Integration of Methane Reforming and Chemical Looping Technologies for Power Generation from Waste Plastic: Technical and Economic Assessment
Abstract
:1. Introduction
2. Process Simulation and Model Development
2.1. Development of Case Studies
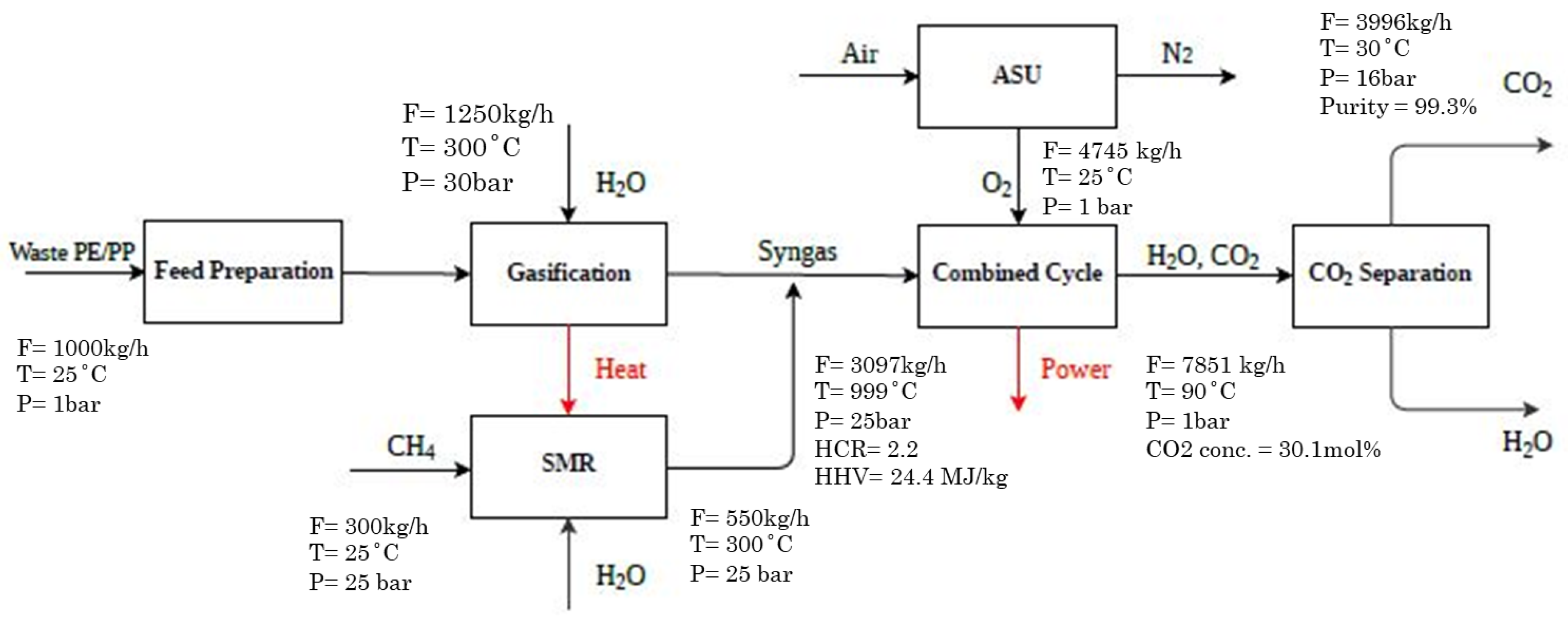
