Determination of Selected Organic Contaminants in the Port of Gdynia Sediments: Towards Cleaner Baltic Ports
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Surface Sediments in the Port of Gdynia
2.2. Laboratory Analysis of Surface Sediments from the Port of Gdynia
2.2.1. Analysis of MPs
2.2.2. Analysis of Dioxins and Furans
3. Results and Discussion
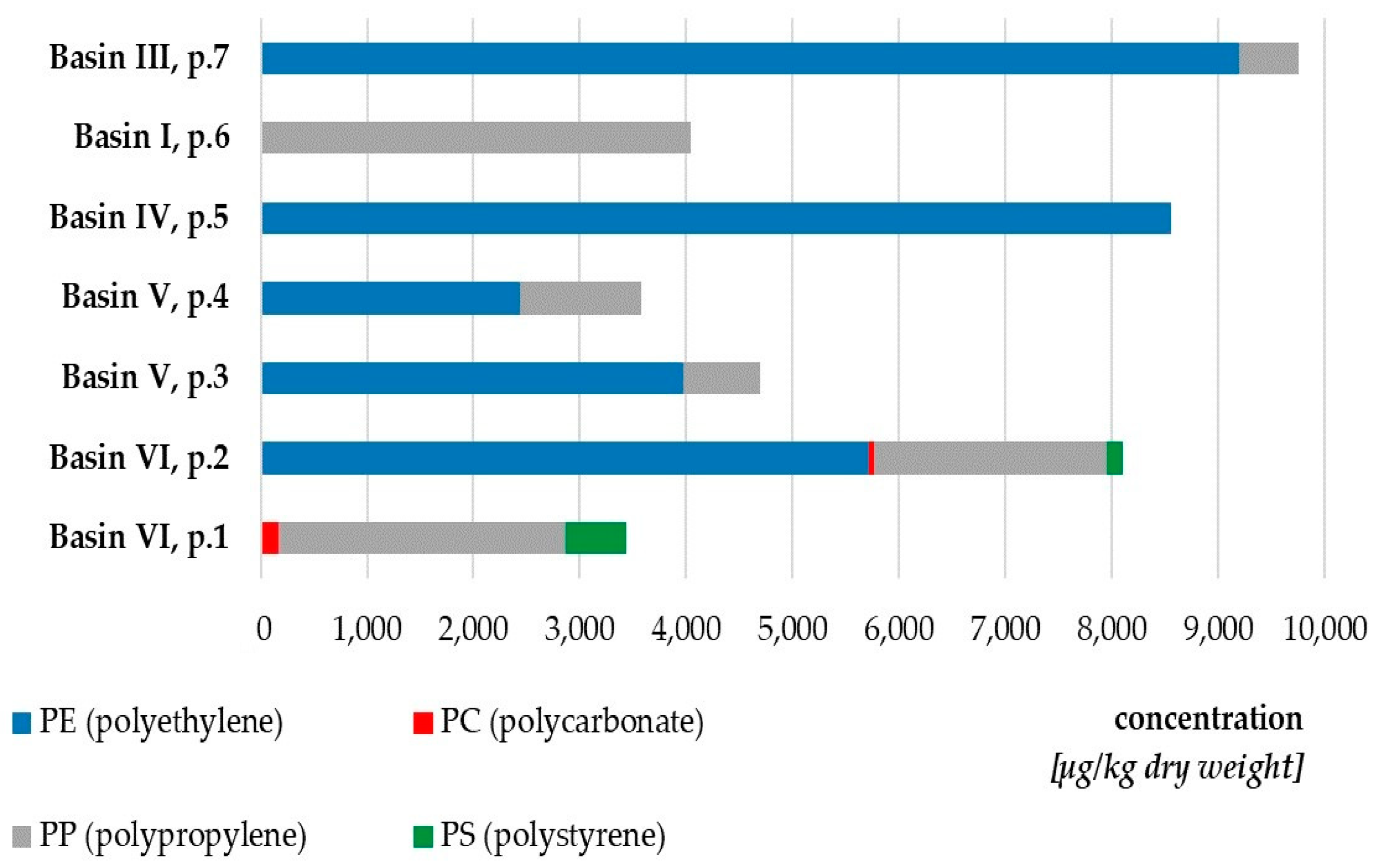
3.1. Microplastics
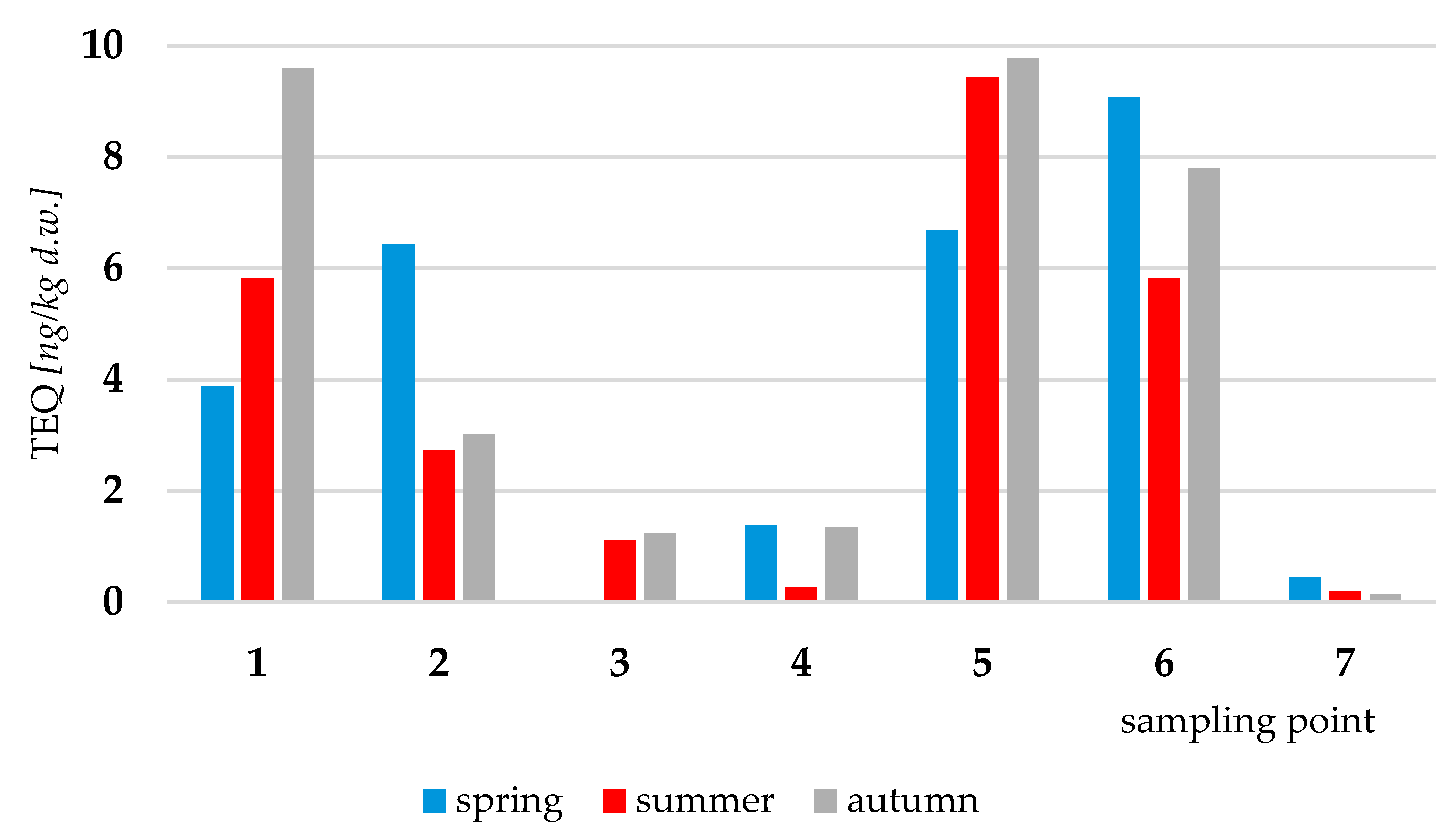
3.2. Dioxins
4. Conclusions and Suggestions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woo, J.K.; Moon, D.S.H.; Lam, J.S.L. The impact of environmental policy on ports and the associated economic opportunities. Transp. Res. Part A Policy Pract. 2018, 110, 234–242. [Google Scholar] [CrossRef]
- Homsombat, W.; Yip, T.L.; Yang, H.; Fu, X. Regional cooperation and management of port pollution. Marit. Pol. Manag. 2013, 40, 451–466. [Google Scholar] [CrossRef]
- Puig, M.; Azarkamand, S.; Wooldridge, C.; Selén, V.; Darbra, R.M. Insights on the environmental management system of the European port sector. Sci. Total Environ. 2022, 806, 150550. [Google Scholar] [CrossRef] [PubMed]
- Notteboom, T.; van der Lugt, L.; van Saase, N.; Sel, S.; Neyens, K. The role of seaports in green supply chain management: Initiatives, attitudes, and perspectives in Rotterdam, Antwerp, North Sea Port, and Zeebrugge. Sustainability 2020, 12, 1688. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Huang, R. Port-based supply chain decisions considering governmental pollution tax. Oper. Res. 2022, 22, 4769–4800. [Google Scholar] [CrossRef]
