Abstract
In the context of climate change and the circular economy, biochar agricultural and environmental applications have attracted a good deal of attention. Biochar has unique characteristics like surface area, porosity, water-holding capacity, pH, surface charge, and nutrients. This study reviews the biochar production from olive pomace (OP) and olive stone (OS) byproducts, its chemical and physical characterization, and its environmental application. The current review highlights the conditions for biochar production, the effects of pyrolysis temperature, and feedstock type on the physicochemical properties of biochar. High pyrolysis temperature (>500 °C) promotes a high specific surface area, high porosity (especially for OS biochars), and pH as well as the content of ash and fixed carbon, but generates low cation exchange capacity (CEC) and electrical conductivity (EC) and high values of O/C and H/C ratio. OP biochar also presents a high C amount, and ash content, i.e., rich in nutrients and high alkalizing capacity. OP biochar serves as an important source of plant nutrients, especially potassium. After adding both types of biochar, aggregate stability and the amount of water held in soil increase, and bulk density and bioavailability of trace elements decrease. Thus, biochar from olive mill wastes can be a potential plant nutrient reservoir, a good amendment to improve soil properties and long-term carbon sequestration. Results presented in this review can be used to build designer biochars from olive mill wastes to help solve environmental issues (water purification and pollutant remediation) and are suitable for improving soil physical chemistry characteristics and crop growth.
1. Introduction
The world production of olives was 21,449 Gg in 2022, with the most producing countries being Spain (29.9%), Italy (13.5%), Greece (12.1%), Turkey (7.4%), Morocco (5.1%), Tunisia (4.4%), and Portugal (2.4%) [1]. In the last 20 years, the annual olive production has increased by 25% due, among other factors, to the increase in the olive-growing area and the intensification of olive farming practices in Mediterranean countries. In 2022 alone, more than 3010 Gg of olive oil were produced worldwide, 15% more than in previous years [2].
This increase in olive oil production has contributed to a large amount of byproducts and effluents being produced, depending on the centrifugation process. In the three-phase continuous centrifugation process, in addition to the oily phase (20%), a solid byproduct (30%), and an aqueous phase (50%) known as olive mill wastewater (OMW) is produced [3]. On the other hand, in the modern two-phase centrifugation process, only one byproduct is created, known as wet olive pomace (OP) (for every kilogram of olive oil produced 4 kg of OP). Therefore, the new effluent comprises a mixture of washes and vegetable solid residue, comprising the skin, pulp, physiological water, and olive stone (OS). In the two-phase centrifugation process, although it produces no OMW, it produces a single effluent stream in semi-solid form, OP, which doubles the amount of “solid” waste requiring disposal [3]. In Spain alone, the world’s main producer, more than 6000 Gt of OP were produced in 2022 [1].
The main reason that has led to the modernization of the oil mills, from three phases to two phases, has been the elimination of OMW, which was a problem in the oil mills due to their high contaminant load. With this industrial change, the water consumption of the oil mills has also been reduced, from 1.5 L to 0.25 L per liter of oil produced [4]. The great advantage has been, without a doubt, reducing the 10–12 × 106 m3 of OMW produced in the 1990s in olive-growing countries, which is compared to an equivalent contamination of 10–16 × 106 inhabitants [5].
However, OP is in sludge form, with about 55–70% moisture content, making the industrial recovery of the residual oil difficult and expensive. The main alternative for economic recovery of OP is the extraction of residual oil (3–4% oleic acid) in seed-oil refineries, where it is necessary to centrifuge and dry the conventional three-phase olive cake, producing a large quantity of OMW. The considerably higher energy requirements than in the three-phase process and, finally, the extraction of residual fat with oil solvents make the industrial recovery of residual oil difficult and expensive [3].
Different treatment processes can be carried out with olive cake. The stone-free OP is obtained after sieving the wet OP and de-oiling and drying [6], which is used as biomass energy given its high energy value. This process is expensive since drying is energy-consuming and highly polluting due to the CO2 emissions generated. It is estimated that OP could provide more than 20.6 Kw kg−1 OP processed electric power by direct burning [7], representing high CO2 emission rates [8]. Furthermore, this industry generates little economic profit [9].
Converting OP and OS into biochar, the solid product of thermochemical conversion under oxygen-limited conditions (pyrolysis), seems a promising approach to address the above-mentioned environmental concerns of olive oil byproduct management. The advantage of pyrolysis is that it does not require O2 for combustion, so there are no CO2 emissions into the atmosphere [10]. In this sense, this type of technology, in addition to its industrial scale, has the advantage of aligning with Green Europe’s decarbonization objectives. The interest in producing biochar from these olive oil byproducts relates to its chemical composition. In the case of OP, the material is made up of a high proportion of C in the form of cellulose, hemicellulose, and lignin (20%); low ash content (8%); and high humidity [11]. In the case of OS, these properties are improved due to low humidity, which is of interest to avoid undesirable combustion in pyrolysis ovens.
In this sense, biochar production is a win–win strategy, considering that it reduces the volume of waste from the olive oil industry while favoring decarbonization compensation for C emissions. Due to its beneficial characteristics, e.g., surface area, porosity, water-holding capacity, pH, surface charge, functional groups, and mineral components, biochar is used as a soil conditioner, a promising alternative for agricultural applications [12,13]. Biochar’s physical and chemical properties largely depend not only on the type of feedstock, but also on the pyrolysis conditions such as temperature and heating rate [14,15,16,17,18]. Therefore, biochar’s pyrolysis conditions are essential when choosing the most suitable biochar for the intended application, which can range from application as an inorganic amendment in soils or remediation of contaminated soils [13,19,20], wastewater treatment [21,22], or carbon capture [16,19], etc.
During biochar production, pyrolysis temperature plays a key role in the thermochemical conversion of biomass [13,16,17,18,19,23,24]. In general, biomass heated to high temperatures (>500 °C) produces biochar with a porous structure, large specific surface area (SSA), aromaticity, C-C content, and high pH, while those made at low temperatures (<400 °C) have a high yield and O/C and H/C ratio, and low electrical conductivity (EC), cation exchange capacity, and surface functional groups [16,17,18,19,25]. In this sense, low-temperature biochar contains a high content of compounds that easily undergo degradation in soil, and, hence, nutrients assimilable by plants so that it could be suitable for agricultural use [26,27], while those produced at high temperatures could be used in more environmentally friendly practices [28].
Pyrolysis of olive waste has been identified as a promising management technique that detoxifies organic contaminants in these byproducts [29], increasing its agricultural value both in terms of nutrient availability and C sequestration [30]. Alotaibi et al. [31] found that the physical properties (pH, EC, SSA) of OP and sewage sludge (SS) biochars increased with increasing pyrolysis temperature, demonstrating the potential of these biochars to store recalcitrant C. Furthermore, when produced only from OP, total C values can be even higher (89.6% C) [32]. Campos and la Rosa [33] showed that biochar produced from OS has physical and chemical properties (water retention capacity greater than 70%, very high surface area (473 m2 g−1), alkaline pH (9.34), low ash content (1%), among others) which may be suitable for pollutant remediation and agricultural soil. Currently, there is a discrepancy in information between biochar types and their efficient use. In this sense, the present study aims to cross-reference existing information (studies published until 2022), to optimize the best production process for the expected purpose. The specific objectives of this review are to discuss: (i) the optimization of the OP and OS biochar production process, (ii) the analysis of factors affecting the process of biochar production, such as temperature and feedstock type, (iii) the physical and chemical characterization of the biochar produced, and finally, (iv) the advantages of the applications of OP and OS biochar to soil, for the crop growth and pollutant remediation. It is expected that this review is believed to serve as a useful tool to define the wide range of OP and OS biochar applications and to establish which pyrolysis process best fits the desired biochar properties (high contaminant retention, high C store).
2. Materials and Methods
Data Survey and Screening
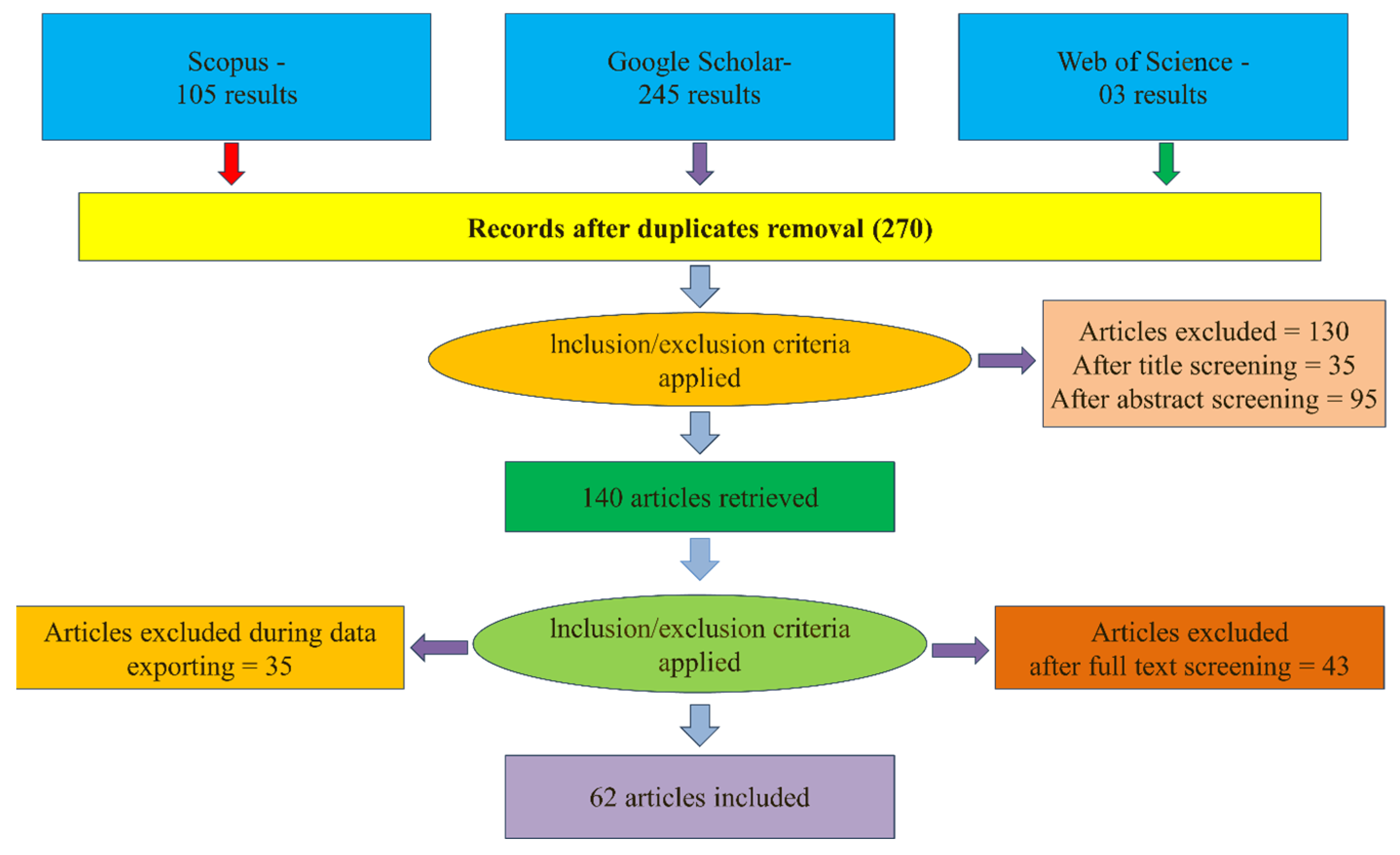
A literature review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework to provide state-of-the-art on biochar produced from both olive pomace (OP) and olive stone (OS) in topics such as production, characterization, and applications. This is accomplished through a five-phase process. The screening process and reasons for exclusion are summarized and identified in Figure 1. Detailed information about this review’s search protocol and steps is presented below.
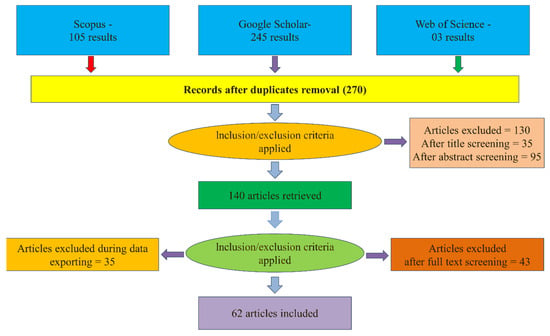
Figure 1.
Flowchart data screening methods according to PRISMA principles.
- (i)
- We formulated the search string using different combinations of the keywords “biochar”, “olive pomace”, and “olive stone”. On this basis, the following search string was designed: (olive pomace biochar OR olive pomace char OR olive pomace charcoal OR olive stone biochar OR olive stone char OR olive stone charcoal OR olive pit biochar OR olive pit char OR olive pit charcoal OR olive mill biochar OR olive mill char OR olive mill charcoal OR olive bagasse biochar OR olive bagasse char OR olive bagasse charcoal) AND (soil application OR soil amendment OR wastewater treatment OR plant growth OR characterization physical chemistry). The references available on the papers that resulted from the mentioned search were added to the paper’s list to ensure that the obtained database comprised a complete survey on this topic.
- (ii)
- Applying exclusion and inclusion criteria—identification: the search included all papers published before May 2023 and available from Scopus, Google Scholar, and Web of Science databases, resulting in 245, 105, and 3 scientific papers, respectively. Results were merged, and duplicated ones were removed, totaling 270 papers. Only peer-reviewed articles in English were considered, and “other” publication types, such as press releases, book chapters, and newsletters, were removed.
