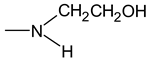
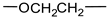Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants
Abstract
:1. Introduction
2. Clay Minerals from the Smectite Group and Organoclays Made from Them
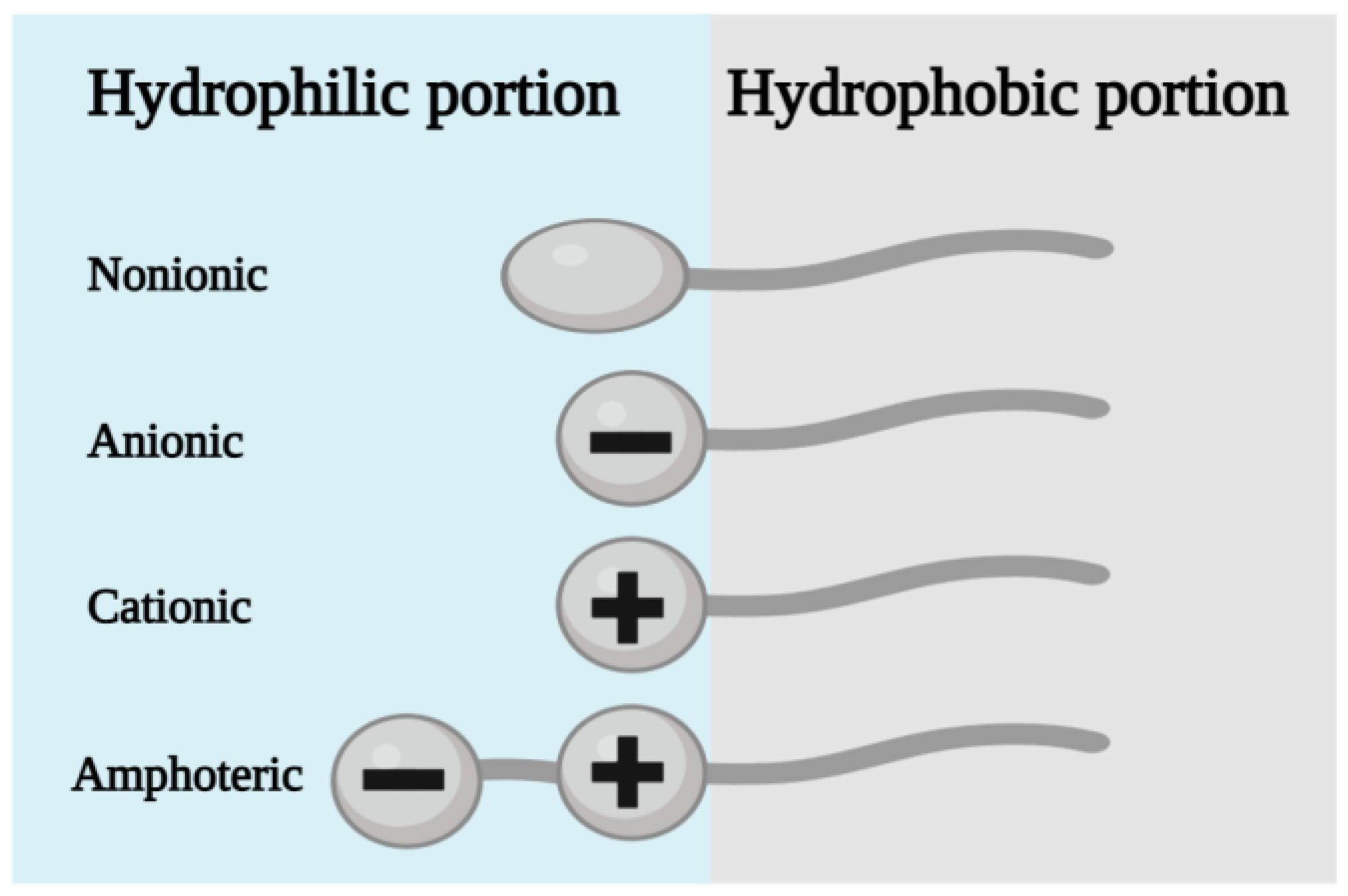
3. Types of Surfactants Used in the Production of Organoclays and Their Sorption Properties
3.1. Cationic Surfactants
- -
- aliphatic and aromatic primary, secondary, and tertiary amines, as well as their salts;
- -
- quaternary ammonium salts (QAS), including salts of pyridine-based compounds;
- -
- oxides of tertiary amines.