- Chiu, R.H.; Lin, L.H.; Ting, S.C. Evaluation of green port factors and performance: A fuzzy AHP analysis. Math. Probl. Eng. 2014, 2014, 802976. [Google Scholar] [CrossRef]
- Teerawattana, R.; Yang, Y.C. Environmental performance indicators for green port policy evaluation: Case study of Laem Chabang Port. Asian J. Shipp. Logist. 2019, 35, 63–69. [Google Scholar] [CrossRef]
- Sogut, M.Z.; Erdoğan, O. An investigation on a holistic framework of green port transition based on energy and environmental sustainability. Ocean Eng. 2022, 226, 112671. [Google Scholar] [CrossRef]
- Lee, P.T.W.; Chung, Y.S.; Lam, J.S.L. Transportation research trends in environmental issues: A literature review of methodology and key subjects. Int. J. Ship. Transp. Logist. 2016, 8, 612–631. [Google Scholar] [CrossRef]
- Schröder-Hinrichs, J.U.; Hebbar, A.A.; Alamoush, A.S. Maritime Risk Research and Its Uptake in Policymaking. J. Mar. Sci. Eng. 2020, 8, 742. [Google Scholar] [CrossRef]
- Stojanovic, T.A.; Smith, H.D.O.; Wooldridge, C.F. The impact of the Habitats Directive on European port operations and management. GeoJournal 2006, 65, 165–176. [Google Scholar] [CrossRef]
