Deactivation Patterns of Potassium-Based γ-Alumina Dry Sorbents for CO2 Capture
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. CO2 Capture Capacity of 5KAlI, 10KAlI, 20KAlI, and 30KAlI Sorbents
3.2. Deactivation Degree According to K2CO3 Loading Amount
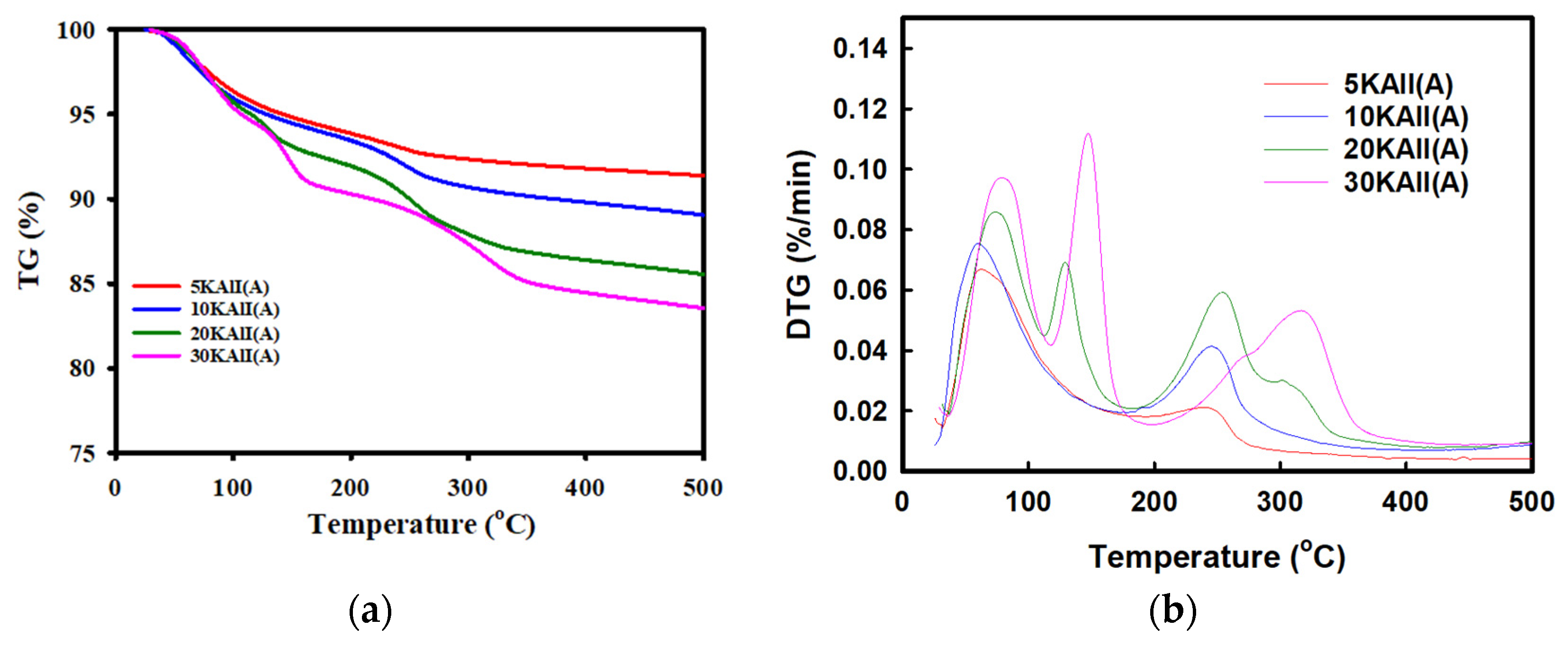
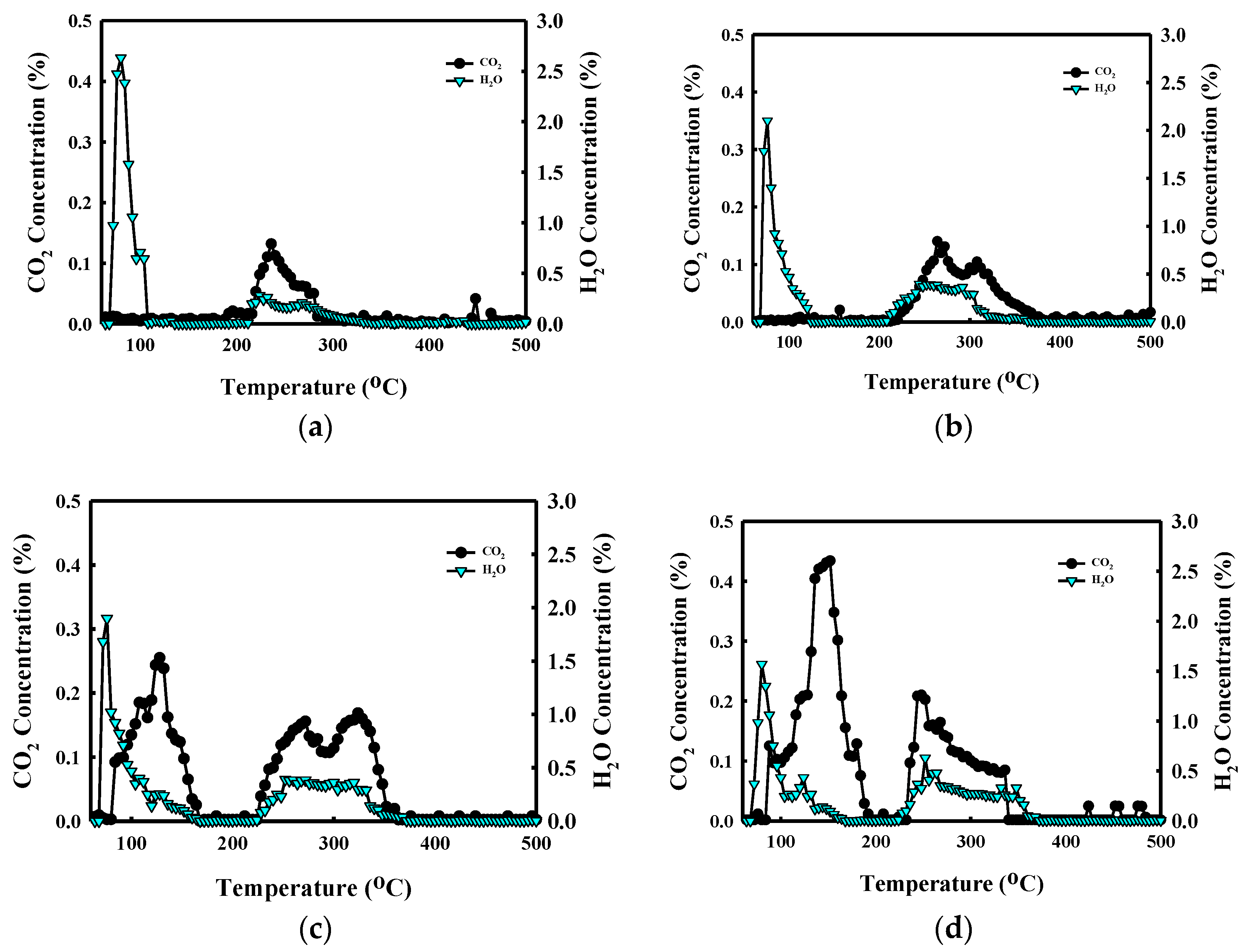
3.3. Structure Identification of the 5–30KAlI Sorbents
3.4. Physical Properities of the 5–30KAlI Sorbents According to BET
3.5. Surface Morphology of the 5–30KAlI Sorbents According to SEM
3.6. Regeneration Properties of the 5–30KAlI Sorbents
3.7. Derivation of the Predictive Model for Capture Capacity Based on K2CO3 Loading Amounts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Wei, K.; Guan, H.; Luo, Q.; He, J. Recent advances in CO2 capture and reduction. nanoscale. Nanoscale 2022, 14, 11869–11891. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Fong, C.; Freeman, B.D.; Hill, M.R.; Xie, Z. Current status and advances in membrane technology for carbon capture. Sep. Purif. Technol. 2022, 5, 121863. [Google Scholar] [CrossRef]
- Gkotsis, P.; Peleka, E.; Zouboulis, A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes 2023, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Siriwardane, R.V.; Stevens, R.W., Jr. Novel regenerable magnesium hydroxide sorbents for CO2 capture at warm gas temperatures. Ind. Eng. Chem. Res. 2009, 48, 2135–2141. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, Y.M.; Chae, H.J.; Jung, S.Y.; Lee, J.B.; Ryu, C.K.; Yi, C.K.; Kim, J.C. Improving regeneration properties of potassium-based alumina sorbents for carbon dioxide capture from flue gas. Fuel 2013, 104, 882–885. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, A.E. Adsorption of carbon dioxide at high temperature—A review. Sep. Purif. Technol. 2002, 26, 195–205. [Google Scholar] [CrossRef]
