Abstract
Stabilizing materials were prepared by different ratios of attapulgite/humic acid composites, and the optimum proportion for the remediation of Cd-polluted soils was found. The results suggested that the bioavailability of Cd in soil was decreased by the application of material prepared with humic acid and attapulgite in a ratio of 1:5. CaCl2-Cd, diethylenetriaminepentaacetic acid (DTPA-Cd) and the toxicity characteristic leaching procedure (TCLP-Cd) were reduced by 34.03%, 26.62% and 43.66%, and the ecological risk was depressed accordingly. The addition of stabilizing materials could transform the acid-soluble and reducible speciation to residue speciation, with a ratio of 1:5, significantly increasing the residue proportion of Cd in soil. The content of the residue state was increased by 63.13%, and the content of the acid-soluble state was significantly decreased by 34.10% compared with the control. The bioavailability, acid-soluble and reducible speciation of Cd had a highly negative correlation with the growth of corn, and the accumulation of Cd in corn had a significantly negative correlation with the residue speciation. Attapulgite/humic acid composites can reduce the bioavailability and increase the ratio of residue Cd in soil effectively, and they have the potential to remediate the pollution of heavy metals in soil.
1. Introduction
The pollution of heavy metals in soil has occurred frequently and is long-term, hidden and irreversible, posing a serious threat to ecosystems [,]. According to current research, the treatment methods for heavy metal pollution in soil can be divided into physical, chemical, ecological and agricultural remediation [,]. Physical remediation includes guest the soil method, soil exchange method, deep plowing method, thermal desorption and vitrification, etc. Chemical remediation includes immobilization, stabilization, chemical leaching and electric remediation [,]. Ecological and agricultural remediation technologies include plant extraction, plant fixation, plant volatilization and animal and microbial remediation. Cd is an important toxic metal; an excessive concentration of Cd will inhibit the physiological processes of plants such as photosynthesis and respiration, resulting in a decrease in crop yield, and finally affecting human health through the food chain. The bioavailability of Cd depends on its exchangeable content in the soil. The soil pH value, clay mineral content, cation exchange capacity and organic matter content always affect the bioavailability of cadmium in soil and the absorption of cadmium by plants [,,].
Attapulgite is a kind of inorganic non-metallic natural clay mineral that is readily available in large quantities. It has a unique 2:1 type chain layered structure, and attapulgite clay contains impurities such as montmorillonite, dolomite and carbonate [,]. The ideal attapulgite crystal chemical formula is Mg5Si8O20(OH)2(OH2)4·4H2O, which is mainly composed of SiO2 and MgO and contains a small amount of Ca, K, Fe, Mn, Na, Al and other metal elements [,,]. The color of attapulgite crystal is mostly white, grey, gray-blue and gray-green. Attapulgite is rod-like or fibrous with a fine and smooth texture, a certain touch of grease, a light texture, low strength and fragility, cracks in the shell shape or sawtooth shape and strong hygroscopic ability [,,,]. The viscosity and plasticity of attapulgite is greatly increased when it is added into water, but the volume changes little after drying, accompanied by a few cracks on the surface. Attapulgite has a large specific surface area and high negative surface charge, so it has good adsorption and ion exchange ability [,,,]. Humic acid is a kind of macromolecular organic polyacid that is black or yellow in appearance and rich in a variety of chemical functional groups. Humic acid is generally formed by animals, plants and microbial remains through microbial degradation and some physical and chemical processes, with a polymer structure and colloidal characteristics of organic matter. The structure of humic acid is very complex, containing rich active functional groups, including an aromatic ring and lipid ring; the ring is also connected with a carboxyl group, hydroxyl group, carbonyl group, quinone group, methoxy group and other active functional groups. Humic acid can be used as an amendment for polluted soil because of its rich active groups. Humic acid can also be used as a soil amendment to promote crop growth and enhance plant resistance through adsorption, ion exchange, acid–base neutralization and complexation of heavy metals [,,,,].
The special functional group on humic acid, after complexing with attapulgite clay, will be adsorbed on attapulgite, increasing its specific surface area increase and making it easier to adsorb heavy metal Cd ions in the soil. Single materials have usually been applied to the remediation of heavy metal pollution. However, there are relatively few studies on the remediation of heavy metal pollution by the combination of multiple materials. In one study, composites prepared with humic acid and tourmaline increased the pH and reduced the bioavailability of vanadium in soil, improving the retention of the available nutrients under the addition of 1% and 5% []. Many research studies claim that attapulgite may be carcinogenic because of its fibrous resemblance to asbestos. Our stabilization agents operate in the soil and have difficulty entering the body through the air, which leads to the production of asbestosis. Secondly, attapulgite contains a variety of mineral elements, and the fiber state similar to asbestos only accounts for a small part, so it does not have carcinogenic hazards. The attapulgite, attapulgite/carbon, and phosphate mineralization bacteria products have been found to stabilize Cd and Pb in soil and decrease the uptake of heavy metals by vegetables under greenhouse and field conditions []. Ren et al. [] used attapulgite and nanoscale zero-valent iron to stabilize Cd-polluted soils. They reported that CaCl2-Cd decreased from 2.24 to 0.95 mg/kg and the residue Cd was significantly increased when attapulgite and nanoscale zero-valent iron were used in a ratio of 2:1, which is similar to this paper.
In this study, humic acid and attapulgite were selected as improved materials. The humic acid and attapulgite were mixed according to different mass ratios, and water was added to stir well and evenly. Then, five different modified attapulgites were prepared by mixing, drying and grinding. A pot experiment was conducted to explore the effects of attapulgite/humic acid composites on plant growth characteristics, plant enrichment and transport of Cd in stabilization soil. Another correlation analysis was conducted to verify the stabilization effect and remediation potential of the composites prepared by humic acid and attapulgite.
