Comparative Study of Pretreatments on Coconut Fiber for Efficient Isolation of Lignocellulosic Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coconut Residue
2.2. Pretreatments
2.2.1. Soxhlet Extraction
2.2.2. Alkaline Extraction
2.2.3. Autohydrolysis
2.2.4. Organosolv
2.3. Biomass Characterization
2.3.1. Lignin Determination
- M = mass of lignin precipitated in liquor (g).
- V = volume of liquor aliquots used to precipitate lignin (mL).
- A = absorbance.
- DF = dilution factor of liquor.
2.3.2. Chemical and Structural Characterization of Lignocellulosic Components
2.3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Pretreatment Conditions
3.2. Chemical Characterization
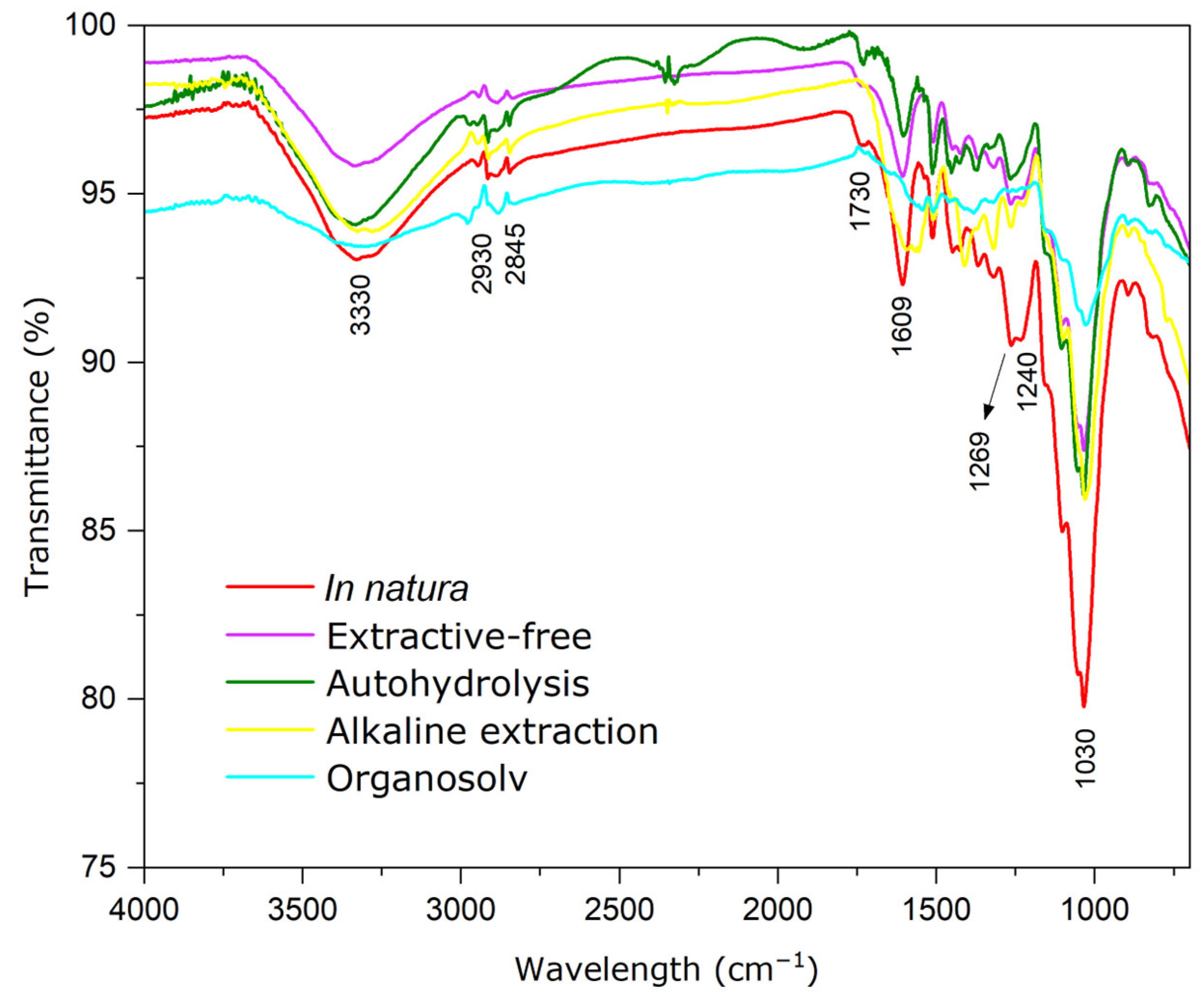
3.3. FT-IR Analysis
Crystallinity Analysis of Samples Using FT-IR
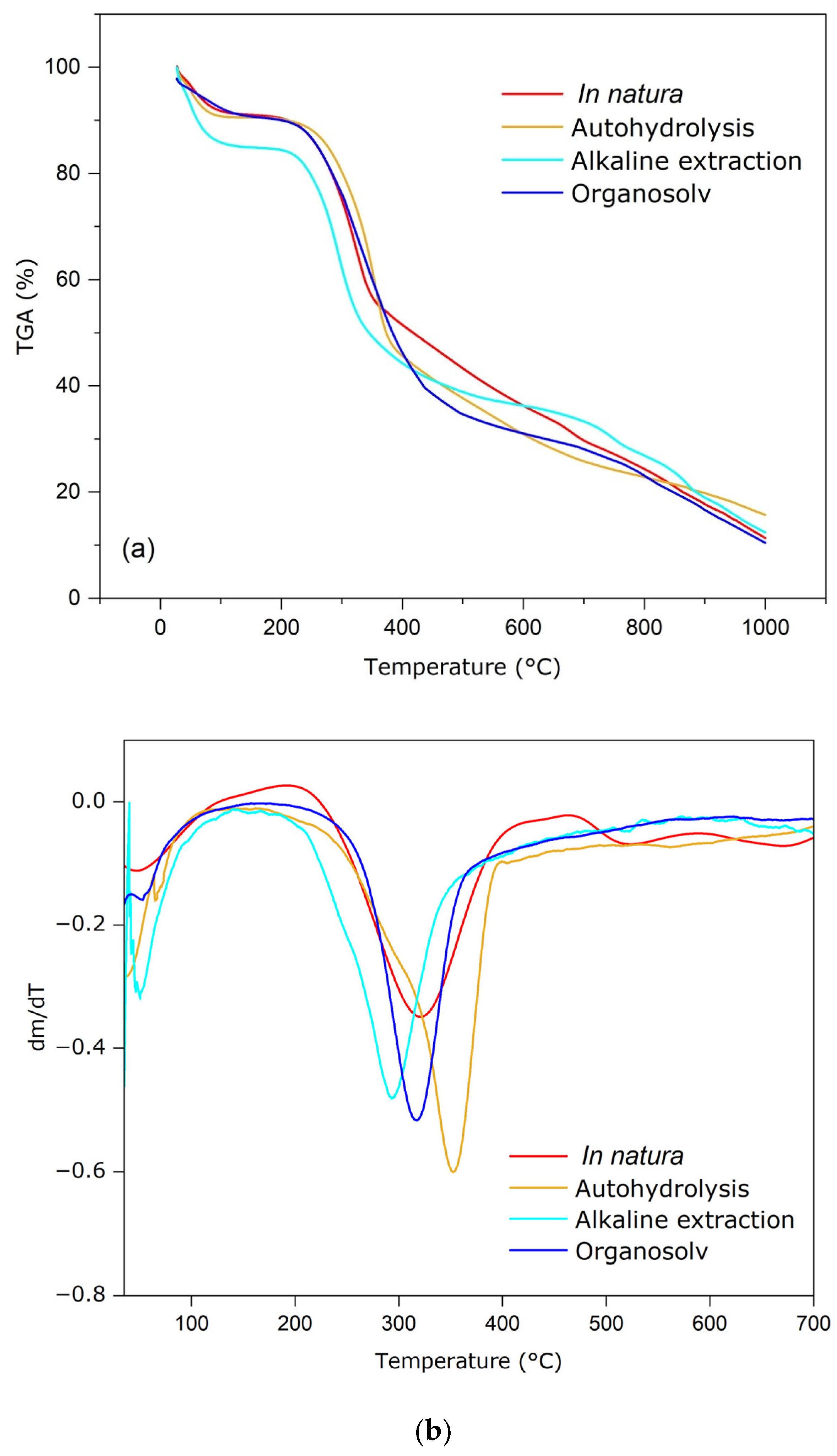
3.4. TGA and DTA Analyses
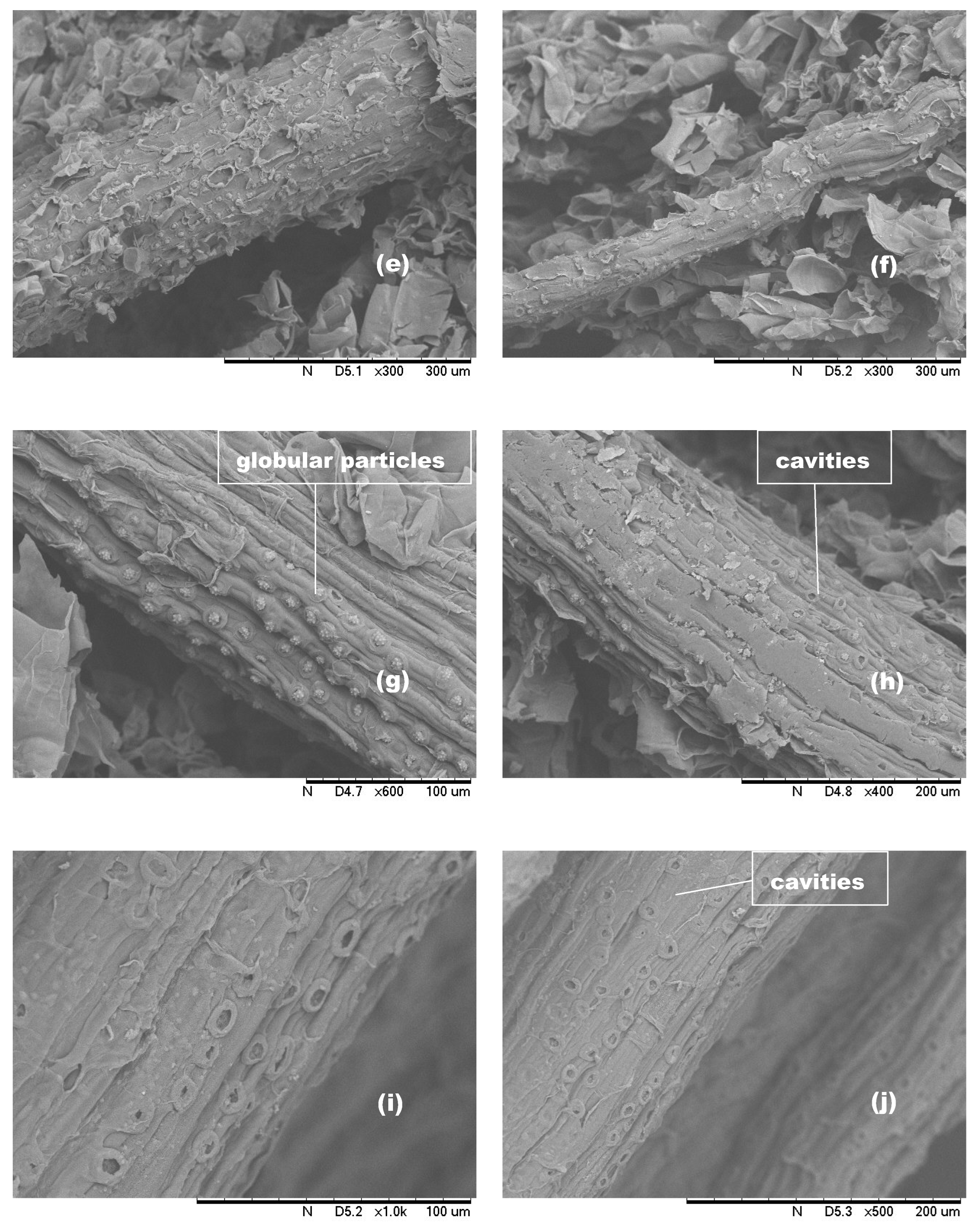
3.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeleye, A.T.; Louis, H.; Akakuru, O.U.; Joseph, I.; Enudi, O.C.; Michael, D.P. A Review on the Conversion of Levulinic Acid and Its Esters to Various Useful Chemicals. AIMS Energy 2019, 7, 165–185. [Google Scholar] [CrossRef]
- Premalatha, N.; Saranya, S.R. Global Research Trends in Biomass as Renewable Energy. In Biomass Energy for Sustainable Development; CRC Press: Boca Raton, FL, USA, 2024; pp. 355–374. [Google Scholar]
- Hayes, D.J.M. Biomass Composition and Its Relevance to Biorefining. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 27–65. ISBN 9780444563309. [Google Scholar]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of Pretreatment of Feedstocks for Optimized Bioethanol Production: Distinct and Integrated Approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Li, M.F.; Fan, Y.M.; Xu, F.; Sun, R.C.; Zhang, X.L. Cold Sodium Hydroxide/Urea Based Pretreatment of Bamboo for Bioethanol Production: Characterization of the Cellulose Rich Fraction. Ind. Crops Prod. 2010, 32, 551–559. [Google Scholar] [CrossRef]
- Mazumder, S.; Zhang, N. Cellulose–Hemicellulose–Lignin Interaction in the Secondary Cell Wall of Coconut Endocarp. Biomimetics 2023, 8, 188. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Khasa, Y.P.; Singh, A.; Zhang, Y.-H.P. Bioethanol Production from Pentose Sugars: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2011, 15, 4950–4962. [Google Scholar] [CrossRef]
- Taiz, L.; Moller, I.M.; Murphy, A.; Zeiger, E. Plant Physiology and Development, 7th ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Barros, D.; Fernandes, É.; Jesus, M.; Barros, L.; Alonso-Esteban, J.I.; Pires, P.; Vaz Velho, M. The Chemical Characterisation of the Maritime Pine Bark Cultivated in Northern Portugal. Plants 2023, 12, 3940. [Google Scholar] [CrossRef]
- FAO, F. and A.O. of the U.N. FAOSTAT—Food and Agriculture Data Database. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize. (accessed on 8 November 2023).
- Leliana, L.; Setyaningsih, W.; Palma, M.; Supriyadi; Santoso, U. Antioxidant Activity of Aqueous and Ethanolic Extracts of Coconut (Cocos Nucifera) Fruit by-Products. Agronomy 2022, 12, 1102. [Google Scholar] [CrossRef]
- Li, N.; Jiang, H.; Yang, J.; Wang, C.; Wu, L.; Hao, Y.; Liu, Y. Characterization of Phenolic Compounds and Anti-Acetylcholinase Activity of Coconut Shells. Food Biosci. 2021, 42, 101204. [Google Scholar] [CrossRef]
- Burns, D.T.; Johnston, E.L.; Walker, M.J. Authenticity and the Potability of Coconut Water-a Critical Review. J. AOAC Int. 2020, 103, 800–806. [Google Scholar] [CrossRef]