3.2. Anionic Surfactants
- -
- Soaps: RCOONa, RCOOK;
- -
- Alkyl sulfates and alkyl phosphates: ROSO3Me, ROPO3Me2;
- -
- Alkylaryl sulfonates (most often alkyl benzene sulfonates, RC6H4SO3Me);
- -
- Alkyl sulfonates and alkyl phosphonates: RSO3Me, RPO3Me2;
- -
- Alkyl sulfosuccinates;
- -
- Alkyl ethoxy sulfates and alkyl ethoxy phosphates.
3.3. Amphoteric Surfactants
3.4. Nonionic Surfactants
- -
- oxyalkylated primary and secondary fatty alcohols;
- -
- polyethylene glycol ethers of alkylcarboxylic acids;
- -
- oxyalkylated alkylphenols;
- -
- pluronics;
- -
- glycerides, glucosides, saccharides, etc. [62].
- -
- Hydrophilic groups in nonionic surfactants can include the following:
- -
- ethylene oxide
- -
- ethanolamine
- -
- diethanolamineet al.

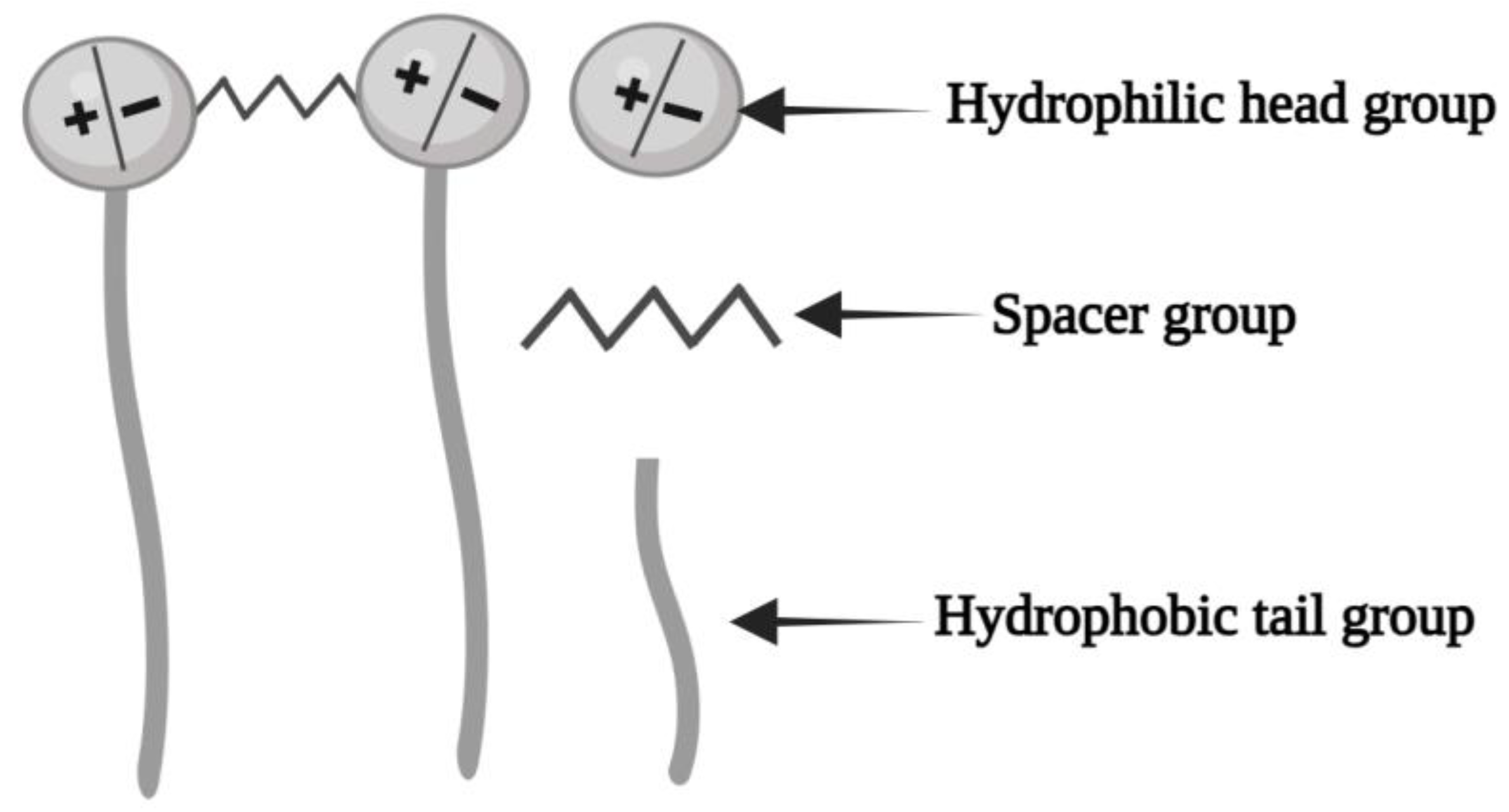
3.5. Gemini Surfactants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobrodeev, O.P. Technogenesis is a powerful geochemical force of the biosphere. Priroda 1978, 11, 88–92. (In Russian) [Google Scholar]
- Vasilyeva, G.; Mikhedova, E.; Zinnatshina, L.; Strijakova, E.; Akhmetov, L.; Sushkova, S.; Ortega-Calvo, J.J. Use of natural sorbents for accelerated bioremediation of grey forest soil contaminated with crude oil. Sci. Total Environ. 2022, 850, 157952. [Google Scholar] [CrossRef] [PubMed]
- Gertsen, M.M.; Perelomov, L.V.; Arlyapov, V.A.; Atroshchenko, Y.M.; Meshalkin, V.P.; Chistyakova, T.B.; Reverberi, A.P. Degradation of oil and petroleum products in water by bioorganic compositions based on humic acids. Energies 2023, 16, 5320. [Google Scholar] [CrossRef]
- Perelomov, L.V.; Atroshchenko, Y.M.; Minkina, T.M.; Perelomova, I.V.; Bauer, T.V.; Pinskij, D.L. Organogliny–novyj klass perspektivnyh sorbentov dlya remediacii himicheski zagryaznennyh ob”ektov okruzhayushchej sredy. Agrohimiya 2021, 8, 82–96. (In Russian) [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007; 561p. [Google Scholar]
- Nafees, M.; Waseem, A. Organoclays as sorbent material for phenolic compounds: A review. Clean-Soil. Air Water 2014, 42, 1500–1508. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Osipov, V.I.; Sokolov, V.H.; Rumyanceva, N.A. Mikrostruktura Glinistyh Porod; Nedra: Moscow, Russia, 1989; 211p. (In Russian) [Google Scholar]
- Pokid’ko, B.V.; Bukanova, E.F.; Tutorskij, I.A.; Il’ina, M.B. Vliyanie ionov kal’ciya na adsorbciyu ionnyh i neionnyh PAV na bentonite. Vestn. MITHT 2009, 4, 77–83. (In Russian) [Google Scholar]
- Fetter, G.; Bosch, P. Microwave effect on clay pillaring. In Pillared Clays and Related Catalysts; Gil, A., Korili, S.A., Trujillano, R., Vicente, M.A., Eds.; Springer: New York, NY, USA, 2010; pp. 1–21. [Google Scholar]
- Ammann, L.; Bergaya, F.; Lagaly, G. Determination of the cation exchange capacity of clays with copper complexes revisited. Clay Miner. 2005, 40, 441–453. [Google Scholar] [CrossRef]
- Theng, B.K.G. Polymer-clay nanocomposites. In Developments in Clay Science; Theng, B.K.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 201–241. [Google Scholar]
- Ray, S.S. Introduction to environmentally friendly polymer nanocomposites. In Environmentally Friendly Polymer Nanocomposites, Types, Processing and Properties, 1st ed.; Ray, S.S., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–24. [Google Scholar] [CrossRef]
- Sokolova, T.A.; Dronova, T.Y.; Tolpeshta, I.I. Glinistye Mineraly v Pochvah. Uchebnoe Posobie; Grif i K.: Tula, Russia, 2005; 336p. (In Russian) [Google Scholar]
