Afteruse development of former landfill sites in Hong Kong. J. Rock. Mech. Geotech. Eng. 2013, 5, 443–451. [Google Scholar] [CrossRef]
- Assef, F.M.; Steiner, M.T.A.; Lima, E.P. A review of clustering techniques for waste management. Heliyon. 2022, 8, e08784. [Google Scholar] [CrossRef]

| Strengths | Weaknesses |
|
|
| Opportunities | Threats |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porowska, D. Carbon and Sulfur Isotope Methods for Tracing Groundwater Contamination: A Review of Sustainable Utilization in Reclaimed Municipal Landfill Areas. Sustainability 2024, 16, 4507. https://doi.org/10.3390/su16114507
Porowska D. Carbon and Sulfur Isotope Methods for Tracing Groundwater Contamination: A Review of Sustainable Utilization in Reclaimed Municipal Landfill Areas. Sustainability. 2024; 16(11):4507. https://doi.org/10.3390/su16114507
Chicago/Turabian StylePorowska, Dorota. 2024. "Carbon and Sulfur Isotope Methods for Tracing Groundwater Contamination: A Review of Sustainable Utilization in Reclaimed Municipal Landfill Areas" Sustainability 16, no. 11: 4507. https://doi.org/10.3390/su16114507
APA StylePorowska, D. (2024). Carbon and Sulfur Isotope Methods for Tracing Groundwater Contamination: A Review of Sustainable Utilization in Reclaimed Municipal Landfill Areas. Sustainability, 16(11), 4507. https://doi.org/10.3390/su16114507







