Carbon Dioxide Gasification of Biochar: A Sustainable Way of Utilizing Captured CO2 to Mitigate Greenhouse Gas Emission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feedstock Analysis
2.2.1. Proximate and Ultimate Analysis
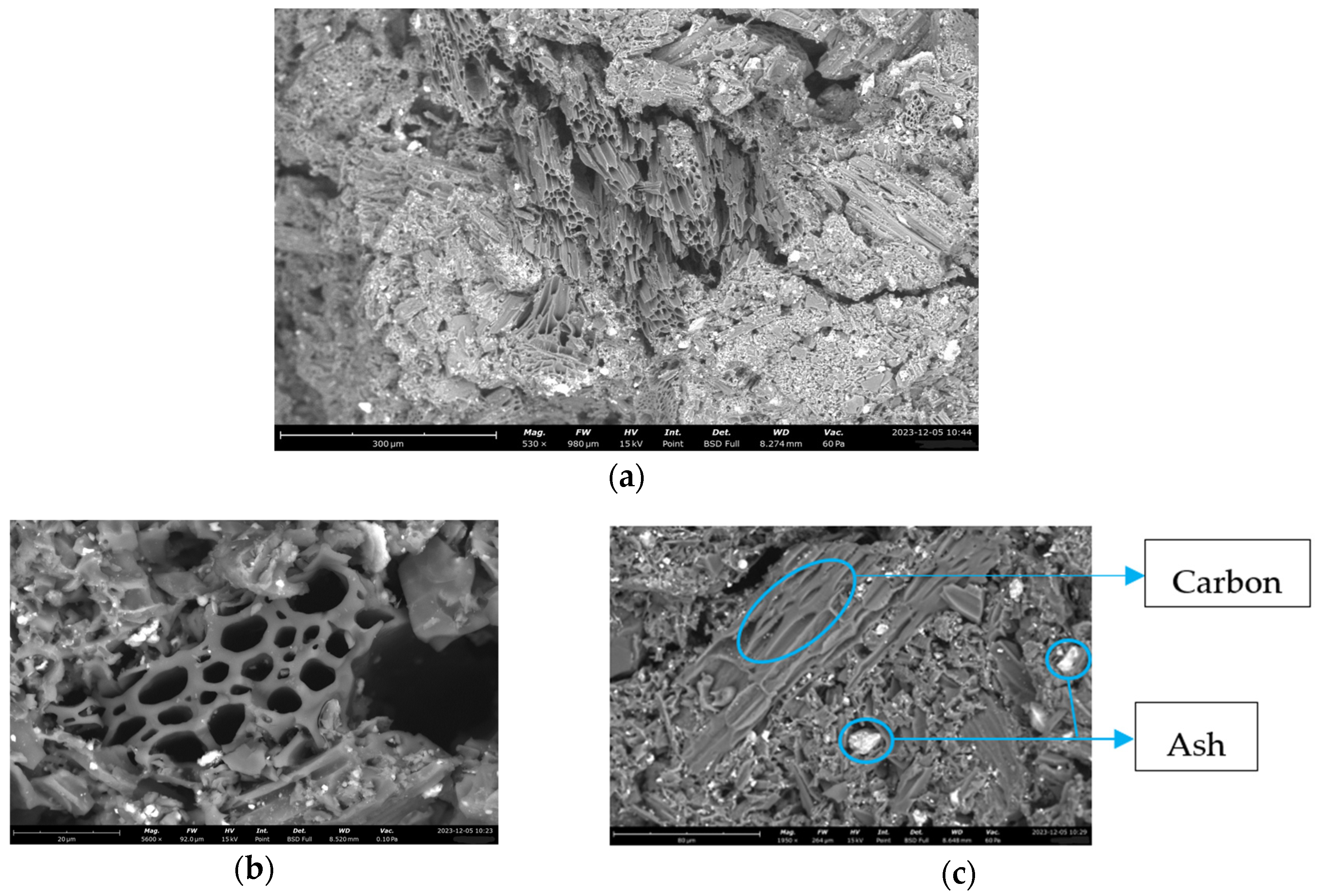
2.2.2. SEM Analysis
2.3. Experimental Setup and Procedure
2.3.1. Experimental Setup
2.3.2. Experimental Design
2.3.3. Operating Procedure
2.4. Kinetic Analysis