2. Materials and Methods
2.1. Cd-Contaminated Soil and Preparation of Stabilizing Material
The soil was taken from the garden soil of Lanzhou Jiaotong University, Lanzhou City, Gansu Province, and the stone and plant residues were separated from the soil. The soil sample of evenly mixed soil was transferred to the laboratory. CdCl2∙2.5H2O was added into the sampling soil to reach 15.5 mg/kg. The Cd-contaminated soil sample was placed at room temperature for one month and retained 70.0% moisture content. The soil was used for heavy metal stabilization experiments and for measuring and analyzing the basic physical and chemical properties.
Attapulgite was provided by Lanzhou Hanxing Environmental Protection Technology (Lanzhou, China), which was collected from Banqiao Town, Linze County, Gansu Province. Its chemical composition consists of 48.38% SiO2, 11.24% Al2O3, 4.78% Fe2O3, 7% MgO and 7.41% CaO. Its mineral composition includes 54.9% attapulgite, 13.1% quartz, 12.7% feldspar, 3.8% dolomite, 2.8% sepiolite, 2.7% chlorite, 2.5% montmorillonite, 2.0% mica and 0.4% gypsum. The attapulgite was crushed, ground and screened through 100 mesh sieves. It was then immersed in 4 mol/L hydrochloric acid for 24 h, rinsed with deionized water until the pH was neutral, put in a drying oven at 105 °C to dry completely and ground to 100 mesh. The humic acid and treated attapulgite were mixed according to the mass ratio of 1:3, 1:4, 1:5, 1:6 and 1:7. Water was added, and the mixture was stirred well, dried and ground to 100 mesh to obtain kinds of composites, which were denoted as HAA13, HAA14, HAA15, HAA16 and HAA17. Contaminated soil without stabilizing agents was used as a control, denoted as CK. Humic acid was purchased from Sour leaf Biotechnology Gansu China Co., Ltd. (Lanzhou, China).
2.2. Incubation and Pot Experiments
First, 1200 g soil samples were put into a plastic bucket and then stabilization materials were added to the Cd-contaminated soil at a 4% ratio. The bucket was placed at room temperature for 30 days and retained 70.0% moisture content. A pot experiment was conducted by planting corn in the control soil and stabilized soil, maintaining 75% moisture content. The corn seedlings were harvested at 20 cm long. The length and Cd content of the roots and stems were determined.
2.3. Analytic Methods
The microscopic morphology of the attapulgite/humic acid composites was characterized by a Gemini 500 high-resolution field emission scanning electron microscope (ZEISS GeminiSEM 500, Carl Zeiss, Oberkochen, Germany). The crystal structure of the attapulgite/humic acid composites was analyzed by an X-ray diffractometer (MiniFlex600, Nikaku, Nara, Japan), and Fourier transform infrared spectroscopy (Bruker, VERTEX 70, Mannheim, Germany) was used to analyze chemical structures and functional groups.
First, a 1 g soil sample was added into 25 mL of 0.01M CaCl2 and shaken at a rate of 180 rpm/min for 2 h at 25 °C in an oscillator. Then, the extracts were separated for 10 min in a 4000 r/min high-speed centrifuge and filtered by a filter membrane with an aperture of 0.45 μm. CaCl2-Cd was determined by a flame atomic absorption spectrophotometer. The diethylenetriaminepentaacetic acid (DTPA) content in the soil was determined by using 20 mL DTPA solution (0.005 M DTPA + 0.01 M CaCl2 + 0.1 M triethanolamine, pH = 7.30) to extract 1g of the soil samples. The concentration of Cd in the filtrate was determined by the toxicity characteristic leaching procedure (TCLP) recommended by the U.S. Environmental Protection Agency.
The acid-soluble, reducible, oxidizable and residual state of Cd in the soil were determined by the European Community Bureau of Reference (BCR) continuous extraction program []. In this method, the heavy metals were divided into exchangeable speciation (EXC), reducible speciation (RED), oxidizable speciation (OX) and residual speciation (RES), and the content of each speciation was determined by a flame atomic absorption spectrophotometer.
The ecological risk index (ERI) of Cd was calculated by Formula (1), where Cb is the bioavailability of Cd in soil. ERI has been commonly used to assess the ecological risks of heavy metals in soil, sludge, bio-char and other materials. It has been widely used to assess the toxicity of heavy metals in environmental science. ERI assesses the availability of heavy metals by applying the percentage of Cd in the bio-availability content. The five classifications of ERI are as follows: no risk, ERI less than 1%; low risk, ERI in the range of 1–10%; moderate risk, ERI in the range of 10% to 30%; high risk, ERI in the range of 30 to 50% and very high risk, ERI higher than 50%.
The bioconcentration factor (BCF) and the transfer factor (TF) were used to evaluate the transfer characteristics of heavy metal Cd from soil to plant. As shown in Formulas (2) and (3) below, BCF is defined as the ratio of the metal concentration in the roots and branches to the soil, and TF is defined as the ratio of the heavy metal concentration in above-ground parts to the heavy metal concentration in underground parts. The transfer coefficient is calculated by Equation (4), where Croot is the concentration of Cd in the root; Cstem is the concentration of Cd in the bud and Csoil is the extractable or usable concentration in the soil.
The environmental risk factor (ERFm) of a heavy metal (m) can be calculated using Equation (5) by comparing the EXC with the sum of EXC, RED, oxidizable speciation (OX) and residual speciation (RES) derived from BCR sequential extraction. Five classification of ERFm were defined as follow: no risk, ERFm lower than 1%; low risk, ERFm in the range of 1~10%; medium risk, ERFm in the range of 10~30%; high risk, ERFm in the range of 30~50% and very high risk, ERFm higher than 50%.
The potential risk index (PRIm) of a heavy metal (m) can be calculated using Equation (6) by comparing the potential mobile speciation (EXC + RED + OX) and stable speciation (RES) of the heavy metal, respectively. PRIm is the potential risk index for an individual heavy metal; Tm is the toxic coefficient of the individual heavy metal, where the values of Tm for Zn, Cr, Cu, Ni, Pb and Cd are in the order of 1, 2, 5, 6, 5 and 30; PRIm is the potential risk index for an individual metal and PRI is the potential risk index of the overall contamination. The values of PRIm can be used to the risk evaluation of heavy metals in soil, sludge, biochar and other materials.