- Hussain, S.T.; Ali, S.A.K. Removal of heavy metal by ion exchange using bentonite clay. J. Ecol. Eng. 2020, 22, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Bhattacharyya, K.G. Treatment of water contaminated with Pb(II) and Cd(II) by adsorption on kaolinite, montmorillonite and their acid-activated forms. Indian J. Chem. Tech. 2009, 16, 457–470. [Google Scholar]
- Liu, H.; Fu, T.; Sarwar, M.T.; Yang, H. Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. App. Clay Sci. 2023, 232, 106796. [Google Scholar] [CrossRef]
- Singh, B.K.; Um, W. Application of clay materials for sorption of radionuclides from waste solutions. Minerals 2023, 13, 239. [Google Scholar] [CrossRef]
- Satouh, S.; Martín, J.; Orta, M.d.M.; Medina-Carrasco, S.; Messikh, N.; Bougdah, N.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of polycyclic aromatic hydrocarbons by natural, synthetic and modified clays. Environments 2021, 8, 124. [Google Scholar] [CrossRef]
- Masini, J.C.; Abate, G. Guidelines to study the adsorption of pesticides onto clay minerals aiming at a straight for evaluation of their removal performance. Minerals 2021, 11, 1282. [Google Scholar] [CrossRef]
- Borchardt, G. Smectites. In Minerals in Soil Environments, 2nd ed.; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 1989; pp. 675–727. [Google Scholar]
- Dehmani, Y.; Ed-Dra, A.; Zennouhi, O.; Bouymajane, A.; Rhazi Filali, F.; Nassiri, L.; Abouarnadasse, S. Chemical characterization and adsorption of oil mill wastewater on Moroccan clay in order to be used in the agricultural field. Heliyon 2020, 6, E03164. [Google Scholar] [CrossRef] [PubMed]
- Guégan, R. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2018, 22, 132–141. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Biswas, J.K.; Vithanage, M.; Bharnagar, A.; et al. Clay-polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2020, 383, 121125. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nano Composite Materials: Environmental and Pharmaceutical Applications; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–127. [Google Scholar]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral-organic interactions. In Handbook of Clay Science. Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505. [Google Scholar]
- Brixie, J.M.; Boyd, S.A. Treatment of contaminated soils with organoclays to reduce leachable pentachlorophenol. J. Environ. Qual. 1994, 23, 1283–1290. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. Dev. Clay Sci. 2006, 1, 1–18. [Google Scholar] [CrossRef]
- Moazed, H.; Viraraghavan, T. Removal of oil from waterby bentonite organoclay. Practic. Period. Hazard. Toxic Radioact. Waste Manag. 2005, 9, 130–134. [Google Scholar] [CrossRef]
- de Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. App. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ion. 1986, 22, 43–51. [Google Scholar] [CrossRef]
- Mansoor, Y.; Atghia, S.V.; Zamanloo, M.R.; Imanzadeh, G.; Sirousazar, M. Polymer–clay nanocomposites: Free-radical grafting of polyacrylamide onto organophilic montmorillonite. Eur. Polym. J. 2010, 46, 1844–1853. [Google Scholar] [CrossRef]
- Herrera, N.N.; Letoffe, J.M.; Putaux, J.L.; David, L.; Lami, E.B. Aqueous dispersions of silane-functionalized laponite clay platelets. A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir 2004, 20, 1564–1571. [Google Scholar] [CrossRef]
- Dean, K.M.; Bateman, S.A.; Simons, R. A comparative study of UV active silane-grafted and ion-exchanged organo-clay for application in photocurable urethane acrylate nano- and micro-composites. Polymer 2007, 48, 2231–2240. [Google Scholar] [CrossRef]