3. Results and Discussion
3.1. SEM Analysis
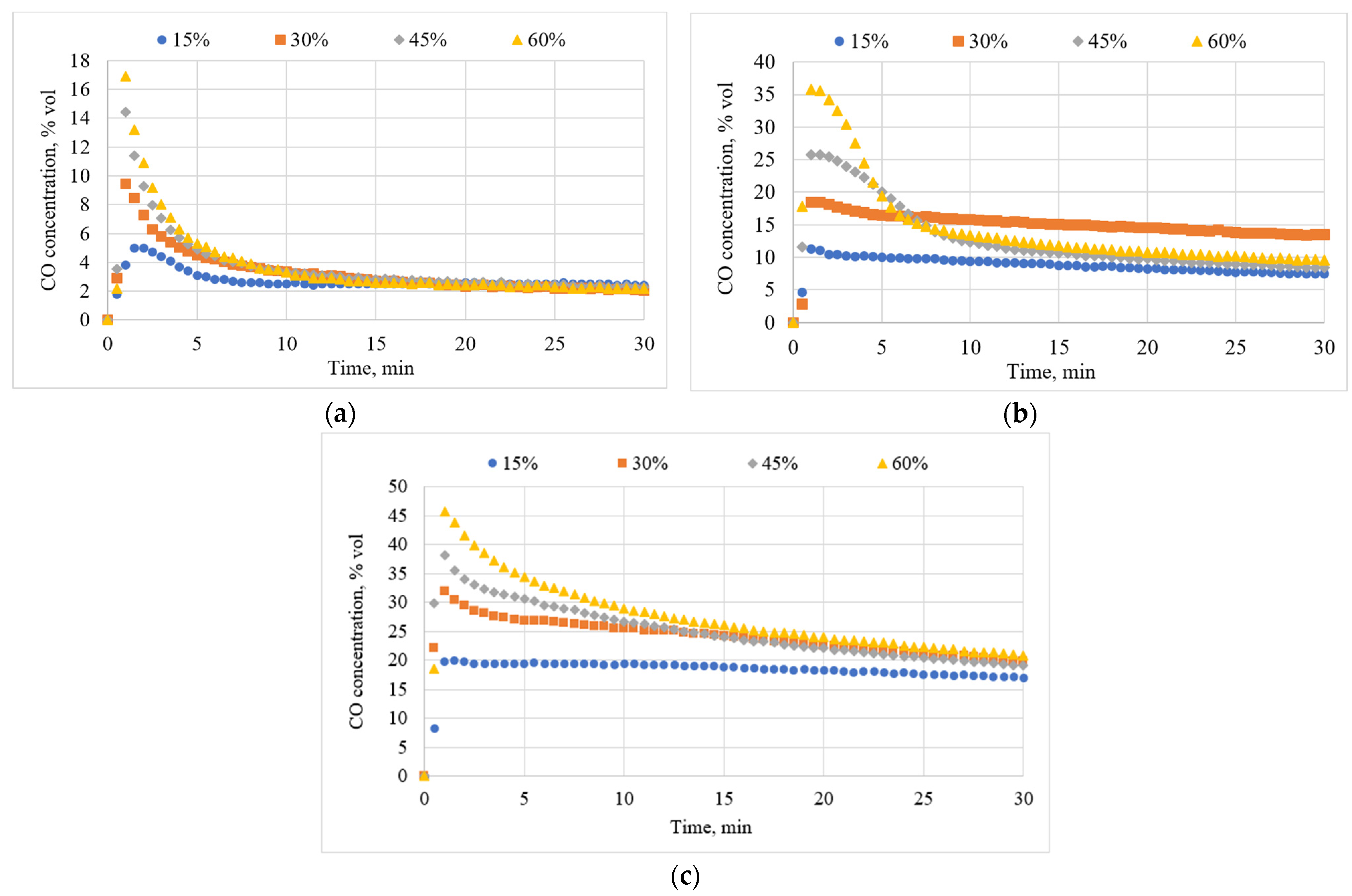
3.2. Effects of Temperature on CO Concentration
3.3. Effects of Inlet CO2 Concentration on CO Concentration
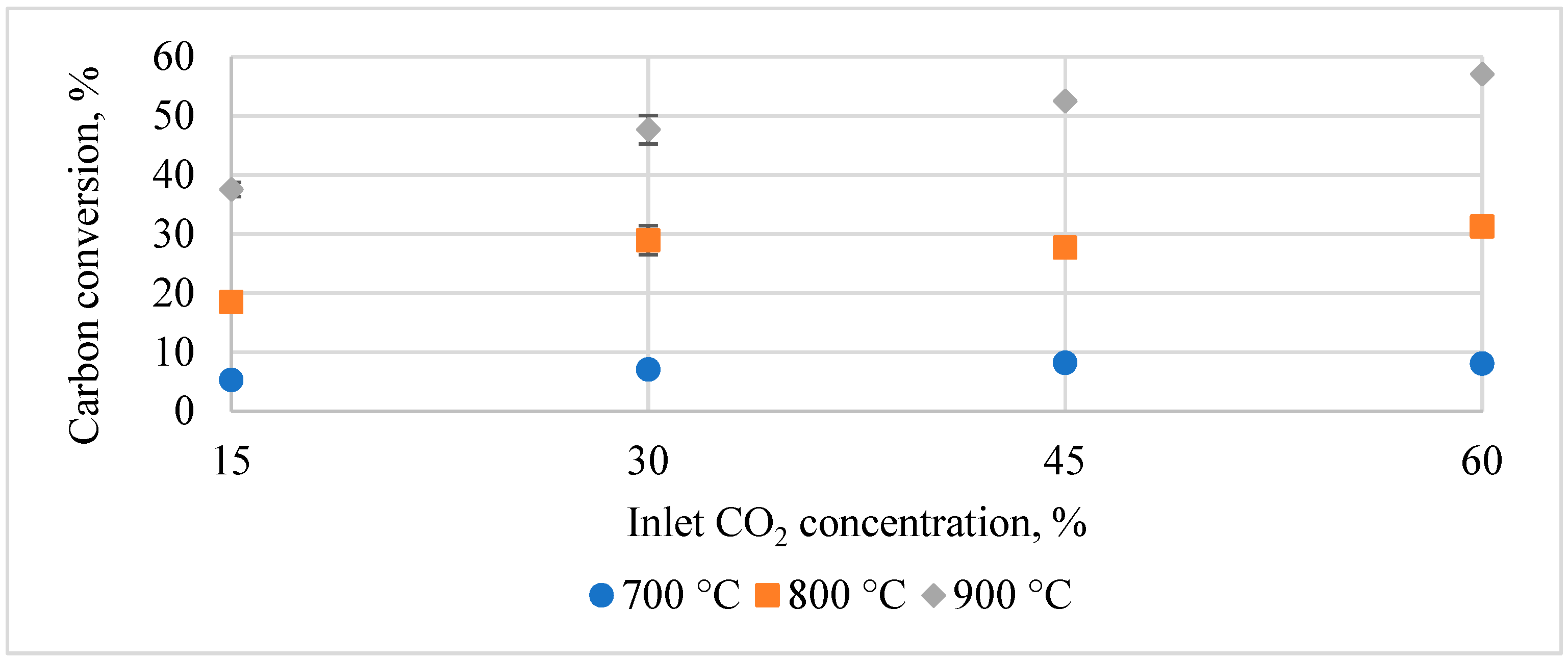
3.4. Effects of Temperature and Inlet CO2 Concentration on Carbon Conversion
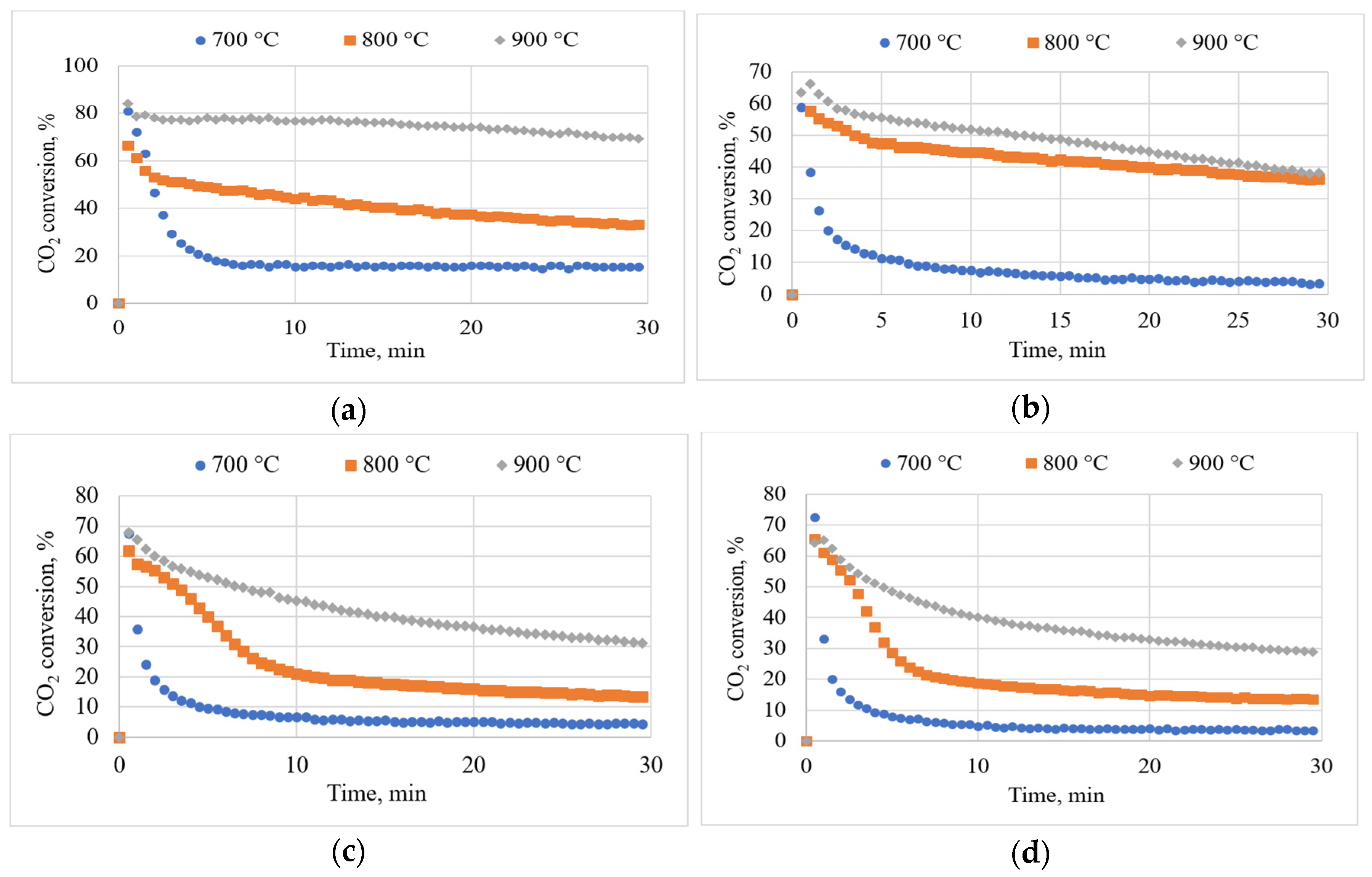
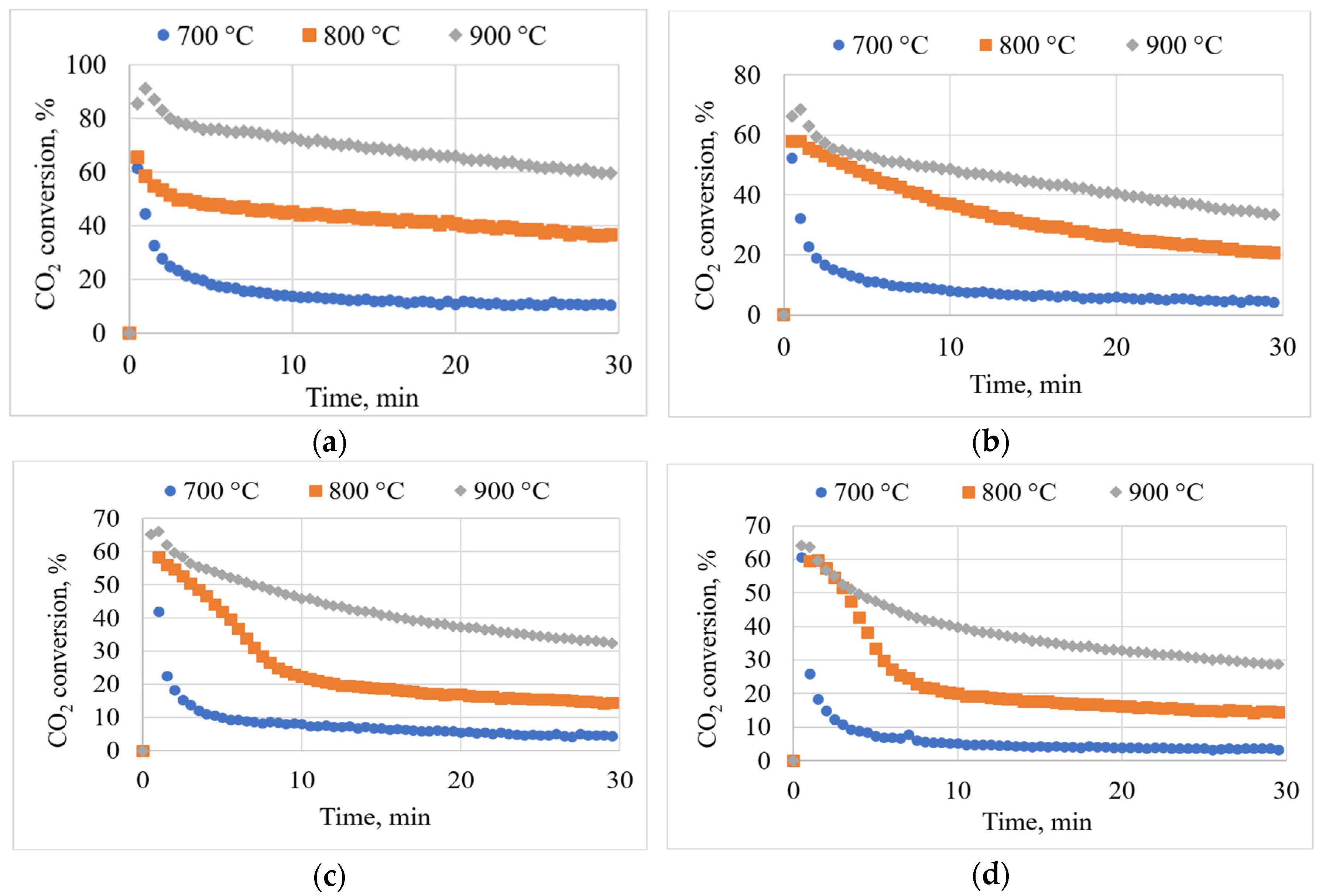
3.5. Effects of Temperature on CO2 Conversion