- Kaur, K.; Chhikara, N.; Sharma, P.; Garg, M.K.; Panghal, A. Coconut Meal: Nutraceutical Importance and Food Industry Application. Foods Raw Mater. 2019, 7, 419–427. [Google Scholar] [CrossRef]
- Vieira, F.; Santana, H.E.P.; Jesus, M.; Santos, J.; Pires, P.; Vaz-Velho, M.; Silva, D.P.; Ruzene, D.S. Coconut Waste: Discovering Sustainable Approaches to Advance a Circular Economy. Sustainability 2024, 16, 3066. [Google Scholar] [CrossRef]
- Qureshi, T.; Farooq, M.; Imran, S.; Munir, M.A.; Javed, M.A.; Sohoo, I.; Sultan, M.; Rehman, A.U.; Farhan, M.; Asim, M. Structural and Thermal Investigation of Lignocellulosic Biomass Conversion for Enhancing Sustainable Imperative in Progressive Organic Refinery Paradigm for Waste-to-Energy Applications. Environ. Res. 2024, 246, 118129. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological Advancements in Lignocellulosic Biomass Pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different Pretreatment Technologies of Lignocellulosic Biomass for Bioethanol Production: An Overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Jesus, M.; Romaní, A.; Mata, F.; Domingues, L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites Following the Concept of Biorefinery: A Review. Polymers 2022, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Yerizam, M.; Jannah, A.M.; Aprianti, N.; Yandriani, Y.; Rendana, M.; Ernas, A.Q.; Tamba, J.L. Bioethanol Production from Coconut Husk Using DES-NADES Pretreatment and Enzymatic Hydrolysis Method. Comptes Rendus Chim. 2023, 26, 1–10. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Bano, K.; Panda, T.K.; Sriariyanun, M.; Bhattacharyya, D. Understanding the Effect of Low-Concentrated Protic Ionic Liquids (PILs) on Coconut (Cocos Nucifera) Residues. Biomass Convers. Biorefin. 2024, 14, 3275–3291. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Pant, K.K. Microwave-Assisted Extraction of Lignin from Coconut Coir Using Deep Eutectic Solvents and Its Valorization to Aromatics. Bioresour. Technol. 2022, 345, 126528. [Google Scholar] [CrossRef] [PubMed]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effective Pretreatment of Lignin-Rich Coconut Wastes Using a Low-Cost Ionic Liquid. Sci. Rep. 2022, 12, 6108. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Mihailof, C.M.; Matis, K.A.; Lappas, A.A.; Triantafyllidis, K.S. The Role of Catalytic Pretreatment in Biomass Valorization Toward Fuels and Chemicals. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 217–260. [Google Scholar]
- Jesus, M.; Mata, F.; Batista, R.A.; Ruzene, D.S.; Albuquerque-Júnior, R.; Cardoso, J.C.; Vaz-Velho, M.; Pires, P.; Padilha, F.F.; Silva, D.P. Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp. Sustainability 2023, 15, 2287. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.P.; Jesus, M.S.; Mata, F.; Prado, A.A.O.S.; Vieira, I.M.M.; Ramos, L.C.; López, J.A.; Vaz-Velho, M.; Ruzene, D.S.; Silva, D.P. Use of Agro-Industrial Waste for Biosurfactant Production: A Comparative Study of Hemicellulosic Liquors from Corncobs and Sunflower Stalks. Sustainability 2023, 15, 6341. [Google Scholar] [CrossRef]
- Zamboni Schiavon, J.; de Oliveira Andrade, J.J. Comparison between Alternative Chemical Treatments on Coir Fibers for Application in Cementitious Materials. J. Mater. Res. Technol. 2023, 25, 4634–4649. [Google Scholar] [CrossRef]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral Valorization of Vine Pruning Residue by Sequential Autohydrolysis Stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; Dos Santos, E.S.; Teixeira, J.A.; De Macedo, G.R. Bioethanol Production from Coconuts and Cactus Pretreated by Autohydrolysis. Ind. Crops Prod. 2015, 77, 1–12. [Google Scholar] [CrossRef]