- Liu, P.; Guo, J. Polyacrylamide grafted attapulgite (PAM-ATP) via surface-initiated atom transfer radical polymerization (SI-ATRP) for removal of Hg(II) ion and dyes. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 498–503. [Google Scholar] [CrossRef]
- Gurses, A. Introduction to Polymer-Clay Nanocomposites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; 360p. [Google Scholar]
- Barrer, R.M.; MacLeod, D.M. Intercalation and sorption by montmorillonite. Transact. Faraday Soc. 1954, 50, 980–989. [Google Scholar] [CrossRef]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Structure of organoclays—An x-ray diffraction and thermogravimetric analysis study. J. Colloids Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef]
- He, H.; Ray, F.L.; Zhu, J. Infrared study of HDTMA+ intercalated montmorillonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; He, H.; Zhu, L.; Wen, X.; Deng, F. Characterization of organic phases in the interlayer of montmorillonite using FTIR and 13C NMR. J. Colloids Interface Sci. 2005, 286, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Dai, Y.; Long, H.; Zhu, N.; Li, P.; Wu, J.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr (II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Borja, M.; Dutta, P. Fatty acids in layered metal hydroxides: Membrane-like structure and dynamics. J. Phys. Chem. 1992, 96, 5434–5444. [Google Scholar] [CrossRef]
- Fröscher, A.; Hasse, H. Adsorption of oleic acid dissolved in isopropanol–water mixtures on hydrotalcite. Adsorpt. Sci. Technol. 2018, 36, 919–926. [Google Scholar] [CrossRef]
- Lin, S.H.; Juang, R.S. Heavy metal removal from water by sorption using surfactant- modified montmorillonite. J. Hazard. Mat. 2002, 92, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Handa, T.; Kuroda, K.; Kato, C. Formation of organoammonium-montmorillonites by solid–solid reactions. Chem. Lett. 1990, 19, 71–74. [Google Scholar] [CrossRef]
- Breakwell, K.I.; Homer, J.; Lawrence, M.A.M.; McWhinnie, W.R. Studies of organophilic clays: The distribution of quaternary ammonium compounds on clay surfaces and the role of impurities. Polyhedron 1995, 14, 2511–2518. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Osashi, F.; Kameyama, T. X-ray diffraction studies of intercalation compounds prepared from aniline salts and montmorillonite by mechanical processing. Solid State Commun. 2005, 136, 251–256. [Google Scholar] [CrossRef]
- Dave, N.; Tejas, J. A Concise Review on Surfactants and Its Significance. Int. J. Appl. Chem. 2017, 13, 663–672. [Google Scholar] [CrossRef]
- Olkowska, E.; Polkowska, Z.; Namiesnik, J. Analytical of surfactants in the environment: Problems and challenges. Chem. Rev. 2011, 111, 5667–5700. [Google Scholar] [CrossRef] [PubMed]
- Fridrihsberg, D.A. Kurs Kolloidnoj Himii, 4th ed.; Lan’: Saint Petersberg, Russia, 2010; 416p. (In Russian) [Google Scholar]
- Mondal, M.H.; Malik, S.; Roy, A.; Saha, R.; Saha, B. Modernization of sur- factant chemistry in the age of gemini and bio-surfactants: A review. RSC Adv. 2015, 5, 92707–92718. [Google Scholar] [CrossRef]
- Myers, D. Surfactant Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; 393p. [Google Scholar]
- Cruz-Guzmán, M.; Celis, R.; Hermosín, M.C.; Koskinen, W.C.; Nater, E.A.; Cornejo, J. Heavy metal adsorption by montmorillonites modified with natural organic cations. Soil. Sci. Soc. Am. J. 2006, 70, 215–221. [Google Scholar] [CrossRef]
