Effect of Common Ions in Agricultural Additives on the Retention of Cd, Cu, and Cr in Farmland Soils
Abstract
:1. Introduction
2. Methods
2.1. Soil Collection and Analyses
2.2. Batch Sorption Experiments
2.2.1. Single-Cation Sorption Experiment
2.2.2. Competitive Cation Effects
2.2.3. Coexisting Anion Effects
2.3. Quenching Titration and Fluorescence Measurements
2.3.1. DOM Extraction
2.3.2. Coexisting Anion Quenching Titration
2.4. Data Analysis and Statistical Analysis
2.4.1. Sorption Experiment Analysis
2.4.2. PARAFAC Analysis and Complexation Modeling
3. Results and Discussion
3.1. Competitive Sorption Experiments
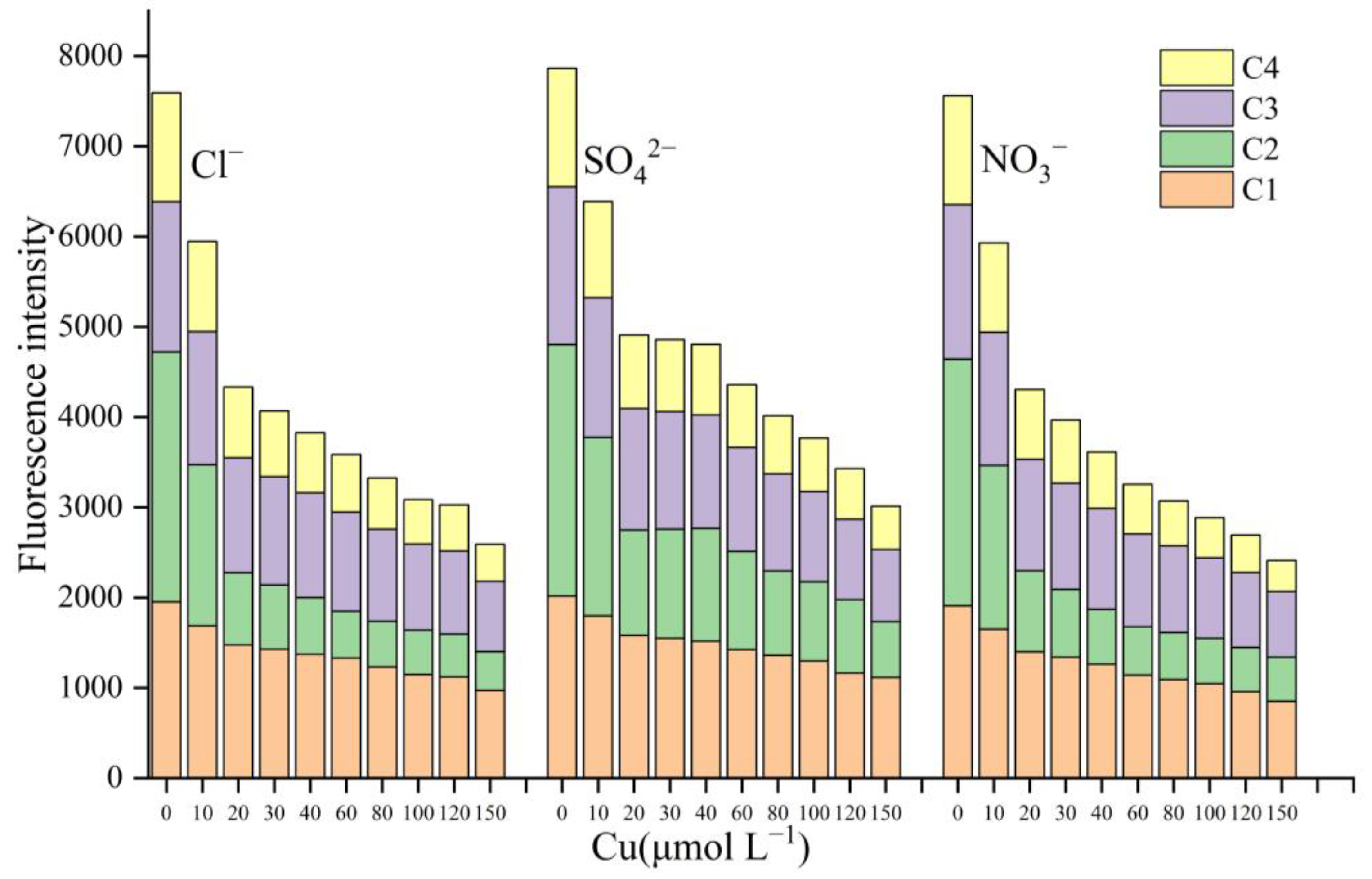
3.2. Coexisting Anion Sorption Experiments
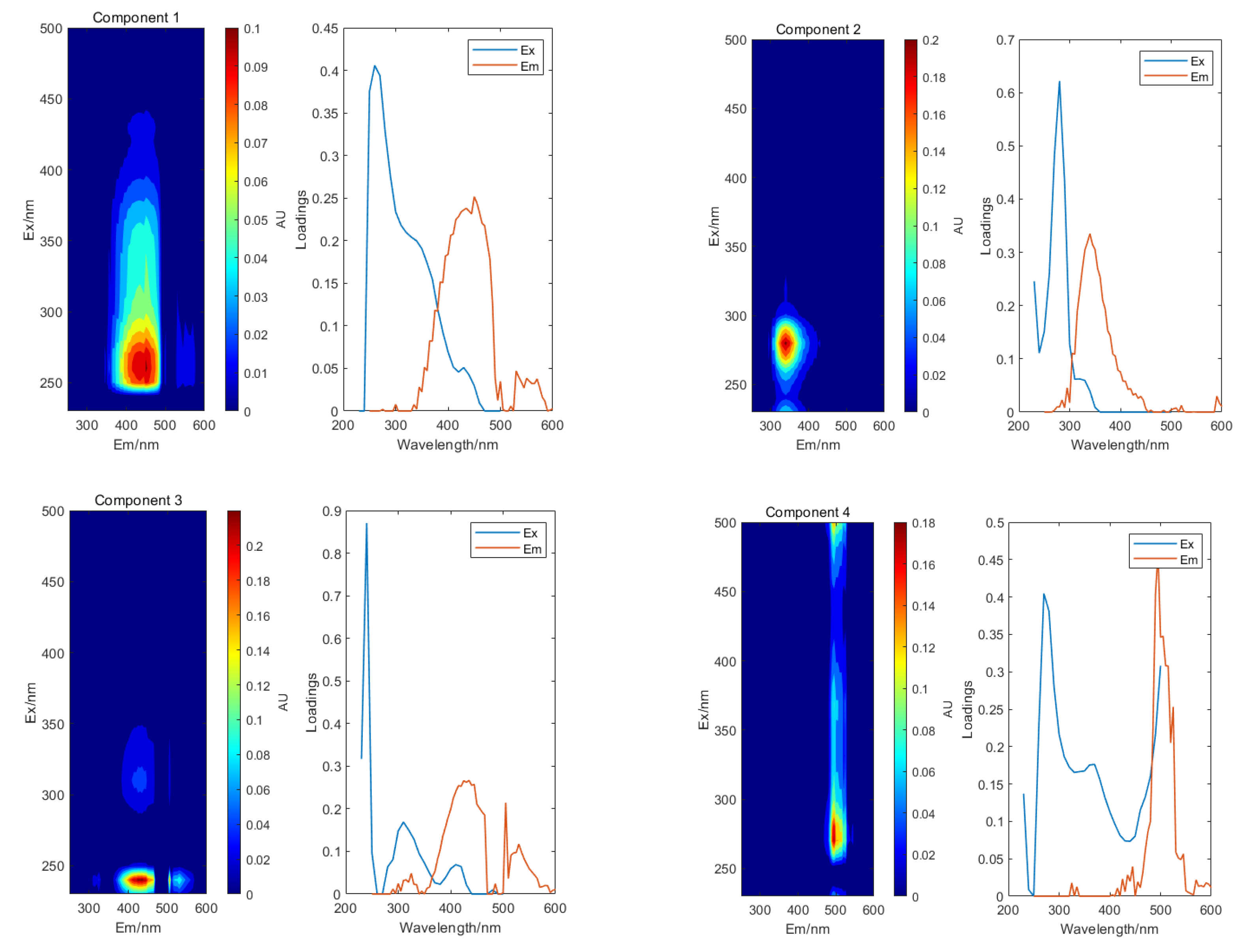
3.3. DOM Characterization Using EEM-PARAFAC
3.4. Behavior of Components with the Addition of Cu2+, Cd2+, and Cr3+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Env. Sci. Pollut R. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, Y.; Weng, L.; Ma, J.; Ma, Y.; Li, Y.; Islam, M.S. Comparisons of heavy metal input inventory in agricultural soils in North and South China: A review. Sci. Total Env. 2019, 660, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Ma, J.; Wu, X.; Ju, T.; Lin, X.; Zhang, Y.; Li, X.; Gong, Y.; Hou, H.; Zhao, L.; et al. Inventories of heavy metal inputs and outputs to and from agricultural soils: A review. Ecotox Env. Safe 2018, 164, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Elbana, T.A.; Magdi Selim, H.; Akrami, N.; Newman, A.; Shaheen, S.M.; Rinklebe, J. Freundlich sorption parameters for cadmium, copper, nickel, lead, and zinc for different soils: Influence of kinetics. Geoderma 2018, 324, 80–88. [Google Scholar] [CrossRef]
- Yan, Y.; Wan, B.; Mansor, M.; Wang, X.; Zhang, Q.; Kappler, A.; Feng, X. Co-sorption of metal ions and inorganic anions/organic ligands on environmental minerals: A review. Sci. Total Env. 2022, 803, 149918. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.K.; Tyagi, U.; Sirohi, S.; Pani, B.; Kumar, K.; Nikita; Kumar, G. Fabrication of biocompatible chitosan/graphene based nanocomposite for the competitive adsorption of heavy metal ions from wastewater in binary and ternary systems: Scale-up and upgradation studies. J. Water Process Eng. 2023, 56, 104555. [Google Scholar] [CrossRef]
- Padilla, J.T.; Selim, H.M.; Gaston, L.A. Modeling the competitive sorption and transport of Ni(II) and Zn(II) in soils: Comparing two multicomponent approaches. J. Contam. Hydrol. 2023, 252, 104108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, R.; Huang, D.; Deng, R.; Li, R.; Chen, Y.; Zhou, W. Three kinds of apatite adsorbents prepared by co-precipitation for Pb (II) and Cd (II) removal from wastewater: Performance, competitive effects and mechanisms. J. Mol. Liq. 2024, 400, 124478. [Google Scholar] [CrossRef]