- Carre, B.; Hebrant, M.; Brosse, N.; Latif, N.H.; Hussin, M.H. Effect of Different Prehydrolysis Processes on Lignin Extractability of Coconut Husk Fibres. J. Phys. Sci. 2019, 30, 207–219. [Google Scholar] [CrossRef]
- Kabakcı, S.B.; Tanış, M.H. Pretreatment of Lignocellulosic Biomass at Atmospheric Conditions by Using Different Organosolv Liquors: A Comparison of Lignins. Biomass Convers. Biorefin. 2021, 11, 2869–2880. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental Review of Organosolv Pretreatment and Its Challenges in Emerging Consolidated Bioprocessing. Biofuels Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the Economy of Lignocellulose-Based Biorefineries with Organosolv Pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- Avelino, F.; da Silva, K.T.; de Souza Filho, M.S.M.; Mazzetto, S.E.; Lomonaco, D. Microwave-Assisted Organosolv Extraction of Coconut Shell Lignin by Brønsted and Lewis Acids Catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Ratanasumarn, N.; Chitprasert, P. Cosmetic Potential of Lignin Extracts from Alkaline-Treated Sugarcane Bagasse: Optimization of Extraction Conditions Using Response Surface Methodology. Int. J. Biol. Macromol. 2020, 153, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, A.; Agustriyanto, R.; Liasari, Y. Enzymatic Hydrolysis of Alkaline Pretreated Coconut Coir. Bull. Chem. React. Eng. Catal. 2013, 8, 34–39. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; dos Santos, E.S.; Teixeira, J.A.; de Macedo, G.R. Valorization, Comparison and Characterization of Coconuts Waste and Cactus in a Biorefinery Context Using NaClO2–C2H4O2 and Sequential NaClO2–C2H4O2/Autohydrolysis Pretreatment. Waste Biomass Valorization 2019, 10, 2249–2262. [Google Scholar] [CrossRef]
- Padilha, C.E.A.; da Costa Nogueira, C.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Organosolv Lignin/Fe3O4 Nanoparticles Applied as a β-Glucosidase Immobilization Support and Adsorbent for Textile Dye Removal. Ind. Crops Prod. 2020, 146, 112167. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dhepe, P.L. Depolymerization of Lignin Using a Solid Base Catalyst. Energy Fuels 2019, 33, 4369–4377. [Google Scholar] [CrossRef]
- Kauldhar, B.S.; Sooch, B.S.; Rai, S.K.; Kumar, V.; Yadav, S.K. Recovery of Nanosized Silica and Lignin from Sugarcane Bagasse Waste and Their Engineering in Fabrication of Composite Membrane for Water Purification. Environ. Sci. Pollut. Res. 2021, 28, 7491–7502. [Google Scholar] [CrossRef]
- Ismail, H.S.; Ibrahim, A.H.; Abidin, C.Z.A.; Ridwan, F.M. Recovery of Nano-Lignin from Anaerobic Treated Palm Oil Mill Effluent (AT-POME). In Proceedings of the IOP Conference Series: Earth and Environmental Science, 2nd International Conference on Civil & Environmental Engineering 20th, Langkawi, Malaysia, 21 November 2019; Volume 476. [Google Scholar]
- Tang, P.L.; Hassan, O.; Md-Jahim, J.; Mustapha, W.A.W.; Maskat, M.Y. Fibrous Agricultural Biomass as a Potential Source for Bioconversion to Vanillic Acid. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Latha, M.; Subramanian, K.S.; Sundara Sharmila, D.J.; Raja, K.; Rajkishore, S.K.; Chitdeshwari, T. Urea-Lignin/Chitosan Nanocomposite as Slow-Release Nanofertilizer. ACS Agric. Sci. Technol. 2023, 3, 463–476. [Google Scholar] [CrossRef]
- Wise, L.E.; Murphy, M.; d’Addieco, A.A. Chlorite Holocellulose, Its Fractionnation and Bearing on Summative Wood Analysis and on Studies on the Hemicelluloses. Tech. Assoc. Pap. 1946, 29, 210–218. [Google Scholar]
