A Mini Review on Liquid Phase Catalytic Exchange for Hydrogen Isotope Separation: Current Status and Future Potential
Abstract
1. Introduction
2. Current Status of Hydrogen Isotope Separation Technologies
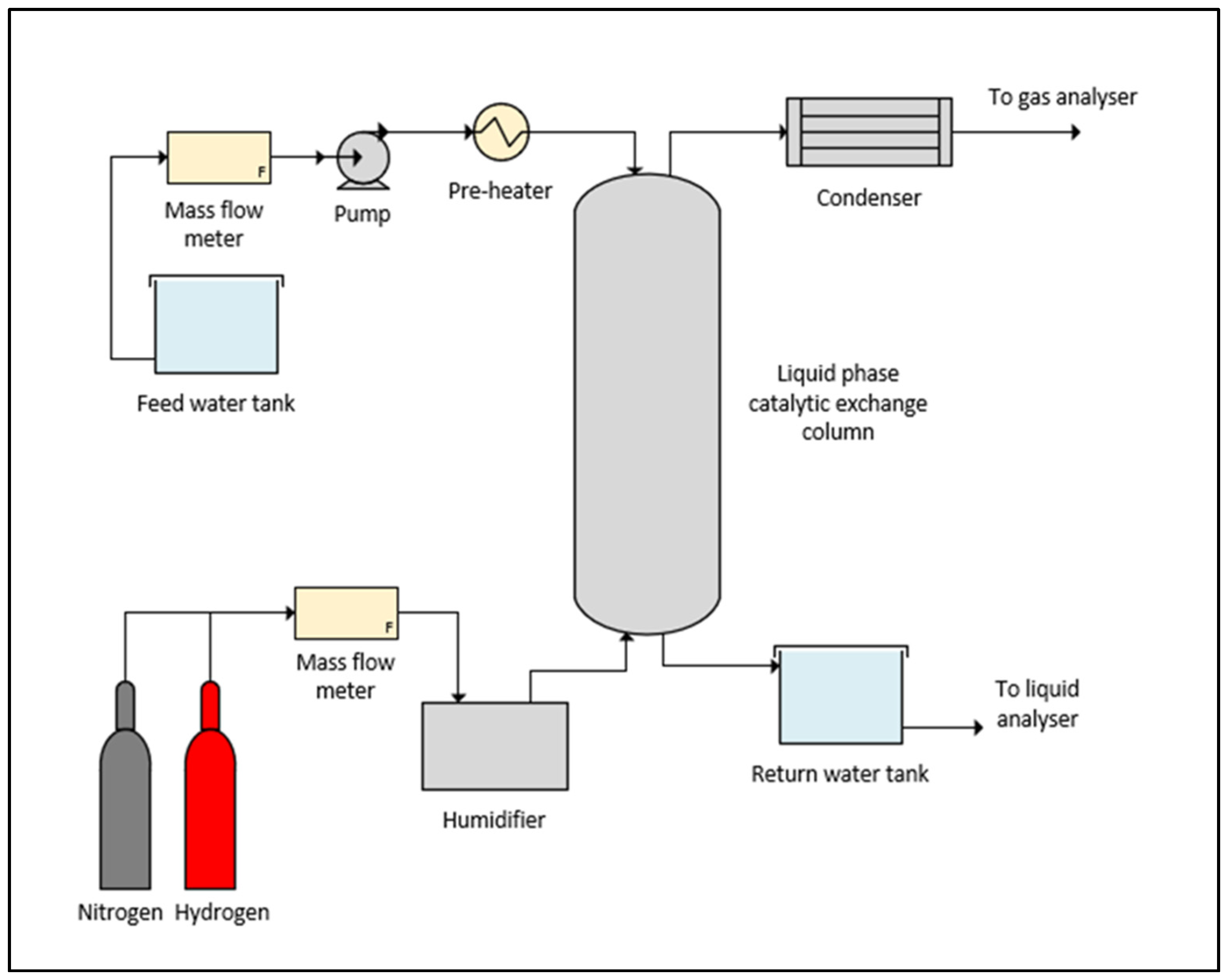
3. Hydrogen Isotope Separation via Liquid Phase Catalytic Exchange
3.1. Fundamentals of Hydrogen Isotope Separation Using LPCE
3.2. Factors Affecting LPCE Performance
3.2.1. Active Metal of Hydrophobic Catalyst
3.2.2. Catalyst Support
| Metal | Support | Coating | Pt Particle Size (nm) | Contact Angle | Specific Surface Area, SBET (m2/g) | Stability | Performance | References |
|---|---|---|---|---|---|---|---|---|
| Pt | Modified carbon nitride (C3N4) | PDMS | Pt/AC: 3.01 ± 0.36 nm Pt/s-C3N4-7: 0.82 ± 0.08 nm | Pt/s-C3N4-7: 159.3° | Pt/s-C3N4-7: 65.7 m2/g | 1.25 day | Pt/AC: ~98% Pt/C3N4-7: 98% at T = 80 °C; G = 12 mL/min; L = 2 mL/h | [48] |
| Pt | 1. Multiwalled carbon nanotubes (MWNTs) 2. Activated carbon (AC) 3. Vulcan XC-72 carbon black (XC) | PTFE | Pt/MWNT: 2.36 ± 0.8 nm Pt/AC: 1.98 ± 0.5 nm Pt/XC:2 ± 0.52 nm | N/A | Pt/MWNT: 233 m2/g Pt/AC: 950 m2/g Pt/XC: 230 m2/g | N/A | Column efficiency: Pt/AC: ~75% Pt/MWNTs: ~88% Pt/XC: ~85% at T = 50 °C; G = 1 L/min | [56] |
| Pt | Chromium-based metal–organic frameworks (MIL-101) | PVDF | MIL-101:pt particle size: 2.9 nm styrene divinylbenzene copolymer (SDB): pt particle size 3.3 nm | Pt/SDB: 132° Pt/MIL-101: 156° | Pt/SDB: 103 m2/g Pt/MIL-101: 289 m2/g | 30 days | Column efficiency: Pt/SDB: ~90% Pt/MIL-101/PVDF: ~98% at T = 60 °C; G = 0.5 L/min | [59] |
| Pt | Santa Barbara 15 (SBA-15)-tetramethyldisilazane | Polyvinylidene fluoride (PVDF) | 2.03 nm | 158° | N/A | 30 days | Column efficiency: Pt/SBA-15-tetramethyldisilazane: ~72% Pt/SDB: ~60% at T = 70 °C; G = 300 mL/min; L = 0.25 mL/min | [62] |
| Pt | Dual modified graphene x-S-NH2-GR (x is amount of trimethoxyoctylsilane) | Poly(dimethylsiloxane) PDMS | 1. Pt/NH2-GR: 1.95 ± 0.29 nm 2. Pt/200-S-NH2-GR: 1.85 ± 0.35 nm | Pt/NH2-GR: 135° Pt/200-S-NH2-GR: 150° | N/A | 600 min | Column efficiency: 5 wt% Pt/C: ~87.5% 3.2 wt% Pt/200-S-NH2-Gr: ~90% at T = 80 °C | [63] |
3.2.3. Operating Temperature
3.2.4. Molar Feed Ratio (G/L)
