Recent Progress in Sludge-Derived Biochar and Its Role in Wastewater Purification
Abstract
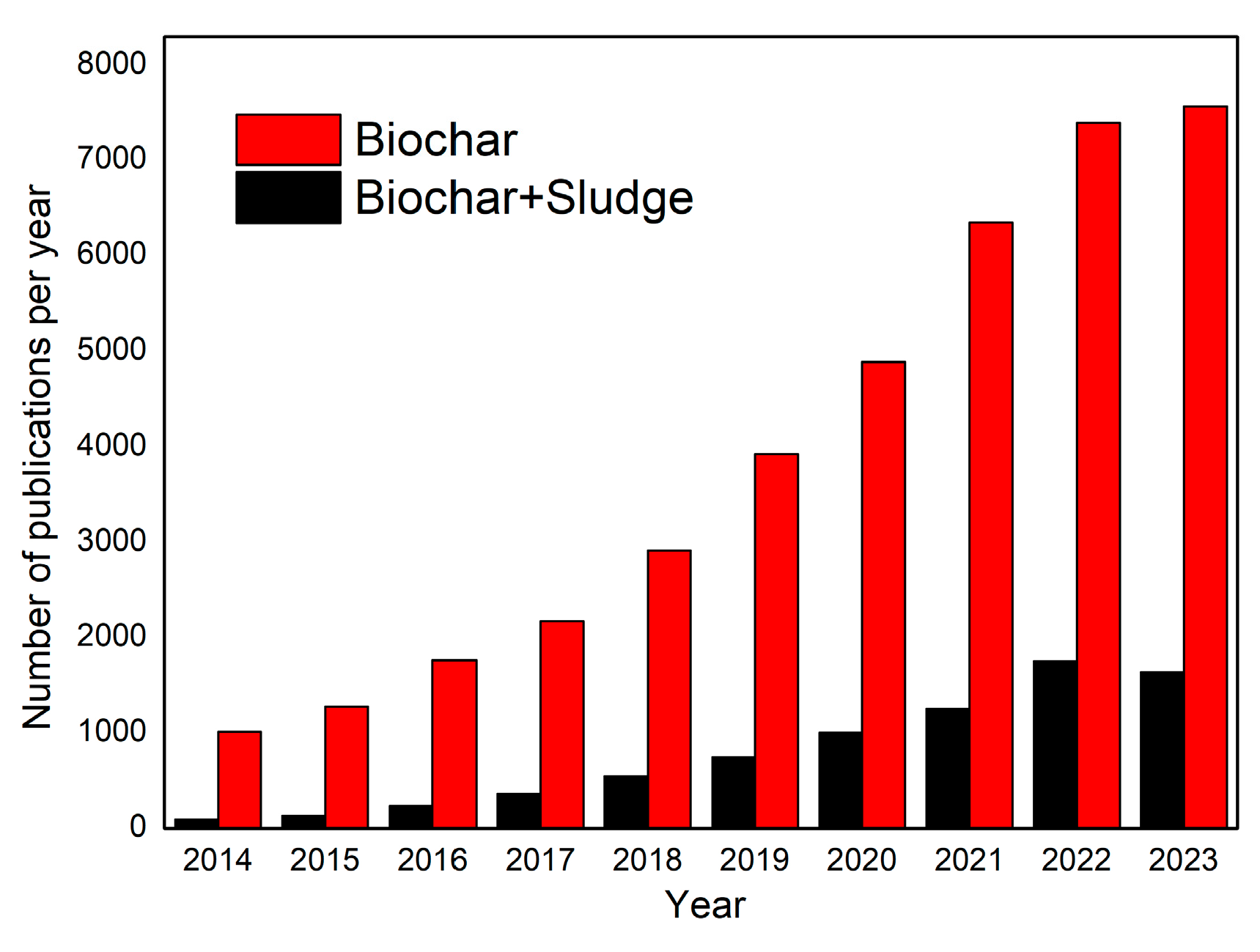
1. Introduction
2. Production of Sludge-Derived Biochar
2.1. Effects of Sludge’s Inherent Properties
2.2. Effects of Production Process Parameters
3. Application of Sludge-Derived Biochar in Wastewater Treatment
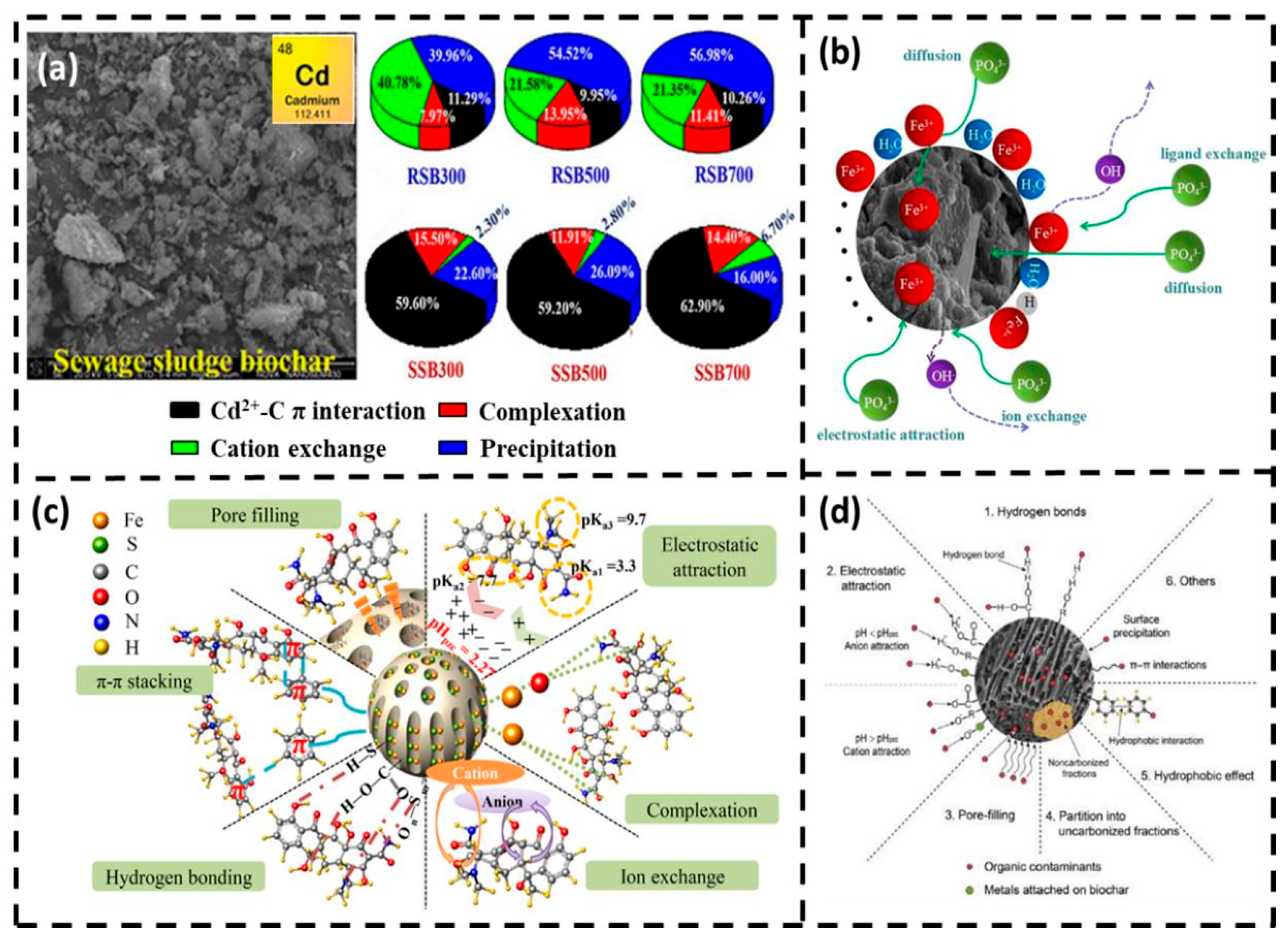
3.1. Adsorption
| Material | Conditions | Type | Contaminants | Specific Surface Area | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|---|
| Sewage sludge modified by hydroxyapatite | Pyrolysis temperature: 550 °C, 2 h, pH 4–8 | Heavy metal | Cu(II); Cd(II). | / | 89.98 mg/g; 114.68 mg/g. | [32] |
| Sewage sludge/MnFe2O4 loaded | Hydrothermal temperature: 180 °C, 10 h, pH 5–6 | Heavy metal | Pb(II) | 129.29 m2/g | 174.22 mg/g | [33] |
| Sewage sludge | Pyrolysis temperature: 600 °C, 2 h, pH 1–2 | Heavy metal | Cr(VI) | 487.59 m2/g | 150.84 mg/g | [34] |
| Sewage sludge activated by ZnCl2 | Pyrolysis temperature: 750 °C, 2 h, pH 5–6 | Dyes | Methylene blue | 461.44 m2/g | 24.83 mg/g | [35] |
| Tannery sludge activated by melamine and KOH | Pyrolysis temperature: 550 °C, 3 h | Dyes | Active red X-3B; direct yellow RS; cationic blue X-GRL; acid blue 2GL. | 47.67 m2/g | 45.13 mg/g; 84.10 mg/g; 154.80 mg/g; 120.92 mg/g. | [36] |
| Industrial sludge activated by ZnCl2 | Pyrolysis temperature: 800 °C, 1 h, pH 5–6 | Dyes | Reactive black 5; green alizarin. | 702.4 m2/g | 256.02 mg/g; 312.69 mg/g. | [37] |
| Dyeing sludge activated by KOH | Pyrolysis temperature: 800 °C, 3 h, pH 5 | Antibiotics | Tetracycline | 1178.4 m2/g | 1081.3 mg/g | [38] |
| Pharmaceutical sludge activated by NaOH | Pyrolysis temperature: 600 °C, pH 3–8 | Antibiotics | Tetracycline | / | 379.8 mg/g | [39] |
| Beverage sludge | Pyrolysis temperature: 800 °C | Pharmaceuticals | Paracetamol; ibuprofen; ketoprofen. | 642.00 m2/g | 145 mg/g (pH 8); 105 mg/g (pH 4); 57 mg/g (pH 6). | [40] |
| Septic tank sludge activated by KOH | Pyrolysis temperature: 800 °C | Inorganic substance | Phosphate | 82.90 m2/g | 42.51 mg/g | [41] |
| Dewatered dry sludge | Pyrolysis temperature: 700 °C, 1 h, pH 11 | Inorganic substance | Phosphate | 20.93 m2/g | 51.79 mg/g | [42] |
| Oil sludge activated by ZnCl2 | Pyrolysis temperature: 800 °C, 1 h, pH 9 | Oil | Gasoil | 110.00 m2/g | 406.8 mg/g | [43] |
| Paper sludge/wheat husks, Austria | Pyrolysis temperature: 500 °C, 20 min, pH 2.8 | Pesticides | 2,4-DCP | 63.80 m2/g | 17.51 mg/g | [44] |
| Activated sludge pretreated by FeCl3·6H2O | Pyrolysis temperature: 600 °C 2 h, pH 3 | Pesticides | Tebuconazole; linuron. | 79.24 m2/g | 12.37 mg/g; 9.06 mg/g. | [45] |
