Abstract
The pursuit of sustainable polyurethane (PU) product development necessitates a profound understanding of precursor materials. Particularly, polyol plays a crucial role, since PU properties are heavily influenced by the type of polyol employed during production. While traditional PUs are solely derived from hydroxyl functionalized polyols, the emergence of amine-hydroxyl hybrid polyols has garnered significant attention due to their potential for enhancing PU product properties. These hybrid polyols are characterized by the presence of both amine and hydroxyl functional groups. However, characterizing these polyols remains a daunting challenge due to the lack of established experimental testing standards for properties, such as fractional hydroxyl and amine moieties and thermo-kinetic parameters for amine reactions with isocyanates. Additionally, characterization methods demand extensive time and resources and pose risks to health and the environment. To bridge these gaps, this study employed computational simulation via MATLAB to determine the moieties’ fractions and thermo-kinetic parameters for hybrid polyols. The computational method integrated energy balance and reaction kinetics analysis for various polyols to elucidate the influence of functional moieties on the thermo-kinetic behavior of PU formations. Validation of the simulated results was conducted by comparing their experimental and simulated prepolymer and foam temperature profiles, highlighting the direct influence of fractional moieties on PU formations. The comparisons revealed an average relative error of less than 5%, indicating the accuracy and credibility of the simulation. Thus, this study represents a pivotal opportunity for advancing knowledge and driving sustainable developments in bio-based polyol characterization for PU production streamlining and formulation optimization.
1. Introduction
Polyurethanes (PU) are prominent polymers globally, owing to their versatility and wide range of applications [1]. Ranked as the sixth most important polymer, PUs are highly favored due to their ability to be tailor-made for specific purposes, well-established synthesis technology, and exceptional properties, such as thermal insulation, diverse densities, and load-bearing capabilities for applications in elastomers, foams, coatings, and adhesives, to name a few [2,3,4]. In 2021, they represented 8.2% of the total polymers demand [5]. Notably, the PU market surged to approximately USD 80 billion in 2022, with projections indicating a rise to USD 110 billion by 2030 [6,7]. Additionally, polyurethanes offer cost-effective solutions, including energy efficiency, durability, and versatility, leading to reduced maintenance costs, lower energy bills, and increased productivity [8,9].
The synthesis of PU involves the reaction between isocyanates and reactive hydrogen from polyols [10]. Several sustainability-focused studies have employed urethane reaction research [11,12,13]. These include the design of self-healing PU materials for application in integrated smart wearable devices, the lever functionalization of cellulose nanocrystal hydroxyls with isocyanate for improved adhesion sensing platforms, and the development of smart PU biocoatings for the enhanced biocompatibility of metal scaffolds.
Moreover, the properties of PUs are intricately linked to the type of polyol used and the specific formulation employed during their synthesis [14,15,16]. In response to the growing need for sustainable production practices, researchers have delved into exploring alternative polyols as replacements for conventional petroleum-based ones [17]. These alternative polyols can be sourced from renewable feedstock such as vegetable oil through various functionalization methods, such as oxirane ring-opening, transesterification, and transamidation [18,19,20]. Notably, transamidation has emerged as one of the most widely used functionalization methods for saturated vegetable oil because it is relatively straightforward, leading to the production of amine–hybrid polyols [21,22].
The increasing demand for these amine hybrid system polyols has underscored the necessity for efficient polyol characterization methods. Researchers have already achieved significant advancements in developing polyols, such as tertiary amine-induced autocatalysis and amino esters in equilibrium with diethanolamide to synthesize PU foam with improved mechanical and thermal properties [23,24]. Polyol has also been produced with diverse hydroxyl and amine moieties, resulting in an improved polyol chain structure and overall reactivity for coating applications [25,26].
With this, analytical techniques for determining fractional moieties offer valuable insights for improving the resulting PU properties, as these factors significantly impact the distinctive characteristics of the resulting PU networks [27]. Primary hydroxyl groups lead to denser PU networks [28]. Secondary hydroxyl groups introduce unfavorable dangling chains that function as plasticizers within the PU structure, while hindered-secondary hydroxyls in the carbon chain cause significant steric hindrance, limiting urethane crosslinking [29]. Furthermore, amines induce more hydrogen in polymer matrix bonding, contributing to providing more intermolecular forces, thereby enhancing the PU mechanical properties [30].
Despite these strides, there are existing challenges in characterizing hydroxyl functional moieties. While the total hydroxyl number, primary amines, and secondary amines in polyol compounds have a standard quantitative characterization procedure, there is a notable gap that exists regarding an established standard method for quantitatively characterizing specific fractions for primary, secondary, and hindered-secondary hydroxyls [31,32,33]. Hence, in exploring both traditional and hybrid polyols, there is a need to delve into comprehensive characterization methods for both hydroxyl and amine moieties.
Furthermore, understanding the reaction kinetics is crucial for tailoring material properties to meet specific requirements in various applications [34,35,36]. Alfeche et al. (2023) and Dingcong et al. (2023) conducted a simulation for the PU polymerization process using traditional hydroxyl-based polyols [27,37]. Their findings revealed that the kinetics of polymerization in terms of the gel time were significantly affected by the chemical characteristic inputs of the polyol, such as the fractions of hydroxyls moieties. However, a gap still exists in the identification of the thermo-kinetic properties associated with hybrid polyols, particularly concerning their distinct amine moieties and reactions with isocyanates.
In addition to technical challenges, the experimental methods for determining the polyol properties and thermo-kinetic information for PU polymerization consume considerable time and financial resources, and may be limited to some laboratories. Moreover, the use of organic solvents and exposure to toxic chemicals during PU formation studies pose health and environmental risks and result in expensive waste management [38,39]. Unsystematic experimental errors may also arise due to the effects of fluctuating environmental conditions, such as ambient temperature and humidity, on the polyurethane polymerization reaction and its rate of heat transfer [40,41,42].
To address these concerns, researchers have turned to computational studies to simulate PU physical formation. Ferkl et al. (2018) simulated PU foam polymerization via the mathematical modeling of reaction kinetics, foam expansion, and wall evolution [43]. Hu et al. (2021) simulated the same process via a numerical study on bubble nucleation and bubble growth [44]. Nonetheless, validating these simulations with accurate experimental results remains a formidable task.
Computational simulations based on heat transfer and reaction kinetics differential equations have been performed on polyurethane foaming processes based on Baser and Kakhar’s models, which employ thermal energy balance and polyurethane reaction kinetics [45]. Zhao et al. (2013) modeled and simulated the temperature profiles during the rigid polyurethane foaming process of hydroxyl-based single and multi-polyol mixture polyurethane formulation systems [46]. Al-Moameri et al. (2015) used the same approach in simulating the effects of different physical blowing agents on the polymerization temperature and height profiles during the polymerization process, with the consideration of parameters based on the type of alcohol moieties present in the polyol [47]. The aforementioned kinetic simulations and modeling utilized only the reaction between hydroxyls and isocyanate for the prepolymerization processes. Considering this, modeling all possible exothermic polymerization kinetics using diverse polyol systems such as amine moieties has been largely unexplored.
The previously mentioned gaps warrant this study, which adopts a computational approach via MATLAB R2021b to simulate temperature profiles that describe the polyurethane prepolymerization and foaming reactions involving hydroxyls and amine-functionalized polyols. It simultaneously solves numerous differential equations based on PU thermodynamic and kinetics principles and heuristics from the established literature [37,46,48,49,50]. Through curve fitting comparisons with experimental data, it determines the fractions of the functional amine and hydroxyl moieties present in the polyol and addresses the lack of available thermo-kinetic parameters for reactions involving secondary amines and isocyanates. The characterization of PU starting materials, particularly polyols, and PU polymerization studies via computational methods using MATLAB requires fewer resources and yields insights in a shorter timeframe in comparison with conventional experimental methods. Furthermore, MATLAB is an appropriate tool for the simultaneous solutions to the multiple differential equations involved in the thermo-kinetic studies of polymerization [51]. Specifically, the current study uses the latest differential equation solver function, ode89 of MATLAB, to enhance the accuracy of previous PU thermo-kinetic simulation studies [24,37,46,52].
Additionally, the study seeks to investigate the effects of different polyol moieties on the reaction kinetics and temperature profiles during polyurethane formations. The insights obtained from this study will contribute to the groundwork for further research, the development of formulations based on existing and developing polyol systems with diverse functionalities, and ultimately, to advancing sustainable polyurethane and polyurea production.
The application of simulations on polyurethane polymerization kinetics using developing polyol systems promises to provide ease and sustainability in improving polyurethane formulations and parameter optimization studies. Understanding the synthesis information obtained through these computational approaches will significantly aid in process development and the engineering of polymeric materials with advanced properties tailored to meet specific application demands, especially when time and resource constraints are critical considerations.
Simulation Description
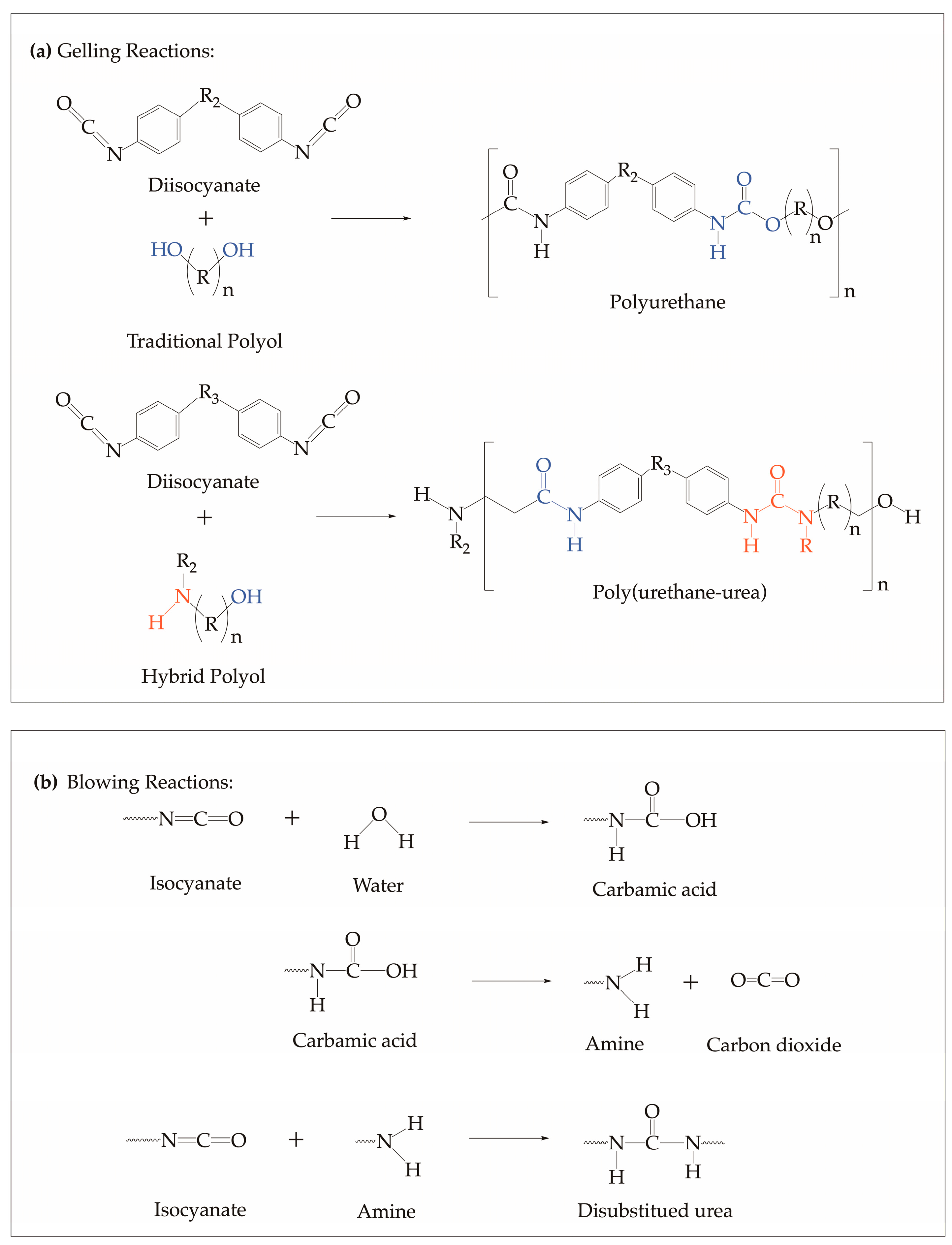
The synthesis of polyurethane prepolymer involves an exothermic reaction between isocyanates and polyol, as shown in Figure 1a. First, isocyanates react with the reactive hydrogen of polyols to form polyurethane prepolymer [1]. For the reaction of traditional polyol (hydroxyl-based polyol) and isocyanates, polyurethanes are formed exclusively. As for the reaction between hybrid polyol (amine-based polyol), polyurea forms along with polyurethane. On the other hand, the synthesis of polyurethane foam involves the addition of water as a blowing agent [53]. Blowing proceeds as the isocyanate reacts with the water present to form an unstable product, carbamic acid, which, consequently, decomposes to amine and carbon dioxide, as shown in Figure 1b.
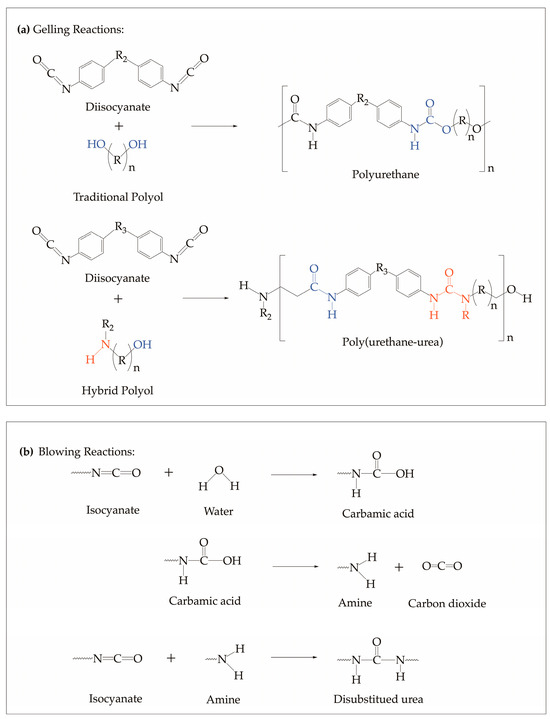
Figure 1.