- Dahlin, A.S.; Eriksson, J.; Campbell, C.D.; Öborn, I. Soil amendment affects Cd uptake by wheat — are we underestimating the risks from chloride inputs? Sci. Total Env. 2016, 554–555, 349–357. [Google Scholar] [CrossRef]
- Guo, J.; Ge, C.; Wang, G.; Zhou, D. Mechanisms of chloride to promote the uptake and accumulation of cadmium in rice (Oryza sativa L.). Sci. Total Env. 2024, 926, 172046. [Google Scholar] [CrossRef]
- Kocik, E.M.; Kim, A.; Aiken, M.L.; Smith, L.; Kim, C.S. Sulfate enhances the adsorption and retention of Cu(II) and Zn(II) to dispersed and aggregated iron oxyhydroxide nanoparticles. Appl. Geochem. 2024, 162, 105929. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Y.; Liu, X.; Miao, T.; Guan, Q.; Yang, R.; Qu, J. Insight into interactions of heavy metals with livestock manure compost-derived dissolved organic matter using EEM-PARAFAC and 2D-FTIR-COS analyses. J. Hazard Mater 2021, 420, 126532. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Quan, G.; Yan, J.; Gao, J.; Zou, X.; Cui, L. Insights into the effects of long-term biochar loading on water-soluble organic matter in soil: Implications for the vertical co-migration of heavy metals. Env. Int 2020, 136, 105439. [Google Scholar] [CrossRef] [PubMed]
- Kasmaei, L.S.A.F. Effect of organic matter on the release behavior and extractability of copper and cadmium in soil. Commun. Soil Sci. Plan 2012, 43, 2209–2217. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, J.W.J.; Kleja, D.B.; Gustafsson, J.P. Acid–base and copper-binding properties of three organic matter fractions isolated from a forest floor soil solution. Geochim. Cosmochim. Ac. 2010, 74, 1391–1406. [Google Scholar] [CrossRef]
- Chen, M.; Ding, S.; Li, C.; Tang, Y.; Fan, X.; Xu, H.; Tsang, D.C.W.; Zhang, C. High cadmium pollution from sediments in a eutrophic lake caused by dissolved organic matter complexation and reduction of manganese oxide. Water Res. 2021, 190, 116711. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Y.; Chen, Y.; Li, Y.; Wang, M.; Wang, G. Biochar reduced soil extractable Cd but increased its accumulation in rice (Oryza sativa L.) cultivated on contaminated soils. J. Soil Sediment 2019, 19, 862–871. [Google Scholar] [CrossRef]