3.3. Potential Future Direction of Hydrogen Isotope Separation via LPCE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osakabe, M.; Takahashi, H.; Yamada, H.; Tanaka, K.; Kobayashi, T.; Ida, K.; Ohdachi, S.; Varela, J.; Ogawa, K.; Kobayashi, M.; et al. Recent results from deuterium experiments on the large helical device and their contribution to fusion reactor development. Nucl. Fusion 2022, 62, 042019. [Google Scholar] [CrossRef]
- Urrestizala, M.; Azkurreta, J.; Alegría, N.; Peñalva, I. Isotope effect of hydrogen and deuterium permeability and diffusivity in fusion reactor materials. A literature review. Fusion Eng. Des. 2023, 194, 113915. [Google Scholar] [CrossRef]
- Hu, X.; Tan, L.; Wang, K.; Massey, C.P.; Hoelzer, D.T.; Katoh, Y. Deuterium retention in advanced steels for fusion reactor structural application. J. Nucl. Mater. 2019, 516, 144–151. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium-and tritium-labelled compounds: Applications in the life sciences. Angew. Chem. Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Kushner, D.; Baker, A.; Dunstall, T. Pharmacological uses and perspectives of heavy water and deuterated compounds. Can. J. Physiol. Pharmacol. 1999, 77, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, R.M.C.; Maxwell, B.D.; Pirali, T. Deuterium in drug discovery: Progress, opportunities and challenges. Nat. Rev. Drug Discov. 2023, 22, 562–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-L.; Zhang, W.; Rao, G.-W. Clinical Application and Synthesis Methods of Deuterated Drugs. Curr. Med. Chem. 2023, 30, 4096–4129. [Google Scholar] [CrossRef] [PubMed]
- Killian, W.G.; Norfleet, A.T.; Lira, C.T. Densities of Selected Deuterated Solvents. J. Chem. Eng. Data 2022, 67, 893–901. [Google Scholar] [CrossRef]
- Paralovo, S.L.; Spruyt, M.; Lauwers, J.; Swinnen, R.; Lazarov, B.; Stranger, M.; Laverge, J. Developing a new passive tracer gas test for air change rate measurement. Int. J. Vent. 2021, 20, 276–286. [Google Scholar] [CrossRef]
- Bowerman, B.; Czajkowski, C. Determination of the Chemical Form of Tritium in Self-Luminous Signs; Nuclear Regulatory Commission: Washington, DC, USA, 1990; Div. of Industrial and Medical Nuclear Safety.