General polyurethane (a) prepolymerization reactions of traditional and hybrid polyols with isocyanate where the OH moieties react with isocyanates to form urethane, and the NH moieties react with isocyanates to form urea. and (b) foaming reaction via water blowing with isocyanate schemes [1,53].
The reactivity of the prepolymerization process is influenced by the degree of hydroxyls, which can be classified as primary, secondary, and hindered-secondary/tertiary, and amines that participated in the reactions [54,55]. Additionally, the heat generated from these exothermic reactions is attributed to the type of functional moieties present in polyol and further plays a critical role in providing the efficient energy needed for curing and cross-linking [56]. With this, the concentrations of the different types of hydroxyl and amine moieties were considered in the computational method to accurately capture the influence of the polyol type on the thermo-kinetic behavior of the PU during the prepolymerization process.
The internal temperature during the polymerization reaction at a given time, t, is computed using an energy balance equation derived from previous work by Zhao et al. (2013) [46], as represented in Equation (1):
where is the summation of the products of the heat and reaction rates of the polymerization reactions, UAT is the product of the overall heat transfer coefficient, the surface area of foam, and temperature difference, and is the summation of the products of the molar concentration and heat capacities of the reactants [46]. Instantaneous molar concentrations of the reactants and products were obtained using the elementary reaction rates presented in Table 1.

Table 1.
Possible poly(urethane-urea) polymerization reactions. A represents isocyanate, B represents polyol, AP represents isocyanate moiety on (growing) polymer, BP represents polyol moieties on (growing) polymer, W represents water, Am represents primary amine, Ur represents urethane, U represents urea, UP represents urea in (growing) polymer, and AmP represents primary amine moiety on (growing) polymer. For the subscripts, P is primary alcohol moiety, S is secondary alcohol moiety, H is hindered-secondary alcohol moiety, and SA is secondary amine [37,57].
Furthermore, research conducted by Zhao et al. (2014) examining the influence of catalysts on PU foam polymerization confirmed that Equation (2) offers an accurate estimation of catalyzed reaction rate constants, with cat denoting the specific catalysts utilized [58].
where ki represents the overall rate constant for every reaction, i, in Table 1, kuncat,i represents the uncatalyzed reaction rate constant, and kcat,i represents the reaction rate constant in the presence of a catalyst.
Additionally, the catalyzed and uncatalyzed rate constants varying at different temperatures, k(T), were calculated via the Arrhenius equation in Equation (3):
where Ea is the activation energy of the reaction, k0 is the pre-exponential factor, R is the gas constant, and T is temperature.
The thermo-kinetic parameters, including the pre-exponential factor (k0) at 25 °C, heat of reactions (ΔH), and activation energy (Ea) related to hydroxyl–isocyanate reactions, were derived from the findings of Ghoreishi et al. (2014) [57]. These thermo-kinetic parameters are summarized in Table 2.

Table 2.
Catalytic thermo-kinetic parameters of isocyanate reaction with primary, secondary, and hindered-secondary hydroxyls [57].
2. Materials and Methods
2.1. Materials
The rigid polyurethane prepolymers were synthesized using the following materials. The isocyanate, methylene diphenyl diisocyanate (MDI) PAPITM 27 with an NCO functionality of 2.7, molecular weight of 340 g/mol, specific heat capacity of 1.8 J/g-K, and density of 1.23 g/mL, was manufactured by Dow Chemical Co., Hayward, CA, USA [59]. The polyol based on petroleum, Voranol® 490 (V490), was also manufactured by Dow Chemical Co. while the amine-based polyol used was Coconut oil Diethanolamine from the Center for Sustainable Polymers of Mindanao State University–Iligan Institute of Technology, Iligan City, Philippines, according to the methods of Dingcong et al. (2023) [24]. The blowing agent used was distilled water. The catalyst, Polycat® 8, was obtained from Evonik Industries AG, Essen, Germany. The surfactant, INV 690, was obtained from Guangzhou Innovate Chemical Co., Ltd., Guangzhou, China. N-tert-butoxycarbonyls, dichloromethane, and anhydrous sodium sulfate were purchased from Merck, Darmstadt, Germany.
2.2. Characterization of Polyol
The hydroxyl and amine values of the amine-based polyol were characterized following the ASTM D4274-11 and ASTM D2074-07 methods, respectively. The specific heat capacity was obtained using the PerkinElmer differential scanning calorimeter (DSC) model 4000 following ASTM E1269-11, using indium metal as a reference and aluminum as heating pans. Moreover, the heating range was from −30 °C to 300 °C while the heating rate was 10 °C/min under a nitrogen gas atmosphere [60]. Additionally, the molecular weight of the polyol was analyzed using the Shimadzu gas permeation chromatography (GPC) assembly (Shimadzu, Kyoto, Japan) based on the ASTM D6474-12 guidelines [61]. Density determination was conducted using the ASTM D4669 [62] methods and the total functionality of the polyol was calculated using Equation (4):
where f is the functionality of the polyol, MW is the molecular weight of the polyol, and OH and NH are the hydroxyl value and amine value, respectively.
The Fourier transform infrared (FTIR) spectra of the amine-based polyol, as well as its raw materials—diethanolamide and glycerol—were collected on a Shimadzu IRTracer-100 FTIR (Shimadzu Corp., Kyoto, Japan) equipped with an attenuated total reflectance (ATR) accessory sampling technique. All data were recorded at room temperature, in the range from 4000 to 500 cm−1, by accumulating 80 scans with a resolution of 4 cm−1. The validation of the amine value involved estimating the integrated peaks in the 1H nuclear magnetic resonance (NMR) spectra of the polyol [4]. 1H NMR spectra were recorded on Bruker Ascend 600MHZ Cryoprobe NMR Spectrometer (Bruker Corp., Billerica, MA, USA) using deuterated chloroform as a solvent. NMR analysis was conducted at the USA DOST-PCHRD Tuklas NMR Laboratory Visayas, University of San Agustin, Iloilo City.
2.3. Experimentation Design
For the gelling, the amine-based polyol was vacuum-dried at 80 °C for 2 h. A polyol mixture comprising amine-based polyol and Voranol 490 was weighed with catalysts and surfactants in a 300 mL paper cup as the B-side. The mixture of B-side components was mixed at 3450 rpm using an electronic mixer blade for 60 s, and was allowed to degas for 120 s. The A-side comprising isocyanate was weighed beforehand and quickly poured into the cup. The reaction mixture was then mixed for 10–15 s at 3450 rpm. The internal temperature of the rising foam was recorded at 5 s intervals. For the foaming process, the same gelling procedure was utilized, with the only difference being that the B-side consisted of a blowing agent, specifically water. It is worth noting that the foaming procedures performed in the present study were adapted from the previous literature [24].
Prepolymerization and foaming reaction experiments using a polyol mixture with varied amine-based polyol replacements were conducted to verify the accuracy of the simulation results. The polyurethane prepolymer samples were labeled according to the percent replacement of the amine-based polyol. The polyol mixture comprised a hydroxyl-based polyol (Voranol 490) (Figure 2a) and amine-based polyol (Figure 2b). Voranol 490 polyol comprised 100 wt. % Voranol 490. The 50% amine-based polyol consisted of 50 wt. % amine-based polyol and 50 wt. % Voranol 490. The 75% amine-based polyol consisted of 75 wt. % amine-based and 25 wt. % Voranol 490. Lastly, the 100% amine-based polyol was an purely amine-based polyol. The same polyol mixture formulation was also used for polyurethane foam sample labeling, that is, 50%, 75%, and 100% amine-based polyols. Table 3 and Table 4 outline the ingredients and proportions used in the prepolymerization and foaming reactions, respectively.
For amine defunctionalization, 1 mol of amine-based polyol was dissolved in 5 mL of dichloromethane. The polyol solution was treated with Boc2O (di-tert-butyl dicarbonate) in a molar ratio of 1.2:1 (Boc2O:amine). The mixture was mixed and maintained at room temperature for 30 min. Afterward, the reaction was quenched with a few drops of water in the mixture. The organic phase was subjected to dehydration using anhydrous sodium sulfate and subsequently concentrated under reduced pressure in a vacuum.
The structure of the formation of polyurethane was confirmed by FTIR spectra using a Fourier transform Shimadzu IRTracer-100 FTIR equipped with an ATR accessory sampling technique. All data were recorded at room temperature, in the range from 4000 to 500 cm−1, by accumulating 128 scans with a resolution of 4 cm−1.
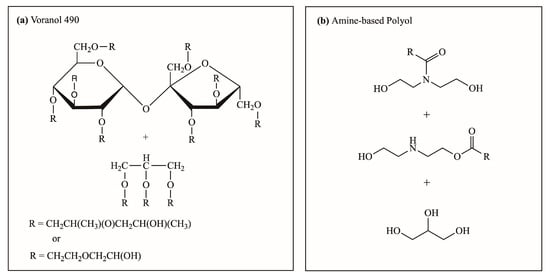
Figure 2.
Chemical structure of representative polyols for polyurethane polymerization thermo-kinetic simulation: (a) Voranol 490 and (b) amine-based polyol [24,63].
Figure 2.
Chemical structure of representative polyols for polyurethane polymerization thermo-kinetic simulation: (a) Voranol 490 and (b) amine-based polyol [24,63].
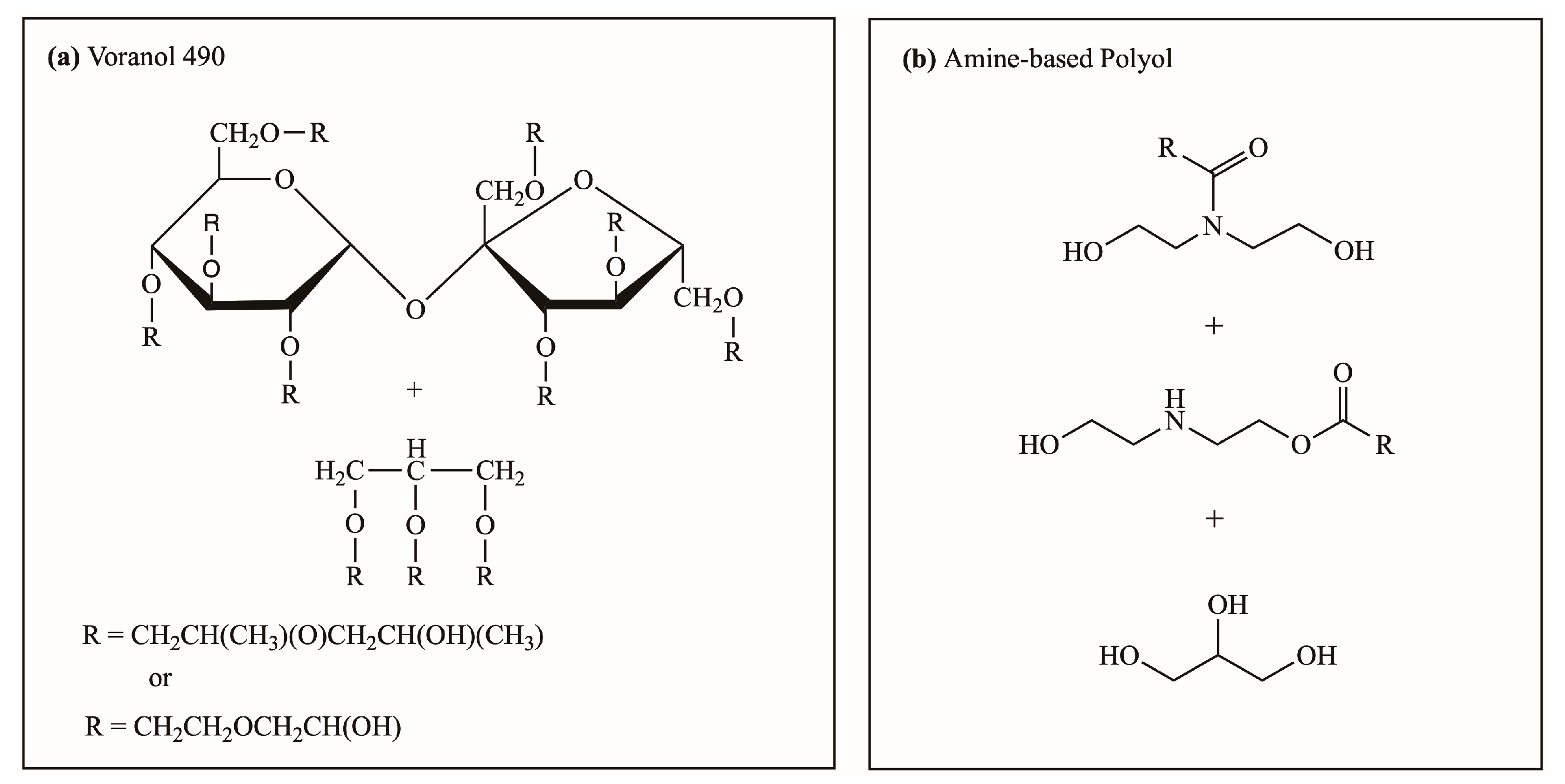

Table 3.
Poly(urethane-urea) prepolymer formulation at different amine-based polyol weight percent replacements.
Table 3.
Poly(urethane-urea) prepolymer formulation at different amine-based polyol weight percent replacements.
| Prepolymer Formulation | Components | 100% V490 | 50% Amine-Based Polyol a | 75% Amine-Based Polyol b | 100% Amine-Based Polyol |
|---|---|---|---|---|---|
| Polyol | V490 (g) | 20 | 10 | 5 | 0 |
| Amine-based polyol (g) | 0 | 10 | 15 | 20 | |
| Catalyst | Polycat 8 (g) | 0.1 | 0.1 | 0.1 | 0.1 |
| Surfactant | INV 690 (g) | 0.2 | 0.2 | 0.2 | 0.2 |
| Isocyanate | MDI PAPI 27 (g) | 25.70 | 21.56 | 19.49 | 17.42 |
| NCO/OH [64] | 1.11 | 1.07 | 1.04 | 1.01 | |
a mixed-polyol composition: 50 wt. % amine-based; 50 wt. % 100% V490. b mixed-polyol composition: 75 wt. % amine-based; 25 wt. % 100% V490.

Table 4.
Poly(urethane-urea) foam formulation at different amine-based polyol weight percent replacements.
Table 4.