2.1.1. Case 1 (Base Case)
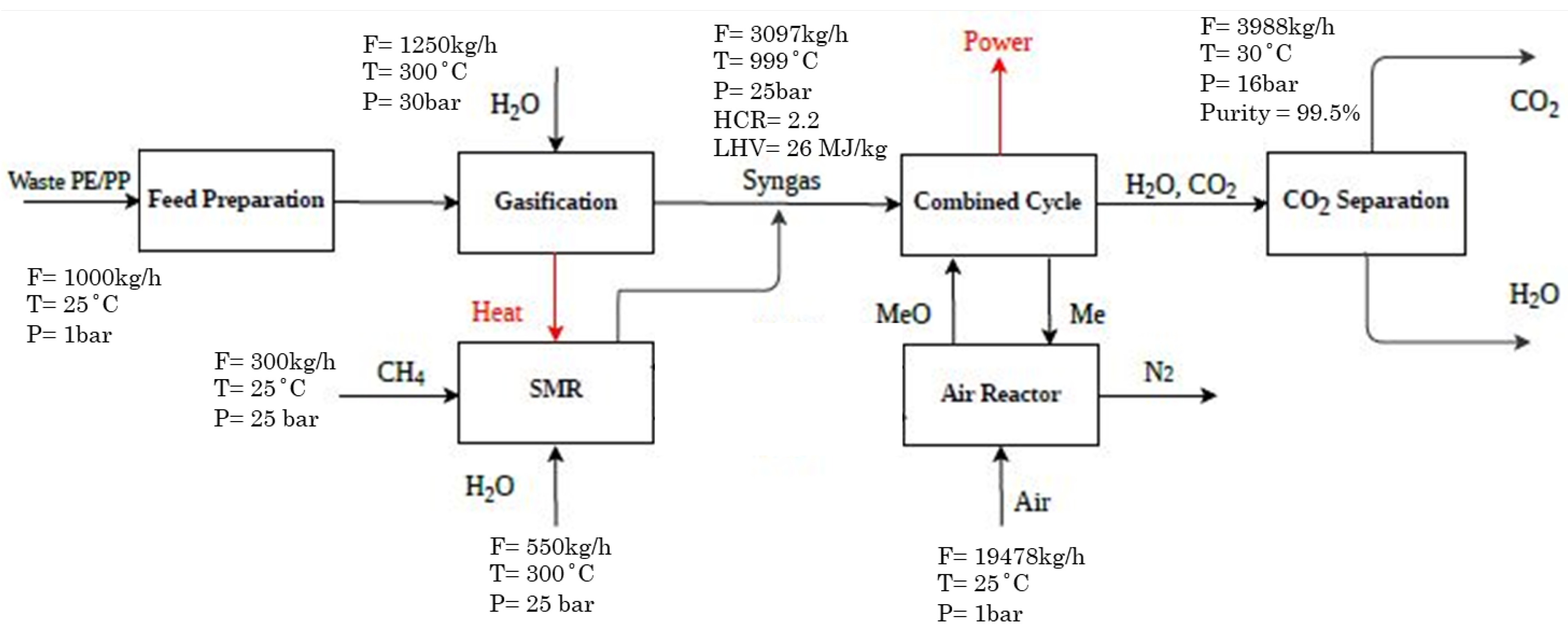
2.1.2. Case 2 (Alternative Case)
2.2. Development of Simulation Model
2.3. Techno-Economic Analysis
3. Results
3.1. Technical Analysis with Stream Compositions
3.2. Process Performance Analysis