- Kim, K.S.; Lee, S.K.; Chung, E.S.; Kim, K.S.; Kim, W.S.; Nam, G.J. Tritium application: Self-luminous glass tube (SLGT). Key Eng. Mater. 2004, 277, 698–702. [Google Scholar]
- Abdou, M.; Riva, M.; Ying, A.; Day, C.; Loarte, A.; Baylor, L.; Humrickhouse, P.; Fuerst, T.F.; Cho, S. Physics and technology considerations for the deuterium–tritium fuel cycle and conditions for tritium fuel self sufficiency. Nucl. Fusion 2020, 61, 013001. [Google Scholar] [CrossRef]
- Kiptily, V. On the core deuterium–tritium fuel ratio and temperature measurements in DEMO. Nucl. Fusion 2015, 55, 023008. [Google Scholar] [CrossRef]
- Miyamae, K.; Yamada, H.; Kasada, R.; Konishi, S.; Sakamoto, R. Fuel flow and stock during deuterium-deuterium start-up of fusion reactor with advanced plasma model. Fusion Eng. Des. 2020, 160, 111794. [Google Scholar] [CrossRef]
- Ananyev, S.S.; Spitsyn, A.V.; Kuteev, B.V. Concept of DT fuel cycle for a fusion neutron source DEMO-FNS. Fusion Eng. Des. 2016, 109, 57–60. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Bhanja, K.; Mohan, S. Simulation studies of the characteristics of a cryogenic distillation column for hydrogen isotope separation. Int. J. Hydrog. Energy 2016, 41, 5003–5018. [Google Scholar] [CrossRef]
- Lazar, A.; Brad, S.; Vijulie, M.; Oubraham, A. Cryogenic distillation experimental stands for hydrogen isotopes separation. Fusion Eng. Des. 2019, 146, 1998–2001. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Ortman, M.S.; Heung, L.K.; Nobile, A.; Rabun, R.L. Tritium processing at the Savannah River Site: Present and future. J. Vac. Sci. Technol. A Vac. Surf. Film. 1990, 8, 2881–2889. [Google Scholar] [CrossRef]
- Bornea, A.; Zamfirache, M.; Stefanescu, I.; Preda, A.; Balteanu, O.; Stefan, I. Investigation related to hydrogen isotopes separation by cryogenic distillation. Fusion Sci. Technol. 2008, 54, 426–429. [Google Scholar] [CrossRef]
- Hammerli, M. CANDU with hydrogen. Int. J. Hydrogen Energy 1983, 8, 269–280. [Google Scholar] [CrossRef]
- Park, D.; Urm, J.J.; Lee, J.-U.; Chang, M.H.; Lee, J.M. Dynamic optimization of cryogenic distillation operation for hydrogen isotope separation in fusion power plant. Int. J. Hydrogen Energy 2021, 46, 24135–24148. [Google Scholar] [CrossRef]
- Ana, R.G.; Cristescu, I.; Dörr, L.; Michling, R.; Welte, S.; Wurster, W. Design and experimental activities in view of Water Detritiation–Isotopic Separation Systems combination in TRENTA facility. Fusion Eng. Des. 2009, 84, 398–403. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Li, Z.; Wang, J. Research Progress of Hydrogen Production Technology and Related Catalysts by Electrolysis of Water. Molecules 2023, 28, 5010. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhang, M.; Wei, F.; Liang, C.; Liang, J.; Li, J.; Cheng, W.; Deng, K.; Liu, W. Gold as an efficient hydrogen isotope separation catalyst in proton exchange membrane water electrolysis. Int. J. Hydrogen Energy 2022, 47, 26842–26849. [Google Scholar] [CrossRef]