Poly(urethane-urea) foam formulation at different amine-based polyol weight percent replacements.
| Foam Formulation | Components | 100% V490 | 50% Amine-Based Polyol a | 75% Amine-Based Polyol b | 100% Amine-Based Polyol |
|---|---|---|---|---|---|
| Polyol | V490 (g) | 20 | 10 | 5 | 0 |
| Amine-based polyol (g) | 0 | 10 | 15 | 20 | |
| Catalyst | Polycat 8 (g) | 0.1 | 0.1 | 0.1 | 0.1 |
| Surfactant | INV 690 (g) | 0.2 | 0.2 | 0.2 | 0.2 |
| Blowing Agent | Water (g) | 0.2 | 0.2 | 0.2 | 0.2 |
| Isocyanate | MDI PAPI 27 (g) | 28.97 | 24.83 | 22.76 | 20.68 |
| NCO/OH [64] | 1.11 | 1.07 | 1.05 | 1.02 | |
a mixed-polyol composition: 50 wt. % amine-based; 50 wt. % 100% V490. b mixed-polyol composition: 75 wt. % amine-based; 25 wt. % 100% V490.
2.4. Computational Method Approach
The MATLAB computational approach is anchored on the solutions of non-stiff ordinary differential equations (ODE) using the Runge–Kutta (8,9) method via the MATLAB ode89 function for a high accuracy.
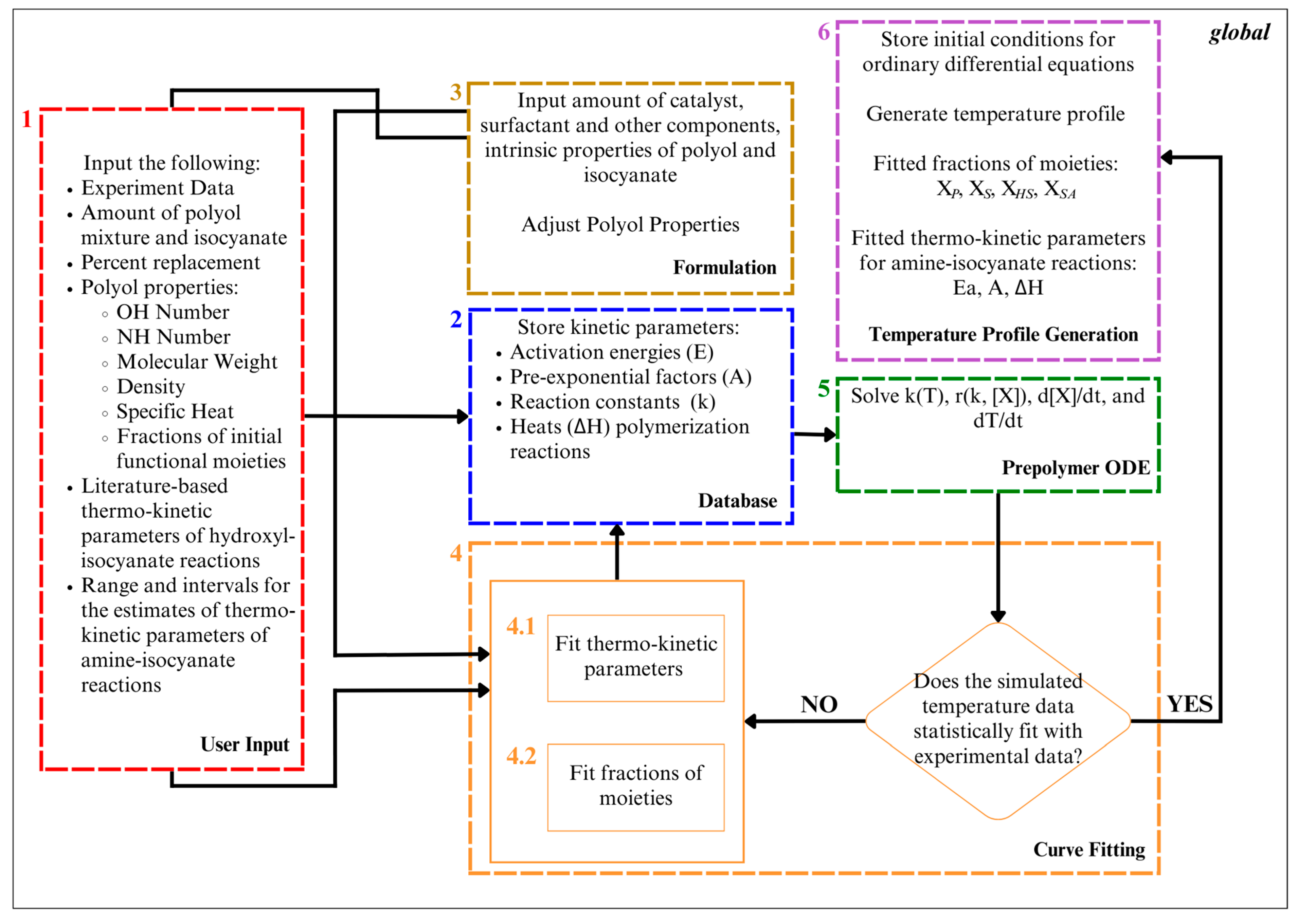
The computational algorithm employed in this study for obtaining the temperature profiles and fitted fractions of the moieties of the polyol system is depicted in Figure 3. The algorithm is composed of six interrelated MATLAB-coded functions provided in Supplementary Materials S2, namely, User Input, Formulation, Database, Curve Fitting, Prepolymer ODE, and Temperature Profile Generation.
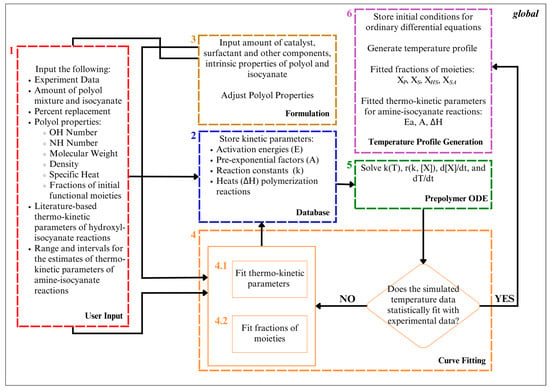
Figure 3.
Algorithm for simulation of polyurethane prepolymerization and foaming temperature profile utilizing both traditional and hybrid polyols and fitting of fractions of moieties of employed polyol system and thermo-kinetic parameters of amine–isocyanate reactions.
The User Input function comprises four prompts that gather key information: (1) the composition of the isocyanate and polyol mixture, and the percentage replacement of the amine-based polyol; (2) property values of the amine-based polyol, encompassing the hydroxyl value, amine value, molecular weights, functionality, density, and specific heat capacities; (3) property values of isocyanate, and (4) experimental data along with the reaction time and range and intervals for the fractions of the functional moieties. With regard to the property values of the amine-based polyol, the hydroxyl value of the polyol serves as a quantifier for the concentration of hydroxyl functional groups, while the amine value allows for the determination of secondary amine concentrations in the amine-based polyol. The molecular weight is necessary for estimating the molar quantities involved in the prepolymerization reactions. Density, on the other hand, aids in calculating the total volume of the reacting mixture, a critical factor for reaction rate determination. Furthermore, specific heat capacity values are crucial for assessing the heat capacities of chemical species, and pivotal for internal temperature calculation during the polymerization process. Lastly, the inputs for ranges and intervals for the functional moiety fractions were crucial for comprehensive exploration, enabling the identification of functional moiety combinations in the polyol system and ensuring robust, accurate simulation results.
The Formulation function stores property values, including those of isocyanate and polyol, along with catalysts and additives, which are vital for the subsequent calculations.
Meanwhile, the Database function stores essential kinetic and thermodynamic parameters. Arrhenius equation parameters, such as activation energy, pre-exponential factor, and heats of reaction for various reactions, are indispensable for calculating the reaction rates across the temperature range of interest, as well as the heat progression of polymerization reactions.
As for the Prepolymer ODE function, it derives the rate of temperature change (Equation (1)), instantaneous molar concentrations of polyol and isocyanate (reaction rate expressions in Table 1), and rate constants (Equations (2) and (3)) by employing values from the Formulation and Database functions and adopting heuristics, outlined in Table 5, based on the established literature.

Table 5.
Computational method heuristics in generating temperature profile [37,46,48,49].
The Curve Fitting function obtains fractions of the hydroxyl and amine moieties according to a minimized average relative error of less than 5.0% between the experimental and simulated results. The experimental data are fitted with ODE-solved temperature-time data based on the minimized average relative error of the residuals of the predicted and experimental data. Simultaneously, the algorithm enables the fitting of thermo-kinetic parameters or fractions of the moieties of the polyols, encompassing primary, secondary, and hindered-secondary hydroxyls, as well as secondary amines. The selection of fitting targets is contingent upon the availability of prior data regarding these parameters or functionalities.
Lastly, the Temperature Profile Generation function stores the initial conditions of the ordinary differential equations and generates a temperature profile using the MATLAB ode89 function. From here, the function also obtains the gel point of polymerization based on the assumption that the increase in temperature is becoming zero [65]. Therefore, the peak temperatures observed during the prepolymerization process of polyurethane foams correlate with the gel point, which is the point at which the polymerization reaction stops and the temperature ceases to increase [37].
3. Results and Discussion
3.1. Polyol Characterization
In this study, a commercial petroleum-based (V490) and amine-based polyol were used as representatives to investigate the prepolymerization and blowing thermo-kinetic behaviors in traditional and hybrid polyurethane systems. The amine-based polyol was derived from coconut oil and prepared via sequential glycerolysis and the amidation reaction pathway, producing multifunctional components, as illustrated in Figure S1 of the Supplementary Materials, such as diethanolamide, free glycerol, and amino ester, which coexisted in equilibrium with diethanolamide [24,66,67]. The in-depth understanding of the distinct compositions of these compounds was then used as the foundation of the thermo-kinetic behavior investigation of prepolymerization and blowing reactions. Particularly, diethanolamide is characterized by primary hydroxyl groups and an intrinsic nonreactive tertiary amine [68]. Conversely, amino esters possess active secondary amine moieties and primary hydroxyl groups, while glycerol comprises primary and secondary hydroxyl functionalities [69,70].
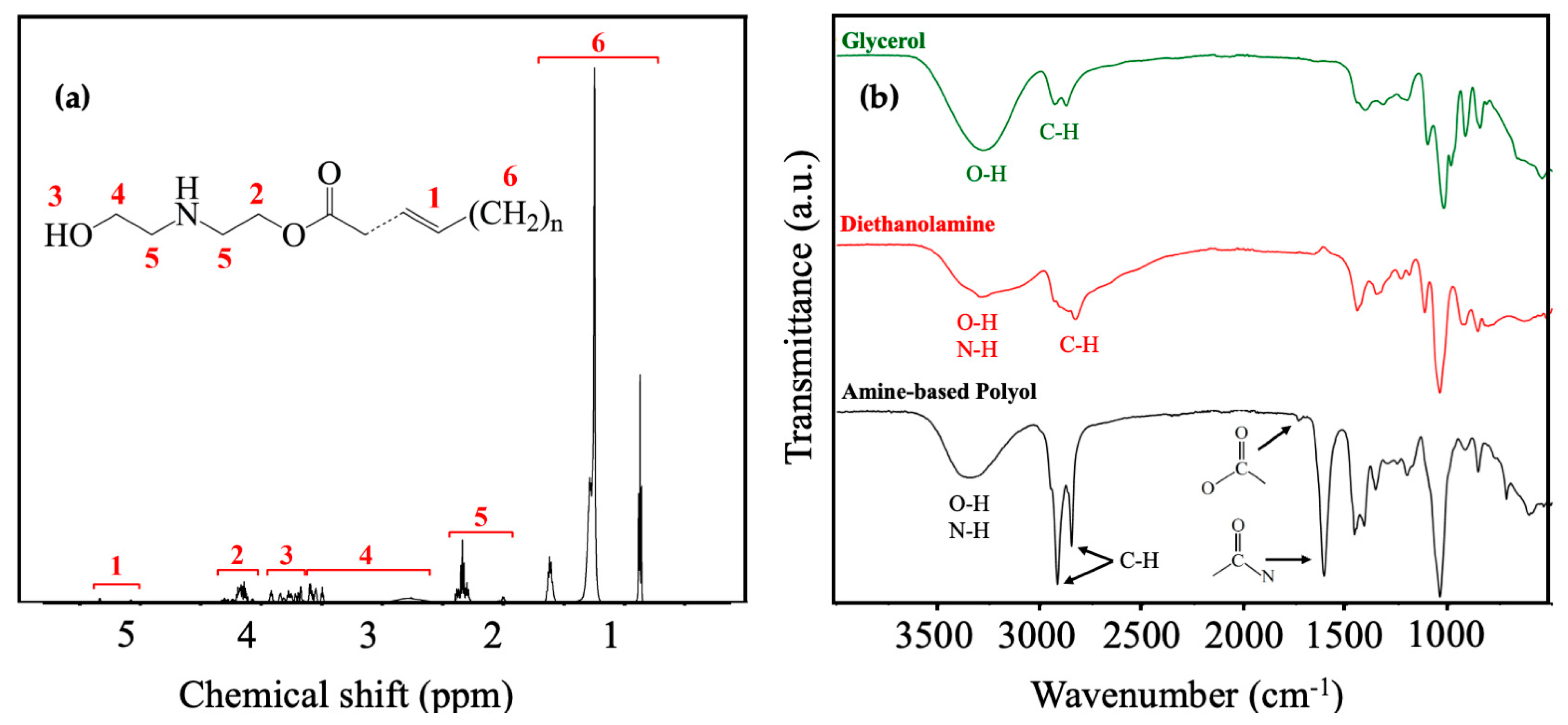
The chemical structure of the resultant product from this sequential reaction was elucidated through a detailed 1HNMR spectroscopic analysis, as illustrated in Figure 4a. The observed peaks in region 1, approximately at (5.3~5.1 ppm), were assigned to the protons of double-bonded carbon [71]. Further structural verification involved the identification of amino ester, manifested by the peaks in region 2 at around 4.15 ppm, signifying protons bonded with ester [72]. Significantly, these features were absent in the 1HNMR spectrum of purified coconut fatty acid diethanolamide, as reported in the literature [23]. Moreover, the absence of these peaks, in comparison to the purified coconut fatty acid diethanolamide of the relevant literature, suggests the presence of amino esters in the amine-based polyol in equilibrium with deithanolamide [24]. Regions 3 (4~3.7 ppm) and 4 (3.7~2.8 ppm) were attributed to the hydroxyls and protons of methylene adjacent to the hydroxyl groups, respectively [73], with a relative decrease in frequency in the same literature due to the presence of the secondary amine in the amino ester in equilibrium with diethanolamide. Additionally, region 5 (2.3 ppm) corresponded to the protons of methylene adjacent to nitrogen, and region 6 (0.8~1.7 ppm) was correlated with the alkyl chain of coconut oil [74,75].