- ISO 14001:2015; Environmental Management System Requirements with Guidance for Use. International Organization for Standardization: Geneva, Switzerland, 2015.
- Alamoush, A.S.; Ballini, F.; Ölçer, A.I. Revisiting port sustainability as a foundation for the implementation of the United Nations Sustainable Development Goals (UN SDGs). J. Shipp. Trd. 2021, 6, 19. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, S.K.; Patil, R.S. Environmental management plan for port and harbour projects. Clean Techn. Environ. Policy 2005, 7, 133–141. [Google Scholar] [CrossRef]
- Herman, S.; Pieters, A.; Glaser, D.; Gregoire, P.; Priez, C.; Desmoulin, D.; Guglielmetti, D. A lustrum of valorisation of contaminated sediments from the Port of Dunkirk (France). In Proceedings of the South Baltic Conference on Dredged Materials in Dike Construction, Rostock, Germany, 10–12 April 2014; pp. 137–144. [Google Scholar]
- Manap, N.; Voulvoulis, N. Environmental management for dredging sediments—The requirement of developing nations. J. Environ. Manag. 2015, 147, 338–348. [Google Scholar] [CrossRef] [PubMed]
- HELCOM. Guidelines for Management of Dredged Material at Sea; HELCOM Reporting Format for Management of Dredged Material at Sea: Helsinki, Finland, 2020; Available online: https://helcom.fi/wp-content/uploads/2016/11/HELCOM-Guidelines-for-Management-of-Dredged-Material-at-Sea.pdf (accessed on 23 January 2024).