- Seo, Y.W.; Jo, S.H.; Ryu, H.J.; Bae, D.H.; Ryu, C.K.; Yi, C.K. Effect of water pretreatment on CO2 capture using a potassium-based solid sorbent in a bubbling fluidized bed reactor. Korean J. Chem. Eng. 2007, 24, 457–460. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Wen, X.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. CO2 capture over K2CO3/MgO/Al2O3 dry sorbent in a fluidized bed. Energy Fuels 2011, 25, 3835–3842. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, J.C. Dry potassium-based sorbents for CO2 capture. Catal. Surv. Asia 2007, 11, 171–185. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.H.A. CO2 capture by solid sorbents: A review of current developments. Ind. Eng. Chem. Res. 2011, 50, 5151–5164. [Google Scholar]
- Lee, K.B.; Lee, J.H.; Lee, C.H.; Kim, J.H.; Yoon, J.H. A study on CO2 breakthrough adsorption capacity of dry sorbents. Chem. Eng. J. 2010, 162, 883–892. [Google Scholar]
- Sanna, A.; Maroto-Valer, M.M. Development of novel dry regenerable sorbent synthesized via ultrasonic spray pyrolysis for CO2 capture. Environ. Sci. Technol. 2012, 46, 8014–8021. [Google Scholar]
- Jo, S.B.; Lee, S.C.; Chae, H.J.; Cho, M.S.; Lee, J.B.; Baek, J.I.; Kim, J.C. Regenerable potassium-based alumina sorbents prepared by CO2 thermal treatment for post-combustion carbon dioxide capture. Korean J. Chem. Eng. 2016, 33, 3207–3215. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Zhao, C.; Wu, Y.; Wu, D. K2CO3/Al2O3 for capturing CO2 in flue gas from power plants. Part 3: CO2 Capture Behaviors of K2CO3/Al2O3 in a Bubbling Fluidized-Bed Reactor. Energy Fuels 2012, 26, 3062–3068. [Google Scholar] [CrossRef]
- Choi, S.H.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, Y.; Wu, Y.; Chen, X.; Cai, T.; Liu, D. Continuous CO2 capture performance of K2CO3/Al2O3 sorbents in a novel integrated bubbling-transport fluidized reactor. Ind. Eng. Chem. Res. 2019, 58, 19733–19740. [Google Scholar] [CrossRef]
- Zhao, C.W.; Chen, X.P.; Zhao, C.S. CO2 absorption using dry potassium-based sorbents with different supports. Energy Fuels 2009, 23, 4683–4687. [Google Scholar] [CrossRef]
- Park, Y.C.; Jo, S.-H.; Ryu, C.K.; Yi, C.-K. Demonstration of pilot scale carbon dioxide capture system using dry regenerable solid sorbents to the real coal-fired power plant in Korea. Energy Procedia 2011, 4, 1508–1512. [Google Scholar] [CrossRef]