- Ferreira, P.F.O.; Pereira, A.L.S.; Rosa, M.F.; de Santiago-Aguiar, R.S. Lignin-Rich Cellulose Nanocrystals from Coir Fiber Treated with Ionic Liquids: Preparation and Evaluation as Pickering Emulsifier. Ind. Crops Prod. 2022, 186. [Google Scholar] [CrossRef]
- Jakka, V.; Goswami, A.; Nallajarla, A.K.; Roy, U.; Srikanth, K.; Sengupta, S. Coconut Coir–Derived Nanocellulose as an Efficient Adsorbent for Removal of Cationic Dye Safranin-O: A Detailed Mechanistic Adsorption Study. Environ. Sci. Pollut. Res. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Abdul Razak, S.; Hanif Mahadi, A.; Abdullah, R.; Mohd Yasin, H.; Ja, F.; Abdul Rahman, N.; Bahruji, H. Biohydrogen Production from Photodecomposition of Various Cellulosic Biomass Wastes Using Metal-TiO 2 Catalysts. Biomass Convers. Biorefin. 2023, 13, 8701–8712. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and Cellulose Derivatives: Different Colloidal States and Food-Related Applications. Carbohydr. Polym. 2021, 255, 117334. [Google Scholar] [CrossRef] [PubMed]
- Lawoko, M.; Samec, J.S.M. Kraft Lignin Valorization: Biofuels and Thermoset Materials in Focus. Curr. Opin. Green Sustain. Chem. 2023, 40, 100738. [Google Scholar] [CrossRef]
- Constant, S.; Basset, C.; Dumas, C.; Di Renzo, F.; Robitzer, M.; Barakat, A.; Quignard, F. Reactive Organosolv Lignin Extraction from Wheat Straw: Influence of Lewis Acid Catalysts on Structural and Chemical Properties of Lignins. Ind. Crops Prod. 2015, 65, 180–189. [Google Scholar] [CrossRef]
- Sujatha, M.; Roy, A. Extraction of Lignin from Agro-Waste Coir Fiber by Mild Alkali Treatment: A Statistical Approach for Process Optimization through Response Surface Methodology. Asian J. Chem. 2024, 36, 81–86. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Caparanga, A.R.; Ordono, E.E.; Villaflores, O.B. Evaluation of Organosolv Pretreatment on the Enzymatic Digestibility of Coconut Coir Fibers and Bioethanol Production via Simultaneous Saccharification and Fermentation. Renew Energy 2017, 109, 41–48. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; Silvino dos Santos, E.; Teixeira, J.A.; de Macedo, G.R. Bioethanol Production by Saccharomyces Cerevisiae, Pichia Stipitis and Zymomonas Mobilis from Delignified Coconut Fibre Mature and Lignin Extraction According to Biorefinery Concept. Renew Energy 2016, 94, 353–365. [Google Scholar] [CrossRef]
- Özyürek, Ö.; van Heiningen, A. Formic Acid Reinforced Autohydrolysis of Wheat Straw for High Yield Production of Monosugars and Minimal Lignin Precipitation. Ind. Crops Prod. 2018, 112, 320–326. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Consonni, R.; Riva, S.; Patel, I. Fractionation of Hemp Hurds by Organosolv Pretreatment and Its Effect on Production of Lignin and Sugars. ChemSusChem 2014, 7, 1991–1999. [Google Scholar] [CrossRef]
- Protásio, T.d.P.; Costa, d.J.S.; Scatolino, V.M.; Lima, M.D.R.; Assis, d.M.R.; Silva, d.M.G.; Bufalino, L.; Junior, A.F.D.; Trugilho, P.F. Revealing the Influence of Chemical Compounds on the Pyrolysis of Lignocellulosic Wastes from the Amazonian Production Chains. Int. J. Environ. Sci. Technol. 2022, 19, 4491–4508. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Sun, Y. Bacterial Cellulose Production by Acetobacter Xylinum CGMCC 1.2378 Using Coconut Shell Acid Hydrolysate as Carbon Source. Bioresources 2021, 16, 1042–1062. [Google Scholar] [CrossRef]
- Parichanon, P.; Matan, N.; Limbo, S.; D’Incecco, P.; Matan, N. Natural Cellulosic Material Characteristics: A Possibility to Develop Antimicrobial Active Fiber-Based Packaging. Bioresources 2021, 16, 5450–5466. [Google Scholar] [CrossRef]
