Short Cold Storage as a Sustainable Postharvest Handling Method for Natural Enrichment in Antioxidants of Fresh and Dried Walnut Kernels—Cultivar Effect
Abstract
1. Introduction
2. Materials and Methods
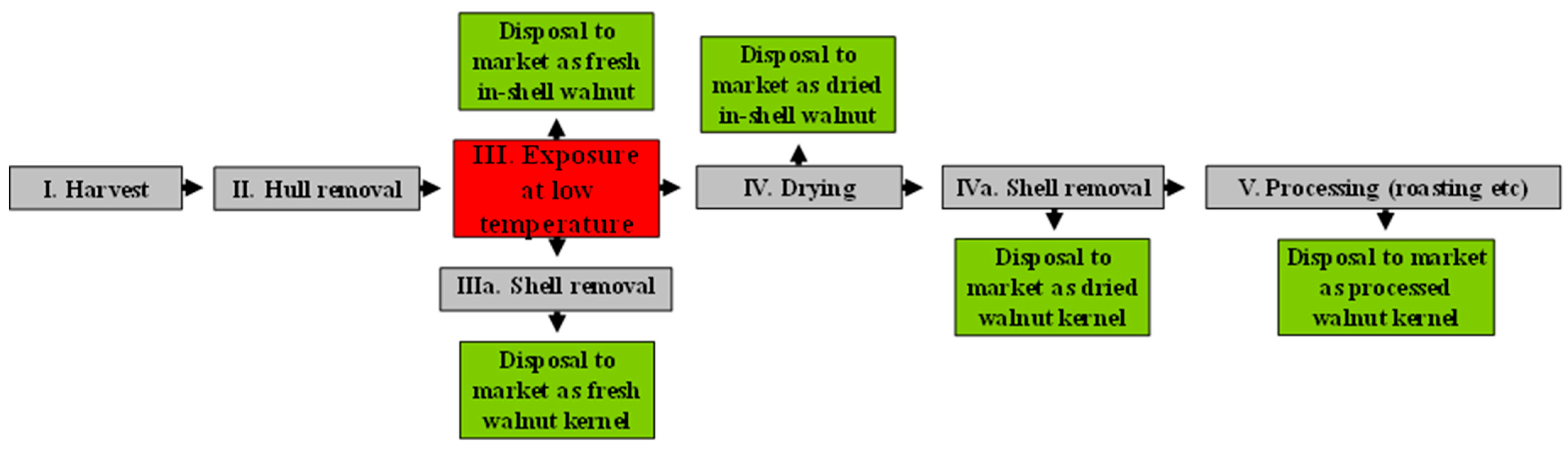
2.1. Plant Material and Experimental Design
2.2. Weight Loss, Respiration and Ethylene Production Rates (Experiment 1)
2.3. Moisture, Color and Shell Rupture Strength (Experiment 1)
2.4. Total Phenolics (TP) and Total Antioxidant Capacity (TAC) (Experiments 1 and 2)
2.5. Data Analyses
3. Results
3.1. Pomological Traits, Weight Loss, Moisture Content, Respiration and Ethylene Production Rates in Fresh Walnuts (Experiment 1)
3.2. Kernel and Shell Color, and Shell Rupture Strength of Fresh Wanuts (Experiment 1)
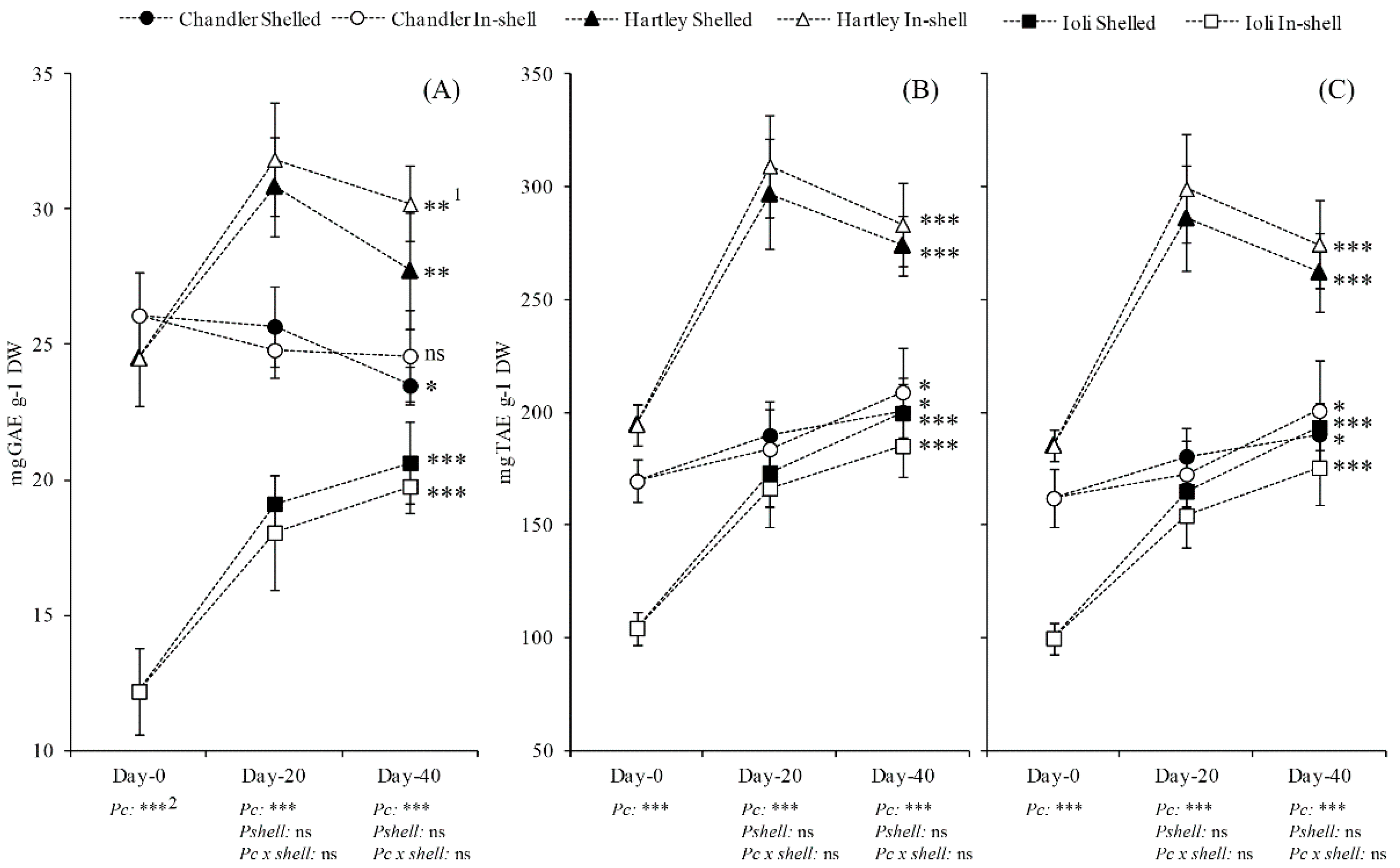
3.3. Total Phenolics (TP) and Total Antioxidant Capacity (TAC) of Fresh Walnuts (Experiment 1)
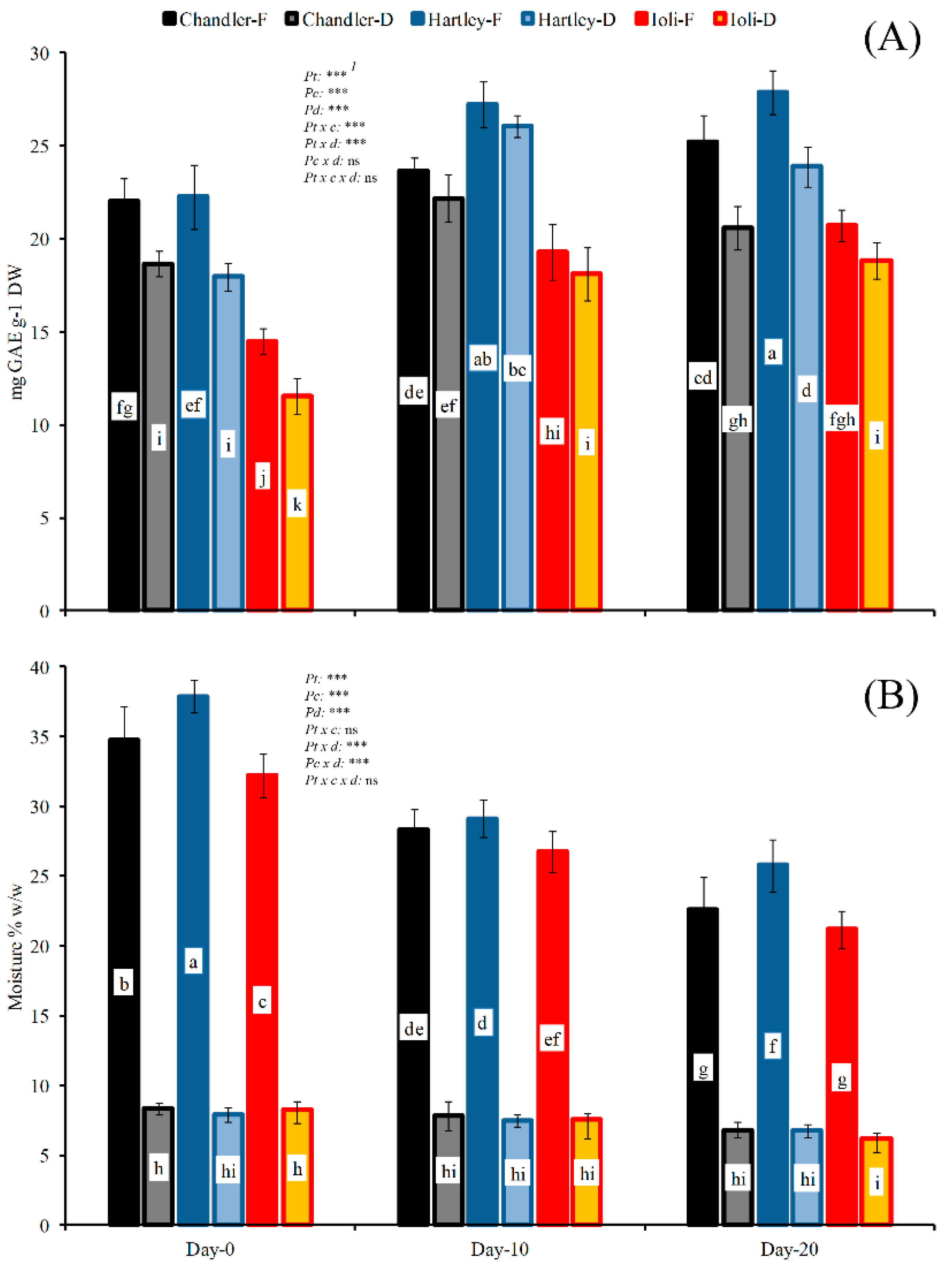
3.4. Drying after Cold Exposure of Fresh Walnuts (Experiment 2)
4. Discussion
4.1. Pomological Traits at Harvest, Weight Loss, Moisture Content, Respiration and Ethylene Production Rates of Fresh Walnuts during Storage
4.2. Kernel and Shell Color, and Shell Rupture Strength of Fresh Wanuts during Storage
4.3. The Total Phenolics (TP) and Total Antioxidant Capacity (TAC) of Fresh Walnuts during Storage
4.4. Drying after Cold Exposure of Fresh Walnuts (Experiment 2)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Zhang, Y.-G.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2022, 62, 5113–5129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ye, N.; Li, M.; Dai, G.; Ma, Y.; Wang, Y.; Liu, C.; Ma, H. Walnut green husk extract enhances the effect of chlorine dioxide on kernel quality and antioxidant properties of fresh-eating walnuts during their shelf life. Food Chem. 2023, 428, 136797. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Tsantili, E. Storage of fresh walnuts (Juglans regia L.)—Low temperature and phenolic compounds. Postharvest Biol. Technol. 2012, 73, 80–88. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Vicente, A.R.; Fields, R.P.; Grilo, F.; Labavitch, J.M.; Donis-Gonzalez, I.; Crisosto, C.H. Walnut (Juglans regia L.) kernel postharvest deterioration as affected by pellicle integrity, cultivar and oxygen concentration. Postharvest Biol. Technol. 2019, 156, 110948. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Liu, X.; Ma, H. Effect of 60Coγ-irradiation doses on nutrients and sensory quality of fresh walnuts during storage. Postharvest Biol. Technol. 2013, 84, 36–42. [Google Scholar] [CrossRef]
- Adiletta, G.; Magri, A.; Albanese, D.; Liguori, L.; Sodo, M.; Di Matteo, M.; Petriccione, M. Overall quality and oxidative damage in packaged freshly shelled walnut kernels during cold storage. J. Food Meas. Charact. 2020, 14, 3483–3492. [Google Scholar] [CrossRef]
- Amini, M.; Ghoranneviss, M. Effects of cold plasma treatment on antioxidants activity, phenolic contents and shelf life of fresh and dried walnut (Juglans regia L.) cultivars during storage. LWT 2016, 73, 178–184. [Google Scholar] [CrossRef]
- Gull, A.; Masoodi, F.A.; Masoodi, L.; Gani, A.; Muzaffar, S. Effect of sodium alginate coatings enriched with α-tocopherol on quality of fresh walnut kernels. Food Chem. Adv. 2023, 2, 100169. [Google Scholar] [CrossRef]
- Habibi, A.; Yazdani, N.; Koushesh Saba, M.; Chatrabnous, N.; Molassiotis, A.; Sarikhani, S.; Vahdati, K. Natural preservation and improving lipid oxidation inhibition of fresh walnut. Hortic. Environ. Biotechnol. 2023, 64, 133–142. [Google Scholar] [CrossRef]
- Habibie, A.; Yazdani, N.; Saba, M.K.; Vahdati, K. Ascorbic acid incorporated with walnut green husk extract for preserving the postharvest quality of cold storage fresh walnut kernels. Sci. Hortic. 2019, 245, 193–199. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Tsantili, E.; Christopoulos, M.V.; Pontikis, C.A.; Kaltsikes, P.; Kallianou, C.; Komaitis, M. Texture and other quality attributes in olives and leaf characteristics after preharvest calcium chloride sprays. HortScience 2008, 43, 1852–1856. [Google Scholar] [CrossRef]