| Sewage sludge | Pyrolysis temperature: 550 °C, 2 h, pH 7.5 | Other organic pollutants | Tetrabromobisphenol A (TBBPA) | / | 87.02 mg/g | [46] |
| Sewage sludge/Layered double hydroxides composited | Pyrolysis temperature: 550 °C, 2 h, pH 3–4 | Other organic pollutants | Benzotriazole (BTA) | 111.74 m2/g | 239.6 mg/g | [47] |
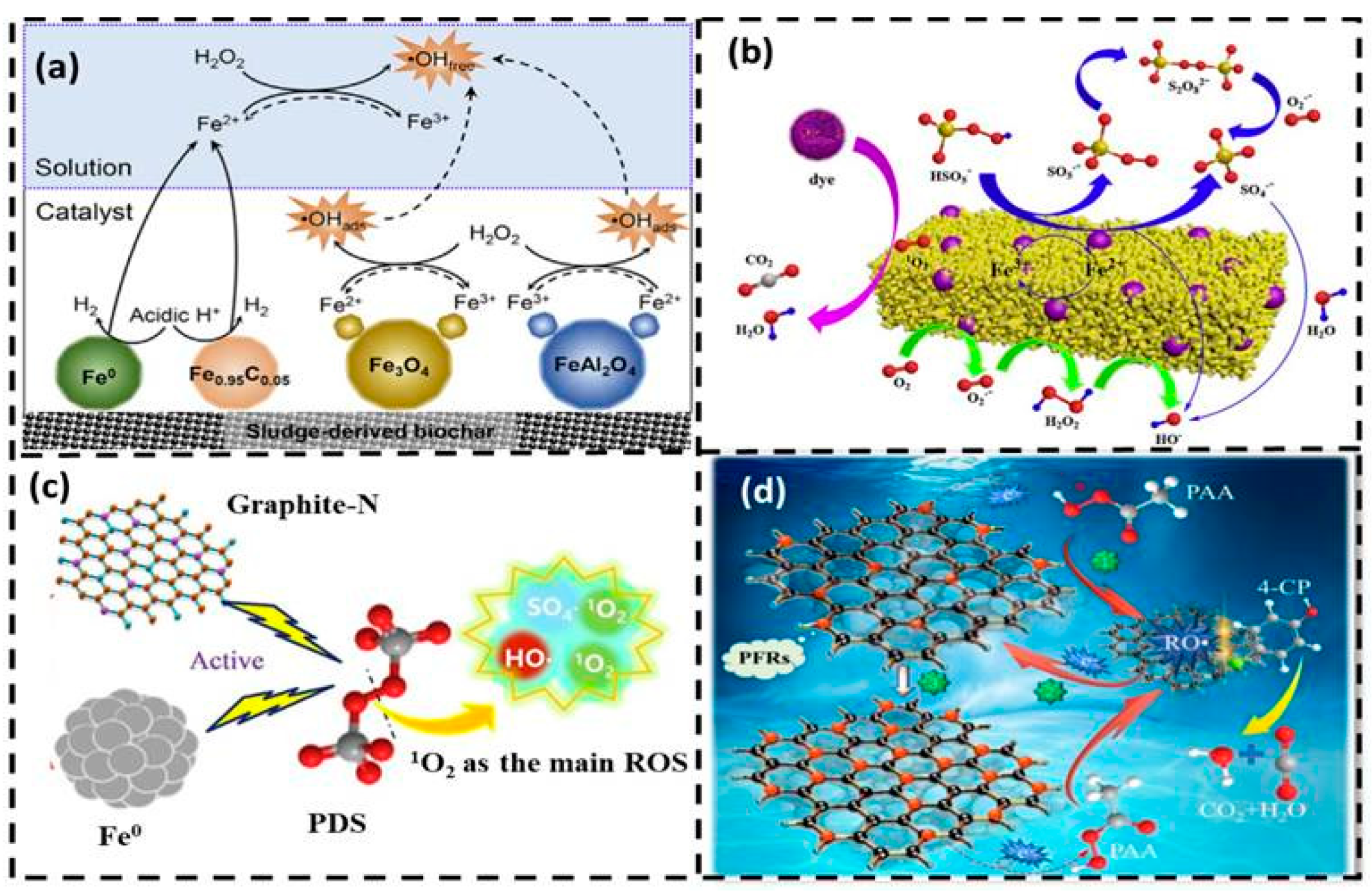
3.2. Advanced Oxidation Processes (AOPs)
| Material | Agents | Contaminants | Experimental Conditions | Removal Kinetics/Efficiency | Ref. |
|---|---|---|---|---|---|
| Fe-rich sludge-derived biochar | H2O2 | 4-chlorophenol | Pollutants 0.78 mM/L, catalysts 2 g/L, pH 2, H2O2 30 mM | 0.51 min−1 | [67] |
| Municipal sewage sludge | H2O2 | Ofloxacin | Pollutants 30 mg/L, catalysts 0.1 g/L, pH 6, H2O2 4 mM | 91.5 ± 1.4%, | [10] |
| Zero-valent iron (ZVI) sludge | PS | Acid orange | Pollutants 0.06 mM, catalysts 0.5 g/L, pH 5.22, PS 0.925 mM | 0.0718 min−1 | [68] |
| Red mud–sewage sludge | PMS | Sulfamethoxazole | Pollutants 0.02 mM, catalysts 1.5 g/L, PMS 0.15 mM | 0.0481 min−1 | [69] |
| Secondary sewage sludge | PDS | Sulfamethoxazole | Pollutants 0.04 mM, catalysts 2.0 g/L, pH 5, PDS 1.5 mM | 0.0145 min−1 | [70] |
| Mixture of primary and secondary sludge | PAA | 4-chlorophenol | Pollutants 5 mg/L, catalysts 0.3 g/L, pH 7, PAA 1.8 mM | 0.051 min−1 | [71] |
3.2.1. AOPs Based on H2O2
3.2.2. AOPs Based on Persulfate
3.2.3. AOPs Based on PAA
4. Challenges and Future Recommendations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Cruz, M.; García-Galán, M.; Guerra, P.; Jelic, A.; Postigo, C.; Eljarrat, E.; Farré, M.; de Alda, M.L.; Petrovic, M.; Barceló, D.; et al. Analysis of selected emerging contaminants in sewage sludge. Trends Anal. Chem. 2009, 28, 1263–1275. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z. Sewage sludge derived biochar for environmental improvement: Advances, challenges, and solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, L.; Li, P.; Yang, L.; He, L.; Chen, S.; Yang, Y.; Gao, F.; Qi, X.; Zhang, Z. A novel, efficient and sustainable magnetic sludge biochar modified by graphene oxide for environmental concentration imidacloprid removal. J. Hazard. Mater. 2021, 407, 124777. [Google Scholar] [CrossRef]
- Chen, J.; Bai, X.; Yuan, Y.; Zhang, Y.; Sun, J. Printing and dyeing sludge derived biochar for activation of peroxymonosulfate to remove aqueous organic pollutants: Activation mechanisms and environmental safety assessment. Chem. Eng. J. 2022, 446, 136942. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic removal of aqueous contaminants on n-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, W.; Liu, C.; Gao, J.; Yang, X.; Xiao, C.; Qi, J.; Zhou, Y.; Zhu, Z.; Yang, Y.; et al. Iron containing sludge-derived carbon towards efficient peroxymonosulfate activation: Active site synergy, performance and alternation mechanism. Sci. Total Environ. 