Sustainable Treatment of Swine Wastewater: Optimizing the Culture Conditions of Tetradesmus cf. obliquus to Improve Treatment Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Isolation, Screening, and Identification
2.2. Optimization of Heterotrophic Culture Conditions for Superior Microalgae Strains
2.3. Effect of Superior Microalgae Strains on Swine Wastewater Treatment
2.4. Flocculation Effect and Mechanism of Microalgae
2.5. Analysis and Testing Methods
2.5.1. Measurement of Microalgal Growth
2.5.2. Determination of Chlorophyll Content of microalgae
2.5.3. Identification of Microalgae
2.5.4. Measurement of Water Quality of Swine Wastewater
2.5.5. Flocculation Efficiency of Microalgae and Extraction and Analysis of EPS
2.6. Data Analyses
3. Results and Discussion
3.1. Microalgae Isolation, Screening, and Identification
3.2. Optimization of the Heterotrophic Culture Model of T. cf. obliquus ZYY1
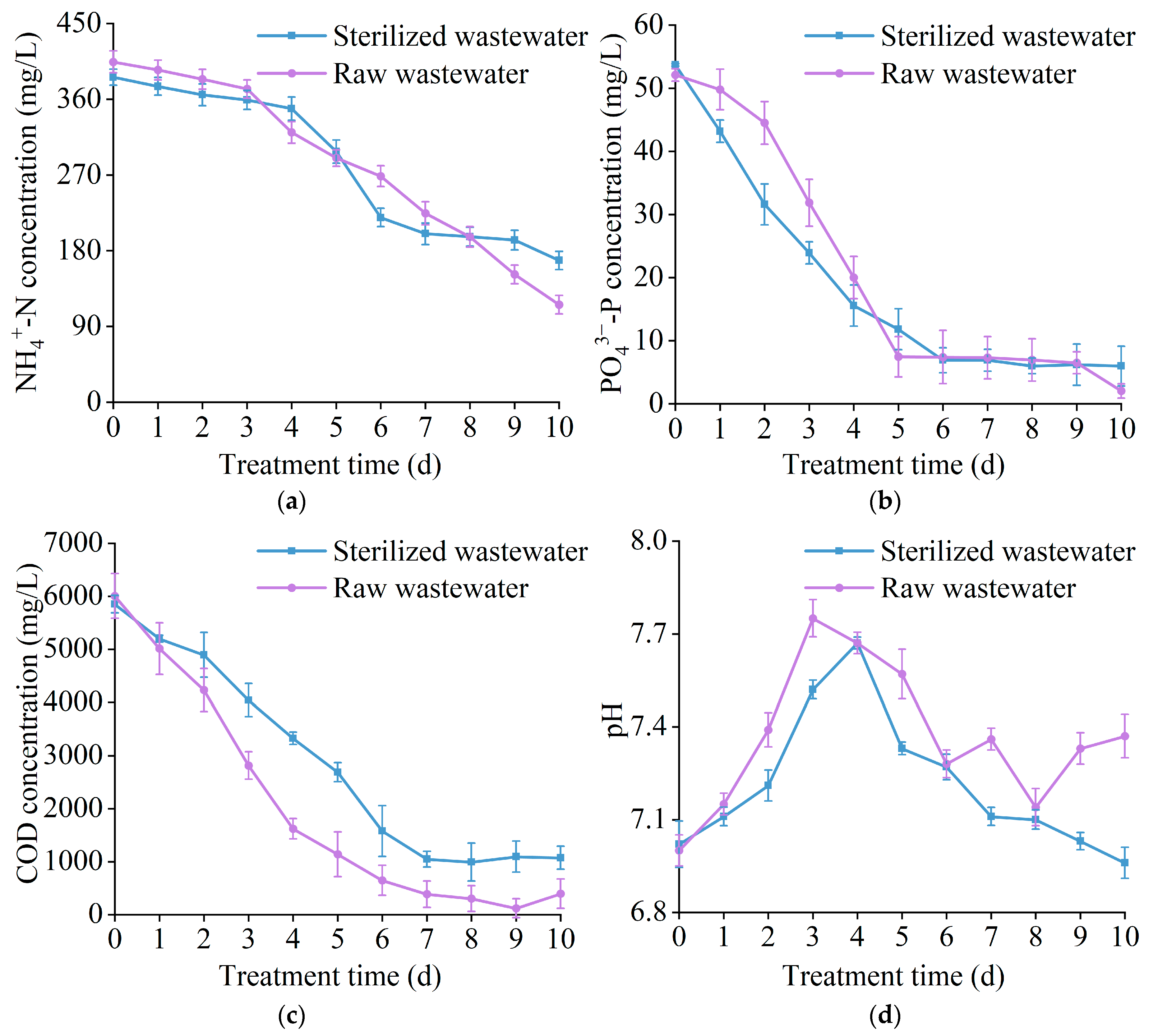
3.3. The Effect of T. cf. obliquus ZYY1 on Treating Swine Wastewater
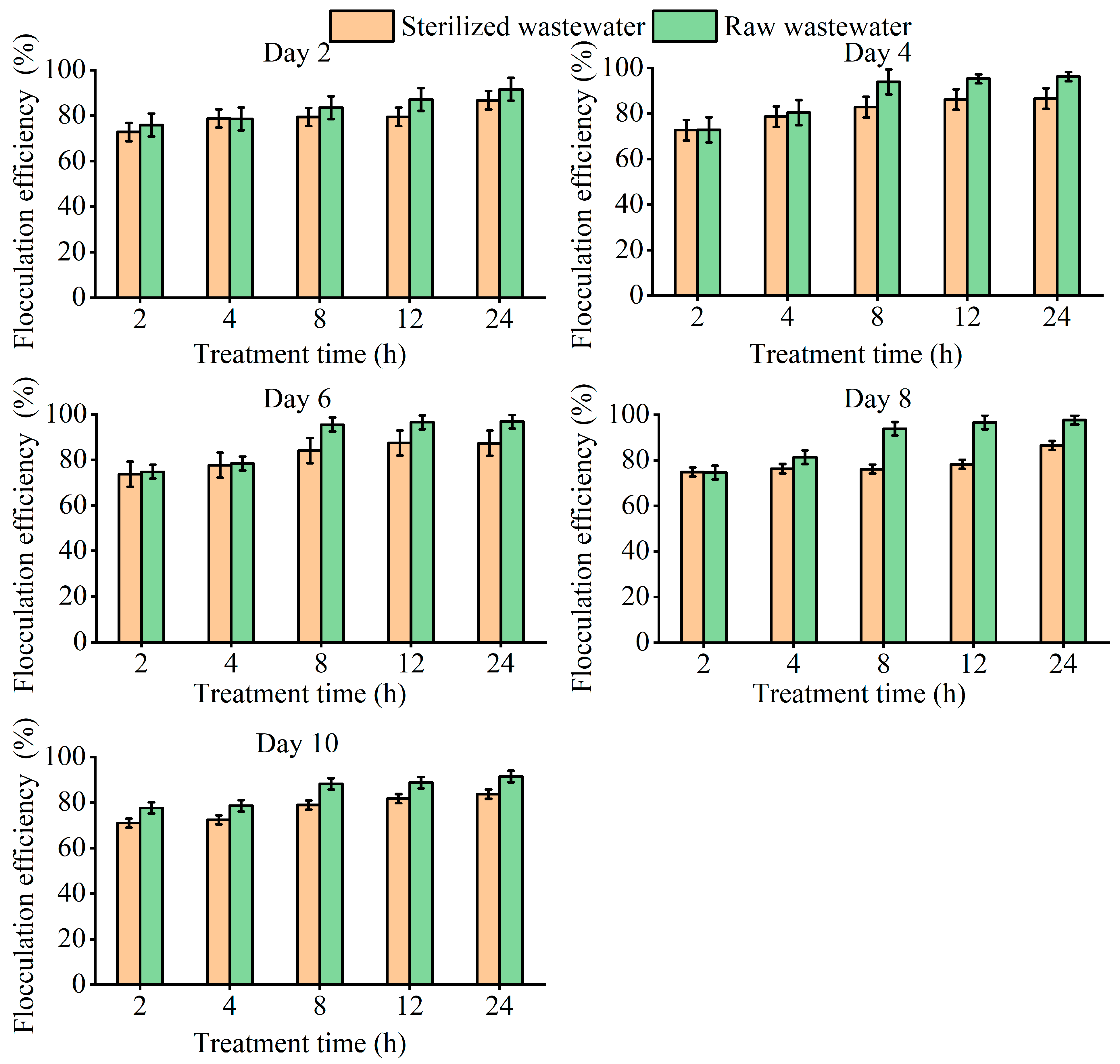
3.4. Flocculation Effect and Mechanism of T. cf. obliquus ZYY1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Liu, Q.; Fang, F.; Luo, R.H.; Lu, Q.; Zhou, W.G.; Huo, S.H.; Cheng, P.F.; Liu, J.Z.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Huang, K.X.; Vadiveloo, A.; Zhou, J.L.; Yang, L.; Chen, D.Z.; Gao, F. Integrated culture and harvest systems for improved microalgal biomass production and wastewater treatment. Bioresour. Technol. 2023, 376, 128941. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Tao, F.; Han, T.; Gao, F.Z.; Dong, T.L.; Jiang, W.Z.; Lu, H.F.; Zhang, Y.H.; Li, B.M. Photoacclimation caused by high frequency flashing light assists Chlorella sp. M-12 wastewater treatment and biomass accumulation in dark color biogas slurry. J. Appl. Phycol. 2022, 34, 2929–2940. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrére, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Chen, Z.P.; Xie, Y.; Qiu, S.; Li, M.T.; Yuan, W.Q.; Ge, S.J. Granular indigenous microalgal-bacterial consortium for wastewater treatment: Establishment strategy, functional microorganism, nutrient removal, and influencing factor. Bioresour. Technol. 2022, 353, 127130. [Google Scholar] [CrossRef]