- UNECE. Standard DDP-01 Concerning the Marketing and Commercial Quality Control of Inshell Walnuts; United Nations Economic Commission: New York, NY, USA; Geneva, Switzerland, 2014; pp. 1–7. [Google Scholar]
- Bayazit, S.; Sümbül, A. Determination of fruit quality and fatty acid composition of Turkish walnut (Juglans regia) cultivars and genotypes grown in subtropical climate of eastern mediterranean region. Int. J. Agric. Biol. 2012, 14, 419–424. [Google Scholar]
- Mihai, B.; Alabedallat, Y.F.; Bucura, F.; Geana, E.I.; Vladu, M. The Productive Capacity and Quality of Several Walnut Cultivars (Juglans regia L.) Grown in North Oltenia, Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 574–579. [Google Scholar] [CrossRef]
- Bujdoso, G.; Vegvari, G.; Hajnal, V.; Ficzek, G.; Magdolna, T. Phenolic profile of the kernel of selected Persian walnut (Juglans regia L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 24–29. [Google Scholar] [CrossRef]
- Ercisli, S.; Sayinci, B.; Kara, M.; Yildiz, C.; Ozturk, I.J.S.h. Determination of size and shape features of walnut (Juglans regia L.) cultivars using image processing. Sci. Hortic. 2012, 133, 47–55. [Google Scholar] [CrossRef]
- Boaghi, E. Walnuts respiration (Juglans regia L) during storage. Ukr. Food J. 2017, 6, 20–27. [Google Scholar] [CrossRef]
- Burton, W.G. Post-Harvest Physiology of Food Crops; Longman Group Ltd.: London, UK, 1982. [Google Scholar]
- Wang, S.; Monzon, M.; Johnson, J.A.; Mitcham, E.J.; Tang, J. Industrial-scale radio frequency treatments for insect control in walnuts: I: Heating uniformity and energy efficiency. Postharvest Biol. Technol. 2007, 45, 240–246. [Google Scholar] [CrossRef]
- Warmund, M.R.; Elmore, J.; Drake, M.; Yates, M.D. Descriptive analysis of kernels of selected black and Persian walnut cultivars. J. Sci. Food Agric. 2009, 89, 117–121. [Google Scholar] [CrossRef]
- Wei, F.; Chen, Q.; Du, Y.; Han, C.; Fu, M.; Jiang, H.; Chen, X. Effects of hulling methods on the odor, taste, nutritional compounds, and antioxidant activity of walnut fruit. LWT 2020, 120, 108938. [Google Scholar] [CrossRef]