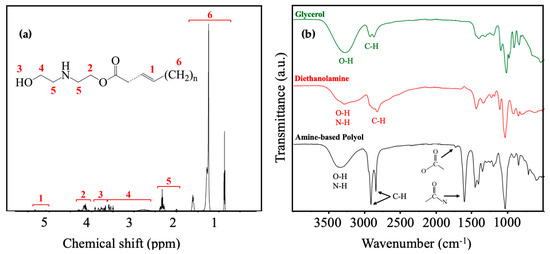
Figure 4.
(a) 1H NMR of coconut-oil amine-based polyol derived from sequential glycerolysis-amidation functionalization; (b) FTIR spectra of glycerol, diethanolamine, and coconut oil amine-based polyol derived from sequential glycerolysis–amidation functionalization.
The validation of the identified functional groups was further supported through FTIR spectra, as illustrated in Figure 4b. The stretching of hydroxyl groups at around 3300 cm−1 was evident in the peaks of all three compounds [76,77]. Particularly, glycerol displayed more intense peaks owing to its higher OH number in comparison to amine-based polyol and diethanolamine. Additionally, N-H stretching, observed at around 3350 cm−1, was present in both the amine-based polyol and diethanolamine [78,79]. Intermolecular bonded O-H usually has a broad range, while N-H, having a weaker polarity than O-H, is positioned in more limited range of 3350–3310 cm−1 [80] Hence, the observed peak located in 3100–3600 cm−1 for diethanolamine and amine-based polyol is attributed to the combined stretching of N-H and O-H [24,81]. The sharp peak for C-H stretching around 2900 cm−1 in the amine-based polyol implies the long-chain R group of amine-based polyol. The distinct peak at 1680 cm−1 in the amine-based polyol spectrum signifies the robust stretching of carbonyls associated with amide [82,83]. Remarkably, this peak is absent in the spectra of glycerol and diethanolamine, confirming the successful formation of the amide functionality in the amine-based polyol [23]. Additionally, the relatively high intensity of this peak suggests that the amide formed from diethanolamine is a major component of the polyol. Furthermore, a small peak at around 1700 cm−1 can be attributed to the carbonyl group from esters. The relative low intensity of this peak may indicate a low concentration of amino esters in the amine-based polyol system, aligning with the subsequent discussion on the input for the fraction of secondary amine moieties.
Furthermore, the prominent physicochemical properties of both the V490 and amine-based polyols were experimentally characterized, as outlined in Table 6. To begin, the total hydroxyl value was determined for both polyols, providing information on the concentration of the hydroxyl functional groups present in the polyol. It was found that the hydroxyl value of V490 was higher than the amine-based polyol due to its higher concentrations of hydroxyl-rich sucrose and glycerol units [84]. These units contribute to multiple secondary hydroxyl groups within a single polyol molecule. In contrast, the amine-based polyol, containing diethanolamide as a major component, had relatively fewer hydroxyl functional groups in one molecule than V490 [69,85]. It is worth noting that the individual hydroxyl moiety values of polyols cannot be experimentally characterized due to the absence of standard testing procedures.
Moreover, the amine moiety characterization of both polyols revealed that V490 did not contain amine moieties, while the amine-based polyol contained a concentration of reactive secondary amine and unreactive tertiary amine values of 10.3 and 19.8 mg KOH/g, respectively. As a result, the fraction of secondary amine moieties (XSA) within the amine-based polyol can be computed as 0.0277, as outlined in S4 of the Supplementary Materials. Employing Liu and Zhu’s methodology [4] for approximating amines through NMR analysis, particularly by integrating NMR signals (as shown in S6 of the Supplementary Material), the signal within region 2 associated with the ester of amino esters was estimated to be 0.0380 relative to the overall integrated signals. Notably, this fraction of active amine moieties approximately aligned with the calculated fraction of secondary amine (XSA = 0.0277), thereby providing a satisfactory input for the estimation of kinetic parameters.
Additionally, the higher molecular weight of the amine-based polyol compared to V490 can be attributed to the relatively high molecular weight of the amine-based polyol components, such as coconut-oil-based diethanolamide, amino esters, and glycerol compared to the sucrose and glycerol main components of V490 [86]. It can also be observed that the functionality of the amine-based polyol was higher than that of the V490. This result can be attributed to the higher molecular weight of the amine-based polyol, thereby inducing higher reactive groups per molecule of amine-based polyol in contrast with V490 [87]. Furthermore, the density for the amine-based polyol was slightly higher than that of V490, which is consistent with the results regarding their molecular weight, implying that amine-based polyol had a greater mass over the volume occupied that constituted the total volume of reacting mixture from which molar concentration was derived. In turn, this may have influenced the calculated reaction rate. Lastly, the specific heat capacity of the amine-based polyol was higher than that of V490. A higher specific heat may lead to higher heat capacity, lowering the increase in heat progression according to Equation (1).

Table 6.
Pertinent physicochemical properties of pure amine-based and Voranol 490 polyols as inputs to the computational method.
Table 6.
Pertinent physicochemical properties of pure amine-based and Voranol 490 polyols as inputs to the computational method.
| Component | Amine-Based Polyol | Voranol 490 (V490) [88] |
|---|---|---|
| Hydroxyl value (mg KOH/g) | 361 ± 2 | 487 ± 3 |
| Primary amine value (mg KOH/g) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Secondary amine value (mg KOH/g) | 10.3 ± 0.6 | 0.0 ± 0.0 |
| Tertiary amine (unreactive) value (mg KOH/g) | 19.8 ± 0.3 | 0.0 ± 0.0 |
| Molecular weight (g/mol) | 755 ± 3 | 490 ± 1 |
| Functionality | 4.9 ± 0.6 | 4.3 ± 0.3 |
| Specific Heat Capacity (J/g-K) | 3.5 ± 0.1 | 1.8 ± 0.1 |
| Density (g/cm3) | 1.08 ± 0.05 | 1.11 ± 0.05 |
3.2. Simulation of Kinetic Parameters of Secondary Amine
The absence of thermo-kinetic parameters for amine moieties has become one of the major limitations for the computational determination of the fractions of hydroxyls and amine moieties present in a polyol, especially in hybrid polyol systems. Since the thermo-kinetic parameters of hydroxyl functionalities are already available in the literature, by defunctionalizing the amine moieties in the amine-based polyol, the fractions of the hydroxyl moieties can now be computationally determined based on the total hydroxyl functionality for each polyol. The amine defunctionalization process, as generally schemed in Figure S2 of the Supplementary Materials, involved the use of Boc Anhydride (Boc2O) for amine-selective N-Boc protection to prevent the participation of the amine moieties in the prepolymerization process [84,89,90].
Subsequent to the amine defunctionalization process of the amine-based polyol is the computational determination of the fractions of the hydroxyl moieties such as primary (XP), secondary (XS), and hindered-secondary hydroxyls (XHS) (based on the total hydroxyl functionality) present in both the amine-defunctionalized and amine-based polyol. Along with the pertinent inputs, the computational method employed the established thermo-kinetics parameters from the literature, as shown in Table 2, in the MATLAB script using the algorithm presented in Figure 3. The result showed that the fractions of the hydroxyl moieties present in the amine-based polyol were composed of XP = 0.885, XS = 0.115, and XHS = 0.000. These results are consistent with the diethanolamide, amino esters, and glycerol components of the amine-based polyol, wherein all of the hydroxyl groups of these components were mainly primary, with only glycerol having secondary and no component bearing hindered-secondary hydroxyl groups.
Following the simulated XP, XS, and XHS (based on the amine-defunctionalized polyol) and the calculated XSA (based on the polyol overall functionality, shown in Supplementary Materials S4), the overall fractions of hydroxyl and amine moieties based on the amine-based polyol functionality can now be calculated, as presented in Supplementary Materials S4. The calculation results show that the amine-based polyol comprised XP = 0.885, XS = 0.115 XHS = 0.000, and XSA = 0.03.
Subsequently, the resulting fractions of moieties were utilized to predict the thermo-kinetic parameters, as presented in Table 7, for the reaction involving isocyanate and the secondary amines during the polyurethane prepolymer formation. These outputs show that the thermo-kinetic parameters, such as the pre-exponential factor and heat of reaction of secondary amine, were higher than those of the hydroxyls, as shown in Table 2 and Table 7, respectively. This observation is consistent with the relative reaction rates of these functional groups with isocyanate, as shown in Table 8, wherein the reaction of secondary amine with isocyanate exhibited a relative reaction rate of 500–1250, which is significantly higher compared to the primary and secondary hydroxyls with relative reaction rates of 2.5 and 0.75, respectively, at 25 °C.
The relatively higher reaction rate of the secondary amine can be attributed to the higher nucleophilicity of amines than with hydroxyl in a reaction [91]. This is consistent with the higher values of the thermo-kinetic parameters of the secondary amine–isocyanate reaction in comparison with that of hydroxyls–isocyanate. Moreover, the reaction between the amines and isocyanate yielded urea bonds involving higher bond energies compared to urethane formation from hydroxyls [92,93]. The formation of urea bonds released a higher energy output, making the reaction with isocyanates highly exothermic and energetically favorable for the secondary amine. Consequently, the secondary amine became more readily available for reactions with isocyanates, leading to its higher pre-exponential factor and heat of reaction, as observed in the simulated thermo-kinetic parameters in Table 8.

Table 7.
Catalytic fitted thermo-kinetic parameters of isocyanate reaction with secondary amine.
Table 7.
Catalytic fitted thermo-kinetic parameters of isocyanate reaction with secondary amine.
| Pre-Exponential Factor (k0) | Activation Energy (Ea), J/mol | Heat of Reaction (∆H), J/mol | |
|---|---|---|---|
| Secondary Amine | 892 | 4.00 × 104 | 7.90 × 104 |

Table 8.
The relative rates of isocyanate reaction against different hydrogen-active compounds [94].
Table 8.
The relative rates of isocyanate reaction against different hydrogen-active compounds [94].
| Hydrogen Active Compound | Formula | The relative Reaction Rate (Noncatalyzed, 25 °C) |
|---|---|---|
| Primary aliphatic amine | R—NH2 | 2.50 × 103 |
| Secondary aliphatic amine | R2—NH | 500–1250 |
| Primary hydroxyl | R–CH2–OH | 2.50 |
| Water | HOH | 2.50 |
| Secondary hydroxyl | R2–CH–OH | 0.750 |
| Tertiary hydroxyl | R3–C–OH | 0.0125 |
3.3. Simulation of Total Fractions of Moieties: XSA, XP, XS, XHS
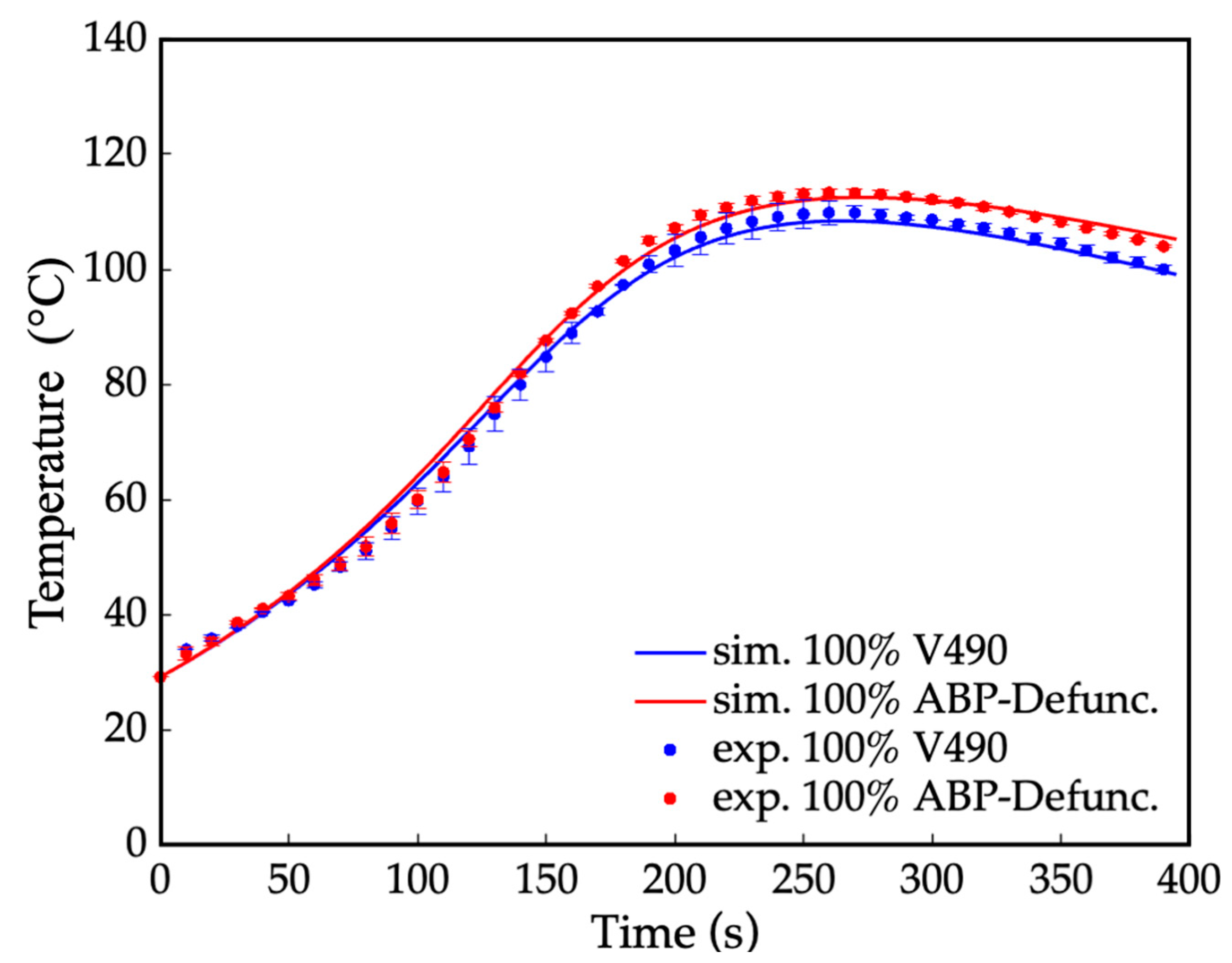
With the availability of the thermo-kinetic parameters and the utilization of the algorithm in Figure 3, the computational method can now directly simulate the overall fractions of hydroxyl and amine moieties, XSA, XP, XS, and XHS, present in the polyol: XP = 0.850, XS = 0.110, XHS = 0.000, and XSA= 0.040. The results show that the simulated fraction of secondary amines (XSA = 0.040) was found to be comparable to the calculated fraction of the secondary amine (XSA = 0.028), as shown in S4 of Supplementary Materials. Thus, this observation validates the accuracy and reliability of the simulated thermo-kinetic parameters for secondary amines.