2024, 915, 170183. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Bu, S.; Huang, Y.; Shao, Y.; Xiao, L.; Shi, X. N-doped hierarchically porous carbon for highly efficient metal-free catalytic activation of peroxymonosulfate in water: A non-radical mechanism. Chemosphere 2019, 216, 545–555. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, J.; Huang, G.; Yu, H. Sludge biochar-based catalyst for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. A 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Yu, C.; Yan, C.; Gu, J.; Zhang, Y.; Li, X.; Dang, Z.; Wang, L.; Wan, J.; Pan, J. In-situ Cu-loaded sludge biochar catalysts for oxidative degradation of bisphenol A from high-salinity wastewater. J. Clean. 2023, 427, 139334. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Lv, Y.; Fu, H.; Liu, D.; Feng, Y.; Xie, H.; Qu, H. Insights into the mechanism of peroxydisulfate activated by magnetic spinel CuFe2O4/SBC as a heterogeneous catalyst for bisphenol S degradation. Chem. Eng. J. 2021, 416, 129162. [Google Scholar] [CrossRef]
- Chen, S.; Qin, C.; Wang, T.; Chen, F.; Li, X.; Hou, H.; Zhou, M. Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: Adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J. Mol. Liq. 2019, 285, 62–74. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Huang, K.; Hu, C.; Tan, Q.; Yu, M.; Shabala, S.; Yang, L.; Sun, X. Highly efficient removal of cadmium from aqueous solution by ammonium polyphosphate-modified biochar. Chemosphere 2022, 305, 135471. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Min, X.; Ge, T.; Li, H.; Shi, Y.; Fang, T.; Sheng, B.; Li, H.; Dong, X. Combining impregnation and co-pyrolysis to reduce the environmental risk of biochar derived from sewage sludge. Chemosphere 2022, 290, 133371. [Google Scholar] [CrossRef]
- Li, K.; Zhang, D.; Niu, X. Insights into CO2 adsorption on KOH-activated biochars derived from the mixed sewage sludge and pine sawdust. Sci. Total Environ. 2022, 826, 154133. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Ding, L.; Yu, J.; Zhou, Q.; Kong, Y.; Ma, J. Novel sodium bicarbonate activation of cassava ethanol sludge derived biochar for removing tetracycline from aqueous solution: Performance assessment and mechanism insight. Bioresour. Technol. 2021, 330, 124949. [Google Scholar] [CrossRef]
- Gu, H.; Lin, W.; Sun, S.; Wu, C.; Yang, F.; Ye, Z.; Chen, N.; Ren, J.; Zheng, S. Calcium oxide modification of activated sludge as a low-cost adsorbent: Preparation and application in Cd(II) removal. Ecotoxicol. Environ. Saf. 2021, 209, 111760. [Google Scholar] [CrossRef]