- Farooq, A.; Hussain, S.Z.; Bhat, T.A.; Naseer, B.; Shafi, F. Walnut fruit: Impact of ethylene assisted hulling on in vitro antioxidant activity, polyphenols, PUFAs, amino acids and sensory attributes. Food Chem. 2023, 404, 134763. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Watkins, C.B.; Ye, N.; Jing, N.; Ma, H.; Zhang, T. Chlorine dioxide and sodium diacetate treatments in controlled atmospheres retard mold incidence and maintain quality of fresh walnuts during cold storage. Postharvest Biol. Technol. 2020, 161, 111063. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Ekinci, K.; Savran, E. Cracking Characteristics of Walnut. Biosyst. Eng. 2004, 87, 305–311. [Google Scholar] [CrossRef]
- Sideli, G.M.; Marrano, A.; Montanari, S.; Leslie, C.A.; Allen, B.J.; Cheng, H.; Brown, P.J.; Neale, D.B.J.P.O. Quantitative phenotyping of shell suture strength in walnut (Juglans regia L.) enhances precision for detection of QTL and genome-wide association mapping. PLoS ONE 2020, 15, e0231144. [Google Scholar] [CrossRef]
- Kafkas, E.; Attar, S.H.; Gundesli, M.A.; Ozcan, A.; Ergun, M. Phenolic and Fatty Acid Profile, and Protein Content of Different Walnut Cultivars and Genotypes (Juglans regia L.) Grown in the USA. Int. J. Fruit Sci. 2020, 20, S1711–S1720. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, S.; Ma, H.; Huang, N.; Ye, N. Differential responses of walnut cultivars to cold storage and their correlation with postharvest physiological parameters. Hortic. Environ. Biotechnol. 2019, 60, 345–356. [Google Scholar] [CrossRef]
- Chatrabnous, N.; Yazdani, N.; Tavallali, V.; Vahdati, K. Preserving quality of fresh walnuts using plant extracts. LWT 2018, 91, 1–7. [Google Scholar] [CrossRef]
- Pakrah, S.; Rahemi, M.; Nabipour, A.; Zahedzadeh, F.; Kakavand, F.; Vahdati, K. Sensory and nutritional attributes of Persian walnut kernel influenced by maturity stage, drying method, and cultivar. J. Food Process. Preserv. 2021, 45, e15513. [Google Scholar] [CrossRef]
- UNECE. Standard DDP-02 Concerning the Marketing and Commercial Quality Control of Walnut Kernels; United Nations Economic Commission: New York, NY, USA; Geneva, Switzerland, 2019; pp. 1–7. [Google Scholar]