- Park, Y.C.; Jo, S.-H.; Kyung, D.-H.; Kim, J.-Y.; Yi, C.-K.; Ryu, C.K.; Shin, M.S. Test operation results of the 10 MWe-scale dry-solid sorbent CO2 capture process integrated with a real coal-fired power plant in Korea. Energy Procedia 2014, 63, 2261–2265. [Google Scholar] [CrossRef]
- Park, Y.C.; Jo, S.-H.; Lee, S.-Y.; Moon, J.-H.; Ryu, C.K.; Lee, J.B.; Yi, C.-K. Performance analysis of K-based KEP-CO2P1 solid solid sorbents in a bench-scale continuous dry-solid sorbent CO2 capture process. Korean J. Chem. Eng. 2016, 33, .73–79. [Google Scholar] [CrossRef]
- Lee, S.C.; Cho, M.S.; Jung, S.Y.; Ryu, C.K.; Kim, J.C. Effects of alumina phases on CO2 sorption and regeneration properties of potassium-based alumina sorbents. Adsorption 2014, 20, 331–339. [Google Scholar] [CrossRef]
- Fernandez-Carrasco, L.; Puertas, F.; Blanco-Varela, M.T.; Vazquez, T.; Rius, J. Synthesis and crystal structure solution of potassium dawsonite: An intermediate compound in the alkaline hydrolysis of calcium aluminate cements. Cem. Concr. Res. 2005, 32, 641–646. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Bararpour, S.T.; Karami, D.; Mahinpey, N. Investigation of the effect of alumina-aerogel support on the CO2 capture performance of K2CO3. Fuel 2019, 242, 124–132. [Google Scholar] [CrossRef]
- Hayashi, H.; Taniuchi, J.; Furuyashiki, N.; Sugiyama, S.; Hirano, S.; Shigemoto, N.; Nonaka, T. Efficient recovery of carbon dioxide from flue gases of coal-fired power plants by cyclic fixed-bed operations over K2CO3-on-carbon. Ind. Eng. Chem. Res. 1998, 37, 185–191. [Google Scholar] [CrossRef]
- Hirano, S.; Shigemoto, N.; Yamaha, S.; Hayashi, H. Cyclic fixed-bed operations over K2CO3-on- carbon for the recovery of carbon dioxide under moist conditions. Bull. Chem. Soc. Jpn. 1995, 68, 1030–1035. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.H.; Huang, W.Y. Synthesis and characterization of potassium-modified alumina superbases. Phys. Chem. Chem. Phys. 2001, 3, 2537–2543. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Zhao, C. Carbonation behavior of K2CO3 with different microstructure used as an active component of dry sorbents for CO2 capture. Ind. Eng. Chem. Res. 2010, 49, 12212–12216. [Google Scholar] [CrossRef]