Similarly, the experimental temperature profiles and the corresponding simulated profile of the resulting fraction of moieties were compared via curve fitting, as presented in Figure 5. The result shows a minimal error of ≤2.0%, implying the accuracy of the simulated values. With further comparison of the amine-based polyol before and after amine defunctionalization in Figure 5, the initial slope of the system containing secondary amine can be observed to be higher than that of the system without reactive amine moieties. This difference in the initial slope was attributed to the relatively higher reaction rate of the secondary amine with isocyanates, as reflected by its higher pre-exponential factor (k0) from the kinetic parameters. Additionally, the peak temperature in the temperature profile was higher for the prepolymerization process before the amine defunctionalization. This higher peak temperature can be attributed to the presence of secondary amines, which exhibit higher exothermicity during their reaction with isocyanates compared to hydroxyls [92,93].
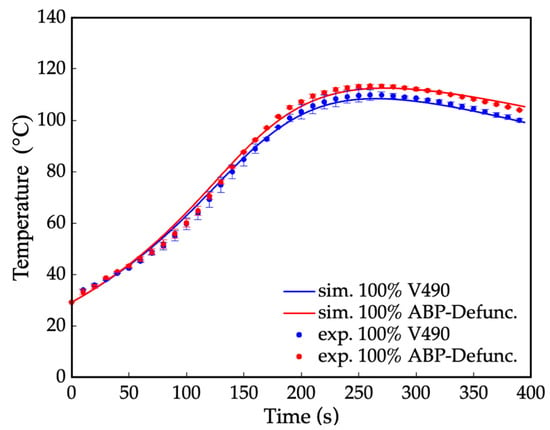
Figure 5.
Temperature profile for the prepolymerization process of amine-based polyol system where: — = simulated (sim.) amine-based polyol before amine defunctionalization (ABP-PreDefunc.), • = experimental (exp.) amine-based polyol before amine defunctionalization, — = simulated amine-based polyol after amine defunctionalization via N-Boc protection (ABP-Defunc.), and • = experimental amine-based polyol after amine defunctionalization via N-Boc protection.
3.4. Prepolymer Reaction Simulation Results for Mixed-Polyol Systems
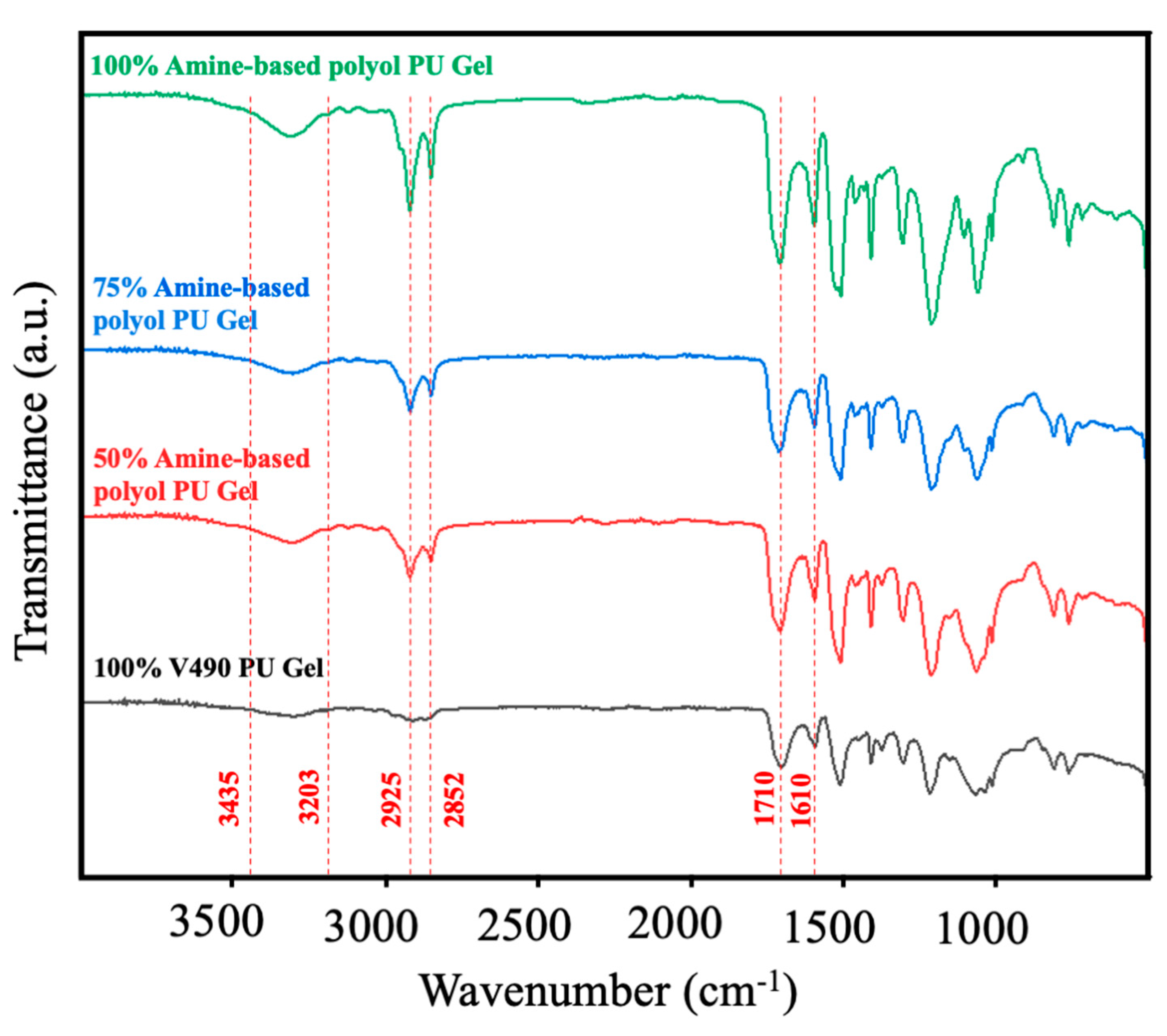
Given the established accuracy of the present computational method in determining the fractions of the hydroxyl and amine moieties of the pure amine-based polyol and V490, the method’s applicability in mixed-polyol systems was subsequently investigated. In this regard, the algorithm in Figure 3 was employed to model the exothermic behavior of polyurethane prepolymer formation using various mixtures of amine-based polyol and V490. Firstly, Figure 6 shows the FTIR spectra of the prepolymer mixtures employed in the simulation to confirm the polyurethane formation. The characteristics of the polyurethane linkage, including –NH– stretching at approximately 3203–3435 cm−1, symmetric CH2 stretching at 2925 cm−1, asymmetric CH2 stretching at 2852 cm−1, and ester carbonyl stretching at 1710 cm−1 and amide carbonyl 1610 cm−1, were successfully identified [95,96,97,98]. With these observations, the formation of polyurethane prepolymer was affirmed. There was a corresponding increase in peak intensities at 2925 cm−1 and 2852 cm−1 as the amine-based concentration increases which can be associated with increasing methylene groups of the amine-based polyol derived from coconut oil [24].
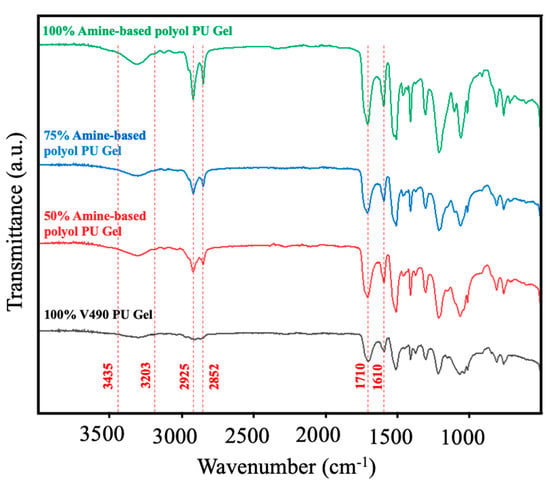
Figure 6.
FTIR spectra of polyurethane prepolymer at different replacement of amine-based polyol.
Table 9 demonstrates the corresponding simulated fractions of the hydroxyls and amine moieties present in the various polyol mixtures. The result shows that with the increase in amine-based polyol concentration, the fractions of primary hydroxyls and secondary amines also increased. Conversely, as the concentration of the amine-based polyol increased, the fraction of hindered-secondary hydroxyls decreased. Moreover, hindered-secondary hydroxyls, which were typically found in the V490, became less dominant as the amine-based polyol concentration increased in the mixture. These observations were consistent with the higher fractions of primary hydroxyls and secondary amines with lower fractions of secondary and hindered-secondary hydroxyls in the pure amine-based polyol compared to pure V490.

Table 9.
Simulation fitted inputs for fraction of moieties of polyol for prepolymerization process.
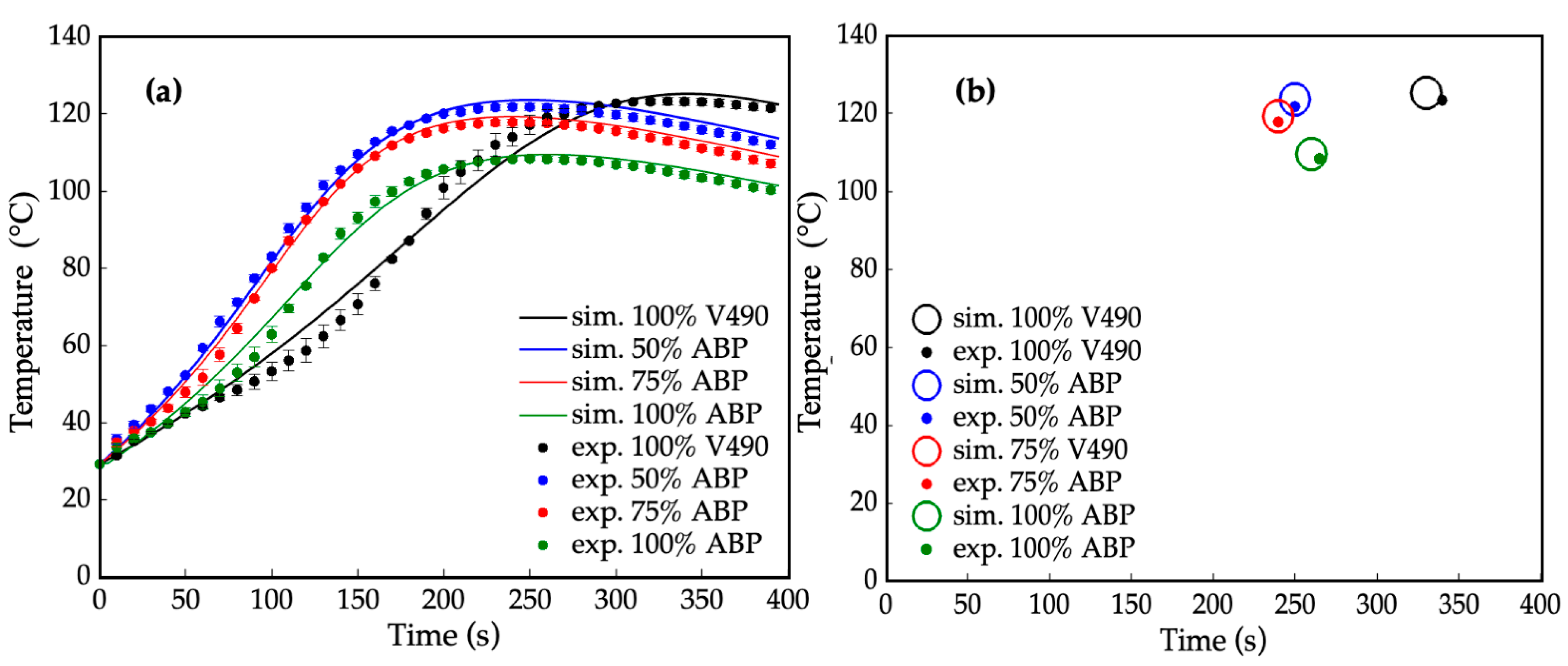
Figure 7a represents the corresponding simulated and experimental temperature profiles of the prepolymerization processes for four polyurethane prepolymer samples: 100% V490, 50%, 75%, and 100% amine-based polyol. It can be observed that the simulated and experimental temperature profiles for both pure and mixed polyol systems were on par with each other with average relative errors of 3.5%, 2.0%, 1.9%, and 2.8%, for the 100% V490, 50%, 75%, and 100% amine-based polyol, respectively. This observation implies that the applicability of the computational method in the present study extends to both pure and mixed polyol systems.
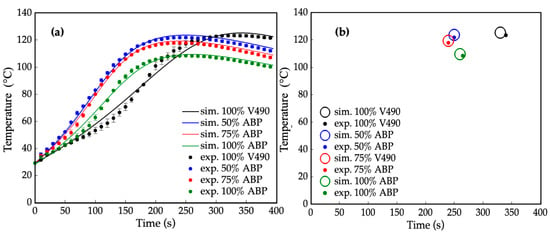
Figure 7.
(a) Internal temperature during prepolymerization reaction at different amine-based polyol percent replacements where: — = simulated (sim.) 100% petroleum-based polyol (V490), — = simulated 50% amine-based polyol (ABP), — = simulated 75% amine-based polyol, and — = simulated 100% amine-based polyol, • = experimental (exp.) 100% petroleum-based polyol (V490) (ave. std. dev. = ±1.92), • = experimental 50% amine-based polyol (ave. std. dev. = ±1.20), • = experimental 75% amine-based polyol (ave. std. dev = ±1.05), and • = experimental 100% amine-based polyol (ave. std. dev. = ±1.33); (b) Gel points of prepolymerization reaction at different purified amine-based polyol percent replacement where ○ = simulated 100% petroleum-based polyol (V490), ○ = simulated 50% amine-based polyol, ○ = simulated 75% amine-based polyol, ○ = simulated 100% amine-based polyol, • = experimental 100% petroleum-based polyol (V490), • = experimental 50% amine-based polyol, • = experimental 75% amine-based polyol, and • = experimental 100% amine-based polyol. Error bars represent standard error.