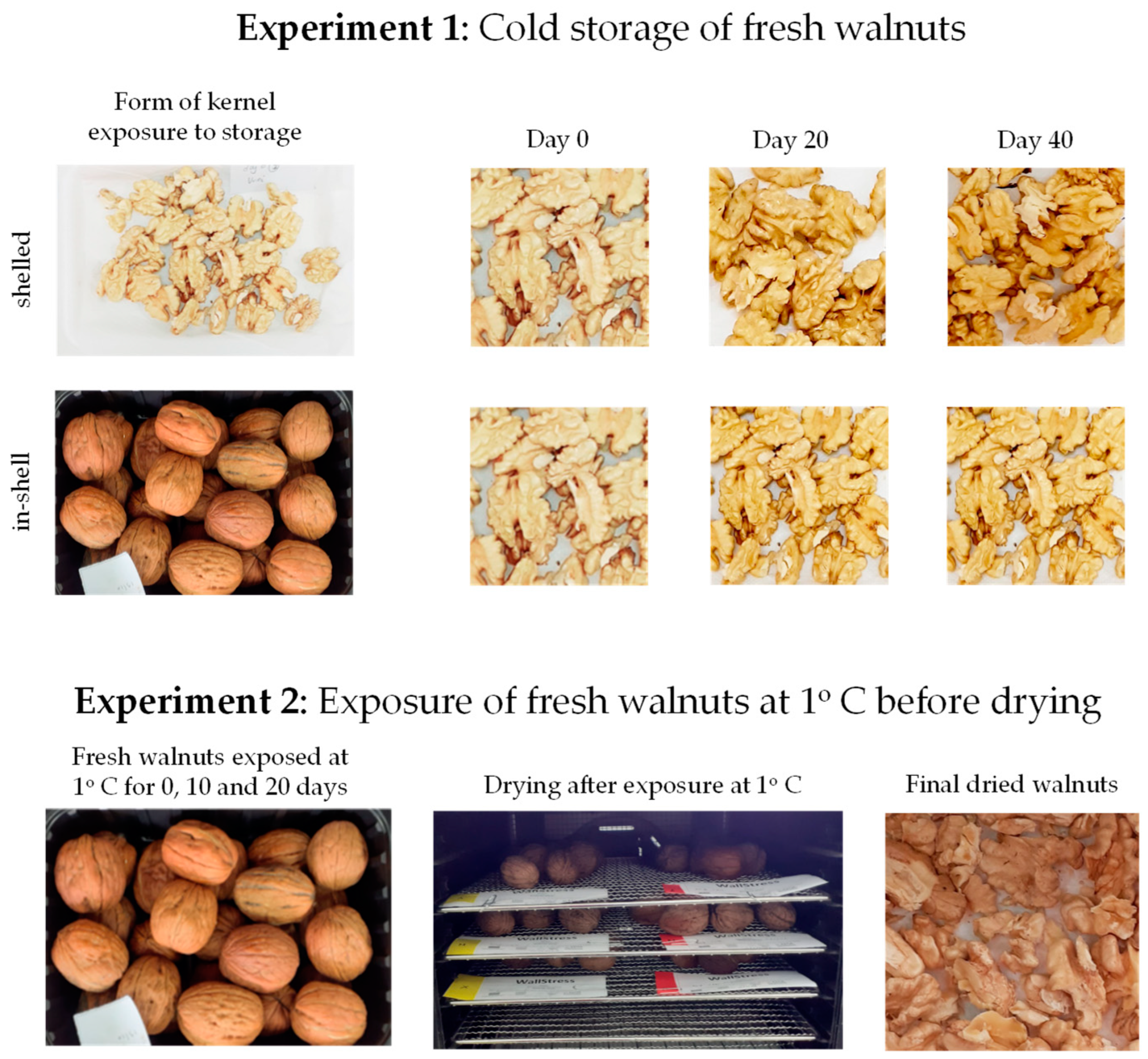
| Cultivar | Length (mm) | Width (mm) | Whole Weight (g) | Kernel Weight (g) | Kernel Percentage (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chandler | 40.02 ± 0.87 | B 1 | 33.36 ± 0.53 | B | 16.73 ± 2.14 | B | 9.17 ± 1.64 | B | 54.59 ± 2.97 | B |
| Hartley | 38.28 ± 0.32 | C | 32.22 ± 0.23 | C | 12.66 ± 0.11 | C | 5.39 ± 0.06 | C | 42.60 ± 0.87 | C |
| Ioli | 41.76 ± 0.55 | A | 38.44 ± 0.57 | A | 19.22 ± 0.72 | A | 11.12 ± 0.64 | A | 57.84 ± 1.30 | A |
| Pc 1 | *** | *** | *** | *** | *** | |||||
| Cultivar | Storage | Day 0 | Day 20 | Day 40 | Pt 1 | |||
|---|---|---|---|---|---|---|---|---|
| Kernel moisture (% w/w) | ||||||||
| Chandler | Shelled | 34.48 ± 1.05 | a 1 A 2 | 21.46 ± 1.29 | bB | 18.82 ± 1.23 | cBCD | *** |
| In-shell | a | 20.82 ± 0.75 | bB | 23.38 ± 2.77 | bA | *** | ||
| Hartley | Shelled | 35.35 ± 0.47 | aA | 24.39 ± 1.86 | bA | 20.36 ± 0.95 | cBC | *** |
| In-shell | a | 25.41 ± 2.97 | bA | 21.00 ± 1.13 | cAB | *** | ||
| Ioli | Shelled | 31.98 ± 1.26 | aB | 20.02 ± 0.59 | bB | 16.85 ± 1.90 | cD | *** |
| In-shell | a | 20.41 ± 1.83 | bB | 18.14 ± 1.99 | bCD | *** | ||
| Pc | ** | *** | ** | |||||
| Pshell | - | ns | ** | |||||
| Pc × shell | - | ns | ns | |||||
| Weight loss (% w/w) | ||||||||
| Chandler | Kernel | - | 13.86 ± 1.07 | bC | 16.66 ± 1.01 | aB | ** | |
| Nut | - | 21.34 ± 0.32 | bA | 32.49 ± 2.65 | aA | *** | ||
| Hartley | Kernel | - | 12.97 ± 1.11 | bC | 17.56 ± 0.59 | aB | *** | |
| Nut | - | 19.52 ± 0.59 | aB | 18.66 ± 2.67 | aB | ns | ||
| Ioli | Kernel | - | 12.75 ± 1.08 | bC | 16.97 ± 1.05 | aB | ** | |
| Nut | - | 21.82 ± 0.99 | bA | 29.82 ± 1.90 | aA | *** | ||
| Pc | - | * | *** | |||||
| Pshell | - | *** | *** | |||||
| Pc × shell | - | * | *** | |||||
| Respiration (μmol CO2 kg−1 h−1) | ||||||||
| Chandler | Kernel | 1160.3 ± 61.0 | aA | 177.0 ± 34.5 | bD | 26.3 ± 3.3 | cD | *** |
| Nut | 866.3 ± 71.7 | bB | 987.3 ± 36.9 | aA | 514.7 ± 55.8 | cA | *** | |
| Hartley | Kernel | 582.1 ± 44.1 | aDE | 45.4 ± 4.8 | bE | 10.7 ± 1.7 | bD | *** |