- (iii)
- Titles and abstracts screening: the titles and abstracts of articles were first checked by two reviewers, and for the doubtful cases, a full-text evaluation was performed. The screening process allowed for a reduction from 270 results to 140 publications.
- (iv)
- Excluding irrelevant papers based on their full text: the selected papers were carefully reviewed, and studies not directly related to this research aim were excluded. A total of 78 papers were excluded in this step. The selected papers thus addressed the characterization of biochar from OP and OS and/or its applications for environmental remediation and soil amendments, which constitute the sample included and analyzed in this systematic review.
- (v)
- Snowballing: we applied backward snowballing (search in the paper’s reference list). This step has the advantage of identifying important papers in the literature review that could not be identified via the systematic review method. Four papers were added by considering this step, resulting in a total of 62 papers.
Data used in this paper were exported directly from the text and tables of selected papers. When the papers’ results were displayed in graph form, the corresponding numerical data were collected by using GIMP software (version 2.10.34). For data interpretations on the effect of increasing temperature on the physicochemical characteristics of biochar, we use specific subsets of each feedstock, along with SigmaPlot 13.0, for graphical interpretations within SigmaPlot 13.0. We utilized regression fitting functions (linear or quadratic) that best fit the presented data (e.g., best R2 values and greatest p values). We avoided data interpretations that lacked logical sense (such as sigmoidal or sinusoidal equations), even if they had higher R2 values compared to regression equations that were logical and interpretable.
3. Results
3.1. Statistical Data
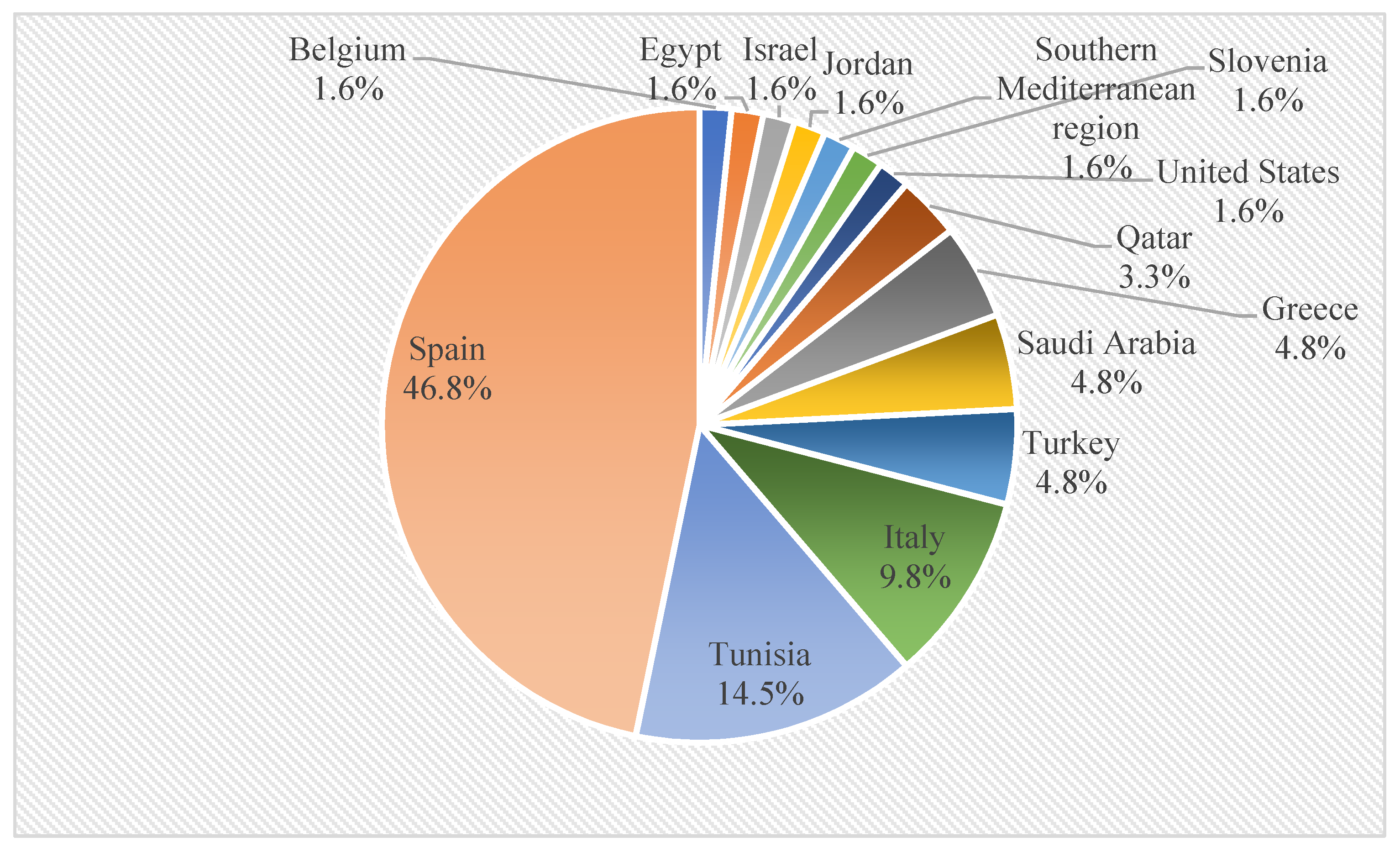
From the selected articles, it was observed that the research institutions responsible for publishing the results on this topic were predominantly from Spain (46.8% of all articles, n = 29), followed by Tunisia (14.5%, n = 9); Italy (9.8%, n = 6); Greece, Saudi Arabia, and Turkey (4.8%, n = 3); and Qatar (3.3%, n = 2) (Figure 2), which is directly related to the fact that Spain is the main world producer of olive oil, contributing with 45% of the world’s olive oil production and 25% of the world’s crop area [34].
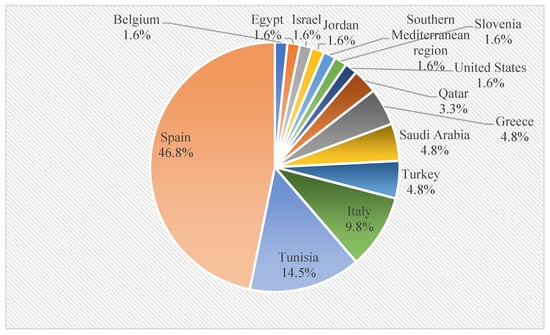
Figure 2.
Global distribution of articles related to OP and OS biochar.
Complete information regarding the pyrolysis process, feedstock type, chemical and physical characterization of biochar, and pyrolysis conditions was considered in 62 papers (Table 1). Papers regarding biochar application on crop/plant productivity, pollutant remediation, and agricultural soil amendments were assessed. The literature review showed that the primary feedstocks for biochar production were OP (56%) or OS (42%). Among the 62 articles selected, 48 experiments reported biochars produced at low temperatures (from 301 °C to 500 °C), 30 trials (from the same article or not) were developed at high temperatures (from 501 °C to 800 °C), and six studies were found in other ranges (up to 300 °C and above 800 °C) (Table 1). In 60% of articles, carbons were synthesized in an N2 atmosphere, 39% in a CO2 atmosphere, while only 1% used He as carrier gas. Most biochars were produced through slow pyrolysis, without activation (74% of the considered data), while the remaining 26% were created either by carbonization and/or physical activation (Table 1 and Table 2).

Table 1.
Summary of biochar from OP and OS research conducted.

Table 2.
Biochar and AC production summary from OP and OS research conducted.
We found that 35%, 29%, and 35% of the studies characterized biochar from a physicochemical, ultimate, and proximate analysis. However, only 16 studies (26%) analyzed all these physicochemical properties simultaneously. In 48% of studies, they measured specific surface area, while 32% of articles reported porous structure results. Finally, 34% of the studies approached crop growth, 45% soil amendments, 29% the amount of nutrients, and 26% pollutant remediation.
3.2. Olive Pomace and Olive Stone Biochar Production Process
The biochar characteristics from olive pomace (OP) and stone (OS) are summarized in Tables S1 and S2. Data indicate highly variable results in activation conditions and associated physicochemical properties. It is confirmed that the biochar quality (which is determined by its composition and properties and is dependent on the purpose-specific applications) and its potential application are greatly affected by the pyrolysis process [13,14,15,16,17,18], as well as the raw material used [14,16,18].
Feedstock plays a critical role in the yield. OS biochar yield is substantially higher than OP biochar (39% versus 33%, means, respectively), which the biomass type may explain. A higher lignin content (50%), in comparison with cellulose (12%) or hemicellulose (24%), probably promotes a higher C-C link formation [91,93,94]. In addition to the variation of content in lignin, cellulose, and hemicellulose, mineral salts in the feedstock will also influence pyrolysis yields [45]. The OS has a lower ash content (Table S3).
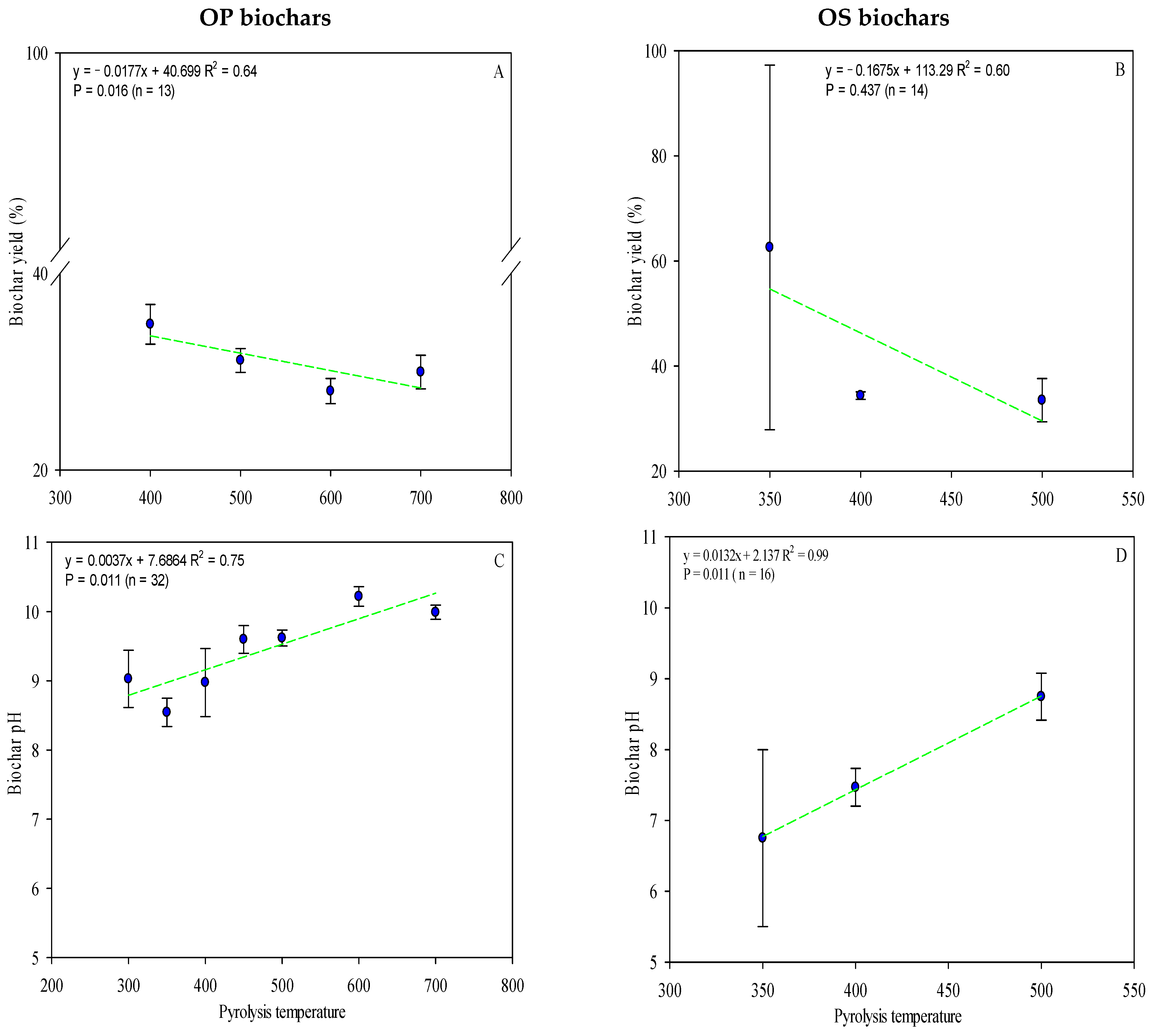
Moreover, biochar yield also decreases with increasing pyrolysis temperature (Figure 3A,B). Both OP biochars (Figure 3A) and OS biochars (Figure 3B) presented a significant correlation between pyrolysis temperature and yield (r2 = 0.64 vs. 0.60, OP vs. OS, respectively). The effect of temperature in combustion has been widely documented in the literature, e.g., at higher temperatures, a decrease in biochar yield is realized [17,18,95,96]. A high and fast pyrolysis temperature intensifies the carbonization of the biomass through rapid dehydrogenation and dehydration of hydroxyl groups, thus reducing the yield [97].