- HELCOM. Hazardous Substances in the Baltic Sea—An Integrated Thematic Assessment of Hazardous Substances in the Baltic Sea; Baltic Sea Environment Proceedings No. 120B; Helsinki Commission: Helsinki, Finland, 2020. Available online: https://www.helcom.fi/wp-content/uploads/2019/08/BSEP120B.pdf (accessed on 23 January 2024).
- Staniszewska, M.; Boniecka, H. Managing dredged material in the coastal zone of the Baltic Sea. Environ. Monit. Assess 2017, 189, 46. [Google Scholar] [CrossRef]
- Verta, M.; Salo, S.; Korhonen, M.; Assmuth, T.; Kiviranta, H.; Koistinen, J.; Ruokojärvi, P.; Isosaari, P.; Bergqvist, P.A.; Tysklind, M.; et al. Dioxin concentrations in sediments of the Baltic Sea—A survey of existing data. Chemosphere 2006, 67, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Bakke, T.; Källqvist, T.; Ruus, A.; Gijs, D.B.; Hylland, K. Development of sediment quality criteria in Norway. J. Soils Sediments 2010, 10, 172–178. [Google Scholar] [CrossRef]
- Ruus, A.; Berge, J.A.; Hylland, K.; Bjerkeng, B.; Bakke, T.; Næs, K. Polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in the Grenland Fjords (Norway)—Disposition, levels, and effects. J. Toxicol. Environ. Health Part A 2006, 69, 185–200. [Google Scholar] [CrossRef]
- Różyński, G.; Bielecka, M. Sediment quality in the Polish part of the trans-boundary Vistula Lagoon: Implications for deposition management. Mar. Policy 2022, 146, 105288. [Google Scholar] [CrossRef]
- Staniszewska, M.; Boniecka, H.; Cylkowska, H. Dredging works in the Polish open sea ports as an anthropogenic factor of de-velopment of sea coastal zones. BMI 2016, 31, 173–180. [Google Scholar] [CrossRef]
- Staniszewska, M.; Boniecka, H. The environmental protection aspects of handling dredged material. BMI 2015, 30, 51–58. [Google Scholar] [CrossRef][Green Version]
- Katsiri, A.; Pantazidou, M.; Damikouka, I.; Kontogiorgi, C.; Tringali, A. Disposal options for dredged marine sediments based on physicochemical and toxicological characterization. Glob. Nest J. 2009, 11, 449–456. [Google Scholar] [CrossRef]
- Sollecito, F.; Vitone, C.; Miccoli, D.; Plötze, M.; Puzrin, A.M.; Cotecchia, F. Marine sediments from a contaminated site: Ge-otechnical properties and chemo-mechanical coupling processes. Geosciences 2019, 9, 333. [Google Scholar] [CrossRef]
- Zalewska, T.; Zdanowska, B. Marine environment status assessment based on macro phytobenthic plants as bio-indicators of heavy metals pollution. Mar. Pollut. Bull. 2017, 118, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycka-Krahel, A.; Bogalecka, M. The Baltic Sea under anthropopressure—The sea of paradoxes. Water 2022, 14, 3772. [Google Scholar] [CrossRef]
- Ogura, I.; Masunaga, S.; Nakanishi, J. Congener—specific characterization of PCDDs/PCDFs in atmospheric deposition: Comparison of profiles between deposition, source and environmental sink. Chemosphere 2001, 45, 173–183. [Google Scholar] [CrossRef]
- Nevalainen, L.; Tuomisto, J.; Haapasaari, P.; Lehikoinen, A. Spatial aspects of the dioxin risk formation in the Baltic Sea: A systematic review. Sci. Total Environ. 2021, 753, 142185. [Google Scholar] [CrossRef]
- Zieliński, M.; Kamińska, J.; Czerska, M.; Ligocka, D.; Urbaniak, M. Levels and sources of PCDDs, PCDFs and dl-PCBs in the water ecosystems of central Poland—A mini review. Int. J. Occup. Med. Environ. Health 2014, 27, 902–918. [Google Scholar] [CrossRef]
- McLachlan, M.; Undeman, E. Dioxins and PCBs in the Baltic Sea; Helcom Baltic Sea Environment Proceedings n°171l; Helsinki Commission: Helsinki, Finland, 2020. Available online: https://helcom.fi/wpcontent/uploads/2020/06/Helcom_171_Dioxins_PCBs.pdf (accessed on 26 May 2023).