- Tohdee, K.; Kaewsichan, L.; Asadullah. Enhancement of adsorption efficiency of heavy metal Cu(II) and Zn(II) onto cationic surfactant modified bentonite. J. Environ. Chem. Eng. 2018, 6, 2821–2828. [Google Scholar] [CrossRef]
- Jin, X.; Zha, S.; Li, S.; Chen, Z. Simultaneous removal of mixed contaminants by organoclays—Amoxicillin and Cu(II) from aqueous solution. Appl. Clay Sci. 2014, 102, 196–201. [Google Scholar] [CrossRef]
- Gorodnov, V.D. Fiziko-Himicheskie Metody Preduprezhdeniya Oslozhnenij v Burenii, 2nd ed.; Nedra: Moscow, Russia, 1984; 229p. (In Russian) [Google Scholar]
- Sanchez-Martin, M.J.; Rodriguez-Cruz, M.S.; Andrades, M.S.; Sanchez-Camazano, M. Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: Influence of clay type and pesticide hydrophobicity. Appl. Clay Sci. 2006, 31, 216–228. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef]
- Ren, S.; Meng, Z.; Sun, X.; Lu, H.; Zhang, M.; Lahori, A.H.; Bu, S. Comparison of Cd2+ adsorption onto amphoteric, amphoteric-cationic and amphoteric-anionic modified magnetic bentonites. Chemosphere 2019, 239, 124840. [Google Scholar] [CrossRef]
- Nikolaev, P.V.; Kozlov, N.A.; Petrova, S.N. Osnovy Himii i Tekhnologii Proizvodstva Sinteticheskih Moyushchih Sredstv; Ivan. gos. him. tekhnol. un-t.: Ivanovo, Russia, 2007; 116p. (In Russian) [Google Scholar]
- Fu, M.; Zhang, Z.P.; Wu, L.M.; Zhuang, G.Z.; Zhang, S.; Yuan, J.Y.; Liao, L.B. Investigation on the co-modification process of montmorillonite by anionic and cationic surfactants. Appl. Clay Sci. 2016, 132–133, 694–701. [Google Scholar] [CrossRef]
- Amirianshoja, T.; Junin, R.; Kamal Idris, A.; Rahmani, O. A comparative study of surfactant adsorption by clay minerals. J. Pet. Sci. Eng. 2013, 101, 21–27. [Google Scholar] [CrossRef]
- Sarkar, R.; Pal, A.; Rakshit, A.; Saha, B. Properties and applications of amphoteric surfactant: A concise review. J. Surf. Deterg. 2021, 24, 709–730. [Google Scholar] [CrossRef]
- Ward, R.S.; Davies, J.; Hodges, G.; Roberts, D.W. Synthesis of quaternary alkylammonium sulfobetaines. Synthesis 2002, 16, 2431–2439. [Google Scholar] [CrossRef]
- Parris, N.; Pierce, C.; Linfield, W.M. Soap based detergent formulation: XXIV. sulfobetaine derivatives of fatty amides. J. Am. Oil Chem. Soc. 1977, 54, 294–296. [Google Scholar] [CrossRef]
- Pambou, E.; Crewe, J.; Yaseen, M.; Padia, F.N.; Rogers, S.; Wang, D.; Xu, H.; Lu, J.R. Structural features of micelles of zwitterionic dodecyl-phosphocholine (C12PC) surfactants studied by small-angle neutron scattering. Langmuir 2015, 31, 9781–9789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Cai, B.X.; Gang, H.Z.; Yang, S.Z.; Mu, B.Z. A family of novel bio-based zwitterionic surfactants derived from oleic acid. RSC Adv. 2014, 4, 38393–38396. [Google Scholar] [CrossRef]
- Gang, H.Z.; Zhang, Q.Q.; Wang, W.; Cai, B.X.; Liu, J.; Yang, S.; Mu, B. Synthesis and interfacial properties of bio-based zwitterionic surfactants derived from different fatty acids in non-edible vegetable oils. J. Renew. Mater. 2020, 8, 417–429. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Cai, B.X.; Gang, H.Z.; Yang, S.Z.; Mu, B.Z. Novel zwitterionic surfactant derived from castor oil and its performance evaluation for oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 87–95. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhou, X.; Liu, H.; Xu, B. Synergistic interactions between zwitterionic surfactants derived from olive oil and an anionic surfactant. J. Dispers. Sci. Technol. 2018, 40, 1308–1316. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Cai, B.X.; Gang, H.Z.; Liu, J.F.; Yang, S.Z.; Mu, B.Z. The Rebirth of Waste Cooking Oil to Novel Bio-based Surfactants. Sci. Rep. 2015, 5, 9971. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 81, 65757–65767. [Google Scholar] [CrossRef]