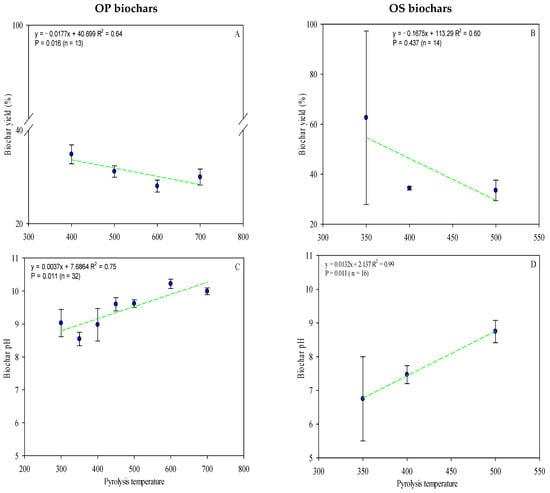
Figure 3.
A linear relationship between pyrolysis temperature and yield (A,B) and pH (C,D) in OP biochar and OS biochar. Complete data for the 62 articles reviewed are provided in Tables S1 and S2. Green lines are mean and the symbol ( ) represents a standard error.
) represents a standard error.
 ) represents a standard error.
) represents a standard error.
Production of Biochar and Activated Carbon
A novel methodology to maximize biochar production involves its transformation into activated carbon (AC) through chemical and physicochemical activations or functionalization.
Functionalizations focus on introducing specific functional groups or chemical moieties onto the OP or OS biochar surface. This process is more targeted and aims to tailor the chemical nature of a material. For example, adding functional groups like -OH, -COOH, or -NH2 to a material’s surface can give specific chemical or biological functionalities. On the other hand, activation generally refers to increasing a material’s reactivity or surface area, making it more porous or chemically reactive. In the context of carbon materials, it often involves exposing the material to gases like steam or carbon dioxide. This process creates pores and increases the surface area, enhancing the material’s adsorption properties [44,63,75,84].
Of the multiple papers reviewed on the activation and functionalization of OP and OS biochar (Table 2), the authors prefer slow pyrolysis processes at different temperatures up to 800–900 °C [41,44,66,75,84,89]. Slow pyrolysis conducted at elevated temperatures is observed to yield optimal results for activating and functionalizing the olive mill byproducts. It is the preferred method to produce activated carbons (ACs), although pyrolysis is still carried out [53,56,71].
Biochar activation processes can be used either before or after slow pyrolysis, with post-treatment being preferred to produce ACs. In El Hanandeh et al. [53], the post-treated method consisted of soaking the pristine biochar in 0.1 M FeCl3 for 1 h, then drying it at 105 °C for 24 h. Due to the acidic digestion by FeCl3, iron oxide was deposited on the biochar’s surface, and the biomass structures opened. This process enhanced the biochar´s porous structure, and the increase in surface area was likely due to the washing of ashes caused by the acidic nature of the solution. This post-treatment can be considered a surface functionalization rather than a direct activation process.
Pre-treatment aims to introduce specific chemical groups or enhance the surface properties of the feedstock, paving the way for a more tailored and controlled pyrolytic process. Omiri et al. [77] incorporated citric acid with copper and nickel nitrates before performing slow pyrolysis to examine the influence of pre-treatment on the nanophysical properties of the biochar, which acts as a support matrix for the deposition of metal nanoparticles. Using citric acid induced the formation of bimetallic copper/nickel nanoparticles exhibiting an agglomerated or even raspberry-shaped morphology. This unexpected outcome can be considered functionalization and activation.
On the other hand, functionalizations can be performed after slow pyrolysis as an additional step to modify and optimize the properties of ACs. This flexibility allows for a comprehensive and customizable approach to meet specific application requirements. The mentioned functionalizations generally involve metallic oxides (e.g., Co3O4) [56], acids (e.g., HNO3, H2SO4, H3PO4) [41,59,66], and metallic salts (e.g., Ni(NO3)2·6H2O, Cu(NO3)2·3H2O), or a mixture of chemical compounds, water vapor, and gases [71,77,84].
3.3. Olive Pomace and Olive Stone Biochar Characteristics
3.3.1. Physical and Chemical Properties of Biochar
OP biochar and OS biochar show pH values varying from 5.33 to 10.7 and 5.50 to 9.80, respectively (Table S2), increasing with pyrolysis temperatures (Figure 3C,D). As expected, a linear correlation exists between pH and biochar pyrolysis temperature (r2 = 0.75 vs. 0.99, OP vs. OS, respectively) (Figure 3C,D). Most of the biochars from olive byproducts have an alkaline pH. In general, high pyrolytic temperatures, high metal oxide, ash content, and the development of heterocyclic nitrogen compounds influence the production of high-pH biochar [98]. Biochar often can have an initially high pH (alkaline), which is desirable when activated with acidic, degraded soils; nonetheless, if soil pH becomes too alkaline, plants may suffer nutrient deficiencies [99].
Although biochar exhibits a considerable cation exchange capacity (CEC), which refers to the capacity to exchange positively charged species, such as Ca2+, Mg2+, K+, and Na+ [18], only five reviewed studies show CEC data.
The highest CECs are observed in OS and OP biochar produced at low temperatures (25.5 and 20.3 cmolc kg−1, respectively), in contrast with biochar produced at relatively high temperatures (>400 °C), ranging from 11.9 to 18.6 cmolc kg−1 [40,79], which may be linked to the loss of some acidic/carboxylic functional groups at high temperatures [100,101]. Moreover, higher CEC biochar may indicate an excellent capacity to adsorb/remove potentially toxic elements [102].
Electrical conductivity (EC) in biochar is mainly influenced by feedstock (Table S2), registering the highest value for OP biochar (3.65 dS m−1, mean value) and the lowest for OS biochar (0.98 dS m−1, mean value). There was no significant correlation between EC and biochar pyrolysis temperature (Figure S1A,B). This could be an important biochar characteristic, limiting its final application in agronomic use. For example, for agronomic applications, a high EC value indicates the presence of cations (e.g., K+, Ca2+, and Mg2+) and anions (SO42− and PO43−), nutrients essential for crop growth. This fact highlights the importance of in-depth biochar production and characterization knowledge, allowing the creation of more versatile biochars with potential socioeconomic impacts and vegetable nutrition.
3.3.2. Proximate Analysis
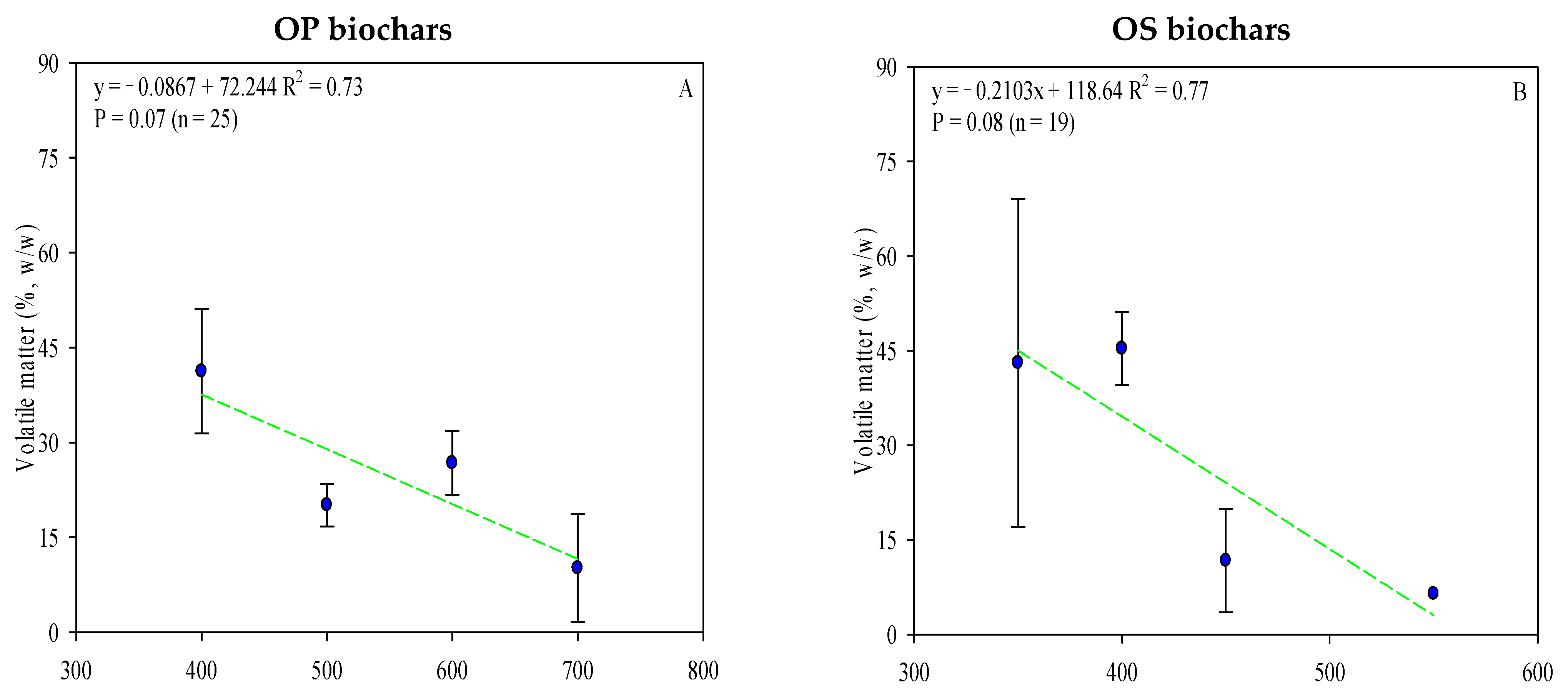
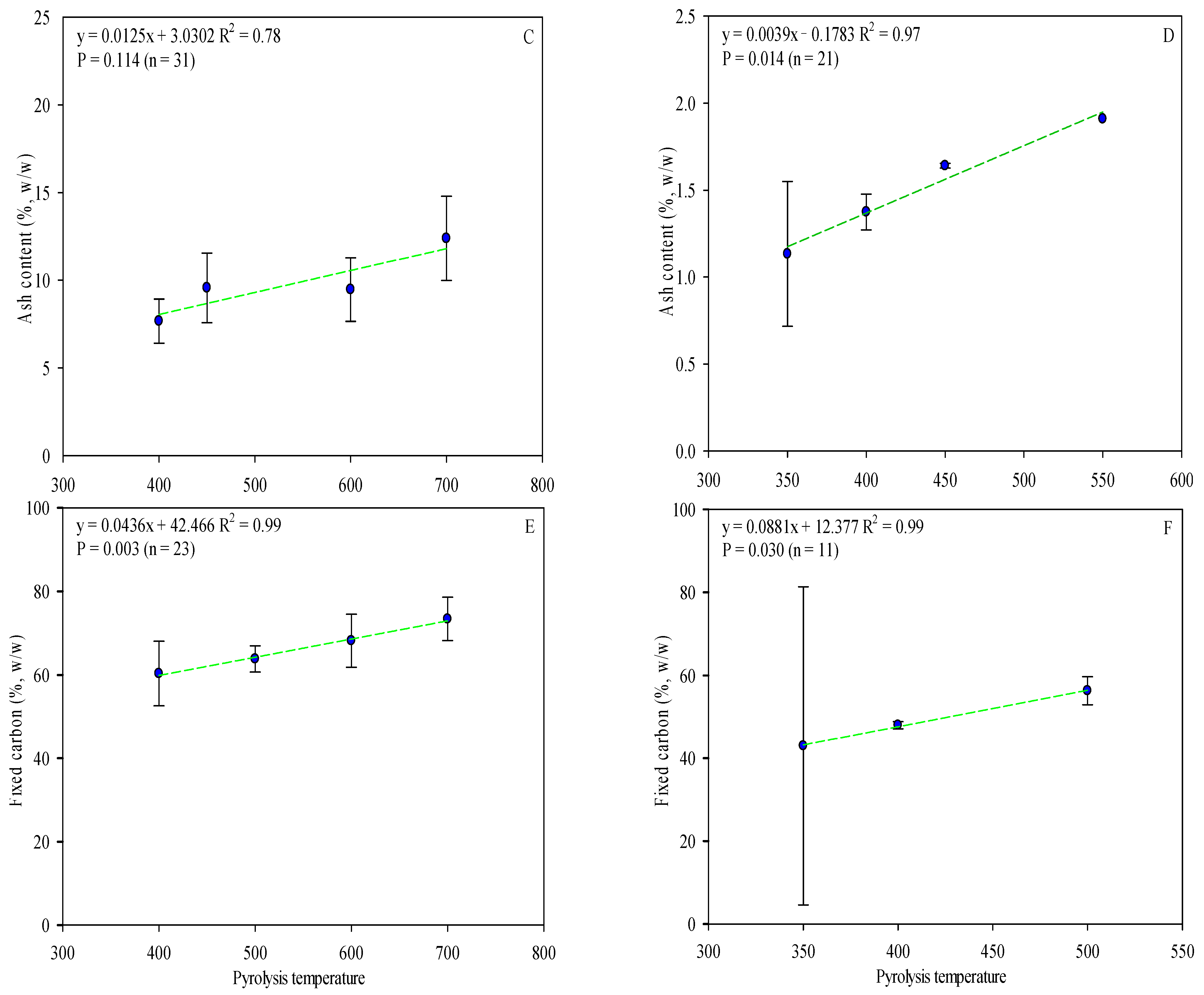
The proximate analysis includes the moisture content, ash content, volatile matter, and fixed carbon. As referred to above, ash content varies according to the type of feedstock used (Table S1) and pyrolysis temperature (Figure 4C,D). OP biochar has a higher ash content, which can be correlated with higher mineral content in olive fruit, mainly potassium (Table S2). Moreover, ash content positively correlated with temperature pyrolysis (Figure 4C,D). It could play an important role in agriculture, where, in addition to reducing soil acidity, it could improve productivity in acid soils. In other cases, it may be more advisable to use OS biochar (with low ash content and pH, when processed under the same conditions [103]) to avoid the concentration of soil mineral salts.