- Crawford, C.B.; Quinn, B. Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bayo, J.; Rojo, D.; Olmos, S. Abundance, morphology and chemical composition of microplastics in sand and sediments from a protected coastal area: The Mar Menor lagoon (SE Spain). Environ. Pollut. 2019, 252, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Zhang, J.; Li, Q.; Wang, Y.; Wang, M.; Huang, X. Distribution and characteristics of microplastics in urban waters of seven cities in the Tuojiang River basin. China. Environ. Res. 2020, 189, 109893. [Google Scholar] [CrossRef]
- Beer, S.; Garm, A.; Huwer, B.; Dierking, J.; Nielsen, T.G. No increase in marine microplastic concentration over the last three decades—A case study from Baltic Sea. Sci. Total Environ. 2018, 621, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Halbach, M.; Vogel, M.; Tammen, J.K.; Rüdel, H.; Koschorreck, J.; Scholz-Böttcher, B.M. 30 years trends of microplastic pollution: Mass-quantitative analysis of archived mussel samples from the North and Baltic Seas. Sci. Total. Environ. 2022, 826, 154179. [Google Scholar] [CrossRef] [PubMed]
- Dereszewska, A.; Krasowska, K.; Popek, M. Microplastics in harbour seawaters: A case study in the Port of Gdynia, Baltic Sea. Sustainability 2023, 15, 6678. [Google Scholar] [CrossRef]
- Narloch, I.; Gackowska, A.; Wejnerowska, G. Microplastic in the Baltic Sea: A review of distribution processes, sources, analysis methods and regulatory policies. Environ. Pollut. 2022, 315, 120453. [Google Scholar] [CrossRef] [PubMed]
- Schernewski, G.; Radtke, H.; Hauk, R.; Baresel, C.; Olshammar, M.; Oberbeckmann, S. Urban microplastics emissions: Effectiveness of retention measures and consequences for the Baltic Sea. Front. Mar. Sci. 2021, 8, 594415. [Google Scholar] [CrossRef]
- Polprasert, C.; Koottatep, T.; Pussayanavin, T.; Jinda, K.; Kamngam, S. Plastic litters and public health. In Marine Plastics: Abatement Challenges, Implications, Assessments and Circularity (Volume 1); Koottatep, T., Winijkul, E., Xue, W., Panuvatvanich, A., Visvanathan, C., Pussayanavin, T., Limphitakphong, N., Polprasert, C., Eds.; IWA Publishing: UK, London, 2023; Chapter 2; pp. 25–84. [Google Scholar] [CrossRef]
- Schmaltz, E.; Melvin, E.C.; Diana, Z.; Gunady, E.; Rittschof, D.; Somarelli, J.; Virdin, J.; Dunphy-Daly, M.M. Plastic pollution solutions: Emerging technologies to prevent and collect marine plastic pollution. Environ. Int. 2020, 144, 106067. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Blazquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of plastic pollution in the environment: A review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Bošković, N.; Joksimović, D.; Perošević-Bajčeta, A.; Peković, M.; Bajt, O. Distribution and characterization of microplastics in marine sediments from the Montenegrin coast. J. Soils Sediments 2022, 22, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.N.; Hahladakis, J.N.; Najib Daly Yahia, M.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-fragmentation of marine plastic waste and their environmental implications: A critical review. Arab. J. Chem. 2022, 15, 04262. [Google Scholar] [CrossRef]
- Doğruyol, P.; Şener, M.; Balkaya, N. Determination of microplastics and large plastics in the sediments of the Golden Horn Estuary (Halic), Istanbul, Turkey. Desalin. Water Treat. 2019, 172, 344–350. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Peković, M.; Perošević-Bajčeta, A.; Bajt, O. Microplastics in surface sediments along the Montenegrin coast, Adriatic Sea: Types, occurrence, and distribution. J. Mar. Sci. Eng. 2021, 9, 841. [Google Scholar] [CrossRef]
- Kerubo, J.O.; Onyari, J.M.; Muthumbi, A.V.N.; Andersson, D.R.; Kimani, E.N. Microplastic polymers in surface waters and sediments in the creeks along the Kenya coast, Western Indian Ocean (WIO). EJOSDR 2022, 6, em0177. [Google Scholar] [CrossRef] [PubMed]
- Belioka, M.P.; Achilias, D.S. Microplastic pollution and monitoring in seawater and harbor environments: A meta-analysis and review. Sustainability 2023, 15, 9079. [Google Scholar] [CrossRef]
- Bakir, A.; Doran, D.; Silburn, B.; Russell, J.; Archer-Rand, S.; Barry, J.; Maes, T.; Limpenny, C.; Mason, C.; Barber, J.; et al. A spatial and temporal assessment of microplastics in seafloor sediments: A case study for the UK. Front. Mar. Sci. 2023, 9, 1093815. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef] [PubMed]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ravit, B.; Cooper, K.; Buckley, B.; Yang, I.; Deshpande, A. Organic compounds associated with microplastic pollutants in New Jersey, U.S.A. surface waters. AIMS Environ. Sci. 2019, 6, 445–459. [Google Scholar] [CrossRef]
- Porter, A.W.; Wolfson, S.J.; Young, L. Pharmaceutical transforming microbes from wastewater and natural environments can colonize microplastics. AIMS Environ. Sci. 2020, 7, 99–116. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Human exposure to microplastics: A study in Iran. J. Hazard Mater. 2021, 403, 123799. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- MSFD 2008/56/EC. Marine Strategy Framework Directive of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy. Official J. Eur. Union 2008, L 164/19–L 164/40. Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32008L0056 (accessed on 9 February 2024).