- Yasuda, S.; Matsushima, H.; Harada, K.; Tanii, R.; Terasawa, T.-O.; Yano, M.; Asaoka, H.; Gueriba, J.S.; Diño, W.A.; Fukutani, K. Efficient hydrogen isotope separation by tunneling effect using graphene-based heterogeneous electrocatalysts in electrochemical hydrogen isotope pumping. ACS Nano 2022, 16, 14362–14369. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Y.; Guo, C.; Sun, W.; Wu, Y.; Xu, Y.; Liu, T.; Wang, Y. Dendritic sp carbon-conjugated benzothiadiazole-based polymers with synergistic multi-active groups for high-performance lithium organic batteries. Angew. Chem. Int. Ed. 2024, 63, e202316208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pham, T.H.M.; Ko, Y.; Li, M.; Yang, S.; Koolen, C.D.; Zhong, L.; Luo, W.; Züttel, A. Tandem effect of Ag@C@Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Koolen, C.D.; Luo, W.; Zuettel, A. From single crystal to single atom catalysts: Structural factors influencing the performance of metal catalysts for CO2 electroreduction. ACS Catal. 2022, 13, 948–973. [Google Scholar] [CrossRef]
- Oh, H.; Hirscher, M. Quantum sieving for separation of hydrogen isotopes using MOFs. Eur. J. Inorg. Chem. 2016, 2016, 4278–4289. [Google Scholar] [CrossRef]
- Cao, D.; Huang, H.; Lan, Y.; Chen, X.; Yang, Q.; Liu, D.; Gong, Y.; Xiao, C.; Zhong, C.; Peng, S. Ultrahigh effective H2/D2 separation in an ultramicroporous metal–organic framework material through quantum sieving. J. Mater. Chem. A 2018, 6, 19954–19959. [Google Scholar] [CrossRef]
- Liu, D.; Wang, W.; Mi, J.; Zhong, C.; Yang, Q.; Wu, D. Quantum sieving in metal–organic frameworks: A computational study. Ind. Eng. Chem. Res. 2012, 51, 434–442. [Google Scholar] [CrossRef]
- Xiong, R.; Chen, J.; Zhang, L.; Li, P.; Yan, X.; Song, Y.; Luo, W.; Tang, T.; Sang, G.; Hirscher, M. Hydrogen isotopes separation in Ag (I) exchanged ZSM-5 zeolite through strong chemical affinity quantum sieving. Microporous Mesoporous Mater. 2021, 313, 110820. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, L.; Li, P.; Luo, W.; Tang, T.; Ao, B.; Sang, G.; Chen, C.; Yan, X.; Chen, J.; et al. Highly effective hydrogen isotope separation through dihydrogen bond on Cu (I)-exchanged zeolites well above liquid nitrogen temperature. Chem. Eng. J. 2020, 391, 123485. [Google Scholar] [CrossRef]
- Jia, Q.; Hu, S.; Liu, Y. Hydrophobically modified ceramic-supported Pt-particle catalyst for enhanced water–hydrogen exchange reactions. Fusion Eng. Des. 2024, 199, 114154. [Google Scholar] [CrossRef]
- Bhartiya, S.; Kohli, D.K.; Singh, A.; Singh, R.; Singh, M.K. Investigation of Pt-Ti doped carbon aerogel as bi-metallic catalyst for H/D exchange process. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017. [Google Scholar]