- Tan, X.-B.; Zhao, Z.-Y.; Gong, H.; Jiang, T.; Liu, X.-P.; Liao, J.-Y.; Zhang, Y.-L. Growth of Scenedesmus obliquus in anaerobically digested swine wastewater from different cleaning processes for pollutants removal and biomass production. Chemosphere 2024, 352, 141515. [Google Scholar] [CrossRef]
- Acebu, P.I.G.; de Luna, M.D.G.; Chen, C.Y.; Abarca, R.R.M.; Chen, J.H.; Chang, J.S. Bioethanol production from Chlorella vulgaris ESP-31 grown in unsterilized swine wastewater. Bioresour. Technol. 2022, 352, 127086. [Google Scholar] [CrossRef] [PubMed]
- Alalawy, A.I.; Yang, Q.; Zhang, M.; Almutairi, F.M.; Al-Jahani, G.M.; Almasoudi, F.M.; Alotaibi, M.A.; Salama, E. Removal of nutrients from salt-rich wastewater via freshwater microalga Tetradesmus obliquus. Biomass Convers. Biorefin. 2023, 12, 1152. [Google Scholar] [CrossRef]
- Vladic, J.; Jazic, J.M.; Ferreira, A.; Maletic, S.; Cvetkovic, D.; Agbaba, J.; Vidovic, S.; Gouveia, L. Application of green technology to extract clean and safe bioactive compounds from Tetradesmus obliquus biomass grown in poultry wastewater. Molecules 2023, 28, 2397. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Figueiredo, D.; Cardeiras, R.; Nabais, R.; Ferreira, F.; Ribeiro, B.; Cordovil, C.; Acién, F.G.; Gouveia, L. Exploring different pretreatment methodologies for allowing microalgae growth in undiluted piggery wastewater. Agronomy 2022, 12, 580. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Guidhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic cultivation of microalgae using aquaculture wastewater: A biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Huntley, M.E.; Johnson, Z.I.; Brown, S.L.; Sills, D.L.; Gerber, L.; Archibald, I.; Machesky, S.C.; Granados, J.; Beal, C.; Greene, C.H. Demonstrated large-scale production of marine microalgae for fuels and feed. Algal Res. 2015, 10, 249–265. [Google Scholar] [CrossRef]
- Shitanaka, T.; Fujioka, H.; Khan, M.; Kaur, M.; Du, Z.Y.; Khanal, S.K. Recent advances in microalgal production, harvesting, prediction, optimization, and control strategies. Bioresour. Technol. 2024, 391, 129924. [Google Scholar] [CrossRef] [PubMed]
- Sero, E.T.; Siziba, N.; Bunhu, T.; Shoko, R. Isolation and screening of microalgal species, native to Zimbabwe, with potential use in biodiesel production. All Life 2021, 14, 256–264. [Google Scholar] [CrossRef]
- Xie, Y.P.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Jing, K.J.; Ng, I.S.; Chen, J.F.; Chang, J.S.; Lu, Y.H. Disruption of thermo-tolerant Desmodesmus sp F51 in high pressure homogenization as a prelude to carotenoids extraction. Biochem. Eng. J. 2016, 109, 243–251. [Google Scholar] [CrossRef]