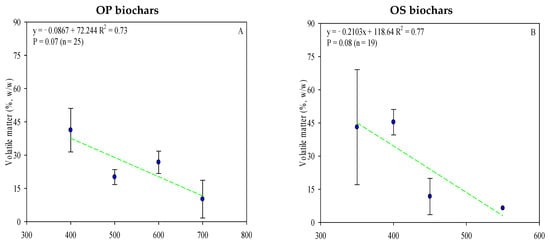
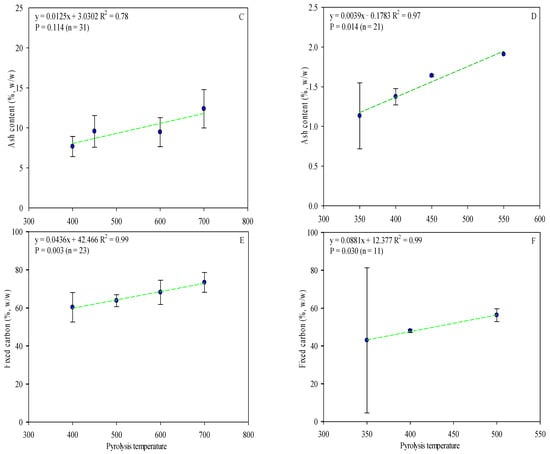
Figure 4.
Linear correlation between pyrolysis temperature and OP and OS biochars’ volatile matter (A,B), ash (C,D), and fixed carbon content (E,F) (w/w). Complete data for the 62 articles reviewed are provided in Table S1. Green lines are mean and the symbol ( ) represents a standard error.
) represents a standard error.
 ) represents a standard error.
) represents a standard error.
Fixed carbon contents for the biochars ranged from 25.5% to 89.0% for OP and 4.60 to 90.2% for OS (Table S1). When pyrolysis temperature increases, the fixed carbon content increases while the volatile matter decreases (Figure 4). The C content fixed in OP and OS biochar increased by 7 and 8% with the temperature increase and, at the same time, the volatile matter decreased by 31–37%, considering the same thermal conditions, resulting in a strong correlation (r2 = >0.7) between volatile matter and pyrolysis temperature (Figure 4). Considering the mentioned data, it is clear that the biochars’ fixed carbon content is affected by both pyrolysis temperature and feedstock. Despite the higher carbon content of OP biochar, the volatile matter level was higher in OS (OP—mean value: 27.7%; O—mean value: 41.7%). Even though previous studies have demonstrated that biochar with greater fixed carbon is obtained from biomasses with greater volatile content [104], this review recorded poor results, which may be explained due to variations in moisture and residence time of pyrolysis. The moisture content significantly reduces the mass loss and release of volatile matter, while extended residence times lead to a significant part of volatile matter being recombined and polymerized within the sample’s structure [105].
The volatile matter is a good indicator of biochar stability and nitrogen availability when applied as a soil amender, considering its potential impact on short-term microbial activity and plant growth [106]. Thus, biochar may constitute a suitable and ecological material for soil amendment, nutrient source, and carbon sequestration.
3.3.3. Ultimate Analysis
The ultimate analysis indicates the elemental constituents of biochar: carbon (C), hydrogen (H), nitrogen (N), sulfur (S), and oxygen (O) [107]. Elemental compositions for biochar obtained with different temperatures are listed in Table S1. OS biochar contains the highest C content (77.2%), while OP biochar contains the highest N content (1.79%). The contents of H and O were similar for the two biochar types. When the pyrolysis temperature increased, C content increased, whereas the H and O decreased, regardless of the type of biomass, indicating an increasing degree of biochars’ carbonization (Table 3).

Table 3.
Elemental composition of OP and OS biochars at different temperatures.
An increase in C concentrations may occur when pyrolysis temperature increases due to cleavage and polymerization reactions that cause easily degradable C compounds to be restructured. At the same time, other elements may be lost to volatilization, and thus, overall total C content increases [19,108]. The great majority of the reviewed literature classifies the OP and OS biochars as class 1 on the International Biochar Initiative (IBI), i.e., C content higher than 60% [109]. In this study, N content in both biochars presented an increasing trend, reaching a peak for an intermediary pyrolysis temperature followed by a slight reduction of N content when subjected to higher temperatures (Table 3), which could be justified by the formation of N compounds, which do not readily volatilize at lower pyrolysis temperatures [110].
The pyrolysis temperature and feedstocks affected the H/C and O/C atomic ratios (Table 3), tending to decrease with pyrolysis temperature and consequently increasing aromaticity [110]. In addition, it was observed that OP-derived biochars generally presented lower H/C and O/C ratios, indicating higher aromaticity and resistance to biodegradation.
Results showed that the C/N ratio increases with the increment of pyrolysis temperature up to 500 °C. It was observed that there is a reduction in the C/N ratio in OP biochar at higher temperatures. Only OP biochar produced at 600 °C may increase N availability in the soil [111]. OS biochar with high C/N is practically inert for microbial activity but could increase the recalcitrant soil organic C pool.
3.3.4. Surface Area and Porosity
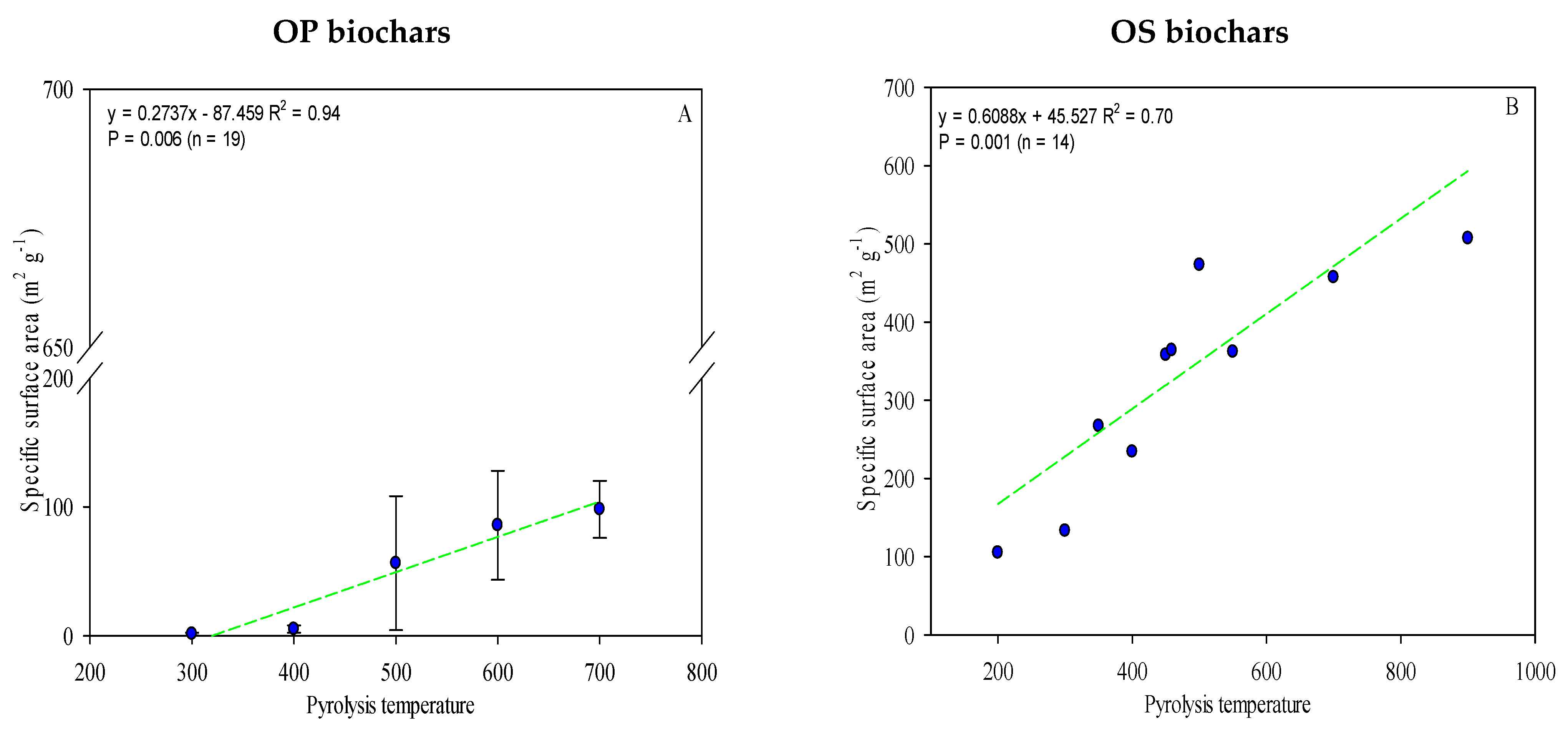
Comparisons of the specific surface area (SSA) and pore volume (PV) of the different biochars indicated that these properties were closely associated with the feedstock type. The reviewed papers recorded that SSA ranged from 0.30 m2 g−1 to 507 m2 g−1 (Table S2), with OS biochars registering much higher values than those produced from OP. As the pyrolysis temperature increases, a gradual increase in the SSA for both types of biochar (OP and OS) was recorded (Figure 5A,B) due to biochar experiencing crystallization and recombination during the carbonization process, leading to an expansion of the total pore volume and, consequently, on the SSA [25,112]. A strong correlation between biochar SSA and pyrolysis temperature was observed (r2 = 0.70 and 0.94 for OS and OP biochars, respectively) (Figure 5A,B).
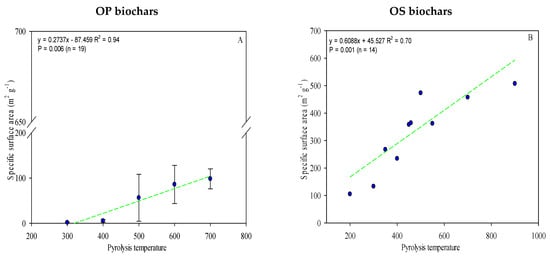
Figure 5.
The linear relationship between pyrolysis temperature and the biochars’ specific surface area (A,B). The complete data is given in Table S2. Green lines are mean and the symbol ( ) represents a standard error.
) represents a standard error.
 ) represents a standard error.
) represents a standard error.
The European Biochar Certificate (EBC) states specified that SSA should preferably be higher than 150 m2 g−1 [113], as with the OS biochars produced over 300 °C. A higher surface area is preferable due to its significant role in soil fertility, leading to improvement of the structure of the soil and provision of ecological niches for soil microorganisms [114]. In addition, a huge SSA of OS biochar makes it an ideal adsorption medium for trace metal elements, organic pollutants, and pesticides.
Alazzaz et al. [38] and Sanginés et al. [85] showed that OP and OS biochars produced under low temperatures (300 °C and 200 °C, respectively) resulted in lower values of total pore volume (0.002 cm3 g−1 and 0.04 cm3 g−1, respectively); as expected, higher pyrolysis temperatures (700 °C and 900 °C, respectively) led to high values of total pore volume (0.083 cm3 g−1 and 0.193 cm3 g−1, OP and OS biochars, respectively) (Table S2). The pores in the C-skeleton of the biochar developed due to the decomposition of lignin, the quick degradation and the release of volatiles from feedstock material, and the reaction of aromatic condensation as the temperature increases [115]. Both the porosity and SSA play key roles in biochar anion and cation exchange capacities, therefore influencing their adsorption abilities [116].
3.3.5. Nutrient Content
Besides C and N, biochar also contains some essential plant nutrients such as P, K, Ca, Mg, and micronutrients, as shown in Table S2. Therefore, biochar incorporation into agricultural soils may reduce the need for chemical fertilizers. In general, OP biochar has a higher nutrient content than OS biochar, reflecting the composition of the feedstocks (Table S3).
Pyrolysis temperature also influenced total nutrient contents, but to a much smaller extent; for example, Delgado-Moreno et al. [32] observed that upon further heating from 300 to 500 °C, OS biochars showed slight enrichment for P, K, Ca, Mg, and Fe (Table S2). On the other hand, Pellera and Gidarakos [78] showed that with the increase in pyrolysis temperature, OP biochars showed a pronounced enrichment for P, K, Ca, Mg, and Fe. It is essential to highlight that the nutrient content of OP may vary between regions, as reported by Fornes et al. [57]. On the other hand, the concentration of trace metals in all biochars was below the maximum concentrations allowed for biochars, according to IBI guidelines [109]. The relatively high nutrient concentrations and low trace metals concentrations in OP biochar show the versatility of its applicability for agricultural use.
3.3.6. Effect of Activation and Functionalization on Activated Carbon Characteristics
Activation focuses on developing the biochar´s surface area, making it more porous, with the aim of application in gas or liquid pollutant adsorption processes. These materials are usually activated by CO2 gasification, steam, demineralization, and others (Table 4).

Table 4.
Correlation of activation and functionalization of biochars with surface area, pore, and micropore volume.
The surface area (SSA) of biochar can vary greatly depending on the selected activation technique, reaching values of up to 2360 m2 g−1, which is an exceptionally large SSA. In this case, the procedure involved carbonizing OS under continuous N2 flow at 500 °C to achieve this elevated surface area. Subsequently, the resulting biochar underwent physical activation with CO2. The gasification temperature was set at 800 °C, and the gas feed was switched to CO2 with a flow rate of 150 cm3 STP min−1 for 7 h, resulting in an AC with high SSA [44]. Guerrero-Pérez et al. [66] employed a methodology similar to Calzado et al. [44], but the material underwent a two-hour CO2 gasification process, obtaining an AC with 1355 m² g−1 of SSA. In these processes, in addition to transforming a byproduct into a high-added value material and a broad spectrum, it also promotes decarbonization by using greenhouse gases such as CO2.