- Alharbi, M.A.H.; Hirai, S.; Tuan, H.A.; Akioka, S.; Shoji, W. Effects of Chemical Composition, Mild Alkaline Pretreatment and Particle Size on Mechanical, Thermal, and Structural Properties of Binderless Lignocellulosic Biopolymers Prepared by Hot-Pressing Raw Microfibrillated Phoenix Dactylifera and Cocos Nucifera. Polym. Test 2020, 84, 106384. [Google Scholar] [CrossRef]
- Fleck, L.C.; Beckum, W.V.; Ritter, G.J. Composition of Coconut Shells. J. Am. Chem. Soc. 1937, 59, 2279–2280. [Google Scholar] [CrossRef]
- Bezerra, P.K.S.d.B.; Silva, d.O.L.; Oliveira, d.S.D.; Padilha, C.E.d.A.; Santos, d.E.S. Cellulolytic Enzymes Behavior in Delignified Green Coconut Residues and Enzymatic Hydrolysis with Enzyme Recovery. Ind. Crops Prod. 2021, 172. [Google Scholar] [CrossRef]
- Din, N.A.S.; Lim, S.J.; Maskat, M.Y.; Zaini, N.A.M. Bioconversion of Coconut Husk Fibre through Biorefinery Process of Alkaline Pretreatment and Enzymatic Hydrolysis. Biomass Convers. Biorefin. 2021, 11, 815–826. [Google Scholar] [CrossRef]
- De Padilha, C.E.A.; Santiago, L.E.P.; de Guilherme, A.A.; Cavalcante, J.D.N.; Thomas, H.Y.; dos Santos, E.S.; de Melo, D.M.A.; Braga, R.M.; de Souza, D.F.S. Effects of Acid and Organosolv Pretreatments on the Analytical Fast Pyrolysis Products of Green Coconut Fiber. Bioenergy Res. 2023. [Google Scholar] [CrossRef]
- Nascimento, R.J.M.; Bezerra, L.C.A.; Almeida, J.S.; de Oliveira Barros, M.; Silva, L.R.R.; Rosa, M.F.; Mazzeto, S.E.; Lomonaco, D.; Pereira, K.R.A.; Avelino, F. Elucidating the Adsorption Mechanism of Rhodamine B on Mesoporous Coconut Coir-Based Biosorbents through a Non-Linear Modeling and Recycling Approach. Environ. Sci. Pollut. Res. 2022, 79920–79934. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Almeida, J.S.; Dias, A.F.; Figueirêdo, M.C.B.; Morais, J.P.S.; Feitosa, J.P.A.; Rosa, M.D.F. A Novel Green Approach for the Preparation of Cellulose Nanowhiskers from White Coir. Carbohydr. Polym. 2014, 110, 456–463. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental Friendly Method for the Extraction of Coir Fibre and Isolation of Nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Gapsari, F.; Purnowidodo, A.; Hidayatullah, S.; Suteja, S. Characterization of Timoho Fiber as a Reinforcement in Green Composite. J. Mater. Res. Technol. 2021, 13, 1305–1315. [Google Scholar] [CrossRef]
- Moshi, A.A.M.; Ravindran, D.; Sundara Bharathi, S.R.; Padma, S.R.; Indran, S.; Divya, D. Characterization of Natural Cellulosic Fiber Extracted from Grewia Damine Flowering Plant’s Stem. Int. J. Biol. Macromol. 2020, 164, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Obi Reddy, K.; Guduri, B.R.; Rajulu, A.V. Structural Characterization and Tensile Properties of Borassus Fruit Fibers. J. Appl. Polym. Sci. 2009, 114, 603–611. [Google Scholar] [CrossRef]
- De Freitas, R.R.M.; do Carmo, K.P.; de Souza Rodrigues, J.; de Lima, V.H.; Osmari da Silva, J.; Botaro, V.R. Influence of Alkaline Treatment on Sisal Fibre Applied as Reinforcement Agent in Composites of Corn Starch and Cellulose Acetate Matrices. Plast. Rubber Compos. 2021, 50, 9–17. [Google Scholar] [CrossRef]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of Lignocellulose of Opuntia (Cactaceae) Species Using FTIR Spectroscopy: Possible Candidates for Renewable Raw Material. Biomass Convers. Biorefin. 2022, 12, 5165–5174. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, I.D.; Shin, Y.; Seo, G. FTIR Analysis of Cellulose Treated with Sodium Hydroxide and Carbon Dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Zhang, Y.; Lang, Y.X.; Yu, M.H. Structural ATR-IR Analysis of Cellulose Fibers Prepared from a NaOH Complex Aqueous Solution. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Guangzhou, China, 23–25 May 2017; Volume 213, p. 012039. [Google Scholar]