- Yu, N.; Wei, Y.L.; Zhang, X.; Zhu, N.; Wang, Y.L.; Zhu, Y.; Zhang, H.P.; Li, F.M.; Yang, L.; Sun, J.Q.; et al. Barcode ITS2: A useful tool for identifying Trachelospermum jasminoides and a good monitor for medicine market. Sci. Rep. 2017, 7, 5037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, W.Q.; Yen, H.W.; Ho, S.H.; Lo, Y.C.; Cheng, C.L.; Ren, N.Q.; Chang, J.S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef]
- Cheng, P.F.; Wang, Y.Z.; Liu, T.H.; Liu, D.F. Biofilm attached cultivation of Chlorella pyrenoidosa is a developed system for swine wastewater treatment and lipid production. Front. Plant Sci. 2017, 8, 1594. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, C.B.; Zhu, Y.A.; Liu, H.; Chen, W.; Zhang, Q. Bioaugmentation with Acinetobacter sp. TAC-1 to enhance nitrogen removal in swine wastewater by moving bed biofilm reactor inoculated with bacteria. Bioresour. Technol. 2022, 359, 127506. [Google Scholar] [CrossRef]
- Yang, L.M.; Li, H.K.; Wang, Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cesário, C.D.; Soares, J.; Cossolin, J.F.S.; Almeida, A.V.M.; Sierra, J.J.B.; Leite, M.D.; Nunes, M.C.; Serrao, J.E.; Martins, M.A.; Coimbra, J.S.D. Biochemical and morphological characterization of freshwater microalga Tetradesmus obliquus (Chlorophyta: Chlorophyceae). Protoplasma 2022, 259, 937–948. [Google Scholar] [CrossRef]
- Valchev, D.; Ribarova, I.; Uzunov, B.; Stoyneva-Gaertner, M. Reclamation potential of onsite wastewater post-treatment with microalgae: Chemical elements perspective. Processes 2023, 11, 1819. [Google Scholar] [CrossRef]
- San Agustin, D.M.; Ledesma, M.T.O.; Ramírez, I.M.; Noguez, I.Y.; Pabello, V.M.L.; Velasquez-Orta, S.B. A non-sterile heterotrophic microalgal process for dual biomass production and carbon removal from swine wastewater. Renew. Energy 2022, 181, 592–603. [Google Scholar] [CrossRef]
- Hoober, J.K.; Maloney, M.A.; Asbury, L.R.; Marks, D.B. Accumulation of chlorophyll a/b-binding polypeptides in Chlamydomonas reinhardtii y-1 in the light or dark at 38 °C. Plant Physiol. 1990, 92, 419–426. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.F.; Xiang, J.Y.; Wu, Q.Y. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Johns, M.R. Relationship between substrate inhibition and maintenance energy of Chlamydomonas reinhardtii in heterotrophic culture. J. Appl. Phycol. 