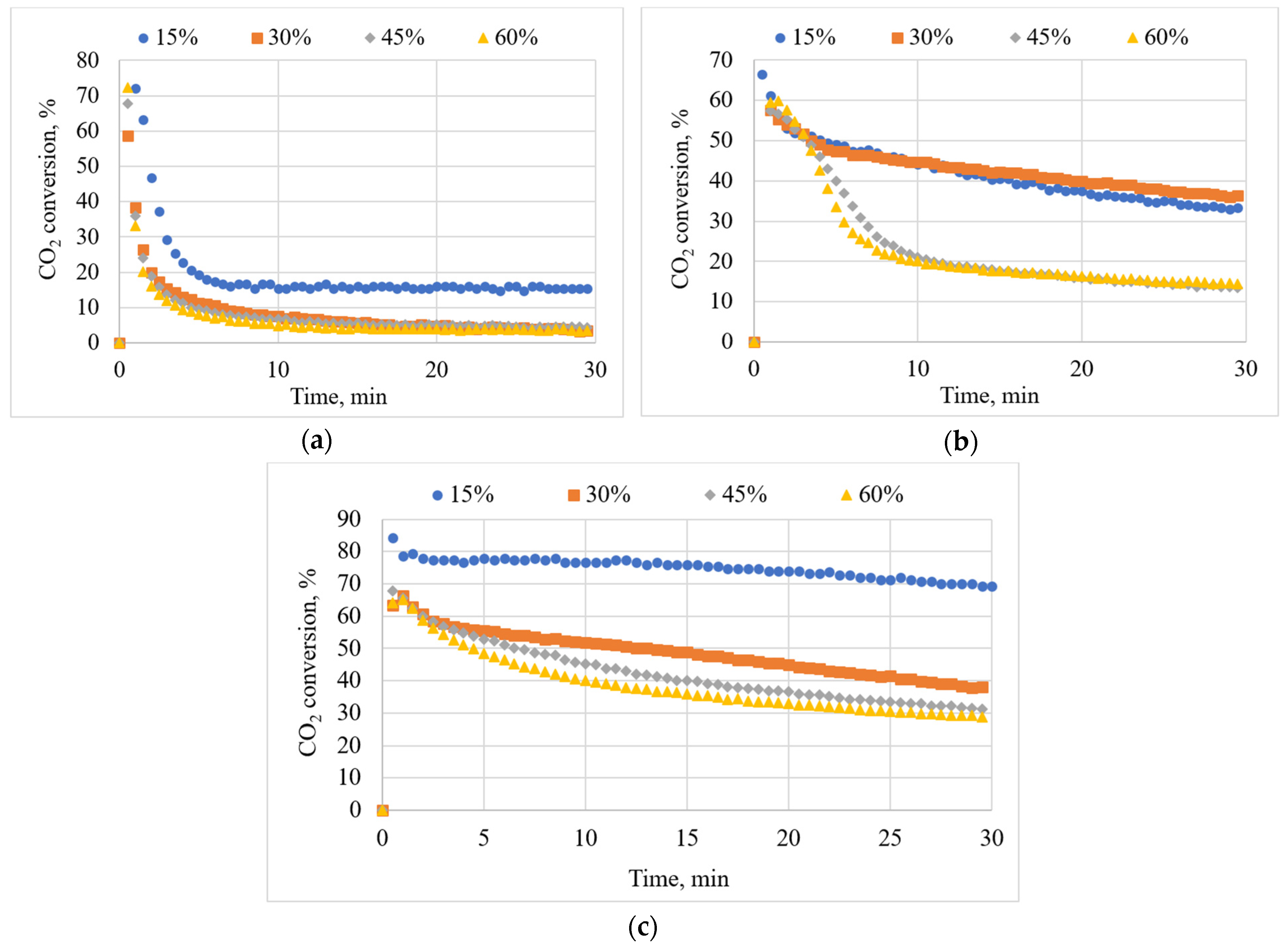
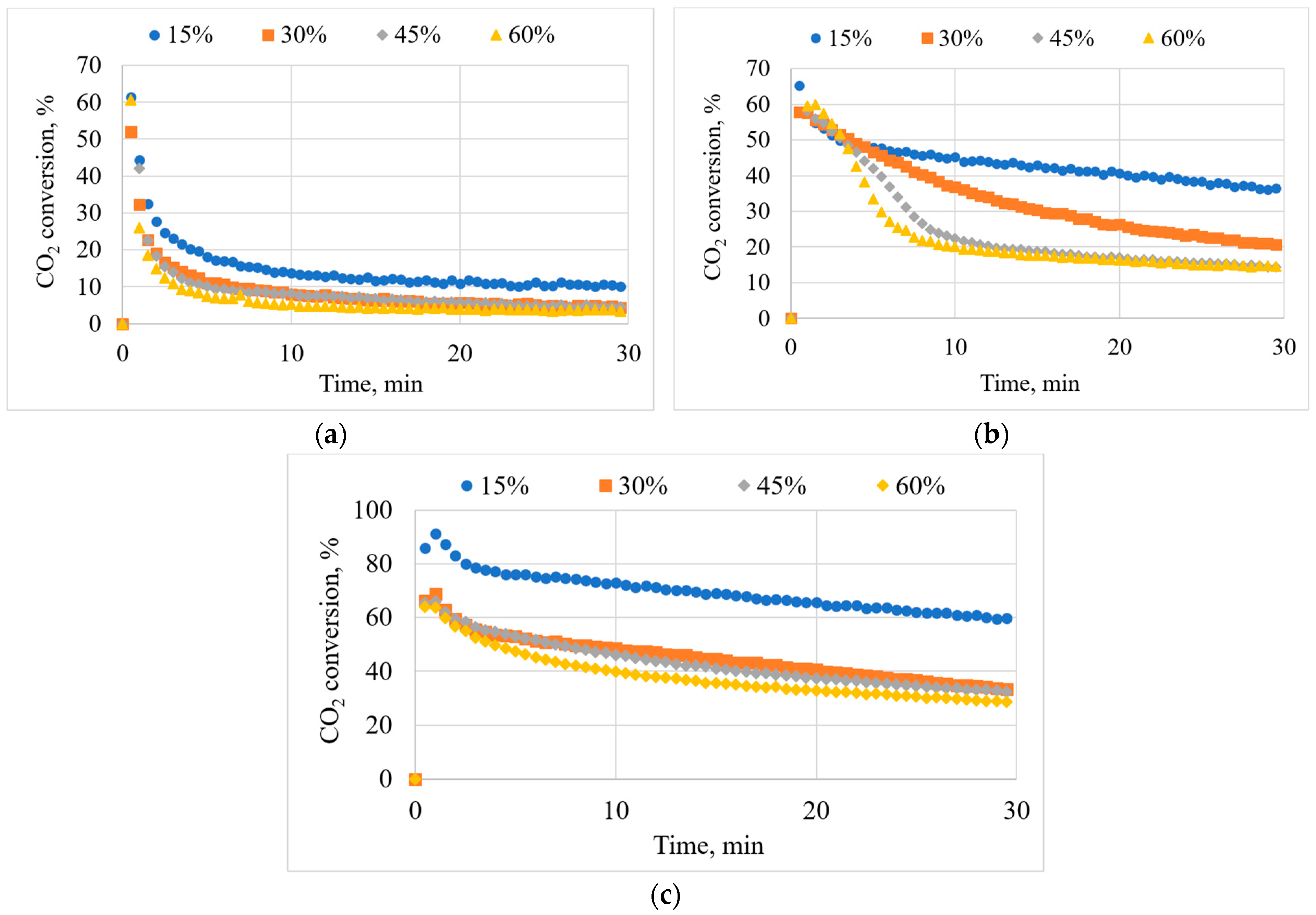
3.6. Effects of Inlet CO2 Concentration on CO2 Conversion
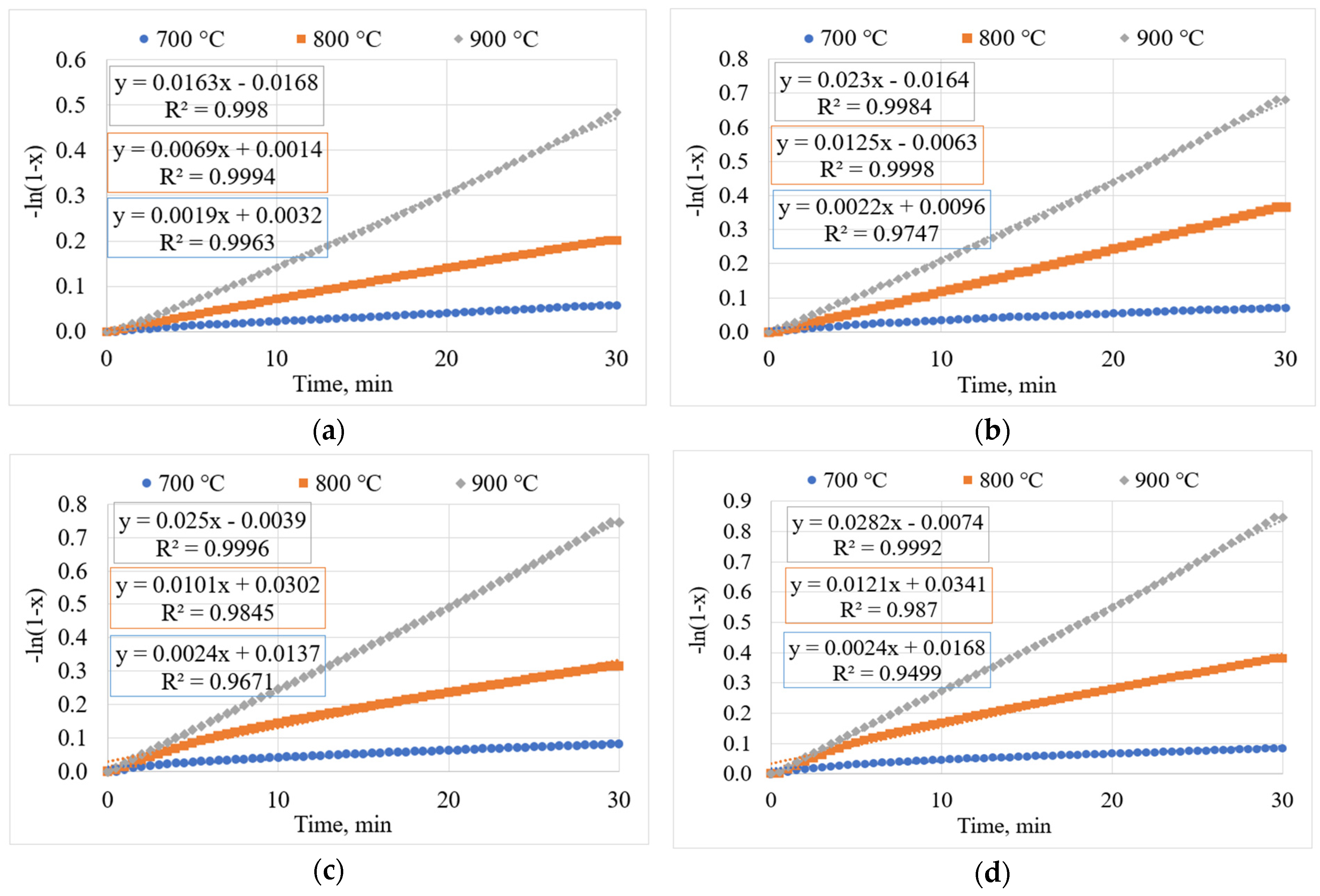
3.7. Determination of Kinetic Parameters
3.7.1. Determining the Gasification Rate Constant (kapp)
3.7.2. Determining the Activation Energy and Pre-Exponential Factor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| FT | Fischer–Tropsch |