The reaction rates between the amine-based polyols exhibited significantly higher reactivity compared to pure V490. To expand this, a significant decreasing trend of gel points of polyurethane prepolymerization, illustrated in Figure 7b, can be observed with an increase in amine-based content compared to V490. This decrease in gel point can be attributed to the presence of intrinsic tertiary amine moieties in the amine-rich amine-based polyol [23,24]. These amine moieties induce autocatalysis in the polymerization process, accelerating the reaction rate and leading to faster prepolymerization.
Additionally, primary hydroxyls react at a relatively higher rate with isocyanates compared to secondary and hindered-secondary hydroxyls [99]. With these, the primary hydroxyls and secondary amines played a significant role in decreasing the gel points of amine-based polyols. In addition, gel points were investigated with the slopes of the temperature profiles. The decreasing of gel points from petroleum-based to amine-based polyols is also consistent with the differences in the initial slope of the temperature profiles, which describe the reaction rate of polymerization in terms of the pre-exponential factor and activation energies [46]. The higher slope observed for the 50% amine-based polyol in Figure 7a indicates a higher reaction rate compared to V490, while the decrease in slope among the pure amine-based polyols can be attributed to their lower hydroxyl values. However, even with a lower hydroxyl number, the initial slope of 100% amine-based polyol is comparable to, if not higher than, that of V490 due to the combined effect of a significant increase in primary hydroxyls and secondary amine content. Notably, the obtained results underscore the current prepolymerization reaction simulation method extends beyond the scope of polyurethane foams used in this study, as prepolymerization encompasses a wide range of polyurethane products, including non-blown polyurethane products such as elastomers, coatings, and adhesives. Therefore, the current method is applicable to various types of prepolymerization reactions, such as adhesives and coatings [100,101,102,103].
3.5. Foaming Reaction Simulation Results for Mixed-Polyol Systems
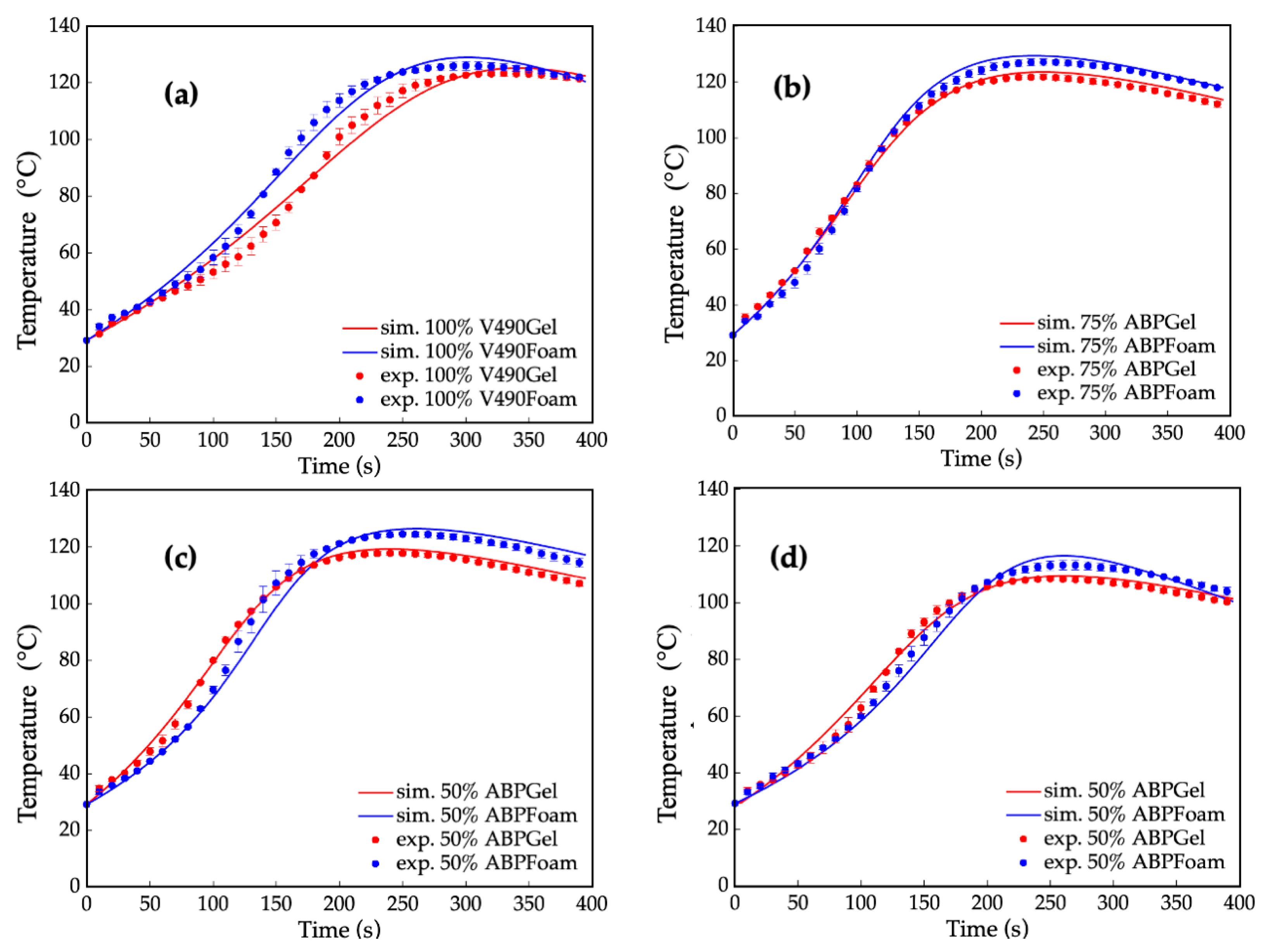
The present simulation method is not limited to prepolymerization reactions, but is also applicable to foaming reactions involving the incorporation of water reacting with isocyanates. Figure 8 shows the superimposed simulation and experimental data of the prepolymerization and foaming reaction using pure and mixtures of amine-based polyol and V490. The observed increase in peak temperatures for the foaming reaction in contrast to the prepolymerization reaction can be attributed to the greater exothermic heat of the reaction between water and isocyanate compared to the reaction between isocyanate and hydroxyl. The reaction of water with isocyanate results in the formation of carbon dioxide gas, leading to the expansion of the foam and a higher exothermic heat release [104]. Moreover, the slope of the temperature profiles for the foaming reaction was higher than that of the prepolymerization temperature due to the significantly higher reaction rate of the isocyanate reaction with both water and primary amine, as indicated in Table 8. In terms of temperature stability, the temperature increases in the prepolymerization curve were more stable compared to the foaming curve due to the stable progression of cross-linking in the prepolymerization process. The foaming curve, on the other hand, showed inflection due to the catalyst activity for the additional reaction of water and amines with isocyanate.
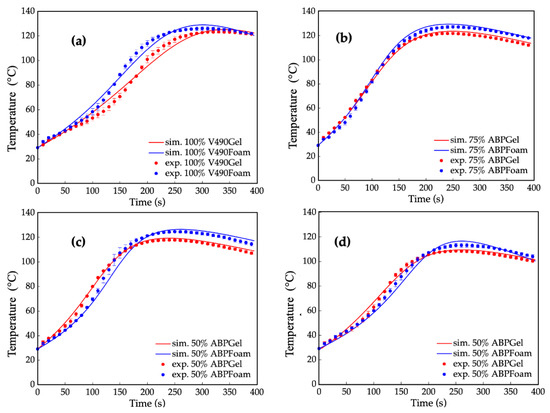
Figure 8.
Temperature profile results of simulated (sim.) (“—”) and experimental (exp.) (“•”) foaming and simulated (“—”) and experimental (“•”) prepolymerization reaction using (a) 100% petroleum-based polyol (V490) (ave. std. dev = ±1.92; ±1.87); (b) 50% amine-based polyol (ABP) (ave. std. dev = ±1.20; ±1.44); (c) 75% amine-based polyol (ave. std. dev = ±1.05; ±1.83); and (d) 100% amine-based polyol (ave. std. dev = ±1.33; ±1.67). Error bars represent standard error.
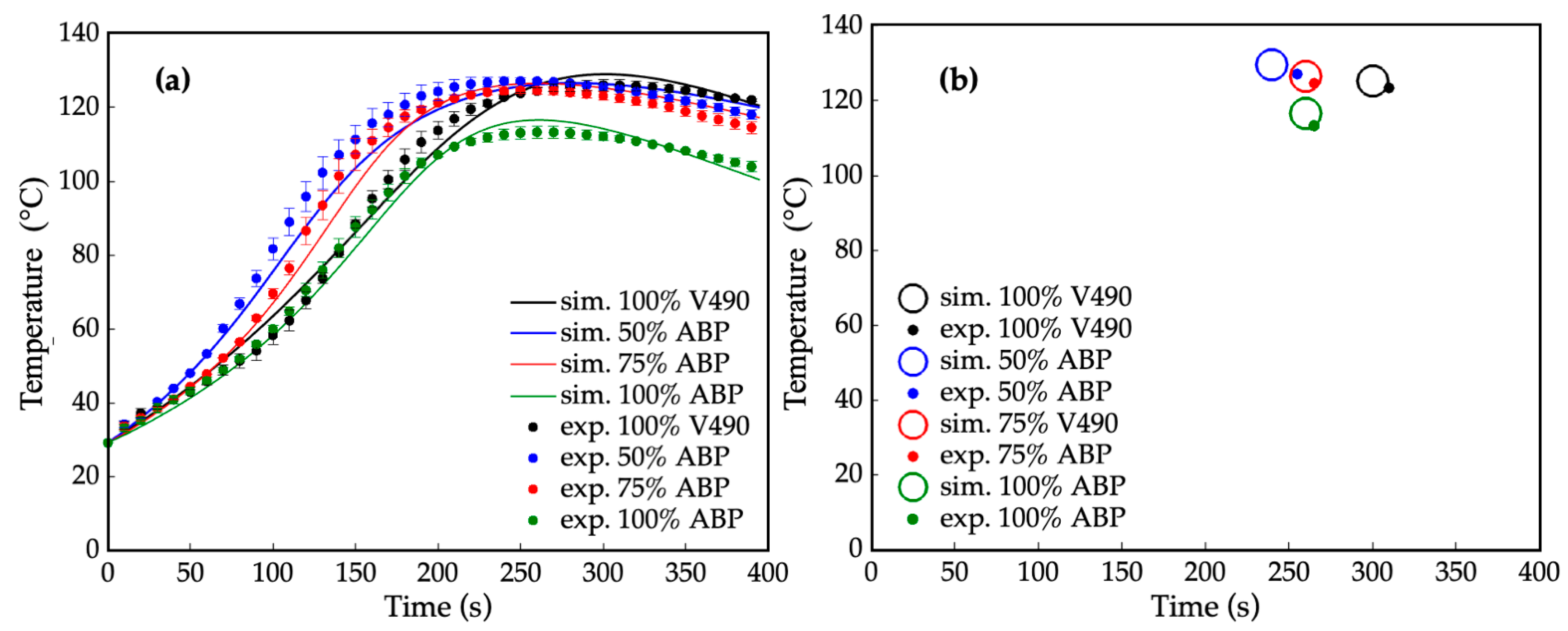
Consistent results in the heat progression of foaming can be noted with prepolymerization, as shown in Figure 9, which presents the superimposed simulated and experimental data of the foaming reaction and their corresponding gel points. Particularly, in the foaming reaction using 100% V490 polyol, the gel point reached 127 °C at around 305 s. On the other hand, the gel point for 50% amine-based polyol peaked at 128 °C. This indicates that the exothermicity in the foaming process of 50% amine-based polyol was higher than that of 100% V490 polyol. For 75% and 100% amine-based polyol foaming processes, the gel points were 123 °C at 265 s and 115 °C at 275 s, respectively. These results, consistent with prepolymerization simulation results, show that as the amine-based polyol content increased, the gel points decreased, indicating a lower heat generation and higher reaction rate during the foaming reaction, which can be attributed to the lowering of hydroxyl value and fractions of increased primary alcohol and secondary amine [105]. Moreover, all polymerization profiles exhibited the same slope between 0 and 40 s of reaction time due to the delayed effect of the catalyst on the reactions between water and isocyanate. Additionally, 100% V490 and 100% amine-based polyol had the same slope between 0 and 140 s of reaction time, indicating that the foaming reaction rate for these two polymerization processes was roughly the same at the beginning because of the intrinsic tertiary amine in the polyol.
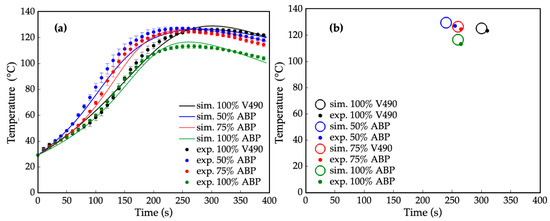
Figure 9.
(a) Internal temperature during foaming reaction at different amine-based polyol percent replacements where: — = simulated (sim.) 100% petroleum-based polyol (V490), — = simulated 50% amine-based polyol (ABP), — = simulated 75% amine-based polyol, and — = simulated 100% amine-based polyol, • = experimental (exp.) 100% petroleum-based polyol (ave. std. dev. = ±1.87), • = experimental 50% amine-based polyol (ave. std. dev. = ±1.44), • = experimental 75% amine-based polyol (ave. std. dev. = ±1.83), and • = experimental 100% amine-based polyol (ave. std. dev. = ±1.67); (b) Gel points of foaming reaction at different amine-rich polyol percent replacement where ○ = simulated 100% petroleum-based polyol (V490), ○ = simulated 50% amine-based polyol, ○= simulated 75% amine-based polyol, ○ = simulated 100% amine-based polyol, • = experimental 100% petroleum-based polyol (V490), • = experimental 50% amine-based polyol, • = experimental 75% amine-based polyol, and • = experimental 100% amine-based polyol. Error bars represent standard error.
Overall, the consistent results obtained from the foaming reaction simulation demonstrate the effectiveness of the present simulation method in predicting the temperature profiles and heat evolution during the foaming process. The ability to accurately simulate foaming reactions is crucial for optimizing foam production and tailoring the properties of polyurethane foams for specific applications.
4. Conclusions
A computational method was developed to further contribute to the characterization of existing polyol systems, an important precursor material for polyurethane. The method encompasses traditional and hybrid polyol systems, that is, both hydroxyl and amine-comprising polyols. Polyol characterization includes the determination of the fraction of functional moieties and amine thermo-kinetic parameters for exothermic reactions with isocyanates. The utilized method showed excellent agreement with existing reaction rates from the literature. The simulated characterization results and corresponding temperature profiles provide valuable insights into the reactivity and heat progression during the polyurethane prepolymerization process. The computational method also captured the influence of secondary amine and other functional groups on the reaction rate and maximum temperature, demonstrating its ability to predict the thermo-kinetic behavior of the polyols’ corresponding prepolymerization processes.