- Gerola, A.P.; Costa, P.F.A.; Nome, F.; Quina, F. Micellization and adsorption of zwitterionic surfactants at the air/water interface. Curr. Opin. Colloid. Interface Sci. 2017, 32, 48–56. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Yu, L.; Jiao, J.; Wang, R.; Sun, L. Surface adsorption and micelle formation of imidazolium-based zwitterionic surface active ionic liquids in aqueous solution. J. Colloid Interface Sci. 2013, 391, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.D.; Souza, B.S.; Tondo, D.W.; Leopoldino, E.C.; Fiedler, H.D.; Faruk, N. Imidazolium-based zwitterionic surfactants: Characterization of normal and reverse micelles and stabilization of nanoparticles. Langmuir 2015, 31, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Storet, Y.; Pourchet, S.; Perchec, P.L. Tensioactive properties of zwitterionic carboxybetaine amphiphiles. Langmuir 1991, 7, 848–853. [Google Scholar] [CrossRef]
- Ren, Z.H. Mechanism of the salt effect on micellization of an aminosulfonate amphoteric surfactant. Ind. Eng. Chem. Res. 2015, 54, 9683–9688. [Google Scholar] [CrossRef]
- Ren, X.Z.; Li, G.Z.; Wang, H.L.; Xu, X.H. Study of betaine solutions by fluorescent probes of pyrene and pyrene-3-carboxaldehyde. Colloids Surf. A Physicochem. Eng. Asp. 1995, 100, 165–172. [Google Scholar] [CrossRef]
- Drinkel, E.; Souza, F.D.; Fiedler, H.D.; Nome, F. The chameleon effect in zwitterionic micelles: Binding of anions and cations and use as nanoparticle stabilizing agents. Curr. Opin. Colloid Interface Sci. 2013, 18, 26–34. [Google Scholar] [CrossRef]
- Sofroniou, C.; Chazapi, I.; Leontidis, E. Binding of lanthanide salts to zwitterionic phospholipid micelles. J. Colloid Interface Sci. 2019, 557, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qing, Y.; Wang, T.; Zhu, R.; Wei, J.; Tao, Q.; Yuan, P.; He, H. Preparation and characterization of zwitterionic surfactant-modified montmorillonites. J. Colloid Interface Sci. 2011, 360, 386–392. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Qing, Y.; Wen, K.; Su, X.; Ma, L.; Wei, J.; Liu, H.; He, H.; Xi, Y. Novel intercalation mechanism of zwitterionic surfactant modified montmorillonites. Appl. Clay Sci. 2017, 141, 265–271. [Google Scholar] [CrossRef]
- Thiebault, T.; Brendle, J.; Auge, G.; Limousy, L. Zwitterionic-surfactant modified Laponites® for removal of ions (Cs+; Sr2+ and Co2+) from aqueous solution as a sustainable recovery of radionuclides from aqueous wastes. Green Chem. 2019, 21, 5118–5127. [Google Scholar] [CrossRef]
- Woodward, G.L.; Peacock, C.L.; Otero-Fariña, A.; Thompson, O.R.; Brown, A.P.; Burke, I.T. A universal uptake mechanism for cobalt (II) on soil constituents: Ferrihydrite, kaolinite, humic acid, and organo-mineral composites. Geochim. Cosmochim. Acta. 2018, 238, 270–291. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Tran, L.; Zhu, N.; Dang, Z. Organo-montmorillonites for efficient and rapid water remediation: Sequential and simultaneous adsorption of lead and bisphenol A. Environ. Chem. 2018, 15, 286–295. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, Y. The Synthesis of Mesoporous Molecular Sieves. In Introduction to Zeolite Science and Practice; Elsevier: Amsterdam, The Netherlands, 2007; pp. 241–300. [Google Scholar] [CrossRef]