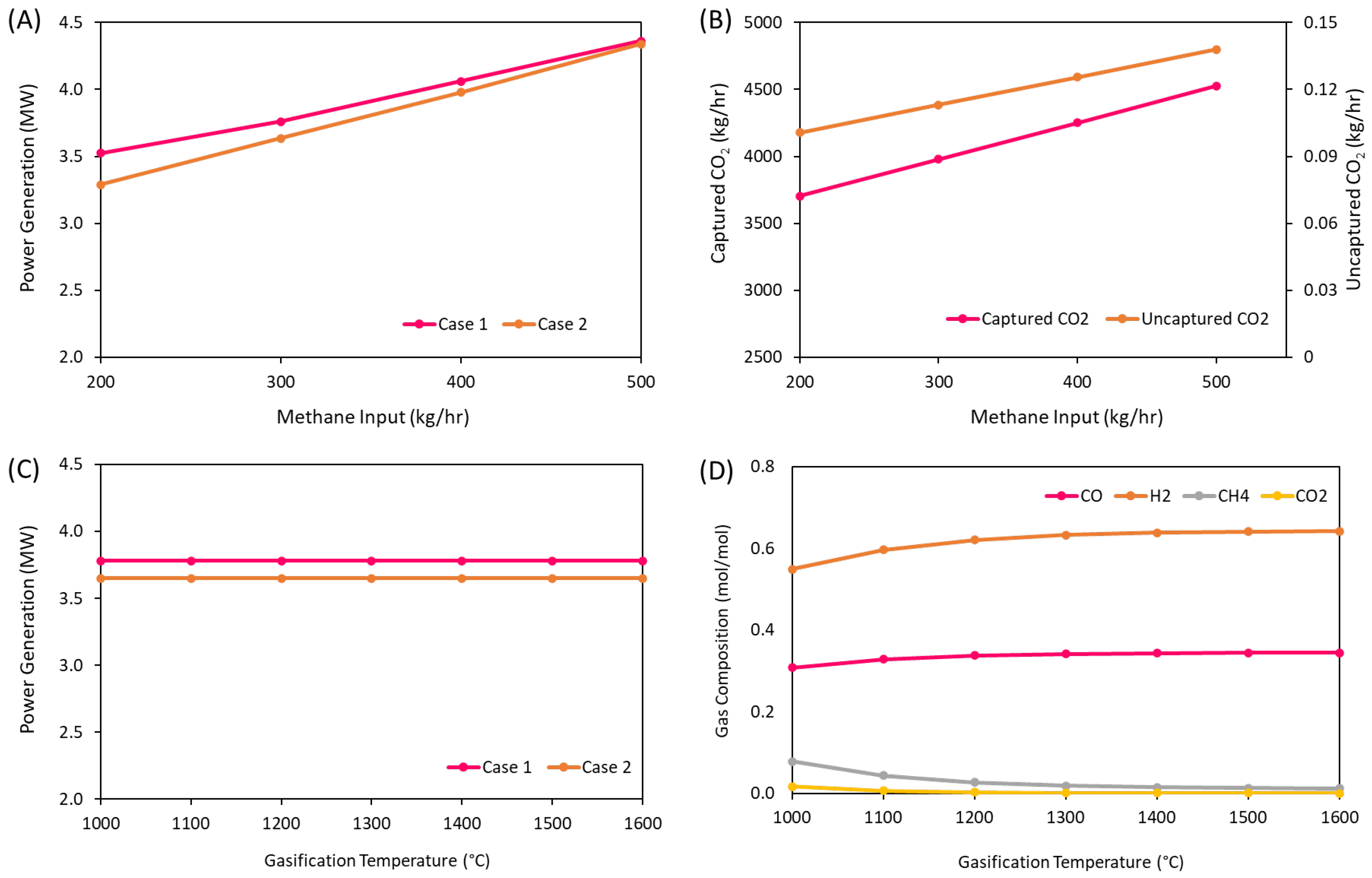
3.3. Impact of Operational Conditions on Process Performance
3.4. Economic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Plastic Pollution. 2018. Available online: https://ourworldindata.org/plastic-pollution#citation (accessed on 24 April 2024).