| Nut | 539.1 ± 20.0 | aE | 248.3 ± 37.3 | bC | 99.4 ± 12.3 | cC | *** | |
| Ioli | Kernel | 752.3 ± 41.3 | aC | 49.8 ± 6.3 | bE | 12.3 ± 2.2 | bD | *** |
| Nut | 621.1 ± 38.6 | bD | 690.6 ± 39.5 | aB | 168.6 ± 29.1 | cB | *** | |
| Pc | *** | *** | *** | |||||
| Pshell | *** | *** | *** | |||||
| Pc × shell | *** | *** | *** | |||||
| Cultivar | Storage | Day 0 | Day 20 | Day 40 | Pt 1 | |||
|---|---|---|---|---|---|---|---|---|
| Kernel L* | ||||||||
| Chandler | Shelled | 65.56 ± 1.13 | a 1A 2 | 65.07 ± 0.96 | aA | 62.95 ± 1.47 | bAB | * |
| In-shell | a | 66.27 ± 1.13 | aA | 63.85 ± 1.66 | aA | ns | ||
| Hartley | Shelled | 62.21 ± 0.71 | aB | 59.81 ± 0.84 | bC | 58.76 ± 2.23 | bC | * |
| In-shell | a | 60.57 ± 1.81 | aBC | 60.49 ± 1.74 | aBC | ns | ||
| Ioli | Shelled | 66.20 ± 0.58 | aA | 61.26 ± 1.28 | bBC | 58.91 ± 0.81 | cC | *** |
| In-shell | a | 62.24 ± 0.92 | bB | 60.09 ± 2.33 | bC | *** | ||
| Pc 2 | *** | *** | *** | |||||
| Pshell | - | ns | ns | |||||
| Pc × shell | - | ns | ns | |||||
| Kernel h° | ||||||||
| Chandler | Shelled | 83.77 ± 0.49 | aA | 82.95 ± 0.56 | aB | 80.07 ± 0.95 | bB | *** |
| In-shell | a | 84.04 ± 0.57 | aA | 81.77 ± 0.93 | bA | ** | ||
| Hartley | Shelled | 81.49 ± 0.67 | aB | 78.52 ± 0.75 | bD | 77.32 ± 0.96 | bD | *** |
| In-shell | a | 78.93 ± 0.36 | bD | 78.42 ± 0.43 | bCD | *** | ||
| Ioli | Shelled | 84.42 ± 0.84 | aA | 81.75 ± 0.48 | bC | 78.11 ± 0.49 | cD | *** |
| In-shell | a | 83.01 ± 0.43 | aB | 79.53 ± 1.20 | bBC | *** | ||
| Pc | *** | *** | *** | |||||
| Pshell | - | *** | *** | |||||
| Pc × shell | - | ns | ns | |||||
| Kernel C* | ||||||||
| Chandler | Shelled | 31.63 ± 0.87 | aB | 32.19 ± 0.66 | aB | 33.05 ± 1.04 | aAB | ns |
| In-shell | a | 30.96 ± 1.19 | aC | 31.24 ± 1.79 | aBC | ns | ||
| Hartley | Shelled | 33.42 ± 0.53 | bA | 34.20 ± 0.31 | abA | 34.87 ± 0.78 | aA | * |
| In-shell | b | 34.23 ± 0.37 | aA | 34.16 ± 0.34 | aA | * | ||
| Ioli | Shelled | 29.55 ± 0.61 | bC | 30.22 ± 0.71 | bC | 31.31 ± 0.70 | aBC | * |
| In-shell | a | 30.37 ± 0.53 | aC | 30.94 ± 2.45 | aC | ns | ||
| Pc | *** | *** | *** | |||||