2.4. Data Analysis
The data obtained in this experiment were expressed as the average of three replicates. Statistic 7.0 was used for data collection and processing. Origin 2021 was used for graphical analysis. Analysis of variance (ANOVA) was applied to all data to determine if there were significant differences between treatments and samples. Multiple comparative analysis based on minimum significant difference (LSD) was used to separate statistically significant means with a mean variance of p < 0.05. The Pearson correlation coefficient was calculated between the two indicators.
3. Results
3.1. Characterization of Attapulgite/Humic Acid Materials
The surface structure of attapulgite/humic acid materials was relatively loose, showing a porous and loose structure, with obvious changes in microscopic morphology. With the increase in the proportion of humic acid composite material, attapulgite clavate crystal elements became significantly shorter and coarser, and more voids were formed on the surface, showing an irregular and loose structure, which increased the physical adsorption function. Many of the particles appeared on the surface of the attapulgite after complexing with humic acid and attapulgite. This is because the special functional groups, such as carbonyl and methyl groups, of the complexation and coprecipitation, thus forming the particles on the surface of the attapulgite. Attapulgite was mainly composed of silica (Q), albite (A), dolomite (D), attapulgite (P) and other minerals. The 2θ values of 20.92°, 26.66°, 39.48°, 50.14°, 60.04° and 68.16° were silica and 8.60°, 19.94° and 33.20° were attapulgite (P), and the characteristic peaks of dolomite (D) appeared at 31.3° and 41.20°. The main component was CaMg (CO3)2. The characteristic diffraction peaks of attapulgite did not change; the crystal structure of attapulgite was not damaged by humic acid (Figure 1).


Figure 1.
Scanning electron micrographs (SEMs) of attapulgite/humic acid composites.
A Fourier infrared spectrometer was used to measure the characteristic absorption spectrum, analyze the stabilization material of attapulgite/humic acid composites and determine the functional groups that may appear in the stabilization material, allowing for an analysis of the active groups of the stabilization material. There was a wide absorption band in the high-frequency range (3700~2800 cm−1), which split into several small peaks with different frequencies, such as 3618 cm−1, 3554 cm−1, 3412 cm−1, 2988 cm−1 and 2833 cm−1. The peaks near 3618 cm−1 and 3554 cm−1 were the absorption peaks of Al-OH and Fe-OH, respectively. The symmetric stretching vibration peak of Al-Mg-OH existed near 3412 cm−1, and the peak near 2988 cm−1 was the C-H stretching vibration peak. The absorption peak near 2833 cm−1 corresponded to the symmetric stretching vibration peak of CH2/CH3. In the intermediate frequency range (1700~1200 cm−1), the absorption peak near 1610 cm−1 was the bending vibration peak of H2O, the absorption peak near 1494 cm−1 and 1380 cm−1 corresponded to the bending vibration absorption peak of C-H2 and the stretching vibration peak near 1380 cm−1 was aliphatic C-H. In the middle- and low-frequency ranges (1100~700 cm−1), the absorption peak near 1034 cm−1 was the Si-O stretching vibration peak in Si-O-Si, and the one near 792 cm−1 was the Si-O stretching vibration peak. The Al-O-Si asymmetric bending vibration peak appeared near 519 cm−1, and the Si-O stretching vibration peak appeared near 477 cm−1. Some new active functional groups were generated by attapulgite/humic acid materials, which may enhance the heavy metal adsorption function of stabilization materials (Figure 2).
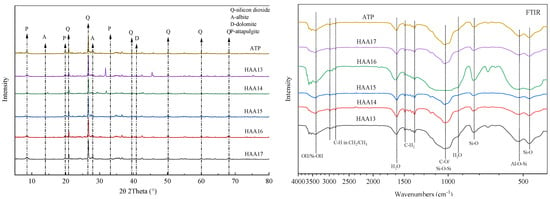
Figure 2.
X-ray diffraction patterns (XRD) and infrared spectrum (FTIR) of attapulgite/humic acid composites.
3.2. Bioavailability of Cd
The ecological risk of heavy metals in soil is not merely influenced by the total amount but is also related to the bioavailable heavy metals that can be absorbed by plants. There was no significant difference among the extracted Cd content of CaCl2 under the HAA14, HAA15 and HAA16 treatments (Table 1), but all of them were significantly lower than CK and the other three treatments, There was no significant difference between the HAA13 and HAA17 treatments, but both of them were significantly lower than attapulgite and CK, and attapulgite was significantly lower than CK. The extracted Cd content of DTPA under the HAA15 treatment was significantly lower than that of CK and the other five treatments. There was no significant difference between the HAA16 and HAA17 treatments, but both of them were significantly lower than the CK, HAA13 and attapulgite treatments and significantly higher than the HAA14 treatment. There was no significant difference between the HAA13 and attapulgite treatments, but both of them were significantly lower than the CK treatment. The extracted Cd content of TCLP under the HAA15 treatment was significantly lower than that of CK and the other five treatments. There was no significant difference between HAA14 and HAA17, but both of them were significantly lower than that of HAA13 and CK and significantly higher than that of the other treatments. The attapulgite treatment was significantly higher than that of HAA16 and significantly lower than that of HAA13 (Table 1). HAA15 presented the best stabilization effect on Cd in soils and reduced the extracted Cd contents of CaCl2, DTPA and TCLP by 34.04%, 26.63% and 43.66% compared with CK.

Table 1.
Bioavailable Cd content in soil treated with attapulgite/humic acid composites (mg/kg).
Humic acid contains a phenolic hydroxyl group, carboxyl group, methoxy group, quinone group and other functional groups and functions in adsorption, ion exchange, acid-base neutralization, etc. []. It can cause heavy metals in soil to be complexed or adsorbed, thus reducing the biological availability of heavy metals. Xu et al. [] used humic acid and ferrihydrite to stabilize Cd in soil. They found that humic acid with ferrihydrite reduced the activity of heavy metals in aqueous solutions. Their results show that a compound stabilizer was obviously better than a single stabilizer, which is consistent with this study.