- Ji, Q.; Yuan, X.; Zhao, Y.; Jiang, L.; Wang, H. Mechanistic insights of removing pollutant in adsorption and advanced oxidation processes by sludge biochar. J. Hazard. Mater. 2022, 430, 128375. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, W.; Qi, Z.; Qian, W.; Yang, L.; Wei, R.; Ni, J. Biochar improved the solubility of triclocarban in aqueous environment: Insight into the role of biochar-derived dissolved organic carbon. Chemosphere 2024, 351, 141172. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Khiari, B.; Jeguirim, M.; Limousy, L.; Bennici, S. Biomass derived chars for energy applications. Renew. Sustain. Energy Rev. 2019, 108, 253–273. [Google Scholar] [CrossRef]
- Liang, F.; Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, Y.; Li, N.; Yan, B.; Chen, G.; Hou, L. Breaking rate-limiting steps in a red mud-sewage sludge carbon catalyst activated peroxymonosulfate system: Effect of pyrolysis temperature. Sep. Purif. Technol. 2022, 299, 121805. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: Performance and mechanism. Chem. Eng. J. 2020, 380, 122519. [Google Scholar] [CrossRef]
- Sharma, H.; Sarmah, A.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Marin-Batista, J.; Mohedano, A.; Rodríguez, J.; de la Rubia, M. Energy and phosphorous recovery through hydrothermal carbonization of digested sewage sludge. Waste Manag. 2020, 105, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, M.; Garg, A. Hydrothermal carbonization of centrifuged sewage sludge: Determination of resource recovery from liquid fraction and thermal behaviour of hydrochar. Waste Manag. 2020, 117, 114–123. [Google Scholar] [CrossRef]
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, F.; Li, H.; Li, Y.; Liu, Y.; Chen, Y.; Chen, L. Simple hydrothermal synthesis of magnetic MnFe2O4-sludge biochar composites for removal of aqueous Pb2+. J. Anal. Appl. Pyrol. 2021, 156, 105173. [Google Scholar] [CrossRef]
- Shi, R.; Liu, T.; Lu, J.; Liang, X.; Ivanets, A.; Yao, J.; Su, X. Fe/C materials prepared by one-step calcination of acidified municipal sludge and their excellent adsorption of Cr (VI). Chemosphere 2022, 304, 135303. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Nie, Y.; Han, Y.; Wang, R.; Zhao, Z. Properties and the application of sludge-based biochar in the removal of phosphate and methylene blue from water: Effects of acid treating. Langmuir 2022, 38, 1833–1844. [Google Scholar] [CrossRef]
- Zhai, S.; Li, M.; Wang, D.; Ju, X.; Fu, S. Cyano and acylamino group modification for tannery sludge bio-char: Enhancement of adsorption universality for dye pollutants. J. Environ. Chem. Eng. 2021, 9, 104939. [Google Scholar] [CrossRef]