- Almohamadi, H.; Alamoudi, M.; Ahmed, U.; Shamsuddin, R.; Smith, K. Producing hydrocarbon fuel from the plastic waste: Techno-economic analysis. Korean J. Chem. Eng. 2021, 38, 2208–2216. [Google Scholar] [CrossRef]
- Ahmed, U.; Zahid, U.; Jeong, Y.S.; Lee, C.-J.; Han, C. IGCC process intensification for simultaneous power generation and CO2 capture. Chem. Eng. Process. Process Intensif. 2016, 101, 72–86. [Google Scholar] [CrossRef]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Ma, D.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Exergy and energy analysis of pyrolysis of plastic wastes in rotary kiln with heat carrier. Process Saf. Environ. Prot. 2020, 142, 203–211. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Barbarias, I.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. HDPE pyrolysis-steam reforming in a tandem spouted bed-fixed bed reactor for H2 production. J. Anal. Appl. Pyrolysis 2015, 116, 34–41. [Google Scholar] [CrossRef]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2022, 1, 140–165. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of plastic waste for synthesis gas production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Rizwan, M.; Saif, Y.; Almansoori, A.; Elkamel, A. Optimal processing route for the utilization and conversion of municipal solid waste into energy and valuable products. J. Clean. Prod. 2018, 174, 857–867. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Ahmed, U.; Jameel, A.G.A.; Zahid, U.; Usman, M.; Ahmad, N. Simulation and Modelling of Hydrogen Production from Waste Plastics: Technoeconomic Analysis. Polymers 2022, 14, 2056. [Google Scholar] [CrossRef]
- Williams, J.M.; Bourtsalas, A.C. Assessment of Co-Gasification Methods for Hydrogen Production from Biomass and Plastic Wastes. Energies 2023, 16, 7548. [Google Scholar] [CrossRef]
- Xu, H.; Shi, B. Design and System Evaluation of Mixed Waste Plastic Gasification Process Based on Integrated Gasification Combined Cycle System. Processes 2022, 10, 499. [Google Scholar] [CrossRef]
- Consonni, S.; Lozza, G.; Pelliccia, G.; Rossini, S.; Saviano, F. Chemical-Looping Combustion for Combined Cycles With CO2 Capture. J. Eng. Gas Turbine Power 2006, 128, 525–534. [Google Scholar] [CrossRef]
- Joshi, A.; Shah, V.; Mohapatra, P.; Kumar, S.; Joshi, R.K.; Kathe, M.; Qin, L.; Tong, A.; Fan, L.S. Chemical looping-A perspective on the next-gen technology for efficient fossil fuel utilization. Adv. Appl. Energy 2021, 3, 100044. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Capone, S.; Anatone, M.; Gallucci, K. Chemical Looping Combustion and Gasification: A Review and a Focus on European Research Projects. Ind. Eng. Chem. Res. 2022, 61, 14403–14432. [Google Scholar] [CrossRef]
- Ghosh, S.K. Energy Recovery Processes from Wastes, 1st ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Ahmed, U.; Jameel, A.G.A.; Ahmad, N.; Zahid, U.; Zein, S.H.; Naqvi, S.R. Process design and techno-economic analysis of dual hydrogen and methanol production from plastics using energy integrated system. Int. J. Hydrogen Energy 2023, 48, 10797–10811. [Google Scholar] [CrossRef]
- The Linde Group, Air Separation Plants. 2018. Available online: https://www.linde-engineering.com/products-and-services/process-plants/air-separation-plants (accessed on 24 April 2024).