- Pandey, K.K.; Theagarajan, K.S. Analysis of Wood Surfaces and Ground Wood by Diffuse Reflectance (DRIFT) and Photoacoustic (PAS) Fourier Transform Infrared Spectroscopic Techniques. Eur. J. Wood Wood Prod. 1997, 55, 383–390. [Google Scholar] [CrossRef]
- Latham, K.G.; Matsakas, L.; Figueira, J.; Kozyatnyk, I.; Rova, U.; Christakopoulos, P.; Jansson, S. Impact of Temperature and Residence Time on the Hydrothermal Carbonization of Organosolv Lignin. J. Anal. Appl. Pyrolysis 2022, 166, 105623. [Google Scholar] [CrossRef]
- Pasang, P.M.; Yunianta; Estiasih, T. Harijono Structure and Morphology of Cellulose from Coconut Coir Fibers. Russ. J. Agric. Socioecon. Sci. 2018, 80, 452–462. [Google Scholar] [CrossRef]
- Cao, C.; Yang, Z.; Han, L.; Jiang, X.; Ji, G. Study on in Situ Analysis of Cellulose, Hemicelluloses and Lignin Distribution Linked to Tissue Structure of Crop Stalk Internodal Transverse Section Based on FTIR Microspectroscopic Imaging. Cellulose 2015, 22, 139–149. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv Fractionating Pre-treatment of Lignocellulosic Biomass for Efficient Enzymatic Saccharification: Chemistry, Kinetics, and Substrate Structures. Biofuels Bioprod. Biorefining 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Zugnmaier, P. Cellulose Derivatives. In Crystalline Cellulose and Derivatives; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 2008; pp. 175–206. [Google Scholar] [CrossRef]
- Wertz, J.-L.; Bédué, O.; Mercier, J.P. Cellulose Science and Technology, 1st ed.; EPFL, Press: Lausanne, Switzerland, 2010. [Google Scholar]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR Analysis and Thermal Characterisation of Lyocell and Viscose-Type Fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of Certain Infrared Bands to Cellulose Crystallinity and Crystal Lattice Type. Part 11. A New Infrared Ratio for Estimation of Crystallinity in Celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- O’connor, R.T.; Dupré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons:Part I: Physical and Crystalline Modifications and Oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Kljun, A.; Benians, T.A.S.; Goubet, F.; Meulewaeter, F.; Knox, J.P.; Blackburn, R.S. Comparative Analysis of Crystallinity Changes in Cellulose i Polymers Using ATR-FTIR, X-Ray Diffraction, and Carbohydrate-Binding Module Probes. Biomacromolecules 2011, 12, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.-A.M.A.; Kamel, S.; El-Sakhaway, M. Thermal Behaviour and Infrared Spectroscopy of Cellulose Carbamates. Polym. Degrad. Stab. 2000, 70, 347–355. [Google Scholar] [CrossRef]
- Spiridon, I.; Teacă, C.-A.; Bodîrlău, R. Structural changes evidenced by FTIR spectroscopy in cellulose materials after pre-treatment with ionic liquid and enzymatic hydrolysis. BioResources 2011, 6, 400–413. [Google Scholar] [CrossRef]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy Analysis of Crystallinity Changes in Lyocell Following Continuous Treatment with Sodium Hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S. Treating Lignocellulosic Biomass with Dilute Solutions at Ambient Temperature: Effects on Cellulose Crystallinity. Biomass Convers. Biorefin 2022. [Google Scholar] [CrossRef]
- Fatmawati, A.; Nurtono, T.; Widjaja, A. Thermogravimetric Kinetic-Based Computation of Raw and Pretreated Coconut Husk Powder Lignocellulosic Composition. Bioresour. Technol. Rep. 2023, 22, 101500. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; de Lira, T.S.; Ataíde, C.H.; de Souza Barrozo, M.A. Thermochemical Conversion of Coconut Waste: Material Characterization and Identification of Pyrolysis Products. J. Therm. Anal. Calorim. 2021, 143, 637–646. [Google Scholar] [CrossRef]