- Sellaoui, L.; Said, S.; Bouzidi, M.; Alshammari, A.S.; Khan, Z.R.; Gandouzi, M.; Erto, A. Highlighting the adsorption mechanism of dyes onto activated carbon derived from sludge by theoretical physical analysis. Environ. Sci. Pollut. Res. 2023, 30, 15789–15796. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Y.; Yang, X.; Yao, Y.; Qi, J.; Zhu, Z.; Yang, Y.; Fang, D.; Zhou, L.; Li, J. Dyeing sludge-derived biochar for efficient removal of antibiotic from water. Sci. Total. Environ. 2024, 912, 169035. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, G.; Li, G. Preparation of porous biochar based on pharmaceutical sludge activated by NaOH and its application in the adsorption of tetracycline. J. Colloid Interface Sci. 2021, 587, 271–278. [Google Scholar] [CrossRef]
- Streit, A.; Collazzo, G.; Druzian, S.; Verdi, R.; Foletto, E.; Oliveira, L.; Dotto, G. Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Zhang, Y.; Lichtfouse, E. Efficient phosphate recycling by adsorption on alkaline sludge biochar. Environ. Chem. Lett. 2023, 21, 21–30. [Google Scholar] [CrossRef]
- Liu, M.; Li, R.; Wang, J.; Liu, X.; Li, S.; Shen, W. Recovery of phosphate from aqueous solution by dewatered dry sludge biochar and its feasibility in fertilizer use. Sci. Total. Environ. 2022, 814, 152752. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; Noreen, A.; Osman, H.; Sammen, S.S.; Al-Ansari, N.; Salman, H.M. Investigation of the viable role of oil sludge-derived activated carbon for oily wastewater remediation. Front. Environ. Sci. 2023, 11, 240. [Google Scholar] [CrossRef]
- Kalderis, D.; Kayan, B.; Akay, S.; Kulaksiz, E.; Gozmen, B. Adsorption of 2,4-dichlorophenol on paper sludge/wheat husk biochar: Process optimization and comparison with biochars prepared from wood chips, sewage sludge and hog fuel/demolition waste. J. Environ. Chem. Eng. 2017, 5, 2222–2231. [Google Scholar] [CrossRef]
- Yang, J.; Sun, H.; Liu, Y.; Wang, X.; Valizadeh, K. The sorption of Tebuconazole and Linuron from an aqueous environment with a modified sludge-based biochar: Effect, mechanisms, and its persistent free radicals study. J. Chem. 2021, 2021, 2912054. [Google Scholar] [CrossRef]
- Li, T.; He, Y.; Peng, X. Efficient removal of tetrabromobisphenol A (TBBPA) using sewage sludge-derived biochar: Adsorptive effect and mechanism. Chemosphere 2020, 251, 126370. [Google Scholar] [CrossRef]