3.3. Ecological Risk Assessment
The combination of humic acid with attapulgite of different proportions can reduce the ecological risk of Cd in soil. The ecological risk of Cd in the extracted state of CaCl2, TCLP and DTPA decreased first and then increased, and all reached a minimum when the ratio of humic acid to attapulgite was 1:5. Among them, the ecological risk of CaCl2 extracted Cd under the HAA14, HAA15 and HAA16 treatments had no significant difference, but they were significantly lower than that under the HAA13, HAA17 and attapulgite treatments. There was no significant difference the between HAA13 and HAA17 treatments, but both were significantly lower than the attapulgite and CK treatments (Figure 3).
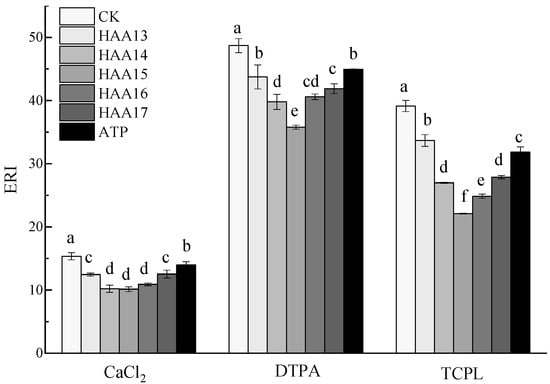
Figure 3.
Ecological risk index (ERI) of Cd in soil stabilized with attapulgite/humic acid composites. Lowercase letters indicate the difference between different stabilization materials, and the same letter indicates no significant difference.
There are many factors that affect the biological availability of heavy metals in soil, such as the total amount of heavy metals, changes in heavy metal forms, soil organic matter content, nutrient status and land use mode. The ecological risk of DTPA-extracted Cd under the HAA15 treatment was significantly lower than CK and the other five treatments. There was no significant difference between the HAA16 and HAA17 treatments, but both of them were significantly lower than the CK, HAA13 and attapulgite treatments and significantly higher than the HAA14 treatment. There was no significant difference between the HAA13 and attapulgite treatments. However, they were significantly lower than the CK treatment. The ecological risk of TCLP-extracted Cd under the HAA15 treatment was significantly lower than that under CK and the other five treatments. There was no significant difference between HAA14 and HAA17, but both of them were significantly lower than those under the HAA13 and attapulgite treatments and significantly higher than those under the HAA15 and HAA16 treatments. The attapulgite treatment was significantly higher than that under the HAA16 treatment and significantly lower than the HAA13 treatment. Compared with CK, the five compound materials and attapulgite treatment significantly reduced the ecological risk of extracted Cd in soil by CaCl2, DTPA and TCLP, among which the HAA15 treatment reduced the risk by 33.94%, 26.55% and 43.51% compared with CK (Figure 3). He et al. [] used attapulgite and oyster shell powder to reduce Cd accumulation in the grains of rice growing in a contaminated acidic paddy field; the utilization of attapulgite and processed oyster shell powder decreased labile fractions but increased stable fractions of Cd in soils through ion exchange, precipitation and complexation.
3.4. BCR Speciation of Cd
The addition of attapulgite significantly reduces EXC speciation of Cd in soil, significantly increases the RES speciation of Cd and promotes the transformation of Cd to RES speciation in soil. There were significant differences between humic acid mixed with attapulgite and RED speciation of heavy metal Cd in stabilized soil. Compared with the attapulgite treatment, the content RES speciation of Cd in the soil of the HAA13, HAA14, HAA15, HAA16 and HAA17 stabilization treatments was significantly higher than that of the control and attapulgite treatments. The content RED speciation of Cd in the soil decreased to varying degrees and showed a trend of decreasing first and then increasing with the increase in the humic acid addition ratio.
The RED-Cd in soils treated by HAA13, HAA14, HAA15, HAA16, HAA17 and attapulgite decreased by 10.27%, 9.13%, 10.82%, 9.15%, 8.19% and 17.69% compared with the control, respectively. The OX of Cd in soil treated with HAA15 was significantly lower than that treated with CK and the other five materials. The RES of Cd treated with all five materials was significantly higher than that of CK, and the RES of Cd treated with HAA15 was significantly higher than that of other treatments. When different proportions of humic acid mixed with attapulgite were added to the Cd-contaminated soil, the RES content of heavy metal Cd in the soil increased to different degrees, and its percentage increased first and then decreased with the increase in the humic acid proportion in the stabilized materials (Figure 4).
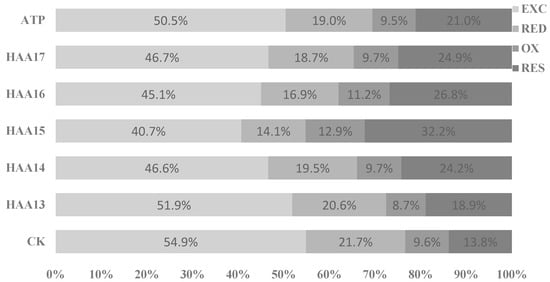
Figure 4.
Chemical speciation distribution of Cd in soils stabilized with attapulgite/humic acid composites.
3.5. Environmental Risk Assessment
Attapulgite/humic acid materials significantly reduced the environmental risk factor (ERF) and potential risk index (PRI) of Cd in the soil. The ERF values of Cd in the HAA14-, HAA15- and HAA16-stabilized soils were significantly lower than those in the CK treatment. The lowest ERF value of Cd in the HAA15-stabilized soil was 10.14%. The PRI values of Cd in soil treated with humic acid mixed with attapulgite were significantly lower than those treated with CK. There was no significant difference between the HAA15 and HAA16 treatments, which were significantly lower than that of the HAA13, HAA14, HAA16 and HAA17 treatments. The PRI values of Cd in the soil of the HAA13, HAA14, HAA15, HAA16, HAA17 and ATP stabilization treatments were 2.15, 1.73, 1.49, 1.62, 1.81 and 1.87, respectively, which were significantly lower than those of the CK treatment. Among them, the PRI value of Cd in the HAA15-stabilized soil decreased to 1.49 (Table 2).