- Ware, A.M.; Waghmare, J.T.; Momin, S.A. Alkylpolyglycoside: Carbo- hydrate based surfactant. J. Dispers. Sci. Technol. 2007, 28, 437–444. [Google Scholar] [CrossRef]
- Dremuk, A.P. Kolloidno-Himicheskie Svojstva Dvojnyh i Trojnyh Smesej PAV Razlichnoj Prirody. Ph.D. Thesis, D. Mendeleev University of Chemical Technology of Russia, Moscow, Russia, 2018. (In Russian). [Google Scholar]
- Chernik, G.G. Phase studies of surfactant-water systems. Curr. Opin. Colloid Interface Sci. 1999, 4, 381–390. [Google Scholar] [CrossRef]
- Lindman, B.; Carlsson, A.; Karlström, G.; Malmsten, M. Nonionic polymers and surfactants—Some anomalies in temperature dependence and in interactions with ionic surfactants. Adv. Colloid Interface Sci. 1990, 32, 183–203. [Google Scholar] [CrossRef]
- Jonsson, B.; Lindman, B.; Holmberg, K.; Kronberg, B. Surfactants and Polymers in Aqueous Solution; Wiley: New York, NY, USA, 2002; 562p. [Google Scholar]
- Ferreira, T.M.; Bernin, D.; Topgaard, D. NMR Studies of Nonionic Surfactants. Annu. Rep. NMR Spectrosc. 2013, 79, 73–127. [Google Scholar] [CrossRef]
- Guégan, R. Intercalation of a Nonionic Surfactant (C10E3) Bilayer into a Na- Montmorillonite Clay. Langmuir 2010, 26, 19175–19180. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H. Preparations of organobentonite using nonionic surfactants. Chemosphere 2001, 44, 989–995. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.B.; White, G.N. Intercalation and surface modification of smectite by two non-ionic surfactants. Clays Clay Miner. 2003, 51, 150–161. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Greenland, D.J. The Adsorption of Poly(Ethylene Glycols) on Clay Minerals. Clay Miner. 1970, 8, 305–315. [Google Scholar] [CrossRef]
- Calabrese, I.; Gelardi, G.; Merli, M.; Rytwo, G.; Sciascia, L.; Liveri, M.L.T. New tailor-made bio-organoclays for the remediation of olive mill waste water. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012040. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, A.; Pickering, W.F. The influence of surfactants on the adsorption of heavy metal ions by clays. Water Res. 1983, 17, 215–225. [Google Scholar] [CrossRef]
- Law, J.P.; Kunze, G.W. Reactions of surfactants with montmorillonite: Adsorption mechanisms. Soil. Sci. Soc. Am. J. 1966, 30, 321–327. [Google Scholar] [CrossRef]
- Mirbaloochzehi, M.R.; Rezvani, A.; Samimi, A.; Shayesteh, M. Application of a Novel Surfactant-Modified Natural Nano-Zeolite for Removal of Heavy Metals from Drinking Water. Adv. J. Chem. A 2020, 3, 612–620. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini-Surfactants: Synthesis and Properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Alami, E.-O.; Holmberg, K. Heterogemini surfactant. Adv. Colloid Interface Sci. 2003, 100–102, 13–46. [Google Scholar] [CrossRef]
- Renouf, P.; Mioskowski, C.; Lebeau, L.; Hebrault, D.; Desmurs, J.-R. Dimeric surfactants: First synthesis of an asymmetrical gemini compound. Tetrahedron Lett. 1998, 39, 1357–1360. [Google Scholar] [CrossRef]