- Cheng, X.; Deng, J.; Li, X.; Wei, X.; Shao, Y.; Zhao, Y. Layered double hydroxides loaded sludge biochar composite for adsorptive removal of benzotriazole and Pb (II) from aqueous solution. Chemosphere 2022, 287, 131966. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Huang, Q.; Wang, C.; Ni, T.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: A critical review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-derived biochars: A review on the influence of synthesis conditions on pollutants removal efficiency from wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, B.; Zhang, H.; Zhang, W. Fe/S modified sludge-based biochar for tetracycline removal from water. Powder Technol. 2020, 364, 889–900. [Google Scholar] [CrossRef]
- Gao, L.; Deng, J.; Huang, G.; Li, K.; Cai, K.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Elkhlifi, Z.; Sellaoui, L.; Zhao, M.; Ifthikar, J.; Jawad, A.; Shahib, I.I.; Sijilmassi, B.; Lahori, A.H.; Selvasembian, R.; Meili, L.; et al. Lanthanum hydroxide engineered sewage sludge biochar for efficient phosphate elimination: Mechanism interpretation using physical modelling. Sci. Total Environ. 2022, 803, 149888. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Tang, J.; Lv, H.; Gong, Y.; Huang, Y. Preparation and characterization of a novel graphene/biochar composite for aqueous phenanthrene and mercury removal. Bioresour. Technol. 2015, 196, 355–363. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, N.; Bian, S.; Li, J.; Xu, S.; Zhang, Y. Enhancing the adsorption capability of areca leaf biochar for methylene blue by K2FeO4-catalyzed oxidative pyrolysis at low temperature. RSC Adv. 2019, 9, 42343–42350. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, Y.; Li, J.; Zhu, Y.; Yu, H.; Peng, Y. Adsorption and co-adsorption of tetracycline and doxycycline by one-step synthesized iron loaded sludge biochar. Chemosphere 2019, 236, 124252. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, P.; Huang, C.; Liu, Y.; Liu, S.; Zhang, K.; Cao, J.; Tan, X.; Liu, S. Effect of dissolved humic acids and coated humic acids on tetracycline adsorption by K2CO3-activated magnetic biochar. Sci. Rep. 2022, 12, 18966. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Pang, Y.; Yang, Q.; Wang, D.; Li, X.; Wang, L.; Lei, M.; Liu, J. Enhanced ciprofloxacin removal by sludge-derived biochar: Effect of humic acid. Chemosphere 2019, 231, 495–501. [Google Scholar] [CrossRef]
- Shen, J.; Huang, G.; An, C.; Zhao, S.; Rosendahl, S. Immobilization of tetrabromobisphenol A by pinecone-derived biochars at solid-liquid interface: Synchrotron-assisted analysis and role of inorganic fertilizer ions. Chem. Eng. J. 2017, 321, 346–357. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Barbarias, I.; Bilbao, J.; Olazar, M. Sewage sludge valorization by flash pyrolysis in a conical spouted bed reactor. Chem. Eng. J. 2015, 273, 173–183. [Google Scholar] [CrossRef]