Table 2.
ERF and PRI of Cd in soils stabilized with attapulgite/humic acid composites.
3.6. Ecotoxicity Assessment
Applying different proportions of attapulgite/humic acid composites significantly changed the growth status of maize plants, and there were significant differences between stem length (F = 14.083, p < 0.001) and root length (F = 14.817, p < 0.001) under different treatments. The HAA15 treatment was significantly higher than the HAA13, HAA16 and HAA17 treatments. There was no significant difference between the HAA14 and HAA15 treatments, but both treatments were significantly higher than the HAA16 treatment, and there was no significant difference among the HAA13, HAA14 and HAA17 treatments. Compared with the CK treatment, the six stabilized agent treatments were significantly higher than the CK treatment. The HAA13, HAA16 and HAA17 treatments and the attapulgite treatment had no significant difference. The HAA14 and HAA15 treatments were significantly higher than the attapulgite treatment. Compared with CK, the lengths of the stem and root parts of corn in the other treatment groups were significantly increased. With the gradual increase in the proportion of attapulgite in stabilization materials, the length of the stems and roots showed a trend of first increasing and then decreasing. The root length of HAA13, HAA14, HAA15, HAA16, HAA17 and attapulgite increased by 9.26%, 20.8%, 27.01%, 13.10%, 13.91% and 9.84%, respectively. The stem length increased by 27.24%, 29.82%, 48.24%, 26.47%, 9.82% and 20.59%, in turn, among which the root length and stem length after the HAA15 treatment were the largest at 25.20 cm and 37.43 cm, respectively (Figure 5).
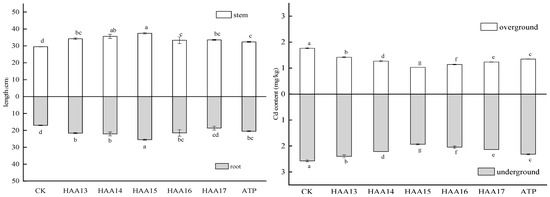
Figure 5.
The length of seeding root and stem, Cd content in the above-ground and underground parts of maize seedlings planted in treated soil. Lowercase letters indicate the difference between different stabilization materials, and the same letter indicates no significant difference.
The application of different treatments with stabilized agents significantly altered the ecotoxicity of maize plants, leading to significant differences in Cd enrichment in both above-ground (F = 87.651, p < 0.001) and underground (F = 561.169, p < 0.001) parts of the maize seedlings under the various treatments. The reduction percentages for the HAA13, HAA14, HAA15, HAA16, HAA17, and attapulgite treatments were 19.48%, 28.07%, 41.53%, 35.43%, 30.03%, and 23.51%, respectively. Among these reductions, the most notable was observed in the HAA15 treatment, while the reduction in the HAA13 treatment was significantly higher than that in the attapulgite treatment. Moreover, the reductions for HAA14, HAA15, HAA16 and HAA17 were all significantly lower than those treated with attapulgite. Compared with the CK treatment, the addition of different proportions of stabilized agents resulted in a significant decrease in Cd enrichment in the underground parts of the maize seedlings, specifically, the reduction percentages for the HAA13, HAA14, HAA15, HAA16, HAA17 and attapulgite treatments were 6.79%, 14.02%, 24.89%, 20.64%, 17.01% and 9.90%, respectively. Among them, the HAA15 treatment had the most obvious reduction, the HAA13 treatment was significantly higher than the attapulgite treatment and the HAA14, HAA15, HAA16 and HAA17 treatments were significantly lower than the attapulgite treatment (Figure 5).
There were significant differences in BCF values between the above-ground and underground parts of the maize seedlings under the different treatments (p < 0.001). The addition of different proportions of stabilized agents significantly reduced the BCF value of the above-ground parts of the maize seedlings. Compared with CK, HAA13, HAA14, HAA15, HAA16, HAA17 and attapulgite decreased by 0.0113, 0.0233, 0.0413, 0.0343, 0.0282 and 0.0164 units, respectively. The HAA13 treatment was significantly higher than the attapulgite treatment, and the HAA14, HAA15, HAA16 and HAA17 treatments were significantly lower than the attapulgite treatment. The BCF value of the underground part of CK was 0.1137, and the BCF value of HAA13, HAA14, HAA15, HAA16, HAA17 and attapulgite were significantly reduced after applying different proportions of stabilized agents. Compared with the attapulgite treatment, the HAA13 treatment was significantly higher, and the HAA14, HAA15, HAA16 and HAA17 treatments were significantly lower. The BCF values of both above- and underground parts of the plants reached minimum values when the ratio of humic acid to attapulgite was 1:5, which were 0.1247 and 0.0665, respectively (Table 3).

Table 3.
The bioconcentration factor (BCF) and transfer factor (TF) of Cd for corn planted in soils stabilized with attapulgite complexed with humic acid.
TF of maize to Cd was significantly decreased, and there was a significant difference among the TF values of the maize seedlings under the different treatments (p < 0.001). There was no significant difference between the HAA15 and HAA16 treatments, but they were significantly lower than the HAA13, HAA14 and HAA17 treatments. There was no significant difference among the HAA14, HAA16 and HAA17 treatments, but they were significantly lower than the HAA13 treatment. The transfer coefficients of the six stabilized agents were significantly lower than the CK treatment. The HAA13 treatment was significantly higher than the attapulgite treatment, and the HAA14, HAA15, HAA16 and HAA17 treatments were significantly lower than the attapulgite treatment. Compared with attapulgite, attapulgite/humic acid materials reduced the TF value more. In general, the enrichment coefficient and transfer coefficient of the HAA15 group were lower than those of the other treatments, which may be because the addition of stabilized materials reduced the activity of Cd2+ in the soil and prevented the absorption of Cd by the roots of maize seedlings (Table 3).