1996, 8, 15–19. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Hong, Y.; Liu, X.; Wang, Q.; Zhai, Q.; Zhang, H. Attached cultivation of microalgae on rational carriers for swine wastewater treatment and biomass harvesting. Bioresour. Technol. 2022, 351, 127014. [Google Scholar] [CrossRef]
- You, K.; Ge, F.R.; Wu, X.D.; Song, K.Y.; Yang, Z.X.; Zhang, Q.; Liu, Y.H.; Ruan, R.G.; Zheng, H.L. Nutrients recovery from piggery wastewater and starch wastewater via microalgae-bacteria consortia. Algal Res. 2021, 60, 102551. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Rostro-Alanís, M.; de la Cruz, R.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Grunewald, C.F.; Llewellyn, C.A.; Olguín, E.J.; et al. Light intensity and nitrogen concentration impact on the biomass and phycoerythrin production by Porphyridium purpureum. Mar. Drugs 2019, 17, 460. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Kumar, S.M. Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour. Technol. 2019, 275, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.Y.; Zhang, C.F.; Chen, X.; Ho, S.H. New concept in swine wastewater treatment: Development of a self-sustaining synergetic microalgae-bacteria symbiosis (ABS) system to achieve environmental sustainability. J. Hazard. Mater. 2021, 418, 126264. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.P.; Duan, P.G.; Wang, F.; Guan, Q.Q. Liquid fuel generation from algal biomass via a two-step process: Effect of feedstocks. Biotechnol. Biofuels 2018, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, R.Q.; Cui, X.Y.; He, M.L.; Zheng, S.Y.; Du, W.J.; Gao, M.; Wang, C. Co-culture of bacteria and microalgae for treatment of high concentration biogas slurry. J. Water Process Eng. 2021, 41, 102014. [Google Scholar] [CrossRef]
- Xie, D.; Ji, X.W.; Zhou, Y.C.; Dai, J.X.; He, Y.J.; Sun, H.; Guo, Z.; Yang, Y.; Zheng, X.; Chen, B.L. Chlorella vulgaris cultivation in pilot-scale to treat real swine wastewater and mitigate carbon dioxide for sustainable biodiesel production by direct enzymatic transesterification. Bioresour. Technol. 2022, 349, 126886. [Google Scholar] [CrossRef] [PubMed]
- GB 18596-2001; Discharge Standard of Pollutants for Livestock and Poultry Breeding. Standardization Administration of the People’s Republic of China (SAC): Beijing, China, 2001.