- Li, P.; Guo, L.; Xiong, R.; Luo, J.; Wen, M.; Yao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Tang, T. Separation process study of liquid phase catalytic exchange reaction based on the Pt/C/PTFE catalysts. Chin. J. Chem. Eng. 2019, 27, 1837–1845. [Google Scholar] [CrossRef]
- Ye, L.; Luo, D.; Tang, T.; Yang, W.; Yang, Y. Hydrogen isotope separation in hydrophobic catalysts between hydrogen and liquid water. Fusion Eng. Des. 2015, 100, 576–580. [Google Scholar] [CrossRef]
- Rolston, J.H.; Hartog, J.D.; Butler, J.P. The deuterium isotope separation factor between hydrogen and liquid water. J. Phys. Chem. 1976, 80, 1064–1067. [Google Scholar] [CrossRef]
- Huang, F. Effects of various factors on the hydrogen isotope separation efficiency of a novel hydrophobic platinum-polytetrafluoroethylene catalyst. Int. J. Hydrogen Energy 2018, 43, 1718–1724. [Google Scholar] [CrossRef]
- Harrison, T. Design of a demonstration tritium recovery plant for Chalk River. In Proceedings of the Tritium Technology in Fission, Fusion, and Isotopic Applications, Dayton, OH, USA, 29 April–1 May 1980. [Google Scholar]
- Holtslander, W.; Harrison, T.; Gallagher, J. The chalk river tritium extraction plant construction and early commissioning. Fusion Technol. 1988, 14, 484–488. [Google Scholar] [CrossRef]
- Holtslander, W.; Harrison, T.; Spagnolo, D. The chalk river tritium extraction plant. Fusion Eng. Des. 1990, 12, 357–363. [Google Scholar] [CrossRef]
- Alekseev, I.A.; Bondarenko, S.D.; Fedorchenko, O.A.; Vasyanina, T.V.; Konoplev, K.A.; Arkhipov, E.A.; Uborsky, V.V. Fifteen years of operation of CECE experimental industrial plant in PNPI. Fusion Sci. Technol. 2011, 60, 1117–1120. [Google Scholar] [CrossRef]
- Mustafa, N.F.A.; Shariff, A.M.; Tay, W.H.; Halim, H.N.A.; Yusof, S.M.M. Mass transfer performance study for CO2 absorption into non-precipitated potassium carbonate promoted with glycine using packed absorption column. Sustainability 2020, 12, 3873. [Google Scholar] [CrossRef]
- Bornea, A.; Zamfirache, M.; Stefanescu, I.; Stefan, L. Two different approaches for performance evaluation of catalytic packing. Smart Energy Sustain. Environ. 2017, 20, 5. [Google Scholar]
- Sagert, N.H.; Pouteau, R.M.L. Pouteau, Hydrogen–water deuterium exchange over unsupported group VIII noble metals. Can. J. Chem. 1974, 52, 2960–2967. [Google Scholar] [CrossRef]
- Fu, Q.; Xin, F.; Yin, X.; Song, Y.; Xu, Y. A highly efficient and stable catalyst for liquid phase catalytic exchange of hydrogen isotopes in a microchannel reactor. Int. J. Hydrogen Energy 2021, 46, 22446–22453. [Google Scholar] [CrossRef]
- Kumar, R.; Mohan, S.; Mahajani, S.M. Reactive stripping for the catalytic exchange of hydrogen isotopes. Ind. Eng. Chem. Res. 2013, 52, 10935–10950. [Google Scholar] [CrossRef]