As an alkaline inorganic modifier, attapulgite can be combined with humic acid for biomass modification to produce nano-composite materials. Tao et al. [] used attapulgite loaded with nanoscale zero-valent iron to stabilize heavy metals in mining soil. They suggested that attapulgite loaded with nanoscale zero-valent iron could combine heavy metal-contaminated soil, reduce the accumulation of heavy metals in the plant dramatically and transform the speciation of heavy metals from the labile fraction to the stable fraction. Zeolite, bentonite and attapulgite, which were also silicate minerals, were very similar to attapulgite in reducing the available state content of heavy metals Cd in soil. He et al. [] used zeolite-supported nanoscale zero-valent iron as a stabilizing agent for As, Cd and Pb co-contaminated soil. They reported that zeolite-supported nanoscale zero-valent iron increased the residual speciation of As, Cd and Pb, and this stabilizing agent showed highly efficient performance in simultaneously reducing As, Cd and Pb availability and mobility in co-contaminated soil. Gao et al. [] found that bentonite reduced mobility and increased the organic matter and residual of Cu and Ni in soils from old mine tailings, indicating that silicate minerals can effectively repair heavy metals in soil.
3.7. Correlation Analysis
The extraction state content of CaCl2 was negatively correlated with root length (p < 0.05) and stem length (p < 0.01) and positively correlated with above-ground and underground enrichment, the enrichment coefficient and the transport coefficient. The content of TCLP was negatively correlated with root length and stem length (p < 0.05), positively correlated with above and below enrichment and enrichment coefficient (p < 0.001) and positively correlated with TF (p < 0.01). The content of extracted DTPA was negatively correlated with root length (p < 0.05) and stem length (p < 0.01) and positively correlated with the above- and underground enrichment coefficient and the transport coefficient (p < 0.01). The results indicated that the bioavailable Cd content was an important factor affecting plant growth. With the decrease in the extracted Cd content, the growth and development of plants would be significantly improved, and the enrichment would also be significantly reduced. The decreased Cd in the stem and root was primarily attributed to increasing RES. Therefore, the Cd in the stem and root was significantly negatively correlated with RES. The increased DTPA, TCLP and CaCl2 directly resulted in a decreased length of the stem. There was a highly negative correlation among DTPA, TCLP, CaCl2 and stem length (Figure 6).
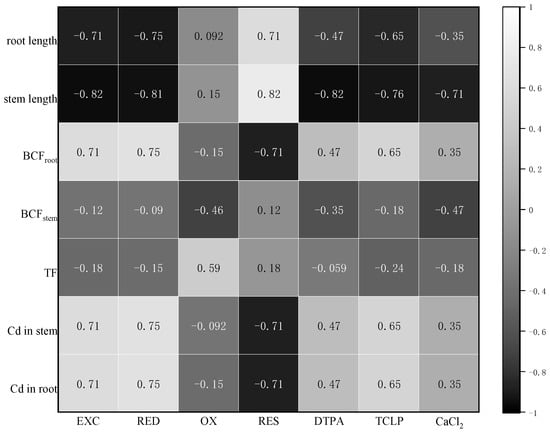
Figure 6.
Correlation among the growth of plants, BCF, TF, the content of Cd and bioavailability and chemical speciation of Cd.
4. Conclusions
The activity of Cd2+ was reduced through physical and chemical reactions after applying different proportions of stabilization materials, which changed the physical and chemical properties of the soil to a certain extent and further affected the form of heavy metals in the soil. The attapulgite/humic acid composite stabilization material contains organic matter, which can significantly increase the biomass of plants, increase the growth indicators of corn seedlings and reduce the enrichment of heavy metal Cd in corn seedlings. The length of the above-ground and underground parts, the fresh weight of the above-ground and underground parts and the dry weight of the above-ground and underground parts of the maize seedlings increased by 27.01%, 32.54%, 20.19%, 31.15%, 30.30% and 63.16%, respectively. The addition of attapulgite/humic acid composites had a significant effect on the decrease in plant BCF above ground, BCF below ground and the TF value of the transport coefficient.
The environmental risk factors of each treatment were significantly lower than the CK treatment, and the environmental risk factors of the HAA15 treatment were the lowest. The effectiveness of the heavy metal immobilization of curing is ranked according to the following order: HAA15 > HAA16 > HAA17 > HAA14 > HAA13. Attapulgite/humic acid composites transformed the Cd in soil from an acid-soluble state to a residue state. The residue proportion of Cd was increased by the material prepared with humic acid and attapulgite in the ratio of 1:5 by 63.13%, and the content of reducible speciation was significantly decreased by 34.10%.
The bioavailability of Cd in soils was significantly negatively correlated with the root length and stem length of corn and significantly positively correlated with the above and below enrichment, the enrichment coefficient and the transport coefficient. The application of attapulgite/humic acid materials reduced the bioavailability of Cd in soils, had a certain promotion effect on the increase in corn plant growth and reduced the accumulation and mobility of Cd. The attapulgite/humic acid composites can be used as stabilizing agents to remediate farmland soil polluted with heavy metals.
Author Contributions
H.R.: visualization, methodology, resources, supervision, and data processing. L.T.: conceptualization, investigation. J.R.: data processing, Formal analysis, visualization, supervision, X.R.: Funding acquisition, methodology. Y.L.: resources, formal analysis. Y.J.: laboratory analyses. M.L.: conceptualization and investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding projects including the Industry Support Plan Project of the Gansu Provincial Department of Education (2021CYZC-31), the Science and Technology Project of Gansu Provincial Science and Technology Department (No. 22CX3GA076), the Special Project of Gansu Science and Technology Commissioner (No. 23CXGA0082) and the Natural Science Foundation of Ningxia Hui Autonomous Region (2023AAC03336).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented within this article.