Other studies examine the combination of water vapor, with or without gases, achieving noteworthy values for SSA [84]. Combining CO2 and steam as activating agents significantly influences the development of AC porous structure. For example, the production process for CS-850-60, which involves CO2 and steam as activating agents at 850 °C for 60 min, exhibits distinctive characteristics from creating micropores (Vmicro = 0.553 cm3 g−1 and SSA = 1187 m2 g−1) and mesopores.
Among the described activation methods, demineralization stands out as a pre-treatment methodology that eliminates minerals from OS biochar, enhancing the resulting biochar’s porosity and SSA. Ball milling, a post-treatment approach, also mechanically crushes and grinds biochar particles, contributing to increased porosity and SSA. These treatments are activation methods as they alter biochar’s physicochemical properties, achieving SSA of up to 438 m2 g−1.
Finally, the wide range of activations can be combined with functionalization. Fourier Transform Infrared analysis (FT-IR) can accurately identify functional groups on the surface of adsorbents (Table S4). The main structures encountered in AC from olive mill residues include hydroxyl (OH), carbonyl (C=O), alkyl, and hydrocarbons. These functional groups exert significant influence over the adsorptive performance of ACs, playing pivotal roles in surface interactions and engagement with reactants [29,41,53,56,66,88].
Moreover, aromatic structures in ACs add an extra layer of significance, contributing to the material’s resilience against decomposition under heat exposure and enhancing its durability when exposed to acidic or alkaline environments [56,63,92].
On the other hand, introducing specific groups on the chemical surface, such as sulfonic acid (SO3H) and sulfates (SO42−), enhances catalytic activity. The catalytic activity of AC is complemented by carbonyl groups, which also serve as active sites for catalytic reactions. Their involvement is attributed to the ability of carbonyl groups to participate in redox reactions and adsorb species on their surface, amplifying the catalytic efficacy of the materials [41].
3.3.7. Elemental Composition and Thermogravimetric Analysis of Activated Carbons from OP and OS
OP is a valuable raw material for synthesizing AC because of its high oxygen concentration and fixed carbon content (41.51% of total carbon in the final product material).
Ayadi et al. [41] carried out an elemental analysis on the ACs and biochar before and after pyrolysis, seeking changes in the material’s composition during the activation and sulfonation processes. Authors identified that the C content increased from 77.95% to 85%, whereas oxygen, nitrogen, and hydrogen contents were reduced by half due to decarboxylation or depolymerization and dehydration reactions.
Azzaz et al. [29] observed that materials that underwent hydrothermal carbonization had higher volatile matter and lower C content compared to un-treatment biochar, which showed higher levels of nutrients (magnesium, potassium, sodium) due to combustion at high temperatures.
Valero-Romero et al. [89] showed that the phosphoric acid activation process increased ash content and oxygen content, accompanied by the integration of phosphorus on the surface. Materials showed an ash value of 0.3–3.7%, a C content of 93.2–87.9%, an oxygen content of 4.7–6.7%, and no detectable phosphorus (Table S5). The authors analyzed the re-oxidation process after a thermal treatment at 900 °C. The authors observed that the carbon surface treated at high temperatures in N2 is unstable and may re-adsorb oxygen even at room temperature. Since no loss of phosphorus was observed on the surface after several reduction/oxidation cycles, it was deduced that the re-oxidation of the phosphoric-acid-ACs at room temperature occurs due to the large number of highly reactive free sites susceptible to re-oxidation, where oxidation from C-P to C-O-P occurs, as this redox reaction is reversible at high temperatures.
On the other hand, Guerrero-Pérez et al. [66] concluded that the presence of phosphorus in the AC material ACP significantly enhanced its thermal stability and oxidation resistance compared to the AC without phosphorus. The TGA results showed that the ACP material remained resistant in air up to 500 °C, while the AC sample began to gasify around 400 °C. This difference in thermal stability indicated that the phosphorus-containing ACP material was more resistant to oxidation at high temperatures and in oxidant atmospheres.
Valero-Romero et al. [89] analyzed that both biochars were thermally stable in the 100–450 °C range. The weight loss observed at other temperature ranges indicated the instability of OP/OS. Additionally, the presence of magnetite on the surface contributed to its enhanced thermal stability, particularly after 550 °C. This finding suggests that incorporating magnetic nanoparticles improved the thermal properties of the biochar, making AC more resistant to high temperatures.
El-Shafie et al. [56] demonstrated that both OS biochar and Co–OSBC (Cobalt-loaded OS biochar) exhibited thermal stability within the 100–450 °C temperature range. The weight loss observed between 50 and 100 °C and 550–800 °C, respectively, was attributed to the vaporization of free water in the first case and to the carbonization of polymeric material in the second.
Zouari et al. [92] showed that the samples subjected to demineralization exhibited higher weight loss values (10%), indicating a reduction in the material’s thermal resistance. It was attributed to the removal of minerals and hemicelluloses during the demineralization. The presence of ashes in the OP biomass was found to have a catalytic effect on the thermal degradation behavior. Ash minerals enhanced biochar formation during thermal decomposition. Demineralization decreased yield, indicating that the high ash content in the OP biomass promoted biochar formation by thermal catalysis.
3.4. Applications of OP and OS Biochars
3.4.1. As Soil Amendments or Conditioners
Due to the physical and chemical characteristics of OP and OS biochar, the soil properties were moderately affected after biochar application, as listed in Table 5.

Table 5.
Summary of papers on the effect of OP- and OS-derived biochar on soil properties.
Biochar’s alkalinity allows it to function as a soil amendment for acidic soils, improving soil health and productivity. Alazzaz et al. [38] reported a slight increase in pH after application of 1% and 3% OP biochar in calcareous soils due to biochar-induced alkalinity as a result of the oxides and carbonates accumulated during the pyrolytic process. Alburquerque et al. [39] attributed the notable increases in soil pH to the soil’s low buffering capacity. The application of OS-derived biochar led to a pH increase of approximately one unit (from 6.5 to 7.5) with a 7% application rate. Moreover, ash-rich biochar led to even higher soil pH values (8.5) for the same biochar application rate. This liming effect is considered positive in acidic soils but can have adverse impacts in non-acidic soils [39].
After applying OP biochars, even more pronounced impacts in soil EC were recorded [38,42,51,55,67]. Hmid et al. [67] reported that the OP biochar application with 15% in sandy soil increased the EC from 0.06 dS m−1 to 0.19 dS m−1, making it unlikely that amended soils have a detrimental effect on microbial activity and crop growth [117]. Consequently, OP or OS biochars could be safely added to soils with relatively high buffering capacity without seriously harming soil salinization and alkalinity.
Nutrients released from biochar into the soil solution vary depending on the element’s specific sorption affinity with either the biochar or the soil [118]. Alazzaz et al. [38] recorded that the application of 3% biochar increased the soil’s exchangeable K content from 40.5 mg kg−1 (control) to 324 mg kg−1. Additionally, the application of 3% biochar exhibited significant increases in the soil’s availability of Na, Ca, Mg, and some micronutrients (Fe and Zn). This suggests that the availability of soil nutrients amended with OP biochar could likely depend on biochar type and application rate [38].
The application of biochar has been proposed as a promising long-term tool to increase soil C sequestration (Figure 6). This is due to biochar’s high stability and aromaticity, whose average residence time is longer than plant biomass, contributing alternatives to promote carbon sequestration [119]. Some studies showed that applying OS and OP biochar significantly increased the total C content on the soil’s top layers [42,47,48,49,72].
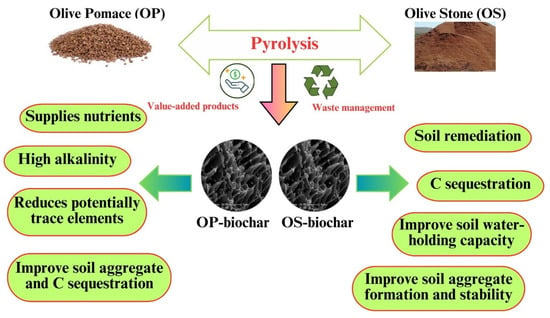
Figure 6.
Positive effects of OP- and OS-derived biochar on soil.
Olive-mill-derived biochars may reduce trace metals in contaminated soils, influencing their mobility and toxicity through diverse physicochemical mechanisms [120]. Despite the few existing OP and OS biochar application studies in contaminated soils, Hmid et al. [67] observed a significant reduction of Cd, Pb, and Zn with increasing rates of OS biochar added to contaminated soil. Contrastingly, Campos et al. [49] did not find that biochar application reduces the bioavailability of trace elements (As, Cu, Pb, Ba, and Zn). However, biochar has a rich pore structure and a huge SSA that can improve soil’s physical characteristics, such as bulk density, surface area, water-holding capacity, and permeability [121,122,123].
In several studies, OS and OP biochar application in different doses (2–30%) decreased soil bulk density and particle density and increased the total pore space and soil water-holding capacity, hence positively impacting plant growth [39,42]. Kavdır et al. [70] found that the application of OP biochar at high temperatures significantly improved soil aggregate stability. In contrast, the proportion of macroaggregates (>4 mm) and mean weight diameter of aggregates decreased. On the other hand, Lilli et al. [72] found that the distribution of water-stable aggregates was significantly increased by the addition of OP biochar (an increase in macroaggregates by 16.4% and a decrease in microaggregates by 3.3%).
Based on previous studies, the variables affecting the microbial response to biochar application were mainly related to biochar properties, application load, and soil properties [124]. However, our current knowledge of the impact of applying OP and OS biochar on the soil’s microbial status remains scarce. Lilli et al. [72] found that in the rhizosphere, the OP biochar-treated plants showed a higher number of unique taxa. On the other hand, Campos et al. [45] found that applying biochar on contaminated soils changed the bacterial diversity, reducing the presence of extremophilic bacteria and increasing the presence of bacteria typical of non-contaminated soils. These authors underlined that changes in the soil bacterial richness and diversity after biochar application were correlated with the increment of soil pH, bacterial diversity, and plant growth.
3.4.2. Plant Growth
Several impacts of biochar application on germination and seedling growth range have been reported. Hormesis is regularly detected, meaning that high biochar rates can harm plants, while low rates can be stimulatory. Both positive [39,40,46,47,51,55,68,72,79,81] and negative [33,42,57,59,60,74] responses have been found in field trials to enhance yield crop as a result of biochar application to soils (Table 6).

Table 6.
Summary of research on the effect of biochar from OP and OS on plant growth.
Plant growth improvements and crop yields due to biochar application are the result of the modification of soil’s physical, chemical, and biological properties. In the research conducted by El-Bassi et al. [55], OP biochar application rates of 2.5% and 5% used as a soil amendment promoted tomato seedling growth. Therefore, the produced biochar had great potential to be used as a biofertilizer in agriculture. Lilli et al. [72] found that the yield of tomato plants treated with biochar was 29% higher than the control, which could be attributed to the minerals contained in the biochar, especially the K, and a direct contribution of C fixed in the biochar [38,55].
An excessive application rate of biochar addition may have an inhibitory effect on seed germination and crop yield. This is due to biochar’s high alkalinity and salinity, mainly caused by the large content of soluble K in the biochar [57,59]. Therefore, a clear understanding of these parameters is essential before the biochar can be used in a plant growth substrate. In this sense, Fornes et al. [57] used seed germination bioassays to prove the produced biochar’s phytotoxic character due to its high salt content. Similarly, Fornes et al. [58] evaluated that the application of 10 and 25% of OP biochars as substrates in soilless cultivation of tomato plants did not affect shoot and root growth; however, higher doses had a negative impact on the production and number of tomato fruits, without consequences on the fruit quality. This negative impact was attributed to one of the biochar-containing substrates being saline. Nevertheless, Fornes and Belda [59] demonstrated that salt excess and alkalinity were easily reduced by treating biochar with diluted nitric acid, thus removing the phytotoxic factors.
Plant stress is one of the major problems encountered in agricultural environments. There are both biotic and abiotic types of plant stresses [125]. Literature has confirmed that adding biochar was generally effective in reducing the hazard of polluted soils and restoring balanced plant mineral nutrition [126]. Campos et al. [47] found that Cd, Cu, and Zn bioavailable contents decreased in soils amended with OS biochars. In addition, biochar application enhanced the germination in acidic, polluted soil, where germination was impossible. Likewise, applying OS biochar to metal-contaminated soils provided environmental benefits, including increased diversity and plant growth [46].
Some studies have shown that biochar might improve the photosynthetic capacity of crop leaves and the activities of antioxidant enzymes [127,128,129]. In this regard, Hmid et al. [68] observed that increasing OP biochar increased 15-day-old bean growth and soluble protein contents and decreased the activities of antioxidant enzymes and leaf Cd, Pb, and Zn contents. Besides, De la Rosa et al. [51] found that olive trees amended with OP biochar had better water and photosynthetic conditions.
The effective application of biochar as a practical soil amendment in agriculture might face limitations due to economic, sustainability, and environmental factors [130]. Collection of waste material, processing of feedstocks (e.g., drying), and pyrolysis operations (i.e., pyrolysis reactor and operating cost), and costs associated with the handling and application of biochar, one must consider and weigh them against the environmental benefits and enhancements in crop production [131]. However, in Europe, just using quantities available of OS and OP as feedstock for the production of biochar could sequester CO2 in the range of 0.93–2.32 MtCO2eq yr−1 and 2.14–2.97 MtCO2eq yr−1, respectively [132].