- Luo, Y.; He, Y.; Zhou, D.; Pan, L.; Wu, Y. Organic amendment application affects the release behaviour, bioavailability, and speciation of heavy metals in zinc smelting slag: Insight into dissolved organic matter. J. Hazard Mater 2024, 465, 133105. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Ly, Q.V.; Hur, J. Further insight into the roles of the chemical composition of dissolved organic matter (DOM) on ultrafiltration membranes as revealed by multiple advanced DOM characterization tools. Chemosphere 2018, 201, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, H.; Yu, H.; Song, Y. Applying EEM-PARAFAC combined with moving-window 2DCOS and structural equation modeling to characterize binding properties of Cu (II) with DOM from different sources in an urbanized river. Water Res. 2022, 227, 119317. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Peng, Y.; Li, N.; Tian, Y.; Dai, L.; Wu, Y.; Huang, Y. Effect of biochar-derived DOM on the interaction between Cu(II) and biochar prepared at different pyrolysis temperatures. J. Hazard Mater 2022, 421, 126739. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhang, W.; Wang, X.; Liu, A.; Li, Z.; Bai, Y.; Wu, F. Leaching behaviors of dissolved organic matter from face masks revealed by fluorescence EEM combined with FRI and PARAFAC. Water Res. 2024, 254, 121399. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Zhuang, W.; Zhu, Z. Unveiling changes in the complexation of dissolved organic matter with Pb(II) by photochemical and microbial degradation using fluorescence EEMs-PARAFAC. Env. Pollut. 2024, 341, 122982. [Google Scholar] [CrossRef]
- Wells, M.J.M.; Funk, D.; Mullins, G.A.; Bell, K.Y. Application of a fluorescence EEM-PARAFAC model for direct and indirect potable water reuse monitoring: Multi-stage ozone–biofiltration without reverse osmosis at Gwinnett County, Georgia, USA. Sci. Total Env. 2023, 886, 163937. [Google Scholar] [CrossRef]
- Liu, F.; Zhuang, W.; Yang, L. Comparing the Pb(II) binding with different fluorescent components of dissolved organic matter from typical sources. Env. Sci. Pollut. R. 2022, 29, 56676–56683. [Google Scholar] [CrossRef]
- Ryan, D.K.; Weber, J.H. Fluorescence quenching titration for determination of complexing capacities and stability constants of fulvic acid. Anal. Chem. (Wash.) 1982, 54, 986–990. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ye, J.; Chen, Z.; Ren, D.; Zhang, S. The spectral characteristics and cadmium complexation of soil dissolved organic matter in a wide range of forest lands. Env. Pollut. 2022, 299, 118834. [Google Scholar] [CrossRef]