Conflicts of Interest
Authors Hanru Ren, Jun Ren and Ling Tao were employed by the company Gansu Hanxing Environmental Protection Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wu, Y.; Wang, S.L.; Ning, X.; Yang, M.; Liu, M.B.; Zang, F.; Nan, Z.R. A promising amendment for the immobilization of heavy metal(loid)s in agricultural soil, northwest China. J. Soils Sediments 2021, 21, 2273–2286. [Google Scholar] [CrossRef]
- Yang, J.; Gao, X.H.; Lia, J.; Zuo, R.; Wang, J.S.; Song, L.T.; Wang, G.Q. The stabilization process in the remediation of vanadium-contaminated soil by attapulgite, zeolite and hydroxyapatite. Ecol. Eng. 2020, 156, 105975. [Google Scholar] [CrossRef]
- Zhang, S.K.; Gong, X.F.; Shen, Z.Y.; Yuan, S.F.; Jiang, L.; Wang, G.H. Study on remediation of Cd-contaminated soil by thermally modified attapulgite combined with ryegrass. Soil Sediment Contam. 2020, 29, 680–701. [Google Scholar] [CrossRef]
- Jiang, K.; Xiang, A.H.; Liu, K.; Peng, Q. Potential of montmorillonite and humus-like substances modified montmorillonite for remediation of Pb and Zn-contaminated soils. Appl. Clay Sci. 2023, 234, 106853. [Google Scholar] [CrossRef]
- Moon, H.D.; An, J.; Park, H.S.; Agamemnon, K. Remediation of heavy metal (Cu, Pb) contaminated fine soil using stabilization with limestone and livestock bone powder. Sustainability 2023, 15, 11244. [Google Scholar] [CrossRef]
- Wei, Y.; Li, R.S.; Lu, N.; Zhang, B.Q. Stabilization of soil co-contaminated with mercury and arsenic by different types of biochar. Sustainability 2022, 14, 13637. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.F.; Yao, D.G.; Wang, W.; Tian, W.; Cui, J.W.; Chen, Y.H.; Cui, J. Effects of organic and inorganic amendments on cadmium fraction in the submersion process of contaminated paddy soil. Environ. Technol. Innov. 2023, 30, 103105. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, C.; Zhang, D.D.; Zhang, F.; Zhou, S.K.; Ma, Y.; Guo, J.D.; Zhang, Y.R.; Xing, B.S. Stabilization of Pb, Cd, and Zn in soil by modified-zeolite: Mechanisms and evaluation of effectiveness. Sci. Total Environ. 2022, 814, 152746. [Google Scholar] [CrossRef]
- Ge, Q.L.; Tian, Q.; Wang, S.F.; Zhu, F. Synergistic effects of phosphoric acid modified hydrochar and coalgangue-based zeolite on bioavailability and accumulation of cadmium and lead in contaminated soil. Chin. J. Chem. Eng. 2022, 46, 150–160. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Cao, Y.Z.; Liang, T.; Wang, P.P.; Luo, H.L.; Yu, J.J.; Zhang, D.D.; Xing, B.S.; Yang, B. Stabilization and remediation of heavy metal-contaminated soil China: Insights from a decade-long national survey. Environ. Sci. Pollut. Res. 2022, 29, 39077–39087. [Google Scholar] [CrossRef]
- Liu, F.Y.; Su, Y.R.; Ma, C.; Xie, P.; Zhao, J.H.; Zhang, H.Z. Remediation of Pb-contaminated soil using a novel magnetic nanomaterial immobilization agent. Bull. Environ. Contam. Toxicol. 2022, 108, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Zou, M.Y.; Zhang, B.W.; Lai, W.B.; Zeng, X.M.; Chen, S.Y.; Wang, M.T.; Yi, X.Y.; Tao, X.Q.; Lu, G.N. Remediation of Cd-, Pb-, Cu-, and Zn-contaminated soil using cow bonemeal and oyster shell meal. Ecotoxicol. Environ. Saf. 2022, 229, 113073. [Google Scholar] [CrossRef] [PubMed]
- Lashen, Z.M.; Shams, M.S.; El-Sheshtawy, H.S.; Slaný, M.; Antoniadis, V.; Yang, X.; Sharma, G.; Rinklebe, J.; Shaheen, S.M.; Elmahdy, S.M. Remediation of Cd and Cu contaminated water and soil using novel nanomaterials derived from sugar beet processing- and clay brick factory-solid wastes. J. Hazard. Mater. 2022, 428, 128205. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.Y.; Huang, W.F.; Wang, Y.; Xu, T.; Zhao, L.W.; Zhang, D.Y.; Luo, Y.M.; Pan, X.L. One stone two birds: Bone char as a cost-effective material for stabilizing multiple heavy metals in soil and promoting crop growth. Sci. Total Environ. 2022, 840, 156163. [Google Scholar] [CrossRef]
- Wang, W.H.; Lu, T.; Liu, L.H.; Yang, X.; Sun, X.C.; Qiu, G.H.; Hua, D.L.; Zhou, D.M. Zeolite-supported manganese oxides decrease the Cd uptake of wheat plants in Cd-contaminated weakly alkaline arable soils. J. Hazard. Mater. 2021, 419, 126464. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W.W.; Miao, L.J.; Ji, T.W.; Wang, Y.F.; Zhang, H.J.; Ding, Y.; Zhu, W.Q. The effects of vermicompost and shell powder addition on Cd bioavailability, enzyme activity and bacterial community in Cd-contaminated soil: A field study. Ecotoxicol. Environ. Saf. 2021, 215, 112163. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Ren, J.; Tao, L. Stabilization of heavy metals in soil using nanoscale zerovalent iron coated with palygorskite. Chem. Ecol. 2021, 37, 234–251. [Google Scholar] [CrossRef]
- Qiu, K.Y.; Zhao, L.L.; An, Y.S.; Li, X.H.; Zhang, Z.J. Stable and efficient immobilization of lead and cadmium in contaminated soil by mercapto iron functionalized nanosilica. Chem. Eng. J. 2021, 426, 128483. [Google Scholar] [CrossRef]