- Khan, A.; Abbas, A.; Dickson, R. Towards a low-carbon future: Exploring green urea synthesis for sustainable agriculture. Green Chem. 2024, 26, 1551–1565. [Google Scholar] [CrossRef]
- Montiel-Bohórquez, N.D.; Agudelo, A.F.; Pérez, J.F. Modelling of an Integrated Plasma Gasification Combined Cycle power plant using Aspen Plus. J. King Saud Univ. Eng. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Bahzad, H.; Shah, N.; Mac Dowell, N.; Boot-Handford, M.; Soltani, S.M.; Ho, M.; Fennell, P.S. Development and techno-economic analyses of a novel hydrogen production process via chemical looping. Int. J. Hydrogen Energy 2019, 44, 21251–21263. [Google Scholar] [CrossRef]
- Kong, F.; Swift, J.; Zhang, Q.; Fan, L.-S.; Tong, A. Biogas to H2 conversion with CO2 capture using chemical looping technology: Process simulation and comparison to conventional reforming processes. Fuel 2020, 279, 118479. [Google Scholar] [CrossRef]
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical Looping Combustion: A Brief Overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Qing, M.; Jin, B.; Ma, J.; Zou, X.; Wang, X.; Zheng, C.; Zhao, H. Thermodynamic and economic performance of oxy-combustion power plants integrating chemical looping air separation. Energy 2020, 206, 118136. [Google Scholar] [CrossRef]
- Wu, W.; Xu, H.; Shi, B.; Kuo, P.-C. Techno-economic analysis of plastic wastes-based polygeneration processes. Chem. Eng. Process. Process Intensif. 2023, 184, 109297. [Google Scholar] [CrossRef]
- Farajollahi, H.; Hossainpour, S. Macroscopic model-based design and techno-economic assessment of a 300 MWth in-situ gasification chemical looping combustion plant for power generation and CO2 capture. Fuel Process. Technol. 2022, 231, 107244. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Khan, M.A.; Abbas, A.; Dickson, R. A strategy for commercialization of macroalga biorefineries. Renew. Sustain. Energy Rev. 2023, 187, 113703. [Google Scholar] [CrossRef]