- Su, M.; Dell’Orto, M.; Scaglia, B.; D’Imporzano, G.; Bani, A.; Adani, F. Growth performance, biochemical composition and nutrient recovery ability of twelve microalgae consortia isolated from various local organic wastes grown on nano-filtered pig slurry. Molecules 2022, 27, 422. [Google Scholar] [CrossRef]
- Zhu, S.N.; Feng, S.R.; Xu, Z.B.; Qin, L.; Shang, C.H.; Feng, P.Z.; Wang, Z.M.; Yuan, Z.H. Cultivation of Chlorella vulgaris on unsterilized dairy-derived liquid digestate for simultaneous biofuels feedstock production and pollutant removal. Bioresour. Technol. 2019, 285, 121353. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Ding, W.Q.; Han, W.; Chen, Y.D.; Jin, W.B.; Zhou, X. A bacterial strain Citrobacter W4 facilitates the bio-flocculation of wastewater cultured microalgae Chlorella pyrenoidosa. Sci. Total Environ. 2022, 806, 151336. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simoes, M.; Pires, J.C.M. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Liu, Y.B.; Zhang, G.Y.; Li, W.R.; Ding, Y.; You, H.; Zhu, J.; Leng, H.R.; Xu, C.; Xing, X.D.; Xu, J.Y.; et al. The characteristic evolution and formation mechanism of hybrid microalgae biofilm and its application in mariculture wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109645. [Google Scholar] [CrossRef]
- Meng, F.G.; Zhou, Z.B.; Ni, B.J.; Zheng, X.; Huang, G.C.; Jia, X.S.; Li, S.Y.; Xiong, Y.; Kraume, M. Characterization of the size-fractionated biomacromolecules: Tracking their role and fate in a membrane bioreactor. Water Res. 2011, 45, 4661–4671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Zhang, C.F.; Liang, F.; Liang, X.; Cui, X.C.; Li, Q.C.; Crittenden, J.C. Responses of the microalga Chlorophyta sp. to bacterial quorum sensing molecules (N-acylhomoserine lactones): Aromatic protein induced self-aggregation. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef]
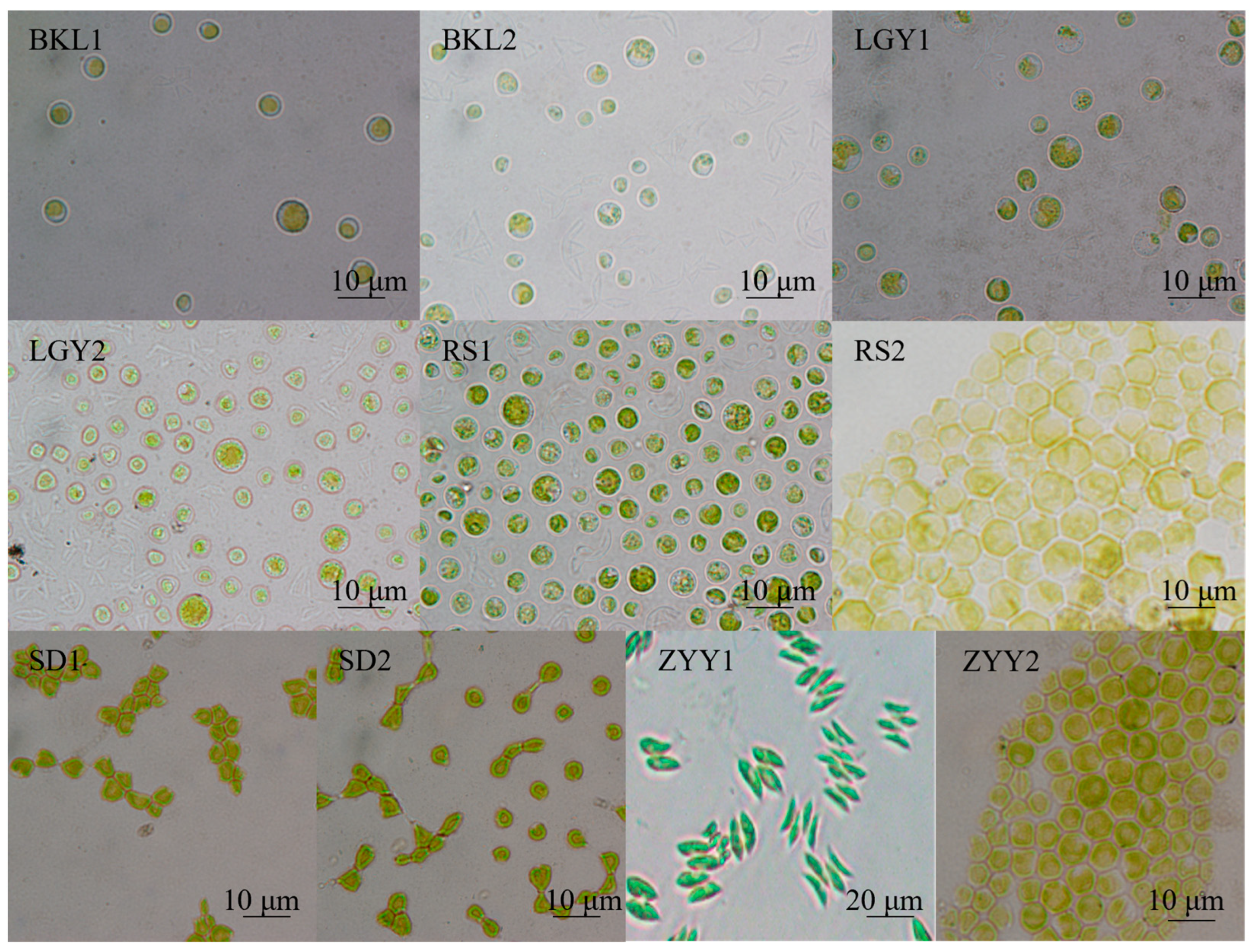
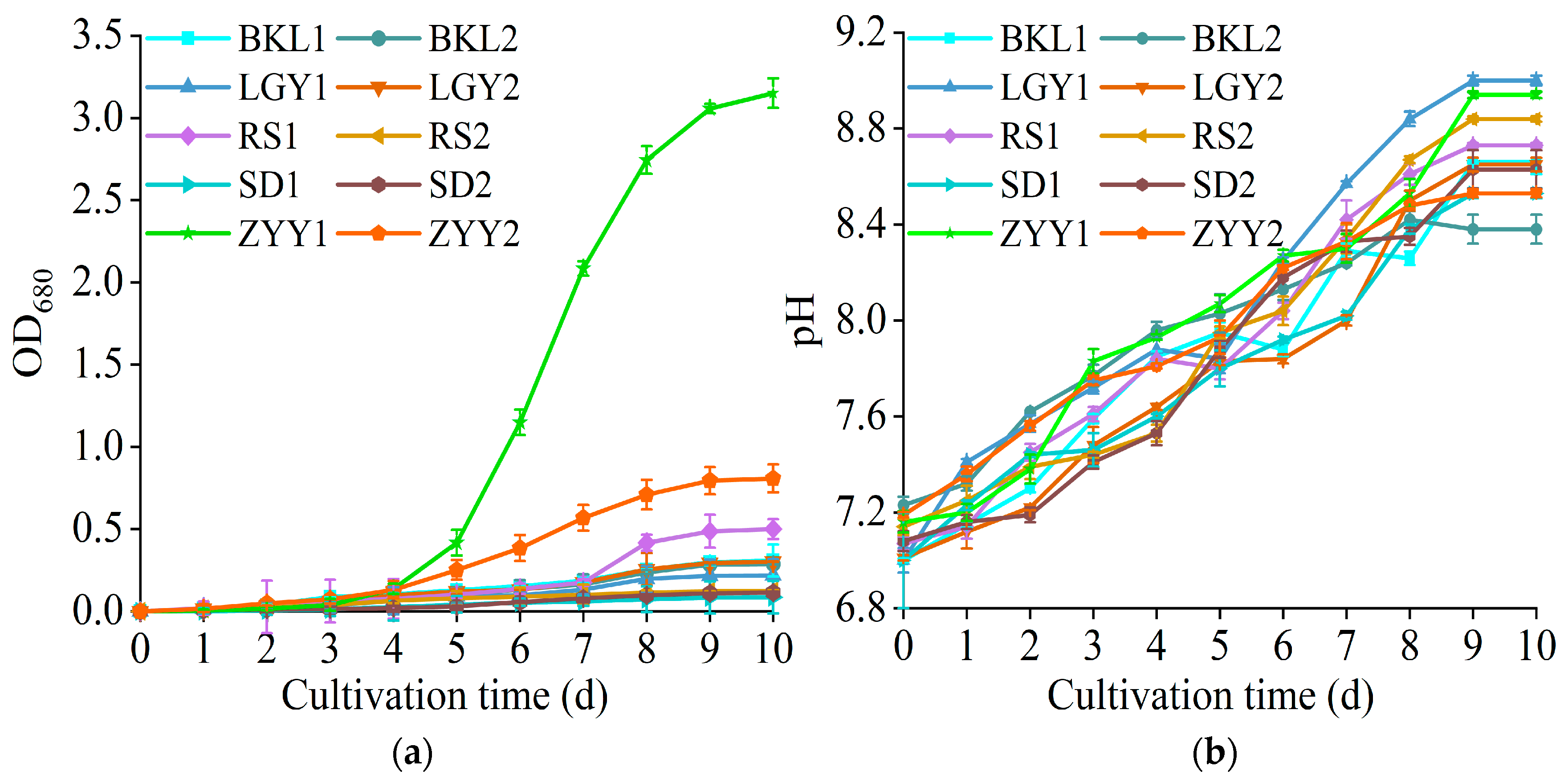

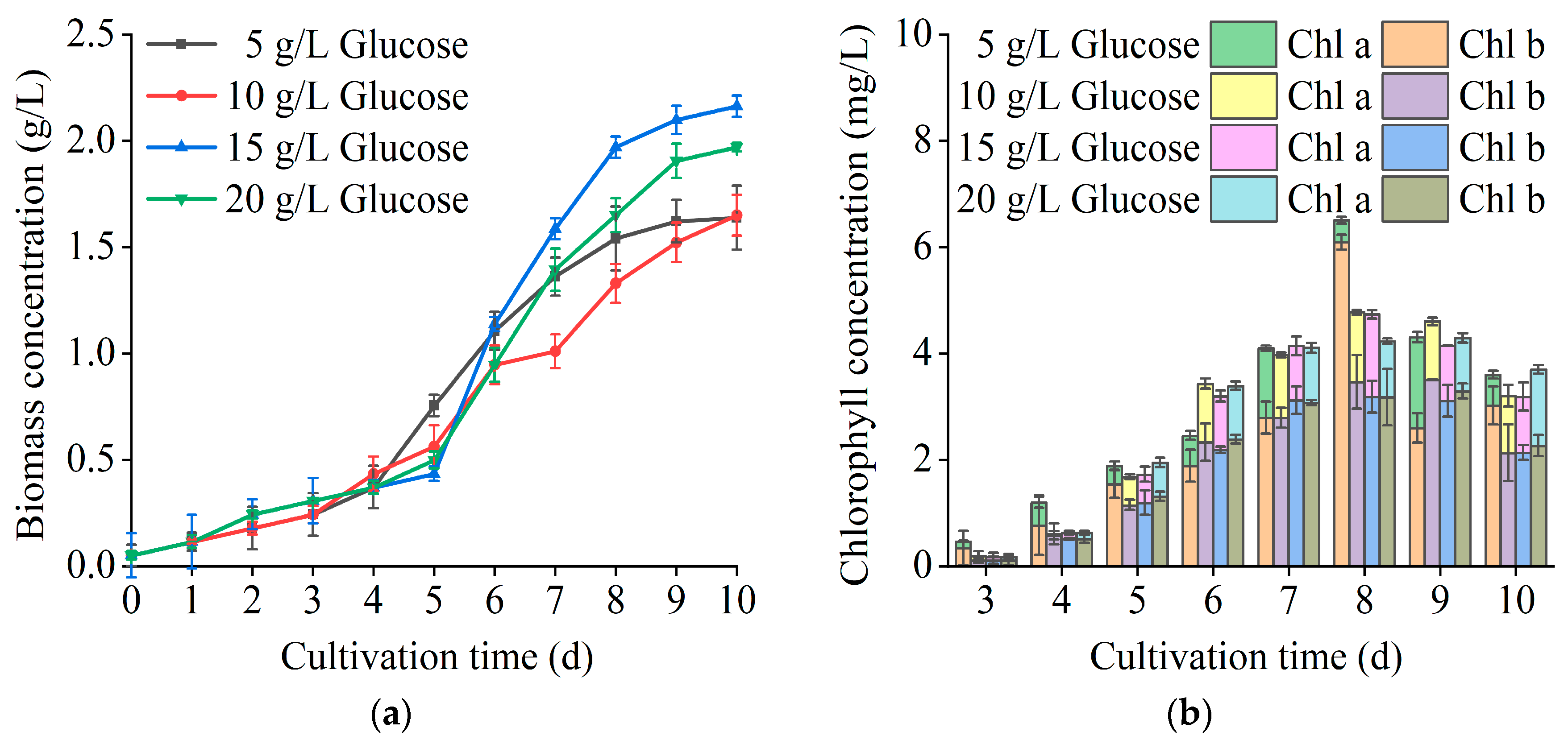
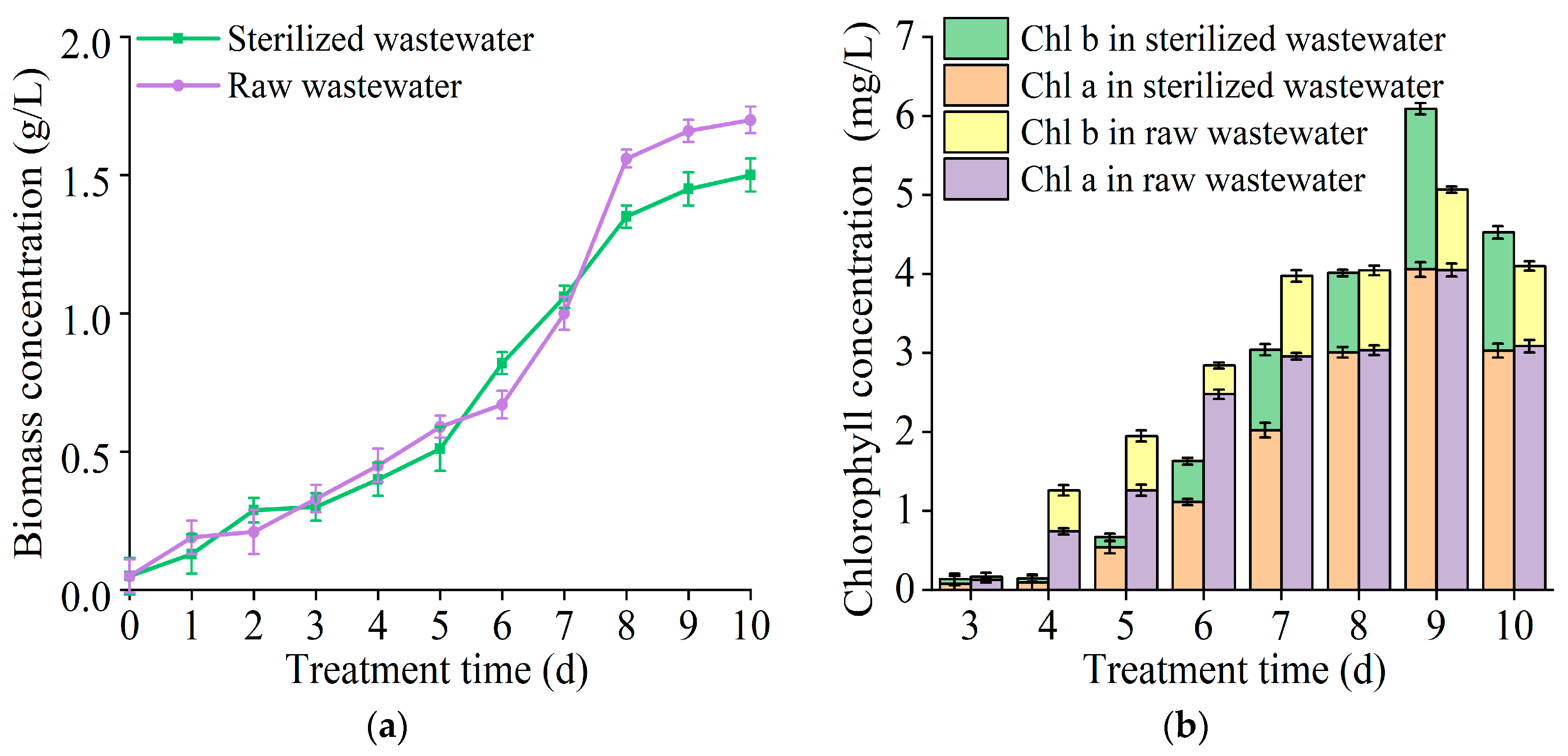


| Cultivation Mode | Biomass Concentration (g/L) | Biomass Productivity (mg/L/d) | Maximum Specific Growth Rate (d−1) |