|---|---|
| CCS | Carbon capture and storage |
| GHG | Greenhouse gas |
| Initial weight of char | |
| Instantaneous weight of char | |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| Gasification rate | |
| Carbon conversion | |
| Apparent reaction rate | |
| Activation energy | |
| Pre-exponential factor | |
| Molar gas constant | |
| Temperature | |
| Gibbs free energy | |
| Enthalpy change | |
| Partial pressure of products | |
| Partial pressure of reactants |


| Instruments | Analysis | Accuracy |
|---|---|---|
| TGA Q50 V20.13 | Weighting | ±0.01% |
| NOVA gas analyzer | CO2, CO, H2, and CH4 gas analysis | 1–2% FS |
| AALBORG mass flow controller | Gas flow rate control | ±1% |
| Omega mass flow meter | Gas flow rate analysis | ±(0.8% of rdg + 0.2% FS) |
References
- EIA. Energy Facts Explained. Available online: https://www.eia.gov/energyexplained/us-energy-facts/ (accessed on 4 February 2024).
- EPA. Climate Change Indicators: U.S. Greenhouse Gas Emissions. Available online: https://www.epa.gov/climate-indicators/climate-change-indicators-us-greenhouse-gas-emissions (accessed on 4 February 2024).
- United Nations. Transforming our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/goals/goal13 (accessed on 12 April 2024).
- United Nations Climate Change. The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 4 February 2024).
- Bobeck, J.; Peace, J.; Ahmad, F.M.; Munson, R. Carbon Utilization-A Vital and Effective Pathway for Decarbonization Summary Report. 2019. Available online: https://www.c2es.org/wp-content/uploads/2019/09/carbon-utilization-a-vital-and-effective-pathway-for-decarbonization.pdf (accessed on 4 February 2024).