- Nascimento, P.F.P.; Neto, E.L.B. Steam Explosion: Hydrothermal Pretreatment in the Production of an Adsorbent Material Using Coconut Husk. Bioenergy Res. 2021, 14, 153–162. [Google Scholar] [CrossRef]
- Rueda-Ordóñez, Y.J.; Tannous, K. Isoconversional Kinetic Study of the Thermal Decomposition of Sugarcane Straw for Thermal Conversion Processes. Bioresour. Technol. 2015, 196, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Sakuri, S.; Surojo, E.; Ariawan, D. Thermogravimetry and Interfacial Characterization of Alkaline Treated Cantala Fiber/Microcrystalline Cellulose-Composite. Procedia Struct. Integr. 2020, 27, 85–92. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, C.; Wang, A.; Wang, Y.; Xu, W. Morphologies and Properties of Juncus Effusus Fiber after Alkali Treatment. Cellulose 2020, 27, 1909–1920. [Google Scholar] [CrossRef]
- Singh, S.; Varanasi, P.; Singh, P.; Adams, P.D.; Auer, M.; Simmons, B.A. Understanding the Impact of Ionic Liquid Pretreatment on Cellulose and Lignin via Thermochemical Analysis. Biomass Bioenergy 2013, 54, 276–283. [Google Scholar] [CrossRef]
- Lopes, F.C.R.; Tannous, K.; de Carmazini, E.B. Thermal Behavior and Kinetic Analysis of Torrefied Coconut Fiber Pyrolysis. Thermochim. Acta 2022, 715, 179275. [Google Scholar] [CrossRef]
- Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Hashim, R.; Hussin, M.H. A Comparison of Alkaline and Organosolv Lignin Extraction Methods from Coconut Husks as an Alternative Material for Green Applications. Bioresources 2022, 17, 469–491. [Google Scholar] [CrossRef]
- Panamgama, L.A.; Peramune, P.R.U.S.K. Coconut Coir Pith Lignin: A Physicochemical and Thermal Characterization. Int. J. Biol. Macromol. 2018, 113, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Egüés, I.; Sanchez, C.; Mondragon, I.; Labidi, J. Effect of Alkaline and Autohydrolysis Processes on the Purity of Obtained Hemicelluloses from Corn Stalks. Bioresour. Technol. 2012, 103, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Klunklin, W.; Hinmo, S.; Thipchai, P.; Rachtanapun, P. Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir. Polymers 2023, 15, 3376. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Su, S.; Chouw, N. Microstructure, Flexural Properties and Durability of Coir Fibre Reinforced Concrete Beams Externally Strengthened with Flax FRP Composites. Compos. B Eng. 2015, 80, 343–354. [Google Scholar] [CrossRef]


| Autohydrolysis | |||||||||
| Temperature (°C) | 175 | 185 | 195 | ||||||
| Time (min) | 20 | 25 | 30 | 20 | 25 | 30 | 20 | 25 | 30 |
| IL (g/L) | 0.75 | 0.81 | 0.90 | 0.62 | 0.85 | 0.83 | 0.68 | 0.73 | 0.70 |
| SL (g/L) | 0.51 | 0.70 | 0.80 | 0.75 | 0.82 | 1.02 | 1.13 | 1.13 | 1.12 |
| TL (g/L) | 1.26 | 1.52 | 1.70 | 1.37 | 1.67 | 1.84 | 1.80 | 1.86 | 1.82 |
| Alkaline extraction | |||||||||
| Time (h) | 1 | 2 | |||||||
| [NaOH] (M) | 0.25 | 0.50 | 0.75 | 0.25 | 0.50 | 0.75 | |||
| IL (g/L) | 1.41 | 2.99 | 3.54 | 1.84 | 5.89 | 6.72 | |||
| SL (g/L) | 0.93 | 1.22 | 1.53 | 2.82 | 3.81 | 3.69 | |||
| TL (g/L) | 2.35 | 4.20 | 5.06 | 4.66 | 9.71 | 10.41 | |||
| Organosolv | |||||||||
| Time (h) | 2 | 3 | 4 | ||||||
| Catalyst (NaOH) | P | A | P | A | P | A | |||
| IL (g/L) | 93.10 | 6.30 | 126.10 | 7.40 | 155.56 | 43.60 | |||
| SL (g/L) | 6.84 | 2.52 | 5.91 | 3.75 | 5.42 | 2.09 | |||
| TL (g/L) | 99.94 | 8.82 | 132.01 | 11.15 | 160.98 | 45.69 | |||
| Residue in natura | Holocellulose (%) | α-Cellulose (%) | Hemicellulose (%) |