3.4.3. Biochar and Activated Carbon Applications
The application of AC represents a dynamic and innovative approach in the field of environmental and agricultural sciences. The use of biochar and AC has increasingly attracted the attention of researchers, given their multiple applications, ranging from soil improvement and carbon sequestration to water purification and pollutant remediation. As researchers delve deeper into the potential of AC, their diverse and sustainable applications continue to unfold, promising solutions to contemporary challenges.
In catalysts, Ayadi et al. [41] demonstrated the potential of different solid AC acid catalysts produced from OP and coconut husks through sulfonation for sustainable and efficient biodiesel production from OP. The solid AC acid catalysts were employed in the esterification of olive pomace oil (OPO), facilitating the conversion of free fatty acids into esters. The results indicated that, after 5 h of reaction, both AC catalysts resulted in acid values lower than 2 mg KOH g−1 and met the requirements of EN14214 standard [133], with a content higher than 97%, indicating the successful esterification process facilitated by the catalysts, highlighting their catalytic properties and applicability in biodiesel production processes. In Guerrero-Pérez et al. [69], the aim was to evaluate the behavior of the vanadium-containing activated carbon catalysts for the partial oxidation of propylene to oxygenates. Furthermore, the study discussed how adding zirconium as a dopant could enhance the catalytic performance. The higher propylene conversion percentages for the vanadium-zirconium-doped catalysts indicate the synergistic effect of incorporating both elements in enhancing the AC catalytic activity, highlighting their catalytic activity and selectivity towards oxygenate products. At last, Omiri et al. [77] deployed biochar as catalysts for methyl orange (MO) degradation.
Among the benefits of AC in adsorption processes, El-Azazy et al. [54] used OS biochar and magnetic OS biochar as adsorbents in the removal of antibiotic ciprofloxacin in aqueous solution, highlighting the strong adsorption capabilities (174.03 mg g−1) of the magnetic OS biochar.
Ghouma et al. [63] analyzed the AC performance in NO2 adsorption under various conditions, finding that the AC, produced through steam activation of OS, proved to be a highly effective adsorbent for NO2 removal. The study highlighted a temperature-dependent variation in the NO2 adsorption capacity of the AC, suffering a substantial reduction in adsorption capacity (39%) when temperature increased from 20 °C to 50 °C.
Finally, Usama et al. [88] aimed to evaluate the effectiveness of biochars in immobilizing lead (Pb) and enhancing nutrient availability in Pb-contaminated sandy loam soil. The silica-embedded (BCSi) and nutrient-loaded (BCSiNP) biochars showed the highest adsorption capacity for Pb (57.78 mg g−1) in BCSiNP followed by BCSi (54.01 mg g−1), indicating their excellent potential to immobilize Pb in the soil. The modified biochars also increased the bioavailability of essential nutrients (P, NO3-, NH4+) in the soil. Overall, the study demonstrated that the modified biochars effectively immobilized Pb and improved nutrient availability in the contaminated soil.
3.5. Challenges and Future Perspectives
The olive industry’s waste constitutes an environmental hazard due to its acidity and high phenol content. Converting these low-value feedstocks into cost-effective materials such as biochar is a sustainable method of waste valorization with several emerging applications. A literature review on biochar production from olive mill wastes revealed academia’s extensive efforts on biochar characterization, property optimization, and applications during the past few decades. Nevertheless, there are several gaps on this topic, requiring deeper knowledge and substantial efforts to understand the biochar production process and how to manipulate certain features in order to obtain functional biochar, answering the needs and challenges presented by the several targets of application, as well as soil and water.
Future studies should focus on the mechanisms involved in agriculture and environmental applications of OP and OS biochars. Although several studies have proven that OP biochar may promote desired effects in both soil and plants, the mechanisms involved and how to control those processes are not well understood.
Since characteristics of biochar are highly controlled by types of feedstocks and pyrolysis conditions (e.g., temperature), it is essential to select appropriate feedstock, i.e., OP or OS, according to the specific physicochemical biochar properties aiming at previously established purposes.
Furthermore, studies related to the use of OP and OS biochar in optimizing the removal of polyphenols from olive mill wastewater can be a cost-effective and profitable alternative, which can be included in conventional wastewater treatment plants. Thus, biochar produced from olive mill byproducts can serve as an ecological alternative and a promising material for future wastewater treatment and residue management strategies, fostering sustainability and a circular economy policy.
When employed in agriculture, biochar is typically applied in significant amounts, which makes it difficult to posteriorly remove it from the soil in case of any unfavorable outcome. Future research should focus on biochar’s effectiveness for different methodologies and time of application, considering as well the cropping system and plant growth stage.
Modified biochar (e.g., acid, base, salt, or steam/air activation) may result in different outcomes imposed by its distinct elemental and chemical composition (e.g., SSA, porosity, CEC, surface functional groups, and pH), which should also be evaluated when considering modified biochar application, not only on soil but also in other environmental compartments.
Regulatory entities, from local to national and European bodies, must provide legal support and financial incentives to produce and use biochar, especially from local waste, such as olive oil byproducts.
4. Conclusions
This systematic review presented a summary of current research developed to produce and characterize OP and other environmental applications. Without a doubt, the pyrolysis temperature and feedstock type have a massive impact on the characteristics of the resultant biochar. Therefore, it is important to have in-depth knowledge of the relation of these two variables in order to optimize the productive process and maximize biochar’s efficiency and its intended uses. OS biochar has advantageous properties such as large surface area, high porosity, low ash content, and high C amount, making it an efficient tool to improve the surface adsorption process. These properties make it an efficient and suitable tool for various applications such as soil remediation, and wastewater treatment, but not to crop growth. However, the OP biochar presents a high C amount and ash content that makes it suitable for amendment in agricultural soils and long-term C sequestration. Biochar alters not only the amended soil’s physical, chemical, and biological properties but also influences the nutrient dynamics, immobilizing trace metals, and thereby improving soil quality and crop yield. However, the application rate of OP biochar may have some inhibitory effect on seed germination and crop yield, which requires further study. Possibly, the phenomenon is related to an overdose or inappropriate production of biochar (toxic polycyclic aromatic hydrocarbons or too aromatic, C-C) that must be previously tested with the quality indicators presented (pH, CEC, SSA, porosity, H/C vs. O/C, ashes). Overall, the use of byproducts from olive oil is a promising feedstock to produce sustainable biochar, though the productive process needs to be further improved and tailored to maximize its characteristics and applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16125004/s1, Table S1: Summary of yield and ultimate and proximate analyses of biochars from OP and OS from different studies; Table S2: Summary of physical and chemical characteristics and nutrients of biochars from OP and OS from different studies; Table S3: Physical and chemical characteristics, elemental composition, and nutrients of the feedstocks (OP and OS) used in this review; Table S4: Correlation of activation and functionalization of biochars and AC with functional groups; Table S5. Elemental analysis of CNHS-O. Figure S1: Linear relationship between pyrolysis temperature and electrical conductivity in OP biochar and OS biochar. Com-plete data for the 62 articles reviewed are provided in Tables S1 and S2. PRISMA 2020 Checklist.
Author Contributions
Conceptualization, J.F.L.F. and Z.H.; methodology, J.F.L.F. and Z.H.; software, J.F.L.F.; formal analysis, J.F.L.F., S.T.C. and Z.H.; investigation, J.F.L.F. and Z.H.; resources, Z.H. and T.d.F.; writing—original draft preparation, J.F.L.F., S.T.C., A.P.F.d.S. and Z.H.; writing—review and editing, J.F.L.F., H.T.G., T.d.F., A.P.F.d.S. and Z.H.; supervision, Z.H.; project administration, Z.H. and T.d.F.; funding acquisition, Z.H. and T.d.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the research carried out by project “EEAGRANT_CALL#5—Innovative nature-based solutions for restoring ecosystem services in areas degraded by the great fire of Picões, Portugal—SOILING” funded by European Economic Area (EEA) Financial Mechanism 2014–2021 Environment, Climate Change and Low Carbon Economy Programme. ‘Environment Programme (*); and Programme 13/REACT-EU/2021 “ForestWaterUp-Nature-based solutions for the ecological restoration of degraded soils in the Sabor Lakes” (POCI-07-62G4-FEDER-181557), funded by COMPETE 2020 Operational Programme. The authors are also grateful to the Foundation for Science and Technology (FCT, Portugal) for the financial support through national funds by FCT/MCTES (PIDDAC): CIMO, UIDB/00690/2020 (DOI: 10.54499/UIDB/00690/2020) and UIDP/00690/2020 (DOI: 10.54499/UIDP/00690/2020); and SusTEC, LA/P/0007/2020 (DOI:10.54499/LA/P/0007/2020), and by the institutional scientific employment program-contract with Zulimar Hernández. Ana Paula Ferreira thanks her doctoral Grant with reference PRT/BD/153090/2021, financed by FCT, with funds from NORTE2020, under Program MIT Portugal. (*) Through the Agreement on the European Economic Area (EEA), Iceland, Liechtenstein, and Norway are partners in the internal market with the Member States of the European Union. The Secretary-General of the Environment operated EEA Grants support to Portugal to promote a continuous and balanced strengthening of economic and trade relations, reduce social and economic disparities in Europe, and strengthen bilateral relations between these three countries and the beneficiary countries.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to acknowledge 11 Call#5-SOILING for funding a full-time Pos-Doctoral Grant (MORE/SOILING/1) for the first author (J.F.L.F.), which allowed the publication of the present research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO Food and Agriculture Organization of the United Nations: FAOSTAT Statistical Database. Available online: https://www.fao.org/statistics/en (accessed on 20 January 2023).
- International Olive Council Prices & Balances. Available online: https://www.internationaloliveoil.org/ (accessed on 20 January 2023).
- Borja, R.; Raposo, F.; Rincón, B. Treatment Technologies of Liquid and Solid Wastes from Two-Phase Olive Oil Mills. Grasas Aceites 2006, 57, 32–46. [Google Scholar] [CrossRef]
- Alba, J.; Hidalgo, F.; Martínez, F.; Ruíz, M.A.; Moyano, M.J.; Borja, R. Evolución Medioambiental de Los Sistemas de Elaboración de Aceite de Oliva En Andalucía. Mercacei 1995, 2, 20–22. [Google Scholar]
- Cabrera, F.; López, R.; Martinez-Bordiú, A.; Dupuy de Lome, E.; Murillo, J.M. Land Treatment of Olive Oil Mill Wastewater. Int. Biodeterior. Biodegrad. 1996, 38, 215–225. [Google Scholar] [CrossRef]
- Giannoutsou, E.; Lambraki, M.; Karagouni, A.D. Microbial Treatment of Olive Mill Effluents. In Proceedings of the Symposium Olive Wastes, Kalamata, Greece, 5–8 November 1997; pp. 89–96. [Google Scholar]
- Vera, D.; Jurado, F.; Carpio, J.; Kamel, S. Biomass Gasification Coupled to an EFGT-ORC Combined System to Maximize the Electrical Energy Generation: A Case Applied to the Olive Oil Industry. Energy 2018, 144, 41–53. [Google Scholar] [CrossRef]
- EEA EMEP/EEA Air Pollutant Emission Inventory Guidebook 2023. Technical Guidance to Prepare National Emission Inventories. Available online: https://www.eea.europa.eu/publications/emep-eea-guidebook-2023 (accessed on 20 January 2023).
- Ministry of Economy Estudios Cadena de Valor Alimentos Frescos. Available online: https://www.mapa.gob.es/es/alimentacion/temas/observatorio-cadena/estudios-e-informes/default.aspx (accessed on 20 May 2023).