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Onrubia, J.A.T.; Djaoudi, K.; Borgogno, F.; Canuto, S.; Angeletti, B.; Besio, G.; Capello, M.; Cutroneo, L.; Stocchino, A.; Mounier, S.; et al. Quantification of microplastics in North-Western Mediterranean harbors: Seasonality and biofilm-related metallic contaminants. J. Mar. Sci. Eng. 2021, 9, 337. [Google Scholar] [CrossRef]
- Agarwala, N.; Saengsupavanich, C. Oceanic environmental impact in seaports. Oceans 2023, 4, 360–380. [Google Scholar] [CrossRef]
- Jahromi, F.A.; Keshavarzi, B.; Moore, F.; Abbasi, S.; Busquets, R.; Hooda, P.S.; Jaafarzadeh, N. Source and risk assessment of heavy metals and microplastics in bivalves and coastal sediments of the Northern Persian Gulf, Hormogzan Province. Environ. Res. 2021, 196, 110963. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Fabi, G.; Grati, F.; Tassetti, A.N.; Polidori, P.; Vega, C.F.; Scarcella, G. Plastic soles: Microplastic litter in the gastrointestinal tract of Solea solea from the Adriatic Sea. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea, Naples, Italy, 26–29 September 2017; Cocca, M., Di Pace, E., Errico, M.E., Gentile, G., Montarsolo, A., Mossotti, R., Eds.; Springer Water (SPWA). Springer: Cham, Switzerland, 2018; pp. 137–149. [Google Scholar] [CrossRef]
- Tien, C.J.; Wang, Z.X.; Chen, C.S. Microplastics in water, sediment and fish from the Fengshan River system: Relationship to aquatic factors and accumulation of polycyclic aromatic hydrocarbons by fish. Environ. Pollut. 2020, 265, 114962. [Google Scholar] [CrossRef]
- Di Cesare, A.; Pjevac, P.; Eckert, E.; Curkov, N.; Miko Šparica, M.; Corno, G.; Orlić, S. The role of metal contamination in shaping microbial communities in heavily polluted marine sediments. Environ. Pollut. 2020, 265, 114823. [Google Scholar] [CrossRef]
- Martellini, T.; Guerranti, C.; Scopetani, C.; Ugolini, A.; Chelazzi, D.; Cincinelli, A. A snapshot of microplastics in the coastal areas of the Mediterranean Sea. Trends Anal. Chem. 2018, 109, 173–179. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in seawater: Sampling strategies, laboratory methodologies, and identification techniques applied to port environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef] [PubMed]
- Tuit, C.B.; Wait, A.D. A review of marine sediment sampling methods. Environ. Forensics 2020, 21, 291–309. [Google Scholar] [CrossRef]
- PN-EN ISO 5667-19:2006; Water Quality—Sampling—Part 19: Guidance on Sampling in Marine Sediments. Polish Committee for Standardization: Geneva, Switzerland, 2006.
- Krasowska, K.; Dereszewska, A.; Popek, M. Preliminary approach to ecological risk assessment of microplastics in selected coastal regions of Baltic Sea. In Proceedings of the Safety and Reliability of Systems and Processes, Summer Safety and Reliability Seminar, Gdynia, Poland, 14–19 July 2022; Kołowrocki, K., Bogalecka, M., Dabrowska, E., Magryta-Mut, B., Eds.; Gdynia Maritime University: Gdynia, Poland, 2022; pp. 133–142. [Google Scholar] [CrossRef]
- Preston-Whyte, F.; Silburn, B.; Meakins, B.; Bakir, A.; Pillay, K.; Worship, M.; Paruk, S.; Mdazuka, Y.; Mooi, G.; Harmer, R.; et al. Meso- and microplastics monitoring in harbour environments: A case study for the Port of Durban, South Africa. Mar. Pollut. Bull. 2021, 163, 111948. [Google Scholar] [CrossRef] [PubMed]
- Lindeque, P.K.; Cole, M.; Coppock, R.L.; Lewis, C.N.; Miller, R.Z.; Watts, A.J.R.; Wilson-McNeal, A.; Wright, S.L.; Galloway, T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020, 265, 114721. [Google Scholar] [CrossRef] [PubMed]
- Aigars, J.; Barone, M.; Suhareva, N.; Putna-Nimane, I.; Dimante-Deimantovica, I. Occurrence and spatial distribution of microplastics in the surface waters of the Baltic Sea and the Gulf of Riga. Mar. Pollut. Bull. 2021, 172, 112860. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kusano, T.; Addai-Arhin, S.; Nugraha, W.C.; Novirsa, R.; Phan Dinh, Q.; Shirosaki, T.; Fujita, E.; Kameda, Y.; Cho, H.S.; et al. Differences in microplastic distributions on the surface freshwater collected using 100- and 355-μm meshes. EMCR 2022, 2, 22–34. [Google Scholar] [CrossRef]
- DIN EN ISO/IEC 17025:2005; General Requirements for the Competence of Testing and Calibration Laboratories. German and English Version EN ISO/IEC 17025:2005; ISO: Geneva, Switzerland, 2005.