- Sadhwani, N.; Adhikari, S.; Eden, M.R. Biomass Gasification Using Carbon Dioxide: Effect of Temperature, CO2/C Ratio, and the Study of Reactions Influencing the Process. Ind. Eng. Chem. Res. 2016, 55, 2883–2891. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Yao, Z.; Cui, X.; Wang, C.H. CO2 gasification of woody biomass: Experimental study from a lab-scale reactor to a small-scale autothermal gasifier. Energy 2019, 170, 497–506. [Google Scholar] [CrossRef]
- Cheng, Y.; Thow, Z.; Wang, C.H. Biomass gasification with CO2 in a fluidized bed. Powder Technol. 2016, 296, 87–101. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, H.; Zhou, F.; Chen, D. A comparison between CO2 gasification of various biomass chars and coal char. Can. J. Chem. Eng. 2019, 97, 1326–1331. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Shao, J.; Lui, Z.; Wang, H.; Li, X.; Zhang, P.; Geng, W.; Zhang, G. Experimental and modeling studies on CO2 gasification of biomass chars. Energy 2016, 114, 143–154. [Google Scholar] [CrossRef]
- Pacioni, T.R.; Soares, D.; Di Domenico, M.; Alves, J.L.F.; Virmond, E.; Moreira, R.P.M.; José, H.J. Kinetic modeling of CO2 gasification of biochars prepared from Brazilian agro-industrial residues: Effect of biomass indigenous mineral content. Biomass Convers. Biorefin. 2023, 13, 6675–6688. [Google Scholar] [CrossRef]
- Beagle, E.; Wang, Y.; Bell, D.; Belmont, E. Co-gasification of pine and oak biochar with sub-bituminous coal in carbon dioxide. Bioresour. Technol. 2018, 251, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bu, J. Kinetic Analysis of Coal and Biomass Co-gasification with Carbon Dioxide. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2009. [Google Scholar] [CrossRef]
- Dutta, S.; Wen, C.Y.; Belt, R.J. Reactivity of Coal and Char. 1. In Carbon Dioxide Atmosphere. Ind. Eng. Chem. 1977, 16, 20–30. [Google Scholar] [CrossRef]
- Ahmed, I.I.; Gupta, A.K. Kinetics of woodchips char gasification with steam and carbon dioxide. Appl. Energy 2011, 88, 1613–1619. [Google Scholar] [CrossRef]
- Mandapati, R.N.; Daggupati, S.; Mahajani, S.M.; Aghalayam, P.; Sapru, R.K.; Sharma, R.K.; Ganesh, A. Experiments and kinetic modeling for CO2 gasification of Indian coal chars in the context of underground coal gasification. Ind. Eng. Chem. 2012, 51, 15041–15052. [Google Scholar] [CrossRef]




| Ultimate Analysis (wt.%, daf) | Proximate Analysis (wt. %) | HHV (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | Volatile Matter (db) | Moisture (wb) | Fixed Carbon (db) | Ash (db) | |
| 57.53 | 2.53 | 0.31 | 23.79 | 0.19 | 35.74 | 4.95 | 46.49 | 17.77 | 17.22 |
| Inlet CO2 Concentration | at Different Temperatures (L/mol·min) | ||
|---|---|---|---|
| 15% | 0.122 | 0.518 | 1.192 |
| 30% | 0.172 | 0.865 | 1.649 |
| 45% | 0.193 | 0.839 | 1.897 |
| 60% | 0.182 | 0.916 | 2.094 |
| Inlet CO2 Concentration | Activation Energy (kJ/mol) | Pre-Exponential Factor (s−1) |
|---|---|---|
| 15 | 109 | 63 |
| 30 | 108 | 87 |
| 45 | 109 | 105 |
| 60 | 117 | 253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofuani, N.; Bhoi, P. Carbon Dioxide Gasification of Biochar: A Sustainable Way of Utilizing Captured CO2 to Mitigate Greenhouse Gas Emission. Sustainability 2024, 16, 5044. https://doi.org/10.3390/su16125044
Ofuani N, Bhoi P. Carbon Dioxide Gasification of Biochar: A Sustainable Way of Utilizing Captured CO2 to Mitigate Greenhouse Gas Emission. Sustainability. 2024; 16(12):5044. https://doi.org/10.3390/su16125044
Chicago/Turabian StyleOfuani, Nnamdi, and Prakashbhai Bhoi. 2024. "Carbon Dioxide Gasification of Biochar: A Sustainable Way of Utilizing Captured CO2 to Mitigate Greenhouse Gas Emission" Sustainability 16, no. 12: 5044. https://doi.org/10.3390/su16125044
APA StyleOfuani, N., & Bhoi, P. (2024). Carbon Dioxide Gasification of Biochar: A Sustainable Way of Utilizing Captured CO2 to Mitigate Greenhouse Gas Emission. Sustainability, 16(12), 5044. https://doi.org/10.3390/su16125044