The computational method employed in the present study realizes the great potential to accelerate polyurethane hybrid foam formulation development and optimization with reaction process simulations, significantly reducing the need for extensive experimental runs. This reduction in experimentation not only saves valuable resources, but also minimizes costs and mitigates exposure to hazardous solvents, promoting a safer and more sustainable research approach.
Furthermore, as new polyol or isocyanate systems are introduced, this method exhibits adaptability in assessing their reactivity and compatibility with other chemical species involved in polyurethane polymerization. By controlling the type and concentration of functional groups in polyols, it is possible to tailor the reactivity and properties of polyurethane materials for specific applications in various industries. The consideration of additional polymeric side reactions, such as the endothermic formations of allophanate and biuret segments in the thermo-kinetic simulation, could potentially improve our understanding in the field while paving a way for future studies on polymeric material development. This adaptability allows for sustainable innovation in polymer chemistry and engineering, driving the field forward.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16114587/s1.
Author Contributions
Conceptualization, R.G.D.J., F.L.A.M.A. and H.H.A.-M.; methodology, L.C.C.M. and R.G.D.J.; software, L.C.C.M., R.G.D.J., F.L.A.M.A., H.H.A.-M. and N.P.B.T.; validation, L.C.C.M. and R.G.D.J.; formal analysis, L.C.C.M., R.G.D.J. and N.P.B.T.; investigation, L.C.C.M., R.G.D.J. and F.L.A.M.A.; resources, L.C.C.M., R.G.D.J., H.H.A.-M., N.P.B.T., R.M.M., A.C.A. and A.A.L.; data curation, L.C.C.M. and R.G.D.J.; writing—original draft preparation, L.C.C.M.; writing—review and editing, L.C.C.M., R.G.D.J., G.G.D., R.M.M. and A.A.L.; visualization, L.C.C.M. and R.G.D.J.; supervision, R.G.D.J., G.G.D., R.M.M. and A.A.L.; project administration, R.G.D.J., R.M.M., A.C.A. and A.A.L.; funding acquisition, G.G.D., R.M.M., A.C.A. and A.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to express their acknowledgment of the financial support from the Philippine Department of Science and Technology (DOST) through the Niche Centers in the Region (NICER)–R&D Center for Sustainable Polymers, grant number 101-02-0194-2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors wish to express gratitude for the support provided by the Philippine Council for Industry, Energy, and Emerging Technology Research and Development (PCIEERD) under the Department of Science and Technology (DOST), specifically through the Niche Centers in the Region (NICER)—R&D Center for Sustainable Polymers and the Engineering Research and Development for Technology (ERDT) Scholarship Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mahanwar, P. A Brief Discussion on Advances in Polyurethane Applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications-a Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, M. Determination of Molar Ratio of Primary Secondary and Tertiary Amines in Polymers by Applying Derivatization and NMR Spectroscopy. Polym. Test. 2016, 56, 174–179. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022 (accessed on 11 May 2024).
- Polyurethane Market Size, Trends, Share, Growth, Report 2032. Available online: https://www.precedenceresearch.com/polyurethane-market (accessed on 11 May 2024).
- Statista Global Polyurethane Market Volume 2015–2030. Available online: https://www.statista.com/statistics/720341/global-polyurethane-market-size-forecast/ (accessed on 11 May 2024).
- Li, X.; Li, J.; Wang, J.; Yuan, J.; Jiang, F.; Yu, X.; Xiao, F. Recent Applications and Developments of Polyurethane Materials in Pavement Engineering. Constr. Build. Mater. 2021, 304, 124639. [Google Scholar] [CrossRef]
- Wiener, J.; Khan, M.Z.; Shah, K. Performance Enhancement of the Solar Still Using Textiles and Polyurethane Rollers. Sci. Rep. 2024, 14, 5202. [Google Scholar] [CrossRef] [PubMed]
- De Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. ACS Symp. Ser. Am. Chem. Soc. 2021, 1380, 1–24. [Google Scholar]
- Kim, S.; Kim, J.W.; Lee, Y.H.; Jeong, Y.R.; Keum, K.; Kim, J.Y.; Lee, H.-C.; Ha, J.S. Tough, Self-Healing Polyurethane with Novel Functionality for Fully Recoverable Layered Sensor Arrays. J. Chem. Eng. 2023, 464, 142700. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Xue, R.; Xu, B.; Qian, X.; Xin, F.; Blank, L.M.; Zhou, J.; Wei, R.; Dong, W.; et al. Biodegradation and Up-Cycling of Polyurethanes: Progress, Challenges, and Prospects. Biotechnol. Adv. 2021, 48, 107730. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguié-Béghin, V. Langmuir-Blodgett Procedure to Precisely Control the Coverage of Functionalized AFM Cantilevers for SMFS Measurements: Application with Cellulose Nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from Seed Oil-Based Polyols: A Review of Synthesis, Mechanical and Thermal Properties. Ind. Crops Prod. 2019, 142. [Google Scholar] [CrossRef]
- Wu, G.; Chen, J.; Yang, Z.; Jin, C.; Liu, G.; Huo, S.; Kong, Z. Preparation and Properties of Autocatalytic Biobased Waterborne Polyol from Rosin Based Epoxy Resin. J. Polym. Environ. 2022, 48, 476–488. [Google Scholar] [CrossRef]
- Echeverria-Altuna, O.; Ollo, O.; Larraza, I.; Gabilondo, N.; Harismendy, I.; Eceiza, A. Effect of the Biobased Polyols Chemical Structure on High Performance Thermoset Polyurethane Properties. Polymer 2022, 263, 125515. [Google Scholar] [CrossRef]
- Sardon, H.; Mecerreyes, D.; Basterretxea, A.; Avérous, L.; Jehanno, C. From Lab to Market: Current Strategies for the Production of Biobased Polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar] [CrossRef]
- Coman, A.E.; Peyrton, J.; Hubca, G.; Sarbu, A.; Gabor, A.R.; Nicolae, C.A.; Iordache, T.V.; Averous, L. Synthesis and Characterization of Renewable Polyurethane Foams Using Different Biobased Polyols from Olive Oil. Eur. Polym. J. 2021, 149, 110363. [Google Scholar] [CrossRef]
- Allauddin, S.; Somisetti, V.; Ravinder, T.; Rao, B.V.S.K.; Narayan, R.; Raju, K.V.S.N. One-Pot Synthesis and Physicochemical Properties of High Functionality Soy Polyols and Their Polyurethane-Urea Coatings. Ind. Crops Prod. 2016, 85, 361–371. [Google Scholar] [CrossRef]
- Alagi, P.; Hong, S.C. Vegetable Oil-Based Polyols for Sustainable Polyurethanes. Macromol. Res. 2015, 23, 1079–1086. [Google Scholar] [CrossRef]
- Bote, S.D.; Narayan, R. Synthesis of Biobased Polyols from Soybean Meal for Application in Rigid Polyurethane Foams. Ind. Eng. Chem. Res. 2021, 60, 5733–5743. [Google Scholar] [CrossRef]
- Frias, C.F.; Fonseca, A.C.; Coelho, J.F.J.; Serra, A.C. Straightforward Synthesis of Amido Polyols from Epoxidized Soybean Oil for Polyurethane Films. Macromol. Mater. Eng. 2021, 306, 2100453. [Google Scholar] [CrossRef]
- Leng, X.; Li, C.; Cai, X.; Yang, Z.; Zhang, F.; Liu, Y.; Yang, G.; Wang, Q.; Fang, G.; Zhang, X. A Study on Coconut Fatty Acid Diethanolamide-Based Polyurethane Foams. RSC Adv. 2022, 12, 13548–13556. [Google Scholar] [CrossRef]
- Dingcong, R.G.; Malaluan, R.M.; Alguno, A.C.; Estrada, D.J.E.; Lubguban, A.A.; Resurreccion, E.P.; Dumancas, G.G.; Al-Moameri, H.H.; Lubguban, A.A. A Novel Reaction Mechanism for the Synthesis of Coconut Oil-Derived Biopolyol for Rigid Poly(Urethane-Urea) Hybrid Foam Application. RSC Adv. 2023, 13, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, Z.; Kessler, M.R.; Brehm-Stecher, B.; Larock, R.C. Antibacterial Soybean-Oil-Based Cationic Polyurethane Coatings Prepared from Different Amino Polyols. ChemSusChem 2012, 5, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Garrison, T.F.; Zhang, Z.; Kim, H.J.; Mitra, D.; Xia, Y.; Pfister, D.P.; Brehm-Stecher, B.F.; Larock, R.C.; Kessler, M.R. Thermo-Mechanical and Antibacterial Properties of Soybean Oil-Based Cationic Polyurethane Coatings: Effects of Amine Ratio and Degree of Crosslinking. Macromol. Mater. Eng. 2014, 299, 1042–1051. [Google Scholar] [CrossRef]
- Dingcong, R.G.; Radjac, D.B.; Alfeche, F.L.A.M.; Dizon, A.C.O.; Tejas, K.J.G.D.; Malaluan, R.M.; Al-Moameri, H.H.; Dumancas, G.G.; Alguno, A.C.; Lubguban, A.A. An Iterative Method for the Simulation of Rice Straw-Based Polyol Hydroxyl Moieties. Sustainability 2023, 15, 12082. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Zheng, B.; Ou, R.; Fan, Q.; Li, L.; Guo, C.; Liu, T.; Wang, Q. Synthesis of Lignin-Based Polyols via Thiol-Ene Chemistry for High-Performance Polyurethane Anticorrosive Coating. Compos. B Eng. 2020, 200, 10895. [Google Scholar] [CrossRef]
- Nagy, L.; Vadkerti, B.; Lakatos, C.; Fehér, P.P.; Zsuga, M.; Kéki, S. Kinetically Equivalent Functionality and Reactivity of Commonly Used Biocompatible Polyurethane Crosslinking Agents. Int. J. Mol. Sci. 2021, 22, 4059. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J. Polyurethanes and Polyureas. In Introduction to Synthetic Methods in Step-Growth Polymers; Rogers, M.E., Long, T.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 197–263. [Google Scholar]
- Ghodke, S.; Dandekar, P.; Jain, R. Simplified Evaluation Aided by Mathematical Calculation for Characterization of Polyols by Hydroxyl Value Determination. Int. J. Polym. Anal. Charact. 2021, 26, 169–178. [Google Scholar] [CrossRef]
- ASTM D4274; Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Number of Polyols. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D2074; Standard Test Methods for Total, Primary, Secondary, and Tertiary Amine Values of Fatty Amines by Alternative Indicator Method. ASTM International: West Conshohocken, PA, USA, 2019.
- Xu, J.; Jiang, Y.; Zhang, T.; Dai, Y.; Yang, D.; Qiu, F.; Yu, Z.; Yang, P. Synthesis of UV-Curing Waterborne Polyurethane-Acrylate Coating and Its Photopolymerization Kinetics Using FT-IR and Photo-DSC Methods. Prog. Org. Coat. 2018, 122, 10–18. [Google Scholar] [CrossRef]
- He, Y.; Xie, D.; Zhang, X. The Structure, Microphase-Separated Morphology, and Property of Polyurethanes and Polyureas. J. Mater. Sci. 2014, 49, 7339–7352. [Google Scholar] [CrossRef]
- Peyrton, J.; Chambaretaud, C.; Avérous, L. New Insight on the Study of the Kinetic of Biobased Polyurethanes Synthesis Based on Oleo-Chemistry. Molecules 2019, 24, 4332. [Google Scholar] [CrossRef]
- Alfeche, F.L.A.M.; Dingcong, R.G.; Mendija, L.C.C.; Al-Moameri, H.H.; Dumancas, G.G.; Lubguban, A.A.; Malaluan, R.M.; Alguno, A.A.; Lubguban, A.A. In Silico Investigation of the Impact of Reaction Kinetics on the Physico-Mechanical Properties of Coconut-Oil-Based Rigid Polyurethane Foam. Sustainability 2023, 15, 7148. [Google Scholar] [CrossRef]
- Mathur, A.K.; Majumder, C.B.; Chatterjee, S.; Roy, P. Biodegradation of Pyridine by the New Bacterial Isolates S. putrefaciens and B. sphaericus. J. Hazard. Mater. 2008, 157, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zareanshahraki, F.; Asemani, H.R.; Skuza, J.; Mannari, V. Synthesis of Non-Isocyanate Polyurethanes and Their Application in Radiation-Curable Aerospace Coatings. Prog. Org. Coat. 2020, 138, 105394. [Google Scholar] [CrossRef]
- Sun, M.; Bi, Y.; Zhuang, W.; Chen, S.; Zhao, P.; Pang, D.; Zhang, W. Mechanism of Polyurethane Binder Curing Reaction and Evaluation of Polyurethane Mixture Properties. Coatings 2021, 11, 1454. [Google Scholar] [CrossRef]
- Galvez, P.; Lopez de Armentia, S.; Abenojar, J.; Martinez, M.A. Effect of Moisture and Temperature on Thermal and Mechanical Properties of Structural Polyurethane Adhesive Joints. Compos. Struct. 2020, 247, 112443. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Deng, Y.; Zhang, P. A Review of Research on the Effect of Temperature on the Properties of Polyurethane Foams. Polymers 2022, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Ferkl, P.; Kršková, I.; Kosek, J. Evolution of Mass Distribution in Walls of Rigid Polyurethane Foams. Chem. Eng. Sci. 2018, 176, 50–58. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, C.; Liu, T.; Chen, Y.; Liu, Z.; Zhao, L. Experimental and Numerical Study of the Polyurethane Foaming Process Using High-Pressure CO2. J. Cell. Plast. 2021, 57, 927–949. [Google Scholar] [CrossRef]
- Baser, S.A.; Khakhar, D.V. Modeling of the Dynamics of Water and R-11 Blown Polyurethane Foam Formation. Polym. Eng. Sci. 1994, 34, 642–649. [Google Scholar] [CrossRef]
- Zhao, Y.; Gordon, M.J.; Tekeei, A.; Hsieh, F.H.; Suppes, G.J. Modeling Reaction Kinetics of Rigid Polyurethane Foaming Process. J. Appl. Polym. Sci. 2013, 130, 1131–1138. [Google Scholar] [CrossRef]
- Al-Moameri, H.; Zhao, Y.; Ghoreishi, R.; Suppes, G.J. Simulation of Liquid Physical Blowing Agents for Forming Rigid Urethane Foams. J. Appl. Polym. Sci. 2015, 132, 42454. [Google Scholar] [CrossRef]
- Ghoreishi, R.; Suppes, G.J. Chain Growth Polymerization Mechanism in Polyurethane-Forming Reactions. RSC Adv. 2015, 5, 68361–68368. [Google Scholar] [CrossRef]
- Al-Moameri, H.H.; Jaf, L.A.; Suppes, G.J. Simulation Approach to Learning Polymer Science. J. Chem. Educ. 2018, 95, 1554–1561. [Google Scholar] [CrossRef]
- Ghoreishi, R.; Suppes, G.J. Modeling of Toluene Sulfonic Acid Catalyzed Oxide Addition Reaction for Soy-Based Polyol. Ind. Eng. Chem. Res. 2015, 54, 91–99. [Google Scholar] [CrossRef]
- Inguva, P.; Bhute, V.J.; Cheng, T.N.H.; Walker, P.J. Introducing Students to Research Codes: A Short Course on Solving Partial Differential Equations in Python. Educ. Chem. Eng. 2021, 36, 1–11. [Google Scholar] [CrossRef]
- Al-Moameri, H.; Ghoreishi, R.; Zhao, Y.; Suppes, G.J. Impact of the Maximum Foam Reaction Temperature on Reducing Foam Shrinkage. RSC Adv. 2015, 5, 17171–17178. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Kurańska, M.; Prociak, A. The Influence of Rapeseed Oil-Based Polyols on the Foaming Process of Rigid Polyurethane Foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Polyurethane Foams Made from Liquefied Bark-Based Polyols. J. Appl. Polym. Sci. 2014, 131, 40599. [Google Scholar] [CrossRef]
- Yeap, P.I.; Yuhana, N.Y.; Fariz, S.; Otoh, M.Z. Temperature on the Mechanical, Thermal and Barrier Property of Polyurethane. IOP Conf. Ser. Mater. Sci. Eng. 2020, 943, 012017. [Google Scholar] [CrossRef]
- Ghoreishi, R.; Zhao, Y.; Suppes, G.J. Reaction Modeling of Urethane Polyols Using Fraction Primary Secondary and Hindered-Secondary Hydroxyl Content. J. Appl. Polym. Sci. 2014, 131, 40388. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, F.; Tekeei, A.; Suppes, G.J. Modeling Impact of Catalyst Loading on Polyurethane Foam Polymerization. Appl. Catal. A Gen. 2014, 469, 229–238. [Google Scholar] [CrossRef]
- DOW Chemical Company. PAPITM 27 Polymeric MDI. Available online: https://www.dow.com/en-us/pdp.papi-27-polymeric-mdi.445799z.html#overview (accessed on 17 July 2022).