| Pshell | - | ns | ns | |||||
| Pc × shell | - | ns | ns | |||||
| WI | ||||||||
| Chandler | Shelled | 53.24 ± 1.32 | aB | 52.49 ± 1.03 | aB | 50.33 ± 0.56 | bAB | ** |
| In-shell | a | 54.21 ± 1.45 | aA | 52.22 ± 2.35 | aA | ns | ||
| Hartley | Shelled | 49.55 ± 0.74 | aC | 47.23 ± 0.51 | bD | 45.99 ± 2.16 | bC | * |
| In-shell | a | 47.77 ± 1.25 | aD | 47.76 ± 1.30 | aBC | ns | ||
| Ioli | Shelled | 55.10 ± 0.80 | aA | 50.86 ± 1.33 | bC | 48.33 ± 0.67 | cBC | *** |
| In-shell | a | 51.53 ± 0.56 | bBC | 49.50 ± 3.27 | bAB | ** | ||
| Pc | *** | *** | ** | |||||
| Pshell | - | * | ns | |||||
| Pc × shell | - | ns | ns | |||||
| BR | ||||||||
| Chandler | Shelled | 0.00 ± 0.00 | b | 1.40 ± 1.94 | bC | 5.46 ± 1.06 | aBC | *** |
| In-shell | a | −1.84 ± 2.71 | aD | 1.92 ± 4.42 | aC | ns | ||
| Hartley | Shelled | 0.00 ± 0.00 | b | 4.69 ± 1.03 | aAB | 7.19 ± 4.35 | aABC | * |
| In-shell | b | 3.59 ± 2.52 | aBC | 3.61 ± 2.61 | aC | * | ||
| Ioli | Shelled | 0.00 ± 0.00 | c | 7.70 ± 2.40 | bA | 12.29 ± 1.21 | aA | *** |
| In-shell | b | 6.48 ± 1.02 | aAB | 10.18 ± 5.93 | aAB | ** | ||
| Pc | - | *** | ** | |||||
| Pshell | - | * | ns | |||||
| Pc × shell | - | ns | ns | |||||
| Cultivar | Day 0 | Day 20 | Day 40 | Pt 1 | |||
|---|---|---|---|---|---|---|---|
| Shell L* | |||||||
| Chandler | 56.54 ± 0.22 | a 1 B 2 | 55.77 ± 0.95 | aB | 54.63 ± 0.27 | bB | ** |
| Hartley | 59.60 ± 0.97 | aA | 58.89 ± 1.87 | aA | 59.03 ± 0.65 | aA | ns |
| Ioli | 50.73 ± 1.09 | aC | 52.20 ± 0.95 | aC | 51.61 ± 0.65 | aC | ns |
| Pc 2 | *** | *** | *** | ||||
| Shell h° | |||||||
| Chandler | 65.93 ± 0.25 | aB | 61.75 ± 1.21 | aB | 66.44 ± 0.70 | bA | *** |
| Hartley | 67.88 ± 0.21 | aA | 66.40 ± 0.63 | bA | 67.34 ± 0.37 | aA | ** |
| Ioli | 61.93 ± 0.67 | aC | 59.74 ± 1.50 | bC | 63.77 ± 1.45 | aB | ** |
| Pc | *** | *** | ** | ||||
| Shell C* | |||||||
| Chandler | 23.05 ± 0.49 | cB | 26.19 ± 0.35 | aA | 24.40 ± 0.48 | bB | *** |
| Hartley | 24.78 ± 0.33 | bA | 26.95 ± 1.37 | aA | 25.84 ± 0.16 | abA | * |
| Ioli | 22.15 ± 0.30 | cC | 26.31 ± 0.56 | aA | 23.22 ± 0.39 | bC | *** |