- Gan, Q.; Hou, H.; Liang, S.; Qiu, J.; Tao, S.; Yang, L.; Yu, W.; Xiao, K.; Liu, B.; Hu, J.; et al. Sludge-derived biochar with multivalent iron as an efficient Fenton catalyst for degradation of 4-Chlorophenol. Sci. Total. Environ. 2020, 725, 138299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, M.; Gong, Q.; Wang, X.; Cai, J.; Wang, S.; Chen, Z. One-step preparation of ZVI-sludge derived biochar without external source of iron and its application on persulfate activation. Sci. Total Environ. 2020, 714, 136728. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, M.; Wang, H.; Du, Y.; Zhou, X.; Liao, Z.; Wang, H.; Chen, Z. Red mud modified sludge biochar for the activation of peroxymonosulfate: Singlet oxygen dominated mechanism and toxicity prediction. Sci. Total Environ. 2020, 740, 140388. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Cheng, P.; She, Y.; Wang, W.; Tian, Y.; Ma, J.; Sun, Z. Efficient activation of peracetic acid by mixed sludge derived biochar: Critical role of persistent free radicals. Water Res. 2022, 223, 119013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, H.; Liu, Y.; Wang, L.; Li, D.; Liu, C.; Gong, M.; Zhang, Z.; Yang, T.; Ma, J. Remarkable enhancement of a photochemical Fenton-like system (UVA/Fe(II)/PMS) at near-neutral pH and low Fe(II)/peroxymonosulfate ratio by three alpha hydroxy acids: Mechanisms and influencing factors. Sep. Purif. Technol. 2019, 224, 142–151. [Google Scholar] [CrossRef]
- Mian, M.; Liu, G.; Fu, B.; Song, Y. Facile synthesis of sludge-derived MnOx-N-biochar as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 255, 117765. [Google Scholar] [CrossRef]
- Zang, T.; Wang, H.; Liu, Y.; Zhou, S.; Ai, S. Fe-doped biochar derived from waste sludge for degradation of rhodamine B via enhancing activation of peroxymonosulfate. Chemosphere 2020, 261, 127616. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yan, S.; Yong, X.; Selvaraj, M.; Ghramh, H.A.; Assiri, M.A.; Zhang, X.; Awasthi, M.K.; Zhou, J. Effective degradation of chloramphenicol in wastewater by activated peroxymonosulfate with Fe-rich porous biochar derived from petrochemical sludge. Chemosphere 2023, 310, 136839. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, S.; Xue, G.; Wang, X.; Sun, P.; Yu, Y.; Zhou, Z.; Wang, Q.; Qian, Y. Enhanced removal of fluoroquinolone antibiotics by peroxydisulfate activated with N-doped sludge biochar: Performance, mechanism and toxicity evaluation. Sep. Purif. Technol. 2023, 305, 122469. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Du, M.; Yang, J.; Lu, Q.; Fu, Q.; He, D.; Zhao, J.; Wang, D. Peracetic acid promotes biohydrogen production from anaerobic dark fermentation of waste activated sludge. Sci. Total. Environ. 