- Xing, J.; Xu, G.; Li, G. Analysis of the complexation behaviors of Cu(II) with DOM from sludge-based biochars and agricultural soil: Effect of pyrolysis temperature. Chemosphere 2020, 250, 126184. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Shao, L.; He, P. Fluorescent characteristics and metal binding properties of individual molecular weight fractions in municipal solid waste leachate. Env. Pollut. 2012, 162, 63–71. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, P.; Shao, L. Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis. Water Res. 2011, 45, 1711–1719. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, K.; Zhang, J.; Ma, C.; Wang, Z.; Tian, X. Removal and Adsorption Mechanisms of Phosphorus, Cd and Pb from Wastewater Conferred by Landfill Leachate Sludge-Derived Biochar. Sustainability 2023, 15, 10045. [Google Scholar] [CrossRef]
- Behroozi, A.; Arora, M.; Fletcher, T.D.; Western, A.W. Sorption and transport behavior of zinc in the soil; Implications for stormwater management. Geoderma 2020, 367, 114243. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the Variability in Dissolved Organic Matter Fluorescence in a Temperate Estuary and Its Catchment Using PARAFAC Analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Yang, L.; Han, D.H.; Lee, B.; Hur, J. Characterizing treated wastewaters of different industries using clustered fluorescence EEM–PARAFAC and FT-IR spectroscopy: Implications for downstream impact and source identification. Chemosphere 2015, 127, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.T.; Selim, H.M. Modeling the kinetics of competitive sorption and desorption of Zn(II), Ni(II), and Pb(II) in an acidic soil: Stirred-flow experiments. Soil Sci. Soc. Am. J. 2021, 85, 560–573. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Adv. Colloid. Interfac. 2013, 201–202, 43–56. [Google Scholar] [CrossRef]
- Mendes, L.A.; Bucater, L.F.P.; Landgraf, M.D.; Rezende, M.O.O. Role of Organic Matter in the Adsorption/Desorption of Cr, Cu and Pb in Competitive Systems in Two Different Soils. Open Access Libr. J. 2014, 1, e1022. [Google Scholar] [CrossRef]
- Cerqueira, B.; Covelo, E.F.; Andrade, L.; Vega, F.A. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma 2011, 162, 20–26. [Google Scholar] [CrossRef]
- López-Chuken, U.J.; Young, S.D. Modelling sulphate-enhanced cadmium uptake by Zea mays from nutrient solution under conditions of constant free Cd2+ ion activity. J. Environ. Sci. (China) 2010, 22, 1080–1085. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Khaliq, M.A.; Xie, T.; Chen, Y.; Wang, G. Chlorine weaken the immobilization of Cd in soil-rice systems by biochar. Chemosphere 2019, 235, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dai, G.; Liu, Z.; He, T.; Zhong, J.; Ma, Y.; Shu, Y. Field-scale fluorescence fingerprints of biochar-derived dissolved organic matter (DOM) provide an effective way to trace biochar migration and the downward co-migration of Pb, Cu and As in soil. Chemosphere 2022, 301, 134738. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor– an online spectral library of auto-fluorescence by organic compounds in the environment. Anal Methods-Uk 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Amaral, V.; Romera-Castillo, C.; Forja, J. Submarine mud volcanoes as a source of chromophoric dissolved organic matter to the deep waters of the Gulf of Cádiz. Sci. Rep. 2021, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Park, M.; Kim, J.; Shinn, Y.J.; Lee, Y.K.; Hur, J. Exploring pore water biogeochemical characteristics as environmental monitoring proxies for a CO2 storage project in Pohang Basin, South Korea. Mar. Pollut. Bull. 2018, 137, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kim, S.; Jung, H.; Hyun, J.; Choi, J.H.; Lee, H.; Huh, I.; Hur, J. Dynamics of dissolved organic matter in riverine sediments affected by weir impoundments: Production, benthic flux, and environmental implications. Water Res. 2017, 121, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Price, R.M.; Yamashita, Y.; Jaffé, R. Comparative study of dissolved organic matter from groundwater and surface water in the Florida coastal Everglades using multi-dimensional spectrofluorometry combined with multivariate statistics. Appl. Geochem. 2010, 25, 872–880. [Google Scholar] [CrossRef]
- Ohno, T.; Bro, R. Dissolved Organic Matter Characterization Using Multiway Spectral. Soil Sci Soc. Am. J. 2006, 70, 2028–2037. [Google Scholar] [CrossRef]
- Hunt, J.F.; Ohno, T. Characterization of Fresh and Decomposed Dissolved Organic Matter Using Excitation−Emission Matrix Fluorescence Spectroscopy and Multiway Analysis. J. Agr. Food Chem. 2007, 55, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Z.; Luo, N.; Yang, R.; Wen, J.; Huang, B.; Zeng, G. Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals. Sci. Total Env. 2019, 646, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Z.; Huang, B.; Luo, N.; Zhang, Q.; Zhai, X.; Zeng, G. Investigating binding characteristics of cadmium and copper to DOM derived from compost and rice straw using EEM−PARAFAC combined with two-dimensional FTIR correlation analyses. J. Hazard Mater 2018, 344, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Shou, W.; Kang, F.; Lu, J. Nature and Value of Freely Dissolved EPS Ecosystem Services: Insight into Molecular Coupling Mechanisms for Regulating Metal Toxicity. Env. Sci. Technol. 2018, 52, 457–466. [Google Scholar] [CrossRef]
- Ali, M.A.; Dzombak, D.A. Effects of simple organic acids on sorption of Cu2+ and Ca2+ on goethite. Geochim. Cosmochim. Ac. 1996, 60, 291–304. [Google Scholar] [CrossRef]