|---|---|---|---|
| This study | 57.81 ± 0.5 | 40.01 ± 0.5 | 17.81 ± 0.5 |
| Protásio et al. [57] | 53.87 | 27.91 | 25.96 |
| Liu et al. [58] | 57.13 | - | - |
| Parichanon et al. [59] | 68.73 | 37.55 | 31.18 |
| Alharbi et al. [60] | - | 46.00 | 16.00 |
| Gonçalves et al. [38] | - | 32.18 | 27.81 |
| Fleck et al. [61] | 61.00 | 44.98 | 16.02 |
| Autohydrolysis | |||
| This study 195 °C—20 min | 58.37 ± 0.5 | 47.97 ± 0.5 | 10.40 ± 0.5 |
| Gonçalves et al. [38] sequential NaClO2-CH4O2/AH 200 °C—50 min | 77.92 | 71.25 | 6.67 |
| Gonçalves et al. [30] 200 °C—30 min | 59.06 | 45.23 | 13.86 |
| Alkaline extraction | |||
| This study 55 °C—2 h—0.50 M [NaOH] | 58.08 ± 0.5 | 48.65 ± 0.5 | 9.43 ± 0.5 |
| Schiavon and Andrade [28] 1 h—5% [NaOH] | - | 40.98 | 8.85 |
| Schiavon and Andrade [28] 2 h—5% [NaOH] | - | 42.75 | 8.71 |
| Bezerra et al. [62] 121 °C—30 min—0.50 M [NaOH] | - | 38.40 | 14.90 |
| Din et al. [63] 121 °C—40 min—1.25 M [NaOH] | - | 40.14 | 12.64 |
| Organosolv | |||
| This study 185 °C—2 h—ethanol:water:NaOH * | 65.91 ± 0.5 | 55.25 ± 0.5 | 10.66 ± 0.5 |
| Padilha et al. [64] 130 °C—2 h—glycerol:water:H2SO4 * | - | 65.99 | 7.29 |
| Nascimento et al. [65] 110 °C—20 min—acetic acid | 55.10 | 38.00 | 17.10 |
| Nascimento et al. [66] 110 °C—3 h—acetic acid:HCl * | - | 52.00 | 23.00 |
| Functional Group | Chemical Structure | |||
|---|---|---|---|---|
| Autohydrolysis | Alkaline Extraction | Organosolv | ||
| 3337 | 3336 | 3336 | stretching O-H | cellulose |
| 2917–2851 | 2983–2920 | 2977–2884 | stretching C-H | cellulose e hemicellulose |
| 1730 | * | 1729 | COOH; C=O | hemicellulose e lignin |
| 1608 | 1604 | * | linkage C=C | lignin |
| 1512 | 1509 | 1509 | aromatic ring vibration | lignin |
| 1373 | * | 1380 | deformation C-H | cellulose e hemicellulose |
| 1328 | 1320 | 1320 | vibration O-H | cellulose |
| 1265 | 1266 | 1268 | carbonyl groups C=O | lignin |
| 1162 | 1162 | 1162 | vibrations C-O-C | cellulose e hemicellulose |
| 1035 | 1032 | 1028 | stretching C-O | cellulose e hemicellulose |
| 893 | 893 | 893 | stretching C-O-C | cellulose |
| Pretreatment | Index | ||
|---|---|---|---|
| HBI | TCI | LOI | |
| In natura | 0.8505 | 1.6206 | 0.8249 |
| Soxhlet | 0.9055 | 1.7606 | 0.8966 |
| Alkaline | 0.8303 | 1.6328 | 0.9372 |
| Organosolv | 1.2324 | 1.0076 | 0.8917 |
| Autohydrolysis | 0.4509 | 98.4246 | 0.7907 |
| Pre-Treatments | 1st Mass Loss (%) | 2nd Mass Loss (%) | 3rd Mass Loss (%) |
|---|---|---|---|
| 27–120 °C | 200–400 °C | >450 °C | |
| In natura | 8.90 | 38.90 | 36.02 |
| Autohydrolysis | 9.39 | 44.59 | 25.62 |
| Alkaline | 14.10 | 40.16 | 28.68 |
| Organosolv | 9.10 | 46.56 | 26.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, F.; Santana, H.E.P.; Jesus, M.; Mata, F.; Pires, P.; Vaz-Velho, M.; Silva, D.P.; Ruzene, D.S. Comparative Study of Pretreatments on Coconut Fiber for Efficient Isolation of Lignocellulosic Fractions. Sustainability 2024, 16, 4784. https://doi.org/10.3390/su16114784
Vieira F, Santana HEP, Jesus M, Mata F, Pires P, Vaz-Velho M, Silva DP, Ruzene DS. Comparative Study of Pretreatments on Coconut Fiber for Efficient Isolation of Lignocellulosic Fractions. Sustainability. 2024; 16(11):4784. https://doi.org/10.3390/su16114784
Chicago/Turabian StyleVieira, Fabrícia, Hortência E. P. Santana, Meirielly Jesus, Fernando Mata, Preciosa Pires, Manuela Vaz-Velho, Daniel Pereira Silva, and Denise Santos Ruzene. 2024. "Comparative Study of Pretreatments on Coconut Fiber for Efficient Isolation of Lignocellulosic Fractions" Sustainability 16, no. 11: 4784. https://doi.org/10.3390/su16114784