- Lee, S.C.; Chae, H.J.; Choi, B.Y.; Jung, S.Y.; Ryu, C.Y.; Park, J.J.; Baek, J.I.; Ryu, C.K.; Kim, J.C. The effect of relative humidity on CO2 capture capacity of potassium-based sorbents. Korean J. Chem. Eng. 2011, in press. [Google Scholar] [CrossRef]







| CO2 Capture | Regeneration | |
|---|---|---|
| Temperature (°C) | 60 | 200 |
| Pressure (atm) | 1 | 1 |
| Flow rate (mL/min) | 60 | 60 |
| Gas composition (vol%) | CO2: 1, H2O: 10, N2: balance | N2: balance |
| Sorbent | Initial Capture Capacity (mg CO2/g Sorbents) | Final Capture Capacity (mg CO2/g Sorbents) | Degree of Deactivation (%) |
|---|---|---|---|
| 5KAlI | 15.8 | 0 | 100 |
| 10KAlI | 30.5 | 2.0 | 26.7 |
| 20KAlI | 60.3 | 24.9 | 62 |
| 30KAlI | 88.2 | 50.4 | 44.6 |
| Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter (nm) | |
|---|---|---|---|
| γ-Al2O3 | 158.74 | 0.296 | 6.529 |
| 5KAlI | 150.5 | 0.27 | 4.9 |
| 10KAlI | 121.25 | 0.212 | 3.834 |
| 20KAlI | 77.237 | 0.151 | 3.832 |
| 30KAlI | 60.559 | 0.117 | 3.828 |
| K2CO3 | 0.592 | 0.001 | 3.058 |
| Sorbent | Weight Loss (%) | CO2 Desorption Capacity (%) | H2O Desorption Capacity (%) | |
|---|---|---|---|---|
| 5KAlI | (I) | 5.21 | 0 | 5.15 |
| (II) | 30.9 | 1.62 | 1.29 | |
| 10KAlI | (I) | 5.59 | 0 | 5.53 |
| (II) | 5.21 | 3.05 | 2.51 | |
| 20KAlI | (I) | 6.94 | 2.1 | 4.82 |
| (II) | 9.76 | 4.01 | 3.29 | |
| 30KAlI | (I) | 8.07 | 4.02 | 4.09 |
| (II) | 7.49 | 4.45 | 3.66 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
In, S.Y.; Min, J.H.; Kim, J.C.; Lee, S.C. Deactivation Patterns of Potassium-Based γ-Alumina Dry Sorbents for CO2 Capture. Sustainability 2024, 16, 5117. https://doi.org/10.3390/su16125117
In SY, Min JH, Kim JC, Lee SC. Deactivation Patterns of Potassium-Based γ-Alumina Dry Sorbents for CO2 Capture. Sustainability. 2024; 16(12):5117. https://doi.org/10.3390/su16125117
Chicago/Turabian StyleIn, Soo Yeong, Ji Hwan Min, Jae Chang Kim, and Soo Chool Lee. 2024. "Deactivation Patterns of Potassium-Based γ-Alumina Dry Sorbents for CO2 Capture" Sustainability 16, no. 12: 5117. https://doi.org/10.3390/su16125117