- Fischer, P.; Rehage, H.; Grüning, B. Linear flow properties of dimer acid betaine solutions with and without changed ionic strength. J. Phys. Chem. 2002, 106, 11041–11046. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Xia, M.; Wang, F.; Zhu, S.; Lei, W. The synergistic effect and microscopic mechanism of co-adsorption of three emerging contaminants and copper ion on gemini surfactant modified montmorillonite. Ecotoxicol. Environ. Saf. 2019, 184, 109610. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Khan, M.A.; Zhu, S.; Xia, M.; Lei, W.; Wang, F.; Xu, Y. Microstructural modification of organo-montmorillonite with gemini surfactant containing four ammonium cations: Molecular dynamics (MD) simulations and adsorption capacity for copper ions. J. Chem. Technol. Biotechnol. 2019, 94, 3585–3594. [Google Scholar] [CrossRef]
- Han, G.; Han, Y.; Wang, X.; Liu, S.; Sun, R. Synthesis of organic rectorite with novel Gemini surfactants for copper removal. Appl. Surf. Sci. 2014, 317, 35–42. [Google Scholar] [CrossRef]
- Fang, X.; Yuan, W.; Xiong, Y.; Qiu, X. Removal of Cr(VI) in aqueous solution using cationic gemini surfactant-modified rectorite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128524. [Google Scholar] [CrossRef]
- Luo, W.; Ouyang, J.; Antwi, P.; Wu, M.; Huang, Z.; Qin, W. Microwave/ultrasoundassisted modification of montmorillonite by conventional and gemini alkyl quaternary ammonium salts for adsorption of chromate and phenol: Structurefunction relationship. Sci. Total Environ. 2019, 655, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Luo, W.; Wei, J.; Yuan, X.; Huang, Q.; Zou, L.; Zhang, M.; Antwi, P.; Zhang, D.; Ren, S. Adsorption of tungstate using cationic gemini surfactant-modified montmorillonite: Influence of alkyl chain length. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127484. [Google Scholar] [CrossRef]
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770. [Google Scholar] [CrossRef]
| Surfactants | Structural Formula |
|---|---|
| Sulfobetaine |  |
| Sulfatobetaine | 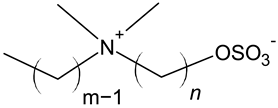 |
| Hydroxypropyl sulfobetaine |  |
| Sulfoimidazolium |  |
| Sulfo-pyridinium | 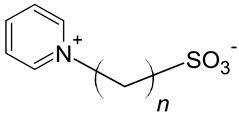 |
| Carboxybetaine | 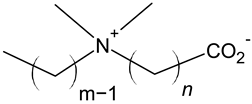 |
| Carboxyimidazolium | 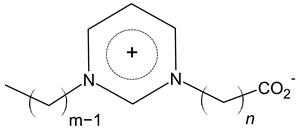 |
| Amidosulfobetaine |  |
| Amidocarboxybetaine |  |
| Phosphocholine | 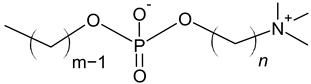 |
| Phenylphosphinatobetaine | 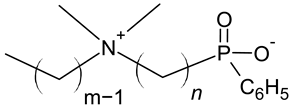 |
| Amine oxide | 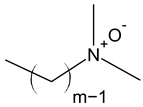 |
| Sulfophosphonium |  |
| Surfactants | Structural Formula |
|---|---|
| Poly(alkylene-oxide) block copolymers | 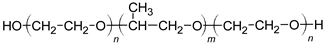 |
 | |
 | |
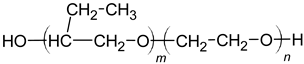 | |
| Oligomeric alkyl-ethylene oxides | 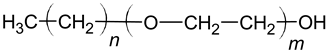 |
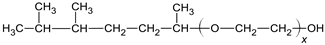 | |
| Alkyl-phenol polyethylenes | 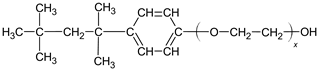 |
| Sorbitan esters |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelomov, L.; Gertsen, M.; Burachevskaya, M.; Hemalatha, S.; Vijayalakshmi, A.; Perelomova, I.; Atroshchenko, Y. Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants. Sustainability 2024, 16, 4804. https://doi.org/10.3390/su16114804
Perelomov L, Gertsen M, Burachevskaya M, Hemalatha S, Vijayalakshmi A, Perelomova I, Atroshchenko Y. Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants. Sustainability. 2024; 16(11):4804. https://doi.org/10.3390/su16114804
Chicago/Turabian StylePerelomov, Leonid, Maria Gertsen, Marina Burachevskaya, S. Hemalatha, Architha Vijayalakshmi, Irina Perelomova, and Yurii Atroshchenko. 2024. "Organoclays Based on Bentonite and Various Types of Surfactants as Heavy Metal Remediants" Sustainability 16, no. 11: 4804. https://doi.org/10.3390/su16114804