- Meyer, E.F.; Burwell, R.L. The Reaction between Deuterium and Dialkylacetylenes on Palladium Catalysts. J. Am. Chem. Soc. 1963, 85, 2877–2880. [Google Scholar] [CrossRef]
- Burwell, R.L.; Tuxworth, R.H. Isotopic Exchange between Deuterium and Hydrocarbons on Nickel–Silica Catalysts. J. Phys. Chem. 1956, 60, 1043–1049. [Google Scholar] [CrossRef]
- Rae, H.K. Selecting Heavy Water Processes. In Separation of Hydrogen Isotopes; American Chemical Society: Washington, DC, USA, 1978; pp. 1–26. [Google Scholar]
- Stevens, W. Process and Catalyst for Enriching A Fluid with Hydrogen Isotopes. Canadian Patent No. 907292, 1972. [Google Scholar]
- Hu, S.; Xiong, L.; Hou, J.; Weng, K.; Luo, Y.; Yang, T. The roles of metals and their oxide species in hydrophobic Pt–Ru catalysts for the interphase H/D isotope separation. Int. J. Hydrogen Energy 2010, 35, 10118–10126. [Google Scholar] [CrossRef]
- Ye, L.; Luo, D.; Yang, W.; Guo, W.; Xu, Q.; Luo, L. Preparation and characterization of hydrophobic carbon-supported Pt3M (M = Fe, Co, Ni and Cr) bimetals for H/D isotope separation between hydrogen and water. Int. J. Hydrogen Energy 2014, 39, 13793–13799. [Google Scholar] [CrossRef]
- Hu, S.; Hou, J.; Xiong, L.; Weng, K.; Yang, T.; Luo, Y. Hydrophobic Pt catalysts with different carbon substrates for the interphase hydrogen isotope separation. Sep. Purif. Technol. 2011, 77, 214–219. [Google Scholar] [CrossRef]
- Vasut, F.; Oubraham, A.; Soare, A.M.; Marinoiu, A.; Ion-Ebrasu, D.; Dragan, M. Platinum supported on graphene-PTFE as catalysts for isotopic exchange in a detritiation plant. Fusion Eng. Des. 2019, 146, 149–152. [Google Scholar] [CrossRef]
- Fu, X.; Hou, J.; Chen, C.; Li, J.; Yue, L.; Chen, X.; Zhao, L.; Ran, G.; Xia, X.; Gong, Y.; et al. Superhydrophobic and superaerophilic hierarchical Pt@ MIL-101/PVDF composite for hydrogen water isotope exchange reactions. J. Hazard. Mater. 2019, 380, 120904. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Ionita, G.; Stefanescu, I.; Varlam, C.; Dobrinescu, D.; Faurescu, I. Improved characteristics of hydrophobic polytetrafluoroethylene–platinum catalysts for tritium recovery from tritiated water. Fusion Eng. Des. 2008, 83, 1392–1394. [Google Scholar] [CrossRef]
- Molina, R.; Vílchez, A.; Canal, C.; Esquena, J. Wetting properties of polystyrene/divinylbenzene crosslinked porous polymers obtained using W/O highly concentrated emulsions as templates. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2009, 41, 371–377. [Google Scholar] [CrossRef]
- Lu, Z.; Fu, X.; Li, J.; Hou, J.; Ran, G.; Xiao, C.; Wang, X. Superhydrophobic Pt@ SBA-15 catalyst for tritium separation in liquid phase catalytic exchange. Int. J. Hydrogen Energy 2023, 48, 1979–1987. [Google Scholar] [CrossRef]
- Ye, L.; Luo, D.; Tang, T.; Yang, W.; Zhao, P. Process simulation for hydrogen/deuterium exchange in a packed column. Int. J. Hydrogen Energy 2014, 39, 6604–6609. [Google Scholar] [CrossRef]