| Characteristics | pH (in Water) | Cd (mg kg−1) | Cu (mg kg−1) | Cr (mg kg−1) | TOC (Water Dissolved, mg kg−1) | Clay % | Silt % | Sand % |
|---|---|---|---|---|---|---|---|---|
| Soil sample | 8.89 | 0.18 ± 0.03 | 20.23 ± 0.38 | 51.03 ± 0.83 | 80.2 ± 8.9 | 45.54 | 29.04 | 25.41 |
| HMs | Coexisting Anions | Single Cations | Multiple Cations | ||||
|---|---|---|---|---|---|---|---|
| Kf (mmol 1−β Lβ kg−1) | β | R2 | Kf (mmol 1−β Lβ kg−1) | β | R2 | ||
| Cd2+ | Cl− | - | - | - | 8.01 | 0.8 | 0.99 |
| NO3− | 27.94 | 0.57 | 0.99 | 9.45 | 0.75 | 0.99 | |
| SO42− | - | - | - | 8.81 | 0.76 | 0.99 | |
| Cr3+ | Cl− | - | - | - | 34.08 | 0.7 | 0.97 |
| NO3− | 47.18 | 0.78 | 0.98 | 43.25 | 0.67 | 0.98 | |
| SO42− | - | - | - | 36.8 | 0.68 | 0.98 | |
| Cu2+ | Cl− | - | - | - | 39.45 | 0.63 | 0.98 |
| NO3− | 35.94 | 0.81 | 0.99 | 40.69 | 0.65 | 0.99 | |
| SO42− | - | - | - | 39.69 | 0.66 | 0.99 | |
| Component | NO3− | SO4− | Cl− | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| logKM | f | R2 | logKM | f | R2 | logKM | f | R2 | ||
| Cd2+ | C1 | FM | FM | FM | ||||||
| C2 | FM | FM | FM | |||||||
| C3 | FM | FM | FM | |||||||
| C4 | FM | FM | FM | |||||||
| Cr3+ | C1 | 5.20 | 59.67 | 0.98 | 5.34 | 85.63 | 0.98 | 5.56 | 58.57 | 0.96 |
| C2 | 4.89 | 70.97 | 0.94 | 6.24 | 85.79 | 0.99 | 4.93 | 81.30 | 0.97 | |
| C3 | 5.22 | 60.27 | 0.96 | 5.26 | 78.20 | 0.98 | 5.37 | 68.51 | 0.98 | |
| C4 | 5.08 | 57.84 | 0.98 | 5.50 | 86.68 | 0.98 | 5.72 | 72.02 | 0.98 | |
| Cu2+ | C1 | 4.75 | 55.41 | 0.97 | 4.7 | 44.68 | 0.94 | 4.66 | 50.26 | 0.95 |
| C2 | 6.00 | 82.15 | 0.99 | 5.01 | 77.80 | 0.95 | 5.82 | 85.4 | 0.99 | |
| C3 | 4.74 | 57.49 | 0.97 | 4.73 | 54.37 | 0.95 | 4.93 | 53.15 | 0.94 | |
| C4 | 4.91 | 71.39 | 0.99 | 4.77 | 63.34 | 0.97 | 4.86 | 66.09 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Cao, H. Effect of Common Ions in Agricultural Additives on the Retention of Cd, Cu, and Cr in Farmland Soils. Sustainability 2024, 16, 4870. https://doi.org/10.3390/su16114870
Zhou X, Cao H. Effect of Common Ions in Agricultural Additives on the Retention of Cd, Cu, and Cr in Farmland Soils. Sustainability. 2024; 16(11):4870. https://doi.org/10.3390/su16114870
Chicago/Turabian StyleZhou, Xu, and Hongbin Cao. 2024. "Effect of Common Ions in Agricultural Additives on the Retention of Cd, Cu, and Cr in Farmland Soils" Sustainability 16, no. 11: 4870. https://doi.org/10.3390/su16114870
APA StyleZhou, X., & Cao, H. (2024). Effect of Common Ions in Agricultural Additives on the Retention of Cd, Cu, and Cr in Farmland Soils. Sustainability, 16(11), 4870. https://doi.org/10.3390/su16114870