- ASTM E1269; Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D6474-12; Standard Test Method for Determining Molecular Weight Distribution and Molecular Weight Averages of Polyolefins By High Temperature Gel Permeation Chromatography. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D4669; Standard Test Method for Polyurethane Raw Materials: Determination of Specific Gravity of Polyols. ASTM International: West Conshohocken, PA, USA, 2018.
- Glittenberg, D. Starch-Based Biopolymers in Paper, Corrugating, and Other Industrial Applications. In Polymer Science: A Comprehensive Reference: Volume 1–10; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1–10, pp. 165–193. [Google Scholar]
- Kurimoto, Y.; Takeda, M.; Doi, S.; Tamura, Y.; Ono, H. Network structures and thermal properties of polyurethane films prepared from liquefied wood. Bioresour. Technol. 2001, 77, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Al-Moameri, H.; Ghoreishi, R.; Suppes, G. Impact of Inter- and Intra-Molecular Movements on Thermoset Polymerization Reactions. Chem. Eng. Sci. 2017, 161, 14–23. [Google Scholar] [CrossRef]
- Palanisamy, A.; Rao, B.S.; Mehazabeen, S. Diethanolamides of Castor Oil as Polyols for the Development of Water-Blown Polyurethane Foam. J. Polym. Environ. 2011, 19, 698–705. [Google Scholar] [CrossRef]
- Stirna, U.; Lazdiņa, B.; Vilsone, D.; Lopez, M.J.; Del Carmen Vargas-Garcia, M.; Suárez-Estrella, F.; Moreno, J. Structure and Properties of the Polyurethane and Polyurethane Foam Synthesized from Castor Oil Polyols. J. Cell. Plast. 2012, 48, 476–488. [Google Scholar] [CrossRef]
- Ma, J.; Shi, J.; Ding, H.; Zhu, G.; Fu, K.; Fu, X. Synthesis of Cationic Polyacrylamide by Low-Pressure UV Initiation for Turbidity Water Flocculation. J. Chem. Eng. 2017, 312, 20–29. [Google Scholar] [CrossRef]
- Bresolin, D.; Valério, A.; de Oliveira, D.; Lenzi, M.K.; Sayer, C.; de Araújo, P.H.H. Polyurethane Foams Based on Biopolyols from Castor Oil and Glycerol. J. Polym. Environ. 2018, 26, 2467–2475. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone, A.; Misane, M.; Vilsone, D. Rapeseed Oil as Renewable Resource for Polyol Synthesis. Environ. Clim. Technol. 2011, 6, 85–90. [Google Scholar] [CrossRef]
- Cevher, D.; Sürdem, S. Polyurethane Adhesive Based on Polyol Monomers BHET and BHETA Depolymerised from PET Waste. Int. J. Adhes. Adhes. 2021, 105, 102799. [Google Scholar] [CrossRef]
- Hou, Z.; Xu, J.; Teng, J.; Jia, Q.; Wang, X. Facile Preparation of Medical Segmented Poly(Ester-Urethane) Containing Uniformly Sized Hard Segments and Phosphorylcholine Groups for Improved Hemocompatibility. Mater. Sci. Eng. C 2020, 109, 110571. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of Polyurethane Scraps via Acidolysis. J. Chem. Eng. 2020, 395, 125102. [Google Scholar] [CrossRef]
- Wu, G.; Bian, J.; Liu, G.; Chen, J.; Huo, S.; Jin, C.; Kong, Z. Self-Catalytic Two-Component Waterborne Polyurethanes with Amino Polyols from Biomass Based Epoxy Resin. J. Polym. Environ. 2020, 28, 713–724. [Google Scholar] [CrossRef]
- Ma, Y.; Song, F.; Kong, Q.; Li, Q.; Jia, P.; Zhou, Y. Preparation and Performance of Bio-Based Polyol Ester from One-Pot Synthesis of Castor Oil as Nontoxic Poly(Vinyl Chloride) Plasticizer. J. Polym. Environ. 2020, 28, 2101–2107. [Google Scholar] [CrossRef]
- Chalasani, S.R.K.; Dewasthale, S.; Hablot, E.; Shi, X.; Graiver, D.; Narayan, R. A Spectroscopic Method for Hydroxyl Value Determination of Polyols. J. Am. Oil Chem. Soc. 2013, 90, 1787–1793. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermal and Mechanical Properties of Poly(Vinyl Alcohol) Plasticized with Glycerol. J. Appl. Polym. Sci. 2011, 122, 3102–3109. [Google Scholar] [CrossRef]
- Slobodinyuk, D.; Slobodinyuk, A.; Strelnikov, V.; Kiselkov, D. Simple and Efficient Synthesis of Oligoetherdiamines: Hardeners of Epoxyurethane Oligomers for Obtaining Coatings with Shape Memory Effect. Polymers 2023, 15, 2450. [Google Scholar] [CrossRef]
- Aydin, Z.; Akbas, F.; Senel, M.; Koc, S.N. Evaluation of Jeffamine®-Cored PAMAM Dendrimers as an Efficient in Vitro Gene Delivery System. J. Biomed. Mater. Res. A 2012, 100, 2623–2628. [Google Scholar] [CrossRef]
- Saif, F.A.; Undre, P.B.; Yaseen, S.A.; Alameen, A.S.; Patil, S.S.; Khirade, P.W. Hydrogen Bonding Interaction between Amide and Alcohols: Dielectric Relaxation and FTIR Study. Integr. Ferroelectr. 2019, 202, 79–88. [Google Scholar] [CrossRef]
- Fawzi, T.; Yu, L.J.; Badri, K.H.; Sajuri, Z.; Al-Talib, A.A.M.; Eh Noum, S.Y. Sodium Hydrogen Bicarbonate and Water as Blowing Agent in Palm Kernel Oil Based Polyol Polyurethane Foam. Mater. Today Proc. 2019, 39, 993–998. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Kulkarni, R.D. Synthesis of Isostearic Acid/Dimer Fatty Acid-Based Polyesteramide Polyol for the Development of Green Polyurethane Coatings. J. Polym. Environ. 2020, 29, 54–70. [Google Scholar] [CrossRef]
- Patil, C.K.; Jirimali, H.D.; Paradeshi, J.S.; Chaudhari, B.L.; Alagi, P.K.; Mahulikar, P.P.; Hong, S.C.; Gite, V.V. Chemical Transformation of Renewable Algae Oil to Polyetheramide Polyols for Polyurethane Coatings. Prog. Org. Coat. 2021, 151, 106084. [Google Scholar] [CrossRef]
- Shrestha, M.L.; Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S.; Upshaw, T. Biobased Aromatic-Aliphatic Polyols from Cardanol by Thermal Thiol-Ene Reaction. J. Renew. Mater. 2018, 6, 87–101. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable Oil Based Polyurethane Coatings—A Sustainable Approach: A Review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- O’connell, A.W. Analysis of Coconut Oil-Diethanolamine Condensates by Gas Chromatography. Anal. Chem. 1977, 49, 835–838. [Google Scholar] [CrossRef]
- Mohd Noor, N.; Noor Maznee Tuan Ismail, T.; Shoot Kian, Y.; Abu Hassan, H. Synthesis of palm-based polyols: Effect of K10 montmorillonite catalys. J. Oil Palm Res. 2013, 25, 92–99. [Google Scholar]
- DOW. VORANOLTM 490 Polyol Technical Data Sheet. Available online: https://www.dow.com/en-us/document-viewer.html?randomVar=5020605142420717484&docPath=/content/dam/dcc/documents/400/400-001/400-00101259en-voranol-490-polyol-tds.pdf (accessed on 17 July 2022).
- Azizi, N.; Shirdel, F. Sustainable and Chemoselective N-Boc Protection of Amines in Biodegradable Deep Eutectic Solvent. Monatsh Chem. 2017, 148, 1069–1074. [Google Scholar] [CrossRef]
- Gulledge, Z.Z.; Carrick, J.D. Deprotection of N-Tert-Butoxycarbonyl (Boc) Protected Functionalized Heteroarenes via Addition–Elimination with 3-Methoxypropylamine. Eur. J. Org. Chem. 2020, 2020, 1817–1822. [Google Scholar] [CrossRef]
- Caraculacu, A.A.; Coseri, S. Isocyanates in Polyaddition Processes. Structure and Reaction Mechanisms. Prog. Polym. Sci. 2001, 26, 799–851. [Google Scholar] [CrossRef]
- Sami, S.; Yildirim, E.; Yurtsever, M.; Yurtsever, E.; Yilgor, E.; Yilgor, I.; Wilkes, G.L. Understanding the Influence of Hydrogen Bonding and Diisocyanate Symmetry on the Morphology and Properties of Segmented Polyurethanes and Polyureas: Computational and Experimental Study. Polymer 2014, 55, 4563–4576. [Google Scholar] [CrossRef]
- Yilgör, E.; Burgaz, E.; Yurtsever, E.; Yilgör, I. ˙ Comparison of Hydrogen Bonding in Polydimethylsiloxane and Polyether Based Urethane and Urea Copolymers. Polymer 2000, 41, 849–857. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Ltd.: Shropshire, UK, 2005; pp. 13–29. [Google Scholar]
- Shaabani, A.; Sedghi, R.; Motasadizadeh, H.; Dinarvand, R. Self-Healable Conductive Polyurethane with the Body Temperature-responsive Shape Memory for Bone Tissue Engineering. J. Chem. Eng. 2021, 411, 128499. [Google Scholar] [CrossRef]
- Liang, H.; Lu, Q.; Liu, M.; Ou, R.; Wang, Q.; Quirino, R.L.; Luo, Y.; Zhang, C. UV Absorption, Anticorrosion, and Long-Term Antibacterial Performance of Vegetable Oil Based Cationic Waterborne Polyurethanes Enabled by Amino Acids. J. Chem. Eng. 2021, 421, 127774. [Google Scholar] [CrossRef]
- Stanzione, M.; Oliviero, M.; Cocca, M.; Errico, M.E.; Gentile, G.; Avella, M.; Lavorgna, M.; Buonocore, G.G.; Verdolotti, L. Tuning of Polyurethane Foam Mechanical and Thermal Properties Using Ball-Milled Cellulose. Carbohydr. Polym. 2020, 231, 115772. [Google Scholar] [CrossRef]
- Wehlack, C.; Possart, W.; Krger, J.K.; Mller, U. Epoxy and Polyurethane Networks in Thin Films on Metals—Formation, Structure, Properties. Soft Mater. 2007, 5, 87–134. [Google Scholar] [CrossRef]
- Ionescu, M. 2. Basic Chemistry of Polyurethanes. In Polyols for Polyurethanes, 3rd ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; Volume 1, pp. 11–26. [Google Scholar]
- Madbouly, S.A.; Otaigbe, J.U. Kinetic Analysis of Fractal Gel Formation in Waterborne Polyurethane Dispersions Undergoing High Deformation Flows. Macromolecules 2006, 39, 4144–4151. [Google Scholar] [CrossRef]
- Jaruchattada, J.; Fuongfuchat, A.; Pattamaprom, C. Rheological Investigation of Cure Kinetics and Adhesive Strength of Polyurethane Acrylate Adhesive. J. Appl. Polym. Sci. 2012, 123, 2344–2350. [Google Scholar] [CrossRef]
- Sonnenschein, M.; Wendt, B.L.; Schrock, A.K.; Sonney, J.M.; Ryan, A.J. The Relationship between Polyurethane Foam Microstructure and Foam Aging. Polymer 2008, 49, 934–942. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone-Girone, A.; Yakushin, V.; Vilsone, D. Processing and Properties of Spray-Applied, 100% Solids Polyurethane Coatings from Rapeseed Oil Polyols. J. Coat. Technol. Res. 2014, 11, 409–420. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of Foam Density on the Properties of Water Blown Rigid Polyurethane Foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 107–117. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).