- van der Roest, E.; van der Spek, M.; Ramirez, A.; van der Zwaan, B.; Rothenberg, G. Converting Waste Toilet Paper into Electricity: A First-Stage Technoeconomic Feasibility Study. Energy Technol. 2017, 5, 2189–2197. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Waste-to-Energy from Municipal Solid Wastes. 2019. Available online: www.energy.gov/eere/bioenergy (accessed on 1 June 2024).
- Pheakdey, D.V.; Van Quan, N.; Xuan, T.D. Economic and Environmental Benefits of Energy Recovery from Municipal Solid Waste in Phnom Penh Municipality, Cambodia. Energies 2023, 16, 3234. [Google Scholar] [CrossRef]
- Nubi, O.; Morse, S.; Murphy, R.J. Prospective Life Cycle Costing of Electricity Generation from Municipal Solid Waste in Nigeria. Sustainability 2022, 14, 13293. [Google Scholar] [CrossRef]

| Components | Polyethylene | Polypropylene |
|---|---|---|
| Ultimate analysis (wt%) | ||
| Moisture content | 0.02 | 0 |
| Ash content | 0.15 | 0.70 |
| Volatile matter | 99.83 | 99.30 |
| Proximate analysis (wt%) | ||
| Carbon content | 85.81 | 86.23 |
| Hydrogen content | 13.86 | 12.28 |
| Nitrogen content | 0.12 | 0.62 |
| Sulphur content | 0.06 | 0.17 |
| Ash content | 0.15 | 0.70 |
| LHV (Lower heating value) MJ/kg | 38.04 | 44.70 |
| Assumed Parameters | Value |
|---|---|
| Costing year | 2023 |
| Plant operational life | 20 years |
| Construction period | 2 years |
| Operating hours/year | 8000 |
| Depreciation of general plant | 7 years |
| Discount rate | 8% per year |
| Tax rate | 15% per year |
| Economic Factors | Values |
|---|---|
| Total direct cost (TDC) | |