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental Advances in Biomass Autothermal/Oxidative Pyrolysis: A Review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Alburquerque, J. Agrochemical Characterisation of “Alperujo”, a Solid by-Product of the Two-Phase Centrifugation Method for Olive Oil Extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for Soil Applications-Sustainability Aspects, Challenges and Future Prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Lustosa Filho, J.F.; Melo, L.C.A.; de Assis, I.R.; de Oliveira, T.S. Classifying the Potential of Biochars from Agricultural and Industrial Waste for the Recovery of Fe and Mg Mining Tailings. J. Anal. Appl. Pyrolysis 2022, 161, 105383. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting Biochar Properties and Functions Based on Feedstock and Pyrolysis Temperature: A Review and Data Syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of Sewage Sludge-Derived Biochars from Different Feedstocks and Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of Change in Biochar Properties Derived from Different Feedstock and Pyrolysis Temperature for Environmental and Agricultural Application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Ferreira, J.; Filho, L.; Carrijo, L.; Melo, A.; Rodrigues De Assis, I.; Senna De Oliveira, T. Influence of Pyrolysis Temperature and Feedstock on the Properties of Biochars Produced from Agricultural and Industrial Wastes. J. Anal. Appl. Pyrolysis 2020, 149, 104839. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Lustosa Filho, J.F.; Melo, L.C.A.; de Assis, I.R.; de Oliveira, T.S. Co-Pyrolysis of Agricultural and Industrial Wastes Changes the Composition and Stability of Biochars and Can Improve Their Agricultural and Environmental Benefits. J. Anal. Appl. Pyrolysis 2021, 155, 105036. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Sanchez-Garcia, M.; Sánchez-Monedero, M.A.; Cayuela, M.L.; Moreno, D.A. Olive Tree Pruning Derived Biochar Increases Glucosinolate Concentrations in Broccoli. Sci. Hortic. 2020, 267, 109329. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment-Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Danesh, P.; Niaparast, P.; Ghorbannezhad, P.; Ali, I. Biochar Production: Recent Developments, Applications, and Challenges. Fuel 2022, 337, 126889. [Google Scholar] [CrossRef]
- Vilas-Boas, A.C.M.; Tarelho, L.A.C.; Oliveira, H.S.M.; Silva, F.G.C.S.; Pio, D.T.; Matos, M.A.A. Valorisation of Residual Biomass by Pyrolysis: Influence of Process Conditions on Products. Sustain. Energy Fuels 2024, 8, 379–396. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of Pyrolysis Temperature on Chemical and Physical Properties of Biochar from Sewage Sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Sarfaraz, Q.; Silva, L.; Drescher, G.; Zafar, M.; Severo, F.; Kokkonen, A.; Molin, G.; Shafi, M.; Shafique, Q.; Solaiman, Z. Characterization and Carbon Mineralization of Biochars Produced from Different Animal Manures and Plant Residues. Sci. Rep. 2020, 10, 955. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A Sustainable Paradigm of Sewage Sludge Biochar: Valorization, Opportunities, Challenges and Future Prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) Ions from Aqueous Solutions by Biochars Derived from Potassium-Rich Biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Ghimbeu, C.M.; Jellai, S.; El-Bassi, L.; Jeguirim, M. Olive Mill By-Products Thermochemical Conversion via Hydrothermal Carbonization and Slow Pyrolysis: Detailed Comparison between the Generated Hydrochars and Biochars Characteristics. Processes 2022, 10, 231. [Google Scholar] [CrossRef]
- Marks, E.A.N.; Kinigopoulou, V.; Akrout, H.; Azzaz, A.A.; Doulgeris, C.; Jellali, S.; Rad, C.; Zulueta, P.S.; Tziritis, E.; El-Bassi, L.; et al. Potential for Production of Biochar-Based Fertilizers from Olive Mill Waste in Mediterranean Basin Countries: An Initial Assessment for Spain, Tunisia, and Greece. Sustainability 2020, 12, 6081. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Arcand, M.; Ziadi, N. Effect of Biochar Addition on Legacy Phosphorus Availability in Long-Term Cultivated Arid Soil. Chem. Biol. Technol. Agric. 2021, 8, 47. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Gasco, G.; Méndez, A.; El Azzouzi, M.; Romero, E. New Insights into the Efficient Removal of Emerging Contaminants by Biochars and Hydrochars Derived from Olive Oil Wastes. Sci. Total Environ. 2021, 752, 141838. [Google Scholar] [CrossRef]
- Campos, P.; De la Rosa, J.M. Assessing the Effects of Biochar on the Immobilization of Trace Elements and Plant Development in a Naturally Contaminated Soil. Sustainability 2020, 12, 6025. [Google Scholar] [CrossRef]
- MAPA. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/aceite-oliva-y-aceituna-mesa (accessed on 26 January 2023).
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of Biochar from Olive Mill Solid Waste for Heavy Metal Removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef]
- Aguirre, J.L.; González-Egido, S.; González-Lucas, M.; González-Pernas, F.M. Medium-Term Effects and Economic Analysis of Biochar Application in Three Mediterranean Crops. Energies 2023, 16, 4131. [Google Scholar] [CrossRef]
- Aissaoui, M.H.; Trabelsi, A.B.H.; Bensidhom, G.; Ceylan, S.; Leahy, J.J.; Kwapinski, W. Insights into Olive Pomace Pyrolysis Conversion to Biofuels and Biochars: Characterization and Techno-Economic Evaluation. Sustain. Chem. Pharm. 2023, 32, 101022. [Google Scholar] [CrossRef]
- Alazzaz, A.; Usman, A.R.A.; Ahmad, M.; Ibrahim, H.M.; Elfaki, J.; Sallam, A.S.; Akanji, M.A.; Al-Wabel, M.I. Potential Short-Term Negative versus Positive Effects of Olive Mill-Derived Biochar on Nutrient Availability in a Calcareous Loamy Sand Soil. PLoS ONE 2020, 15, e0232811. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of Biochars Produced from Different Feedstocks on Soil Properties and Sunflower Growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Sánchez, M.E.; Mora, M.; Barrón, V. Slow Pyrolysis of Relevant Biomasses in the Mediterranean Basin. Part 2. Char Characterisation for Carbon Sequestration and Agricultural Uses. J. Clean. Prod. 2016, 120, 191–197. [Google Scholar] [CrossRef]
- Ayadi, M.; Awad, S.; Villot, A.; Abderrabba, M.; Tazerout, M. Heterogeneous Acid Catalyst Preparation from Olive Pomace and Its Use for Olive Pomace Oil Esterification. Renew. Energy 2021, 165, 1–13. [Google Scholar] [CrossRef]
- Belda, R.M.; Lidón, A.; Fornes, F. Biochars and Hydrochars as Substrate Constituents for Soilless Growth of Myrtle and Mastic. Ind. Crops Prod. 2016, 94, 132–142. [Google Scholar] [CrossRef]
- Caballero, B.M.; López-Urionabarrenechea, A.; Pérez, B.; Solar, J.; Acha, E.; de Marco, I. Potentiality of “Orujillo”(Olive Oil Solid Waste) to Produce Hydrogen by Means of Pyrolysis. Int. J. Hydrogen Energy 2020, 45, 20549–20557. [Google Scholar] [CrossRef]
- Calzado, M.; Valero-Romero, M.J.; Garriga, P.; Chica, A.; Guerrero-Pérez, M.O.; Rodríguez-Mirasol, J.; Cordero, T. Lignocellulosic Waste-Derived Basic Solids and Their Catalytic Applications for the Transformation of Biomass Waste. Catal. Today 2015, 257, 229–236. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Knicker, H.; Costa-Pereira, M.F.; Merino, A.; De la Rosa, J.M. Chemical, Physical and Morphological Properties of Biochars Produced from Agricultural Residues: Implications for Their Use as Soil Amendment. Waste Manag. 2020, 105, 256–267. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Prats, S.A.; Knicker, H.; Hagemann, N.; De la Rosa, J.M. Biochar Amendment Increases Bacterial Diversity and Vegetation Cover in Trace Element-Polluted Soils: A Long-Term Field Experiment. Soil Biol. Biochem. 2020, 150, 108014. [Google Scholar] [CrossRef]
- Campos, P.; Knicker, H.; López, R.; De la Rosa, J.M. Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica Rapa Growth. Agronomy 2021, 11, 1394. [Google Scholar] [CrossRef]
- Campos, P.; Knicker, H.; Velasco-Molina, M.; De la Rosa, J.M. Assessment of the Biochemical Degradability of Crop Derived Biochars in Trace Elements Polluted Soils. J. Anal. Appl. Pyrolysis 2021, 157, 105186. [Google Scholar] [CrossRef]
- Campos, P.; Knicker, H.; Miller, A.Z.; Velasco-Molina, M.; De la Rosa, J.M. Biochar Ageing in Polluted Soils and Trace Elements Immobilisation in a 2-Year Field Experiment. Environ. Pollut. 2021, 290, 118025. [Google Scholar] [CrossRef] [PubMed]
- Cavoski, I.; Al Chami, Z.; Jarrar, M.; Mondelli, D. Solutions for Soil Fertility Management to Overcome the Challenges of the Mediterranean Organic Agriculture: Tomato Plant Case Study. Soil Res. 2016, 54, 125–133. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Campos, P.; Diaz-Espejo, A.; Rosa Arranz, J.M.; Campos Díaz de Mayorga, P.; Díaz-Espejo, A.; De la Rosa, J.M.; Campos, P.; Diaz-Espejo, A.; Rosa Arranz, J.M.; et al. Soil Biochar Application: Assessment of the Effects on Soil Water Properties, Plant Physiological Status, and Yield of Super-Intensive Olive Groves under Controlled Irrigation Conditions. Agronomy 2022, 12, 2321. [Google Scholar] [CrossRef]
- del Pozo, C.; Rego, F.; Puy, N.; Bartrolí, J.; Fàbregas, E.; Yang, Y.; Bridgwater, A.V. The Effect of Reactor Scale on Biochars and Pyrolysis Liquids from Slow Pyrolysis of Coffee Silverskin, Grape Pomace and Olive Mill Waste, in Auger Reactors. Waste Manag. 2022, 148, 106–116. [Google Scholar] [CrossRef]
- El Hanandeh, A.; Albalasmeh, A.; Gharaibeh, M.; Alajlouni, M. Modification of Biochar Prepared from Olive Oil Processing Waste to Enhance Phenol Removal from Synthetic and Olive Mill Wastewater. Sep. Sci. Technol. 2021, 56, 1659–1671. [Google Scholar] [CrossRef]
- El-Azazy, M.; Nabil, I.; Hassan, S.S.; El-Shafie, A.S. Adsorption Characteristics of Pristine and Magnetic Olive Stones Biochar with Respect to Clofazimine. Nanomaterials 2021, 11, 963. [Google Scholar] [CrossRef]
- El-Bassi, L.; Azzaz, A.A.; Jellali, S.; Akrout, H.; Marks, E.A.N.; Ghimbeu, C.M.; Jeguirim, M. Application of Olive Mill Waste-Based Biochars in Agriculture: Impact on Soil Properties, Enzymatic Activities and Tomato Growth. Sci. Total Environ. 2021, 755, 142531. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Ahsan, I.; Radhwani, M.; Al-Khangi, M.A.; El-Azazy, M. Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance. Nanomaterials 2022, 12, 379. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Lidón, A. Analysis of Two Biochars and One Hydrochar from Different Feedstock: Focus Set on Environmental, Nutritional and Horticultural Considerations. J. Clean. Prod. 2015, 86, 40–48. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assessment of Biochar and Hydrochar as Minor to Major Constituents of Growing Media for Containerized Tomato Production. J. Sci. Food Agric. 2017, 97, 3675–3684. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Belda, R.M. Acidification with Nitric Acid Improves Chemical Characteristics and Reduces Phytotoxicity of Alkaline Chars. J. Environ. Manag. 2017, 191, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Belda, R.M. Biochar versus Hydrochar as Growth Media Constituents for Ornamental Plant Cultivation. Sci. Agric. 2018, 75, 304–312. [Google Scholar] [CrossRef]
- Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Soil Modification with Organic Amendments and Organo-Clays: Effects on Sorption, Degradation, and Bioactivity of the Allelochemical Scopoletin. J. Environ. Manag. 2022, 302, 114102. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; Pignatello, J.J.; Cox, L.; Hermosín, M.C.; Celis, R. Environmental Fate of the Fungicide Metalaxyl in Soil Amended with Composted Olive-Mill Waste and Its Biochar: An Enantioselective Study. Sci. Total Environ. 2016, 541, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Ghimbeu, C.M.; Ouederni, A. Activated Carbon Prepared by Physical Activation of Olive Stones for the Removal of NO2 at Ambient Temperature. C. R. Chim. 2015, 18, 63–74. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Guizani, C.; Ouederni, A.; Limousy, L. Pyrolysis of Olive Pomace: Degradation Kinetics, Gaseous Analysis and Char Characterization. Waste Biomass Valorization 2017, 8, 1689–1697. [Google Scholar] [CrossRef]
- Gómez, N.; Rosas, J.G.; Cara, J.; Martínez, O.; Alburquerque, J.A.; Sánchez, M.E. Slow Pyrolysis of Relevant Biomasses in the Mediterranean Basin. Part 1. Effect of Temperature on Process Performance on a Pilot Scale. J. Clean. Prod. 2016, 120, 181–190. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O.; Valero-Romero, M.J.; Hernández, S.; Nieto, J.M.L.; Rodríguez-Mirasol, J.; Cordero, T. Lignocellulosic-Derived Mesoporous Materials: An Answer to Manufacturing Non-Expensive Catalysts Useful for the Biorefinery Processes. Catal. Today 2012, 195, 155–161. [Google Scholar] [CrossRef]
- Hmid, A.; Mondelli, D.; Fiore, S.; Fanizzi, F.P.; Al Chami, Z.; Dumontet, S. Production and Characterization of Biochar from Three-Phase Olive Mill Waste through Slow Pyrolysis. Biomass Bioenergy 2014, 71, 330–339. [Google Scholar] [CrossRef]
- Hmid, A.; Al Chami, Z.; Sillen, W.; De Vocht, A.; Vangronsveld, J. Olive Mill Waste Biochar: A Promising Soil Amendment for Metal Immobilization in Contaminated Soils. Environ. Sci. Pollut. Res. 2015, 22, 1444–1456. [Google Scholar] [CrossRef]
- İlay, R. Short-Lived Effects of Olive Pomace Biochar Produced at Different Temperatures on Nitrate (NO3−), Bromide (Br−), Sulfate (SO42−) and Phosphate (PO43−) Leaching from Sandy Loam Soils. Commun. Soil Sci. Plant Anal. 2020, 51, 2223–2243. [Google Scholar] [CrossRef]