2022, 844, 156991. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, C.; Xie, Z.; Li, L.; Xiong, Z.; Zhang, H.; Zhou, P.; Jiang, F.; Mu, Y.; Lai, B. Efficient activation of PAA by FeS for fast removal of pharmaceuticals: The dual role of sulfur species in regulating the reactive oxidized species. Water Res. 2022, 217, 118402. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C. UV/Peracetic acid for degradation of pharmaceuticals and reactive species evaluation. Environ. Sci. Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Asgodom, M.; Liu, D.; Xie, H.; Qu, H. Study on the degradation of accumulated bisphenol S and regeneration of magnetic sludge-derived biochar upon microwave irritation in the presence of hydrogen peroxide for application in integrated process. Bioresour. Technol. 2019, 293, 122072. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zeng, C.; Ding, Y.; Tang, J.; Masek, O.; Deng, Z.; Mu, R.; Zhang, Z. Facile synthesis of ball milling and magnetization co-modified sludge-derived biochar for efficient adsorbing environmental concentration sulfamethoxazole from various waters: Performance and mechanism. Sep. Purif. Technol. 2024, 331, 125584. [Google Scholar] [CrossRef]
| Production Method | Operation Conditions | Advantages |
|---|---|---|
| Gasification | >700 °C, <1 min | Rapid, stable, low volatile matter content |
| Conventional pyrolysis | 300–1000 °C, >1 h | Easy operation, rapid heat, most mature technology |
| Microwave pyrolysis | 0.3–300 GHz | Flexibility, not affected by sludge moisture |
| HTC | 180–250 °C, 1–4 MPa | No pretreatment, low energy consumption |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Gao, J.; Yang, X.; Ni, H.; Qi, J.; Zhu, Z.; Yang, Y.; Fang, D.; Zhou, L.; Li, J. Recent Progress in Sludge-Derived Biochar and Its Role in Wastewater Purification. Sustainability 2024, 16, 5012. https://doi.org/10.3390/su16125012
Zhou Y, Gao J, Yang X, Ni H, Qi J, Zhu Z, Yang Y, Fang D, Zhou L, Li J. Recent Progress in Sludge-Derived Biochar and Its Role in Wastewater Purification. Sustainability. 2024; 16(12):5012. https://doi.org/10.3390/su16125012
Chicago/Turabian StyleZhou, Yujun, Jiamin Gao, Xuran Yang, Hao Ni, Junwen Qi, Zhigao Zhu, Yue Yang, Di Fang, Lixiang Zhou, and Jiansheng Li. 2024. "Recent Progress in Sludge-Derived Biochar and Its Role in Wastewater Purification" Sustainability 16, no. 12: 5012. https://doi.org/10.3390/su16125012
APA StyleZhou, Y., Gao, J., Yang, X., Ni, H., Qi, J., Zhu, Z., Yang, Y., Fang, D., Zhou, L., & Li, J. (2024). Recent Progress in Sludge-Derived Biochar and Its Role in Wastewater Purification. Sustainability, 16(12), 5012. https://doi.org/10.3390/su16125012