| Warehouse | 4% of ISBL |
| Site development | 9% of ISBL |
| Additional piping | 4.5% of ISBL |
| Total indirect cost (TIDC) | |
| Field expenses | 10% of TDC |
| Prorateable costs | 10% of TDC |
| Project contingency | 10% of TDC |
| Home office and construction | 10% of TDC |
| Other costs | 10% of TDC |
| Capital expenditures (CAPEX) | |
| Working capital (WC) | 5% of FCI |
| Fixed capital investment (FCI) | TDC + TIDC |
| Land | 6% of (Installed costs) |
| Plastic Feed | Steam to Gasifier | Gasifier Outlet | Methane to SMR | Reformer Outlet | GT Outlet (Flue Gas) | ST Outlet (Steam) | CO2 Purification | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1/2 | Case 1/2 | Case 1/2 | Case 1/2 | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | |
| T (°C) | 25 | 300 | 1300 | 25 | 900 | 900 | 756 | 728 | 49 | 49 | 30 | 30 |
| P (bar) | 1 | 30 | 25 | 25 | 30 | 30 | 1 | 1 | 0.1 | 0.1 | 16 | 16 |
| Mass flow (kg/hr) | 1000 | 1250 | 2250 | 300 | 850 | 850 | 7633 | 7633 | 3070 | 2870 | 3996 | 3996 |
| Mole (%) | ||||||||||||
| PE | 50 | - | - | - | - | - | - | - | - | - | - | - |
| PP | 50 | - | - | - | - | - | - | - | - | - | - | - |
| H2 | - | - | 63.3 | - | 67.2 | 67.2 | - | - | - | - | - | - |
| CO | - | - | 34.1 | - | 19.1 | 19.1 | - | - | - | - | - | - |
| CO2 | - | - | 0.11 | - | 2.4 | 2.4 | 29.4 | 30.1 | - | - | 99.5 | 99.5 |
| H2O | - | 100 | 0.57 | - | 11.2 | 11.2 | 68.2 | 69.8 | 100 | 100 | 0.3 | 0.3 |
| O2 | - | - | - | - | - | - | 2.3 | - | - | - | 0.2 | 0.2 |
| CH4 | - | - | 1.84 | 100 | - | - | - | - | - | - | - | - |
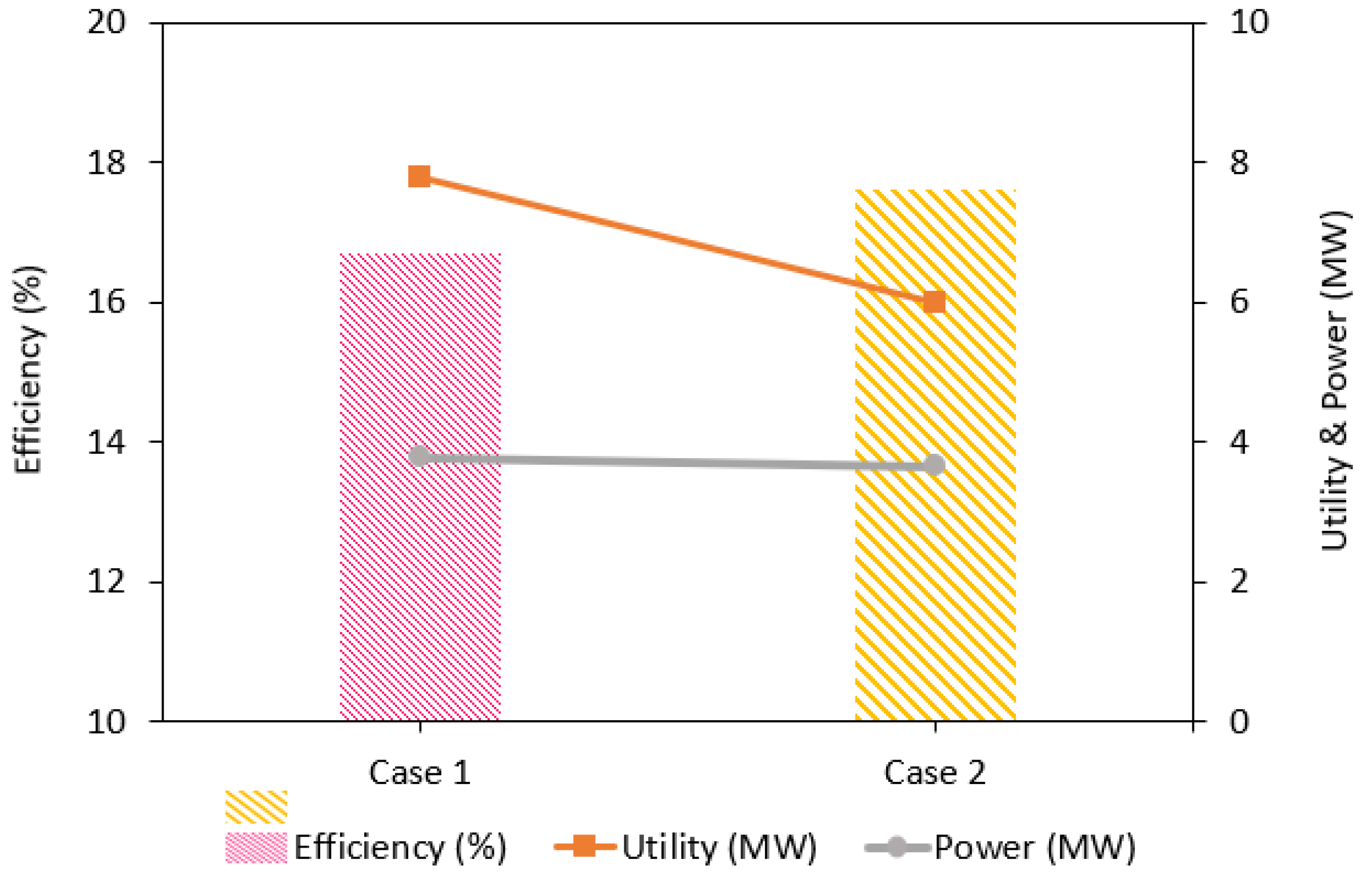
| Process Parameters | Case 1 | Case 2 |
|---|---|---|
| Syngas production (kg/h) | 3097 | 3097 |
| Syngas HCR | 2.2 | 2.2 |
| Syngas HHV (MJ/kg) | 25.9 | 25.9 |