- Qin, X.; Liu, Y.T.; Wang, L.; Li, B.Y.; Wang, H.Y.; Xu, Y.M. Remediation of heavy metal–polluted alkaline vegetable soil using mercapto-grafted palygorskite: Effects of field-scale application and soil environmental quality. Environ. Sci. Pollut. Res. 2021, 28, 60526–60536. [Google Scholar] [CrossRef]
- Yang, D.Z.; Chu, Z.T.; Zheng, R.J.; Wei, W.F.; Feng, X.Z.; Zhang, J.; Li, C.Y.; Zhang, Z.T.; Chen, H. Remediation of Cu-polluted soil with analcime synthesized from engineering abandoned soils through green chemistry approaches. J. Hazard. Mater. 2021, 406, 124673. [Google Scholar] [CrossRef]
- Zhao, H.H.; Huang, X.Y.; Liu, F.H.; Hu, X.F.; Zhao, X.; Wang, L.; Gao, P.C.; Li, X.Y.; Ji, P.H. Potential of using a new aluminosilicate amendment for the remediation of paddy soil co-contaminated with Cd and Pb. Environ. Pollut. 2021, 269, 116198. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Ren, H.R.; Tong, Y.L.; Ren, J.; Tao, L. Palygorskite loaded with nanoscale zero-valent iron as an effective stabilizer for remediation of soil contaminated by Cd and Pb. Environ. Stud. 2021, 30, 5629–5641. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.G.; Sun, Y.B.; Wang, L.; Liang, X.F.; Xu, Y.M. In-situ stabilization of Cd by sepiolite co–applied with organic amendments in contaminated soils. Ecotoxicol. Environ. Saf. 2021, 208, 111600. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Xu, Y.M. Influence of clay application and water management on ability of rice to resist cadmium stress. Environ. Eng. Sci. 2021, 38, 695–702. [Google Scholar] [CrossRef]
- Rizwan, M.S.; Imtiaz, M.; Zhu, J.; Yousaf, B.; Hussain, M.; Ali, L.; Ditta, A.; Ihsan, M.Z.; Huang, G.Y.; Ashraf, M.; et al. Immobilization of Pb and Cu by organic and inorganic amendments in contaminated soil. Geoderma 2021, 385, 114803. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, J.J.; Liu, W.; Yan, Y.B.; Wang, Y.H. Hydroxyapatite as a passivator for safe wheat production and its impacts on soil microbial communities in a Cd-contaminated alkaline soil. J. Hazard. Mater. 2021, 404, 124005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Xu, Y.M.; Liang, X.F.; Sun, Y.B.; Huang, Q.Q.; Qin, X.; Zhao, L.J. Effects of mercapto-palygorskite on Cd distribution in soil aggregates and Cd accumulation by wheat in Cd contaminated alkaline soil. Chemosphere 2021, 271, 129590. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.B.; Lin, H.; Zhao, Y.M.; Menzembere, E.R.G.Y. Remediation of vanadium-contaminated soils by the combination of natural clay mineral and humic acid. J. Clean. Prod. 2021, 279, 123874. [Google Scholar] [CrossRef]
- Wang, H.F.; Hu, W.Y.; Wu, Q.M.; Huang, B.; Zong, L.; Wang, A.Q.; Siebecker, M.G. Effectiveness evaluation of environmentally friendly stabilizers on remediation of Cd and Pb in agricultural soils by multi-scale experiments. J. Clean. Prod. 2021, 311, 127673. [Google Scholar] [CrossRef]
- Ren, J.; Mi, X.; Tao, L. Stabilization of cadmium in polluted soil using palygorskite-coated nanoscale zero-valent iron. Soils Sediments 2021, 21, 1001–1009. [Google Scholar] [CrossRef]
- Wen, J.; Yi, Y.J.; Zeng, G.G. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction. J. Environ. Manag. 2016, 178, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lia, Y.; Wang, X.J.; Lia, J.; Wang, Y.; Song, J.K.; Xia, S.Q.; Jing, H.P.; Zhao, J.F. Effects of struvite-humic acid loaded biochar/bentonite composite amendment on Zn (II) and antibiotic resistance genes in manure-soil. Chem. Eng. J. 2019, 375, 122013. [Google Scholar] [CrossRef]
- Xu, M.M.; Zhao, Z.J.; Shi, M.; Yao, L.W.; Fan, T.F.; Wang, Z.M. Effect of humic acid on the stabilization of cadmium in soil by coprecipitating with ferrihydrite. Environ. Sci. Pollut. Res. 2019, 26, 27330–27337. [Google Scholar] [CrossRef]
- He, L.Z.; Meng, J.; Wang, Y.; Tang, X.J.; Liu, X.M.; Tang, C.X.; Ma, L.Q.; Xu, J.M. Attapulgite and processed oyster shell powder effectively reduce cadmium accumulation in grains of rice growing in a contaminated acidic paddy field. Ecotoxicol. Environ. Saf. 2021, 209, 111840. [Google Scholar] [CrossRef]
- Tao, L.; Mi, X.; Ren, H.R.; Tong, Y.L.; Wang, Y.R.; Ren, J. Stabilization of heavy metals in mining soil using palygorskite loaded by nanoscale zero-valent iron. Int. J. Environ. Sci. Technol. 2022, 19, 6789–6802. [Google Scholar] [CrossRef]
- He, Y.H.; Fang, T.T.; Wang, J.; Liu, X.Y.; Yan, Z.G.; Lin, H.; Li, F.S.; Guo, G.L. Insight into the stabilization mechanism and long-term effect on As, Cd, and Pb in soil using zeolite-supported nanoscale zero-valent iron. J. Clean. Prod. 2022, 355, 131634. [Google Scholar] [CrossRef]
- Gao, Y.P.; Li, X.J. Effects of bentonite addition on the speciation and mobility of Cu and Ni in soils from old mine tailings. Sustainability 2022, 14, 10878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).