- Butler, J. Hydrogen isotope separation by catalyzed exchange between hydrogen and liquid water. Sep. Sci. Technol. 1980, 15, 371–396. [Google Scholar] [CrossRef]
- Kim, K.; Lee, M.; Paek, S.; Yim, S.; Ahn, D.; Chung, H. Operational analysis of a liquid phase catalytic exchange column for a detritiation of heavy water. Sep. Purif. Technol. 2007, 54, 410–414. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, L.; Ren, X.; Wang, C.; Luo, Y. Pt–Ir binary hydrophobic catalysts: Effects of Ir content and particle size on catalytic performance for liquid phase catalytic exchange. Int. J. Hydrogen Energy 2009, 34, 8723–8732. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, F.; Jiang, Y.; Yin, X. Liquid-phase catalytic exchange of hydrogen isotopes over platinum on dual-modified graphene. ACS Appl. Mater. Interfaces 2021, 13, 31660–31667. [Google Scholar] [CrossRef] [PubMed]
- Vauche, L.; Guillemaud, G.; Barbosa, J.-C.L.; Di Cioccio, L. Cradle-to-Gate Life Cycle Assessment (LCA) of GaN Power Semiconductor Device. Sustainability 2024, 16, 901. [Google Scholar] [CrossRef]
- Smit, R.; Helmers, E.; Schwingshackl, M.; Opetnik, M.; Kennedy, D. Greenhouse Gas Emissions Performance of Electric, Hydrogen and Fossil-Fuelled Freight Trucks with Uncertainty Estimates Using a Probabilistic Life-Cycle Assessment (pLCA). Sustainability 2024, 16, 762. [Google Scholar] [CrossRef]
- Go, Y.-J.; Kang, D.-H.; Park, H.-J.; Lee, J.-H.; Shim, J.-K. Meta-Analysis of Life Cycle Assessment Studies for Polyethylene Terephthalate Water Bottle System. Sustainability 2024, 16, 535. [Google Scholar] [CrossRef]
- Seo, Y.; An, J.; Park, E.; Kim, J.; Cho, M.; Han, S.; Lee, J. Technical–Economic Analysis for Ammonia Ocean Transportation Using an Ammonia-Fueled Carrier. Sustainability 2024, 16, 827. [Google Scholar] [CrossRef]
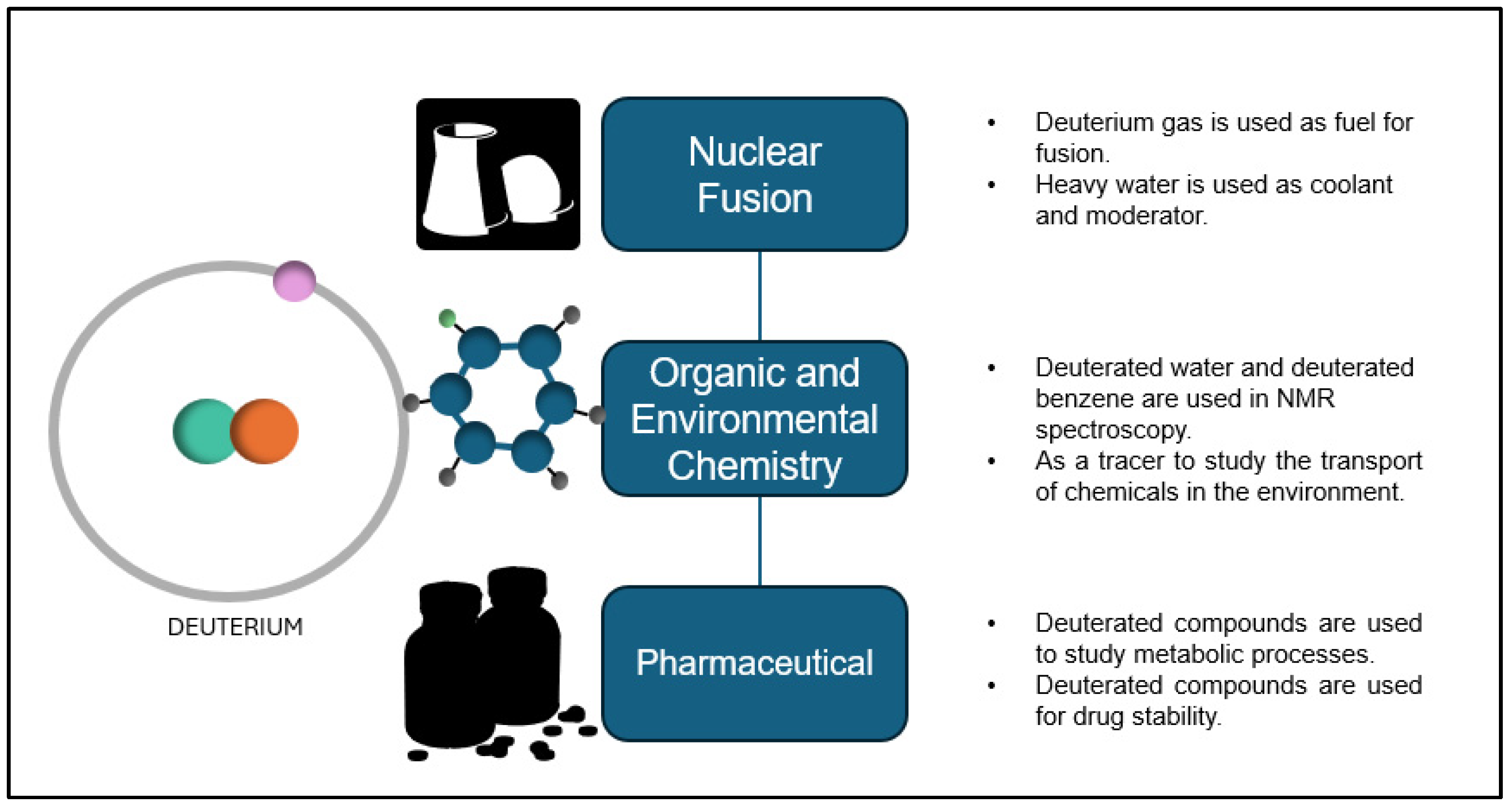



| Technologies | Cryogenic Distillation | Quantum Sieving | Electrolysis | Liquid Phase Catalytic Exchange |
|---|---|---|---|---|
| Advantages | 1. Produces high-purity isotopes. 2. Capable of processing large quantities of feed. 3. Can be performed at ambient pressure. | 1. Can be performed at ambient pressure. 2. More energy-efficient as compared to cryogenic distillation. | 1. Easy to scale up since it is a simple process. 2. High precision of separation. | 1. High separation efficiency with high purity. 2. Can be performed at ambient pressure and low temperature, resulting in low energy consumption. 3. Capable of processing large quantities of feed. |