| Syngas temperature (°C) | 1500 | 1454 |
| Minimum utility required (MW) | 7.8 | 6.0 |
| Power production (MW) | 3.78 | 3.65 |
| Process efficiency (%) | 16.7 | 17.6 |
| CO2 captured (kg/h) | 3996 | 3996 |
| Case 1 | Case 2 | |
|---|---|---|
| CAPEX calculation (M$) | ||
| Equipment and installation cost | 23.70 | 10.51 |
| Direct cost | 4.15 | 1.84 |
| Indirect cost | 13.92 | 6.17 |
| Working and land cost | 3.51 | 1.56 |
| Total CAPEX | 45.28 | 20.08 |
| OPEX calculation (M$/yr) | ||
| Labor Cost | 0.59 | 0.59 |
| Raw material cost | 0.01 | 0.01 |
| Utility cost | 1.07 | 1.09 |
| Total OPEX | 1.68 | 1.70 |
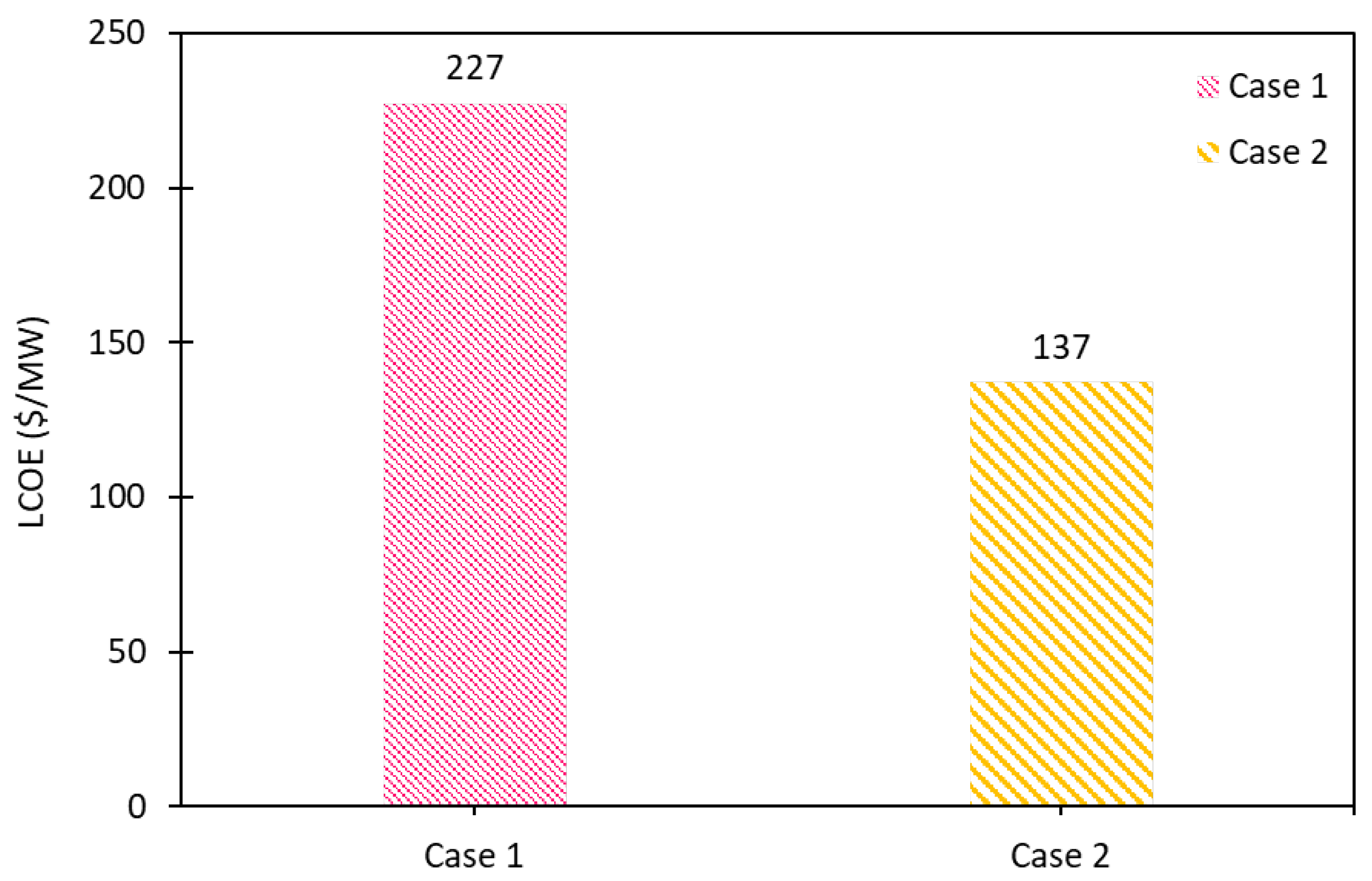
| LCOE ($/MW) | 227 | 137 |
| T | Waste Type | LCOE ($/MW) | Recycling Process | Reference |
|---|---|---|---|---|
| 1 | Waste toilet paper | 203 | Gasification | [31] |
| 2 | MSW | 170 | Anaerobic digestion | [32] |
| 3 | MSW | 93 | Anaerobic digestion | [33] |
| 4 | MSW | 160 | LFGTE | [34] |
| 5 | Waste plastic | 137 | Gasification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarzaee, F.; Ahmed, U. Integration of Methane Reforming and Chemical Looping Technologies for Power Generation from Waste Plastic: Technical and Economic Assessment. Sustainability 2024, 16, 5082. https://doi.org/10.3390/su16125082
Alqarzaee F, Ahmed U. Integration of Methane Reforming and Chemical Looping Technologies for Power Generation from Waste Plastic: Technical and Economic Assessment. Sustainability. 2024; 16(12):5082. https://doi.org/10.3390/su16125082
Chicago/Turabian StyleAlqarzaee, Faisal, and Usama Ahmed. 2024. "Integration of Methane Reforming and Chemical Looping Technologies for Power Generation from Waste Plastic: Technical and Economic Assessment" Sustainability 16, no. 12: 5082. https://doi.org/10.3390/su16125082