|---|---|---|---|
| photoheterotrophy | 1.58 ± 0.04 | 153 | 0.67 |
| chemoheterotrophy | 1.94 ± 0.01 | 189 | 1.26 |
| Glucose Concentration (g/L) | Biomass Concentration (g/L) | Biomass Productivity (mg/L/d) | Maximum Specific Growth Rate (d−1) |
|---|---|---|---|
| 5 | 1.64 ± 0.01 | 164 | 0.82 |
| 10 | 1.65 ± 0.01 | 178 | 0.82 |
| 15 | 2.16 ± 0.01 | 216 | 0.96 |
| 20 | 1.97 ± 0.02 | 197 | 0.82 |
| Wastewater Type | pH | -N (mg/L) | -P (mg/L) | COD (mg/L) |
|---|---|---|---|---|
| Raw wastewater | 7.00 ± 0.01 | 404.40 ± 13.04 | 52.10 ± 1.00 | 6005.84 ± 422.24 |
| Sterilized wastewater | 7.02 ± 0.08 | 386.20 ± 9.63 | 53.70 ± 0.20 | 5849.65 ± 162.40 |
| Parameter | Raw Wastewater | Sterilized Wastewater | |
|---|---|---|---|
| effluent concentration (mg/L) | -N | 115.84 ± 11.04 | 168.48 ± 10.84 |
| -P | 2.03 ± 0.13 | 5.97 ± 0.13 | |
| COD | 392.32 ± 276.08 | 1070.96 ± 16.24 | |
| removal efficiency (%) | -N | 71.36 ± 3.57 | 56.37 ± 2.82 |
| -P | 96.09 ± 4.81 | 88.88 ± 4.44 | |