- Kavdır, Y.; İlay, R.; Güven, O.B.; Sungur, A. Characterization of Olive Pomace Biochar Produced at Different Temperatures and Their Temporal Effects on Soil Aggregation and Carbon Content. Biomass Convers. Biorefinery. 2023. [Google Scholar] [CrossRef]
- Khalil, A.M.; Michely, L.; Pires, R.; Bastide, S.; Jlassi, K.; Ammar, S.; Jaziri, M.; Chehimi, M.M. Copper/Nickel-Decorated Olive Pit Biochar: One Pot Solid State Synthesis for Environmental Remediation. Appl. Sci. 2021, 11, 8513. [Google Scholar] [CrossRef]
- Lilli, M.A.; Paranychianakis, N.V.; Lionoudakis, K.; Kritikaki, A.; Voutsadaki, S.; Saru, M.L.; Komnitsas, K.; Nikolaidis, N.P. The Impact of Sewage-Sludge- and Olive-Mill-Waste-Derived Biochar Amendments to Tomato Cultivation. Sustainability 2023, 15, 3879. [Google Scholar] [CrossRef]
- López-Cabeza, R.; Gámiz, B.; Cornejo, J.; Celis, R. Behavior of the Enantiomers of the Herbicide Imazaquin in Agricultural Soils under Different Application Regimes. Geoderma 2017, 293, 64–72. [Google Scholar] [CrossRef]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; D’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from Olive Mill Waste Have Contrasting Effects on Plants, Fungi and Phytoparasitic Nematodes. PLoS ONE 2018, 13, e0198728. [Google Scholar] [CrossRef] [PubMed]
- Molina-Sabio, M.; Sánchez-Montero, M.J.; Juarez-Galan, J.M.; Salvador, F.; Rodríguez-Reinoso, F.; Salvador, A. Development of Porosity in a Char during Reaction with Steam or Supercritical Water. J. Phys. Chem. B 2006, 110, 12360–12364. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Mänttäri, M.; López, S. Insights into the Adsorption of CO2 Generated from Synthetic Urban Wastewater Treatment on Olive Pomace Biochar. J. Environ. Manag. 2023, 339, 117951. [Google Scholar] [CrossRef]
- Omiri, J.; Snoussi, Y.; Bhakta, A.K.; Truong, S.; Ammar, S.; Khalil, A.M.; Jouini, M.; Chehimi, M.M. Citric-Acid-Assisted Preparation of Biochar Loaded with Copper/Nickel Bimetallic Nanoparticles for Dye Degradation. Colloids Interfaces 2022, 6, 18. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Gidarakos, E. Effect of Dried Olive Pomace—Derived Biochar on the Mobility of Cadmium and Nickel in Soil. J. Environ. Chem. Eng. 2015, 3, 1163–1176. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Regkouzas, P.; Manolikaki, I.; Diamadopoulos, E. Biochar Production from Waste Biomass: Characterization and Evaluation for Agronomic and Environmental Applications. Detritus 2021, 17, 15–29. [Google Scholar] [CrossRef]
- Piscitelli, L.; Malerba, A.D.; Mezzapesa, G.N.; Dumontet, S.; Mondelli, D.; Miano, T.; Bruno, G.L. Potential Microbial Remediation of Pyrene Polluted Soil: The Role of Biochar. Soil Res. 2019, 57, 807–813. [Google Scholar] [CrossRef]
- Piscitelli, L.; Rasse, D.P.; Malerba, A.D.; Miano, T.; Mondelli, D. Slow Pyrolysis of Olive Mill Solid Residues as a Sustainable Valorization Strategy for Waste Biomass. J. Mater. Cycles Waste Manag. 2023, 25, 1688–1698. [Google Scholar] [CrossRef]
- Reda, R.M.; El Gaafary, N.M.; Rashwan, A.A.; Mahsoub, F.; El-Gazzar, N. Evaluation of Olive Stone Biochar as Valuable and Inexpensive Agro-waste Adsorbent for the Adsorption and Removal of Inorganic Mercury from Nile Tilapia Aquaculture Systems. Aquac. Res. 2022, 53, 1676–1692. [Google Scholar] [CrossRef]
- Rittenhouse, J.L.; Rice, P.J.; Spokas, K.A.; Koskinen, W.C. Assessing Biochar’s Ability to Reduce Bioavailability of Aminocyclopyrachlor in Soils. Environ. Pollut. 2014, 189, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; González, J.F.; González-García, C.M.; Zamora, F. Control of Pore Development during CO2 and Steam Activation of Olive Stones. Fuel Process. Technol. 2008, 89, 715–720. [Google Scholar] [CrossRef]
- Sanginés, P.; Domínguez, M.P.; Sánchez, F.; San Miguel, G. Slow Pyrolysis of Olive Stones in a Rotary Kiln: Chemical and Energy Characterization of Solid, Gas, and Condensable Products. J. Renew. Sustain. Energy 2015, 7, 043103. [Google Scholar] [CrossRef]
- Senbayram, M.; Saygan, E.P.; Chen, R.; Aydemir, S.; Kaya, C.; Wu, D.; Bladogatskaya, E. Effect of Biochar Origin and Soil Type on the Greenhouse Gas Emission and the Bacterial Community Structure in N Fertilised Acidic Sandy and Alkaline Clay Soil. Sci. Total Environ. 2019, 660, 69–79. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Chouchène, A.; Valatkevičius, P.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. [Google Scholar] [CrossRef]
- Usama, M.; Rafique, M.I.; Ahmad, J.; Ahmad, M.; Al-Wabel, M.I.; Al-Farraj, A.S.F. A Sustainable Approach towards the Restoration of Lead-Contaminated Soils through Nutrient-Doped Olive Waste-Derived Biochar Application. Sustainability 2023, 15, 2606. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T. Role of Surface Phosphorus Complexes on the Oxidation of Porous Carbons. Fuel Process. Technol. 2017, 157, 116–126. [Google Scholar] [CrossRef]
- Volpe, M.; D’Anna, C.; Messineo, S.; Volpe, R.; Messineo, A. Sustainable Production of Bio-Combustibles from Pyrolysis of Agro-Industrial Wastes. Sustainability 2014, 6, 7866–7882. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of Biochars from Crop Residues: Potential for Carbon Sequestration and Soil Amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef]
- Zouari, M.; Marrot, L.; DeVallance, D.B. Effect of Demineralization and Ball Milling Treatments on the Properties of Arundo Donax and Olive Stone-Derived Biochar. Int. J. Environ. Sci. Technol. 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Blanco López, M.; Blanco, C.; Martínez-Alonso, A.; Tascón, J.M. Composition of Gases Released during Olive Stones Pyrolysis. J. Anal. Appl. Pyrolysis 2002, 65, 313–322. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A Study of Lignocellulosic Biomass Pyrolysis via the Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- dos Santos, S.R.; Lustosa Filho, J.F.; Vergütz, L.; Melo, L.C.A. Biochar Association with Phosphate Fertilizer and Its Influence on Phosphorus Use Efficiency by Maize. Ciênc. Agrotecnol. 2019, 43, e025718. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, Thermochemical, and Infrared Spectral Characterization of Feedstocks and Biochar Derived at Different Pyrolysis Temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.H.; Kirkham, M.B.; et al. Multifunctional Applications of Biochar beyond Carbon Storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent Advances in Biochar Applications in Agricultural Soils: Benefits and Environmental Implications. Clean.-Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Guo, D.; Jogi, Q.; et al. Recent Developments in Biochar as an Effective Tool for Agricultural Soil Management: A Review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of Biochar Properties as a Function of Feedstock Sources and Production Temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Conductivity and Liming Potential. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: New York, NY, USA, 2017; pp. 23–38. [Google Scholar]
- Chaturvedi, S.; Singh, S.V.; Dhyani, V.C.; Govindaraju, K.; Vinu, R.; Mandal, S. Characterization, Bioenergy Value, and Thermal Stability of Biochars Derived from Diverse Agriculture and Forestry Lignocellulosic Wastes. Biomass Convers. Biorefinery 2023, 13, 879–892. [Google Scholar] [CrossRef]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of Fruit Wastes: A Promising Technique for Generating Hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef]
- Nagel, K.; Hoilett, N.O.; Mottaleb, M.A.; Meziani, M.J.; Wistrom, J.; Bellamy, M. Physicochemical Characteristics of Biochars Derived From Corn, Hardwood, Miscanthus, and Horse Manure Biomasses. Commun. Soil Sci. Plant Anal. 2019, 50, 987–1002. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Yang, H.; Doran, J.; Rooney, D.W. Industrial Biochar Systems for Atmospheric Carbon Removal: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Available online: https://biochar-international.org/wp-content/uploads/2023/01/IBI_Biochar_Standards_V2.1_Final2.pdf (accessed on 22 October 2023).
- Wang, W.; Bai, J.; Lu, Q.; Zhang, G.; Wang, D.; Jia, J.; Guan, Y.; Yu, L. Pyrolysis Temperature and Feedstock Alter the Functional Groups and Carbon Sequestration Potential of Phragmites Australis- and Spartina Alterniflora-Derived Biochars. GCB Bioenergy 2021, 13, 493–506. [Google Scholar] [CrossRef]
- Wiedner, K.; Rumpel, C.; Steiner, C.; Pozzi, A.; Maas, R.; Glaser, B. Chemical Evaluation of Chars Produced by Thermochemical Conversion (Gasification, Pyrolysis and Hydrothermal Carbonization) of Agro-Industrial Biomass on a Commercial Scale. Biomass Bioenergy 2013, 59, 264–278. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Xian, Q.; Shen, F.; Wu, J.; Zhang, Y. Removal of Congo Red and Methylene Blue from Aqueous Solutions by Vermicompost-Derived Biochars. PLoS ONE 2016, 11, e0154562. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar’. 2012. Available online: http://www.european-biochar.org/en/download (accessed on 9 October 2023).
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Soil Microbial Responses to Biochars Varying in Particle Size, Surface and Pore Properties. Pedosphere 2015, 25, 770–780. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Ajibade, F.O.; Atakpa, E.O.; Baquy, M.A.-A.; Mia, S.; Odii, E.C.; Xu, R. Reduction of Heavy Metal Uptake from Polluted Soils and Associated Health Risks through Biochar Amendment: A Critical Synthesis. J. Hazard. Mater. Adv. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F.; Besharati, H.; Khalaj, M.A. The Effects of Biochar on Soil Nutrients Status, Microbial Activity and Carbon Sequestration Potential in Two Calcareous Soils. Biochar 2021, 3, 105–116. [Google Scholar] [CrossRef]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.W.; Rinklebe, J.; Ok, Y.S. Biochar Composition-Dependent Impacts on Soil Nutrient Release, Carbon Mineralization, and Potential Environmental Risk: A Review. J. Environ. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Almatrafi, E.; Tan, X.; Luo, S.; Xiong, W.; Zhou, C.; Qin, M.; Liu, Y.; Cheng, M.; Zeng, G.; et al. Biochar-Based Agricultural Soil Management: An Application-Dependent Strategy for Contributing to Carbon Neutrality. Renew. Sustain. Energy Rev. 2022, 164, 112529. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Biochar Assisted Phytoremediation and Biomass Disposal in Heavy Metal Contaminated Mine Soils: A Review. Int. J. Phytoremediat. 2021, 23, 559–576. [Google Scholar] [CrossRef]
- Igaz, D.; Šimanský, V.; Horák, J.; Kondrlová, E.; Domanová, J.; Rodný, M.; Buchkina, N.P. Can a Single Dose of Biochar Affect Selected Soil Physical and Chemical Characteristics? J. Hydrol. Hydromech. 2018, 66, 421–428. [Google Scholar] [CrossRef]
- Hussain, R.; Garg, A.; Ravi, K. Soil-Biochar-Plant Interaction: Differences from the Perspective of Engineered and Agricultural Soils. Bull. Eng. Geol. Environ. 2020, 79, 4461–4481. [Google Scholar] [CrossRef]
- Toková, L.; Igaz, D.; Horák, J.; Aydin, E. Effect of Biochar Application and Re-application on Soil Bulk Density, Porosity, Saturated Hydraulic Conductivity, Water Content and Soil Water Availability in a Silty Loam Haplic Luvisol. Agronomy 2020, 10, 1005. [Google Scholar] [CrossRef]
- Xu, W.; Whitman, W.B.; Gundale, M.J.; Chien, C.; Chiu, C. Functional Response of the Soil Microbial Community to Biochar Applications. GCB Bioenergy 2021, 13, 269–281. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and Limitations of Biochar Amendment in Agricultural Soils: A Review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.; Munir, N.; Abideen, Z.; Zulfiqar, F.; Koyro, H.W.; El-Naggar, A.; Caçador, I.; Duarte, B.; Rinklebe, J.; Yong, J.W.H. Biochar-Plant Interaction and Detoxification Strategies under Abiotic Stresses for Achieving Agricultural Resilience: A Critical Review. Ecotoxicol. Environ. Saf. 2023, 249, 114408. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of Biochar on Alleviation of Cadmium Toxicity in Wheat (Triticum aestivum L.) Grown on Cd-Contaminated Saline Soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Ibrahim, M.; Li, G.; Chan, F.K.S.; Kay, P.; Liu, X.X.; Firbank, L.; Xu, Y.Y. Biochars Effects Potentially Toxic Elements and Antioxidant Enzymes in Lactuca sativa L. Grown in Multi-Metals Contaminated Soil. Environ. Technol. Innov. 2019, 15, 100427. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of Rice Straw Biochar on Growth, Antioxidant Capacity and Copper Uptake in Ramie (Boehmeria nivea L.) Grown as Forage in Aged Copper-Contaminated Soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.-K.; Koutsospyros, A.; Moon, D.H. Impacts of Biochar Application on Upland Agriculture: A Review. J. Environ. Manag. 2019, 234, 52–64. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management; Routledge: London, UK, 2015; ISBN 9780203762264. [Google Scholar]
- Galán-Martín, Á.; Contreras, M.d.M.; Romero, I.; Ruiz, E.; Bueno-Rodríguez, S.; Eliche-Quesada, D.; Castro-Galiano, E. The Potential Role of Olive Groves to Deliver Carbon Dioxide Removal in a Carbon-Neutral Europe: Opportunities and Challenges. Renew. Sustain. Energy Rev. 2022, 165, 112609. [Google Scholar] [CrossRef]
- EN 14214:2008; Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2008.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).