| Pc | *** | ns | *** | ||||
| Shell WI | |||||||
| Chandler | 50.80 ± 0.27 | aB | 45.54 ± 1.48 | bB | 48.48 ± 0.43 | cB | *** |
| Hartley | 52.60 ± 0.85 | aA | 50.83 ± 1.86 | aA | 51.56 ± 0.55 | aA | ns |
| Ioli | 45.98 ± 0.97 | aC | 43.38 ± 0.65 | bB | 46.32 ± 0.52 | aC | *** |
| Pc | *** | *** | *** | ||||
| Shell BR | |||||||
| Chandler | 0.00 ± 0.00 | cA | 10.36 ± 2.91 | bA | 4.57 ± 0.84 | aA | *** |
| Hartley | 0.00 ± 0.00 | aA | 3.37 ± 3.53 | aB | 1.98 ± 1.04 | aB | ns |
| Ioli | 0.00 ± 0.00 | bA | 5.65 ± 1.42 | aB | −0.76 ± 1.14 | bC | *** |
| Pc | - | * | *** | ||||
| Rupture strength (N) | |||||||
| Chandler | 248.9 ± 17.0 | aB | 260.9 ± 11.0 | aB | 243.7 ± 16.7 | aB | ns |
| Hartley | 288.3 ± 14.2 | aA | 299.2 ± 30.3 | aA | 284.8 ± 11.6 | aB | ns |
| Ioli | 236.5 ± 10.5 | aB | 233.3 ± 7.4 | aB | 222.1 ± 9.3 | aC | ns |
| Pc | *** | ** | *** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulos, M.V.; Kafkaletou, M.; Velliou, A.; Tsantili, E. Short Cold Storage as a Sustainable Postharvest Handling Method for Natural Enrichment in Antioxidants of Fresh and Dried Walnut Kernels—Cultivar Effect. Sustainability 2024, 16, 4727. https://doi.org/10.3390/su16114727
Christopoulos MV, Kafkaletou M, Velliou A, Tsantili E. Short Cold Storage as a Sustainable Postharvest Handling Method for Natural Enrichment in Antioxidants of Fresh and Dried Walnut Kernels—Cultivar Effect. Sustainability. 2024; 16(11):4727. https://doi.org/10.3390/su16114727
Chicago/Turabian StyleChristopoulos, Miltiadis V., Mina Kafkaletou, Anna Velliou, and Eleni Tsantili. 2024. "Short Cold Storage as a Sustainable Postharvest Handling Method for Natural Enrichment in Antioxidants of Fresh and Dried Walnut Kernels—Cultivar Effect" Sustainability 16, no. 11: 4727. https://doi.org/10.3390/su16114727
APA StyleChristopoulos, M. V., Kafkaletou, M., Velliou, A., & Tsantili, E. (2024). Short Cold Storage as a Sustainable Postharvest Handling Method for Natural Enrichment in Antioxidants of Fresh and Dried Walnut Kernels—Cultivar Effect. Sustainability, 16(11), 4727. https://doi.org/10.3390/su16114727