| Disadvantages and Challenges | 1. Energy-intensive. 2. Requires expensive equipment such as cryogenic distillation columns and cold boxes. | 1. Complex and expensive synthesis methodologies of quantum sieving materials, which limits its scalability. 2. The selectivity of quantum sieving is temperature-dependent. 3. The practical application of quantum sieving is still in the early stage of development. | 1. High energy consumption. 2. Complex and expensive equipment. 3. Highly dependent on the availability and cost of electricity. | 1. The separation efficiency is highly affected by the performance of the catalyst. 2. The cost of the commercial catalyst used, which is platinum, is quite expensive. |
| First Metal | Second Metal | Support | Hydrophobic Coating | Pt Particle Size (nm) | Performance | Main Findings | References |
|---|---|---|---|---|---|---|---|
| Pt | Ru | C | PTFE | Pt/C: 1.9 ± 0.4 nm Pt0.5Ru0.5/C: 1.9 ± 0.5 nm Pt/RuO2/C: 2.5 ± 0.6 nm | Column efficiency: Pt/C: ~77% Pt0.5Ru0.5/C and Pt/RuO2/C: ~85% at G = 2 L/min |
| [54] |
| Pt | 1. Fe 2. Co 3. Ni 4. Cr | C | Teflon | 1. Pt/Fe: 2.3 nm 2. Pt/Co: 2.6 nm 3. Pt/Ni: 2.2 nm 4. Pt/Cr: 2.4 nm 5. Pt/C: 2.5 nm | Column efficiency: 1. Pt/Fe: ~59% 2. Pt/Co: ~52% 3. Pt/Ni: ~53% 4. Pt/Cr: ~67% 5. Pt/C: ~41% at G = 2 L/min |
| [55] |
| Pt | Fe | C | PTFE | Pt/C: 1.9 nm Pt3Fe/C: 2.0 nm | Column efficiency: Pt/C: ~58% Pt3Fe/C: ~75% at G = 2 L/min |
| [56] |
| Pt | Ir | C | PTFE | Pt/C: 2.1 nm Pt4Ir1/C: 2.2 nm | Column efficiency: Pt/C: ~73% Pt4Ir1/: ~87% at G = 2 L/min |
| [57] |
| Catalyst | Operating Pressure | Operating Temperature | G/L | Optimized Condition Identified | Main Findings | References |
|---|---|---|---|---|---|---|
| 0.8 wt% Pt/C/PTFE | 1 atm | 30–90 °C | 0.8–3.5 | Temperature: 60–80 °C G/L: 1.5–2.5 |
| [37] |
| 0.8 wt% Pt/PTFE | N/A | 30–90 °C | N/A | Temperature: 80 °C |
| [40] |
| 10 wt% Pt/PTFE | 1 atm | 20–80 °C | 0.5–4 | Temperature: 70 °C |
| [64] |
| 0.37 wt% Pt/C/Teflon | 1 atm | 25–60 °C | N/A | Temperature: 60 °C |
| [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhd Yusof, S.M.; Lock, S.S.M.; Abdul Talib, N.N.; Seng, L.C. A Mini Review on Liquid Phase Catalytic Exchange for Hydrogen Isotope Separation: Current Status and Future Potential. Sustainability 2024, 16, 4796. https://doi.org/10.3390/su16114796
Mhd Yusof SM, Lock SSM, Abdul Talib NN, Seng LC. A Mini Review on Liquid Phase Catalytic Exchange for Hydrogen Isotope Separation: Current Status and Future Potential. Sustainability. 2024; 16(11):4796. https://doi.org/10.3390/su16114796
Chicago/Turabian StyleMhd Yusof, Siti Munirah, Serene Sow Mun Lock, Nur Najwa Abdul Talib, and Liew Chin Seng. 2024. "A Mini Review on Liquid Phase Catalytic Exchange for Hydrogen Isotope Separation: Current Status and Future Potential" Sustainability 16, no. 11: 4796. https://doi.org/10.3390/su16114796
APA StyleMhd Yusof, S. M., Lock, S. S. M., Abdul Talib, N. N., & Seng, L. C. (2024). A Mini Review on Liquid Phase Catalytic Exchange for Hydrogen Isotope Separation: Current Status and Future Potential. Sustainability, 16(11), 4796. https://doi.org/10.3390/su16114796