| COD | 93.13 ± 4.67 | 81.69 ± 4.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, K.; Qu, W.; Song, D.; Li, J.; Ho, S.-H. Sustainable Treatment of Swine Wastewater: Optimizing the Culture Conditions of Tetradesmus cf. obliquus to Improve Treatment Efficiency. Sustainability 2024, 16, 4633. https://doi.org/10.3390/su16114633
Bai K, Qu W, Song D, Li J, Ho S-H. Sustainable Treatment of Swine Wastewater: Optimizing the Culture Conditions of Tetradesmus cf. obliquus to Improve Treatment Efficiency. Sustainability. 2024; 16(11):4633. https://doi.org/10.3390/su16114633
Chicago/Turabian StyleBai, Kailong, Wenying Qu, Duo Song, Junfeng Li, and Shih-Hsin Ho. 2024. "Sustainable Treatment of Swine Wastewater: Optimizing the Culture Conditions of Tetradesmus cf. obliquus to Improve Treatment Efficiency" Sustainability 16, no. 11: 4633. https://doi.org/10.3390/su16114633
APA StyleBai, K., Qu, W., Song, D., Li, J., & Ho, S.-H. (2024). Sustainable Treatment of Swine Wastewater: Optimizing the Culture Conditions of Tetradesmus cf. obliquus to Improve Treatment Efficiency. Sustainability, 16(11), 4633. https://doi.org/10.3390/su16114633