- Safe, S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and related compounds: En-vironmental and mechanistic considerations which support the development of toxic equivalent factors (TEFs). Crit. Rev. Toxicol. 1990, 21, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 277/2012 of 28 March 2012 Amending Annexes I and II to Directive 2002/32/EC of the Eu-ropean Parliament and of the Council as Regards Maximum Levels and Action Thresholds for Dioxins and Polychlorinated bi-Phenyls. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:091:0001:0007:EN:PDF (accessed on 10 February 2024).
- Manning, T.; Batley, G.E. A guideline value for dioxin-like compounds in marine sediments. Environ. Toxicol. Chem. 2023, 42, 257–271. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2015. [Google Scholar]
- Alomar, C.; Sureda, A.; Capo, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary. China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Park, J.W.; Eo, S.; Choi, J.; Song, Y.K.; Cho, Y.; Hong, S.H.; Shim, W.J. Ecological risk assessment of microplastics in coastal, shelf, and deep sea waters with a consideration of environmentally relevant size and shape. Environ. Pollut. 2021, 270, 116217. [Google Scholar] [CrossRef] [PubMed]
- Montoya, D. Pollution in Sydney Harbour: Sewage, Toxic Chemicals and Microplastics; Briefing Paper 03/2015; NSW Parliamentary Research Service: Sydney, Australia, 2015. [Google Scholar]
- Su, L.; Sharp, S.M.; Pettigrove, V.J.; Craig, N.J.; Nan, B.; Du, F.; Shi, H. Superimposed microplastic pollution in a coastal metropolis. Water Res. 2020, 168, 115140. [Google Scholar] [CrossRef] [PubMed]
- Noren, F. Small Plastic Particles in Coastal Swedish Waters; KIMO: Billdal, Sweden, 2007; pp. 1–11.
- Ferreira, M.; Thompson, J.; Paris, A.; Rohindra, D.; Rico, C. Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state. Mar. Pollut. Bull. 2020, 153, 110991. [Google Scholar] [CrossRef] [PubMed]
- Yoswaty, D.; Effendi, I.; Mardalisa, M.; Efriyeldi, E.; Makwa, A.M.M.; Dzikri, M.F. The threat of microplastic waste in Dumai waters, Province of Riau, Indonesia. Carpathian J. Earth Environ. Sci. 2021, 16, 383–390. [Google Scholar] [CrossRef]
- Castro, L.J.A.; Monsada, A.M.; Cruz, K.D. Occurrence of microplastics in the sediments of Baseco Port area at Manila Bay, Philippines. IOP Conf. Ser. Earth Environ. Sci. 2022, 958, 012009. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Guidelines for the Monitoring & Assessment of Plastic Litter in the Ocean; Report and Studies No. 99; Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019. [Google Scholar]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Gray, A.D.; Wertz, H.; Leads, R.R.; Weinstein, J.E. Microplastic in two South Carolina Estuaries: Occurrence, distribution, and composition. Mar. Pollut. Bull. 2018, 128, 223–233. [Google Scholar] [CrossRef]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ. 2020, 738, 139866. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jaramillo, M.; Soledad Islas, M.; Gonzalez, M. Spatial distribution patterns and identification of microplastics on intertidal sediments from urban and semi-natural SW Atlantic estuaries. Environ. Pollut. 2021, 273, 116398. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the Changjiang Estuary, China. Environ. Pollut. 2017, 225, 283–290. [Google Scholar] [CrossRef]
- Andrade, J.M.; Ferreiro, B.; Lopez-Mahía, P.; Muniategui-Lorenzo, S. Standardization of the minimum information for publication of infrared-related data when microplastics are characterized. Mar. Pollut. Bull. 2020, 154, 111035. [Google Scholar] [CrossRef] [PubMed]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Abidli, S.; Antunes, J.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; El Menif, N.T. Microplastics in sediments from the littoral zone of the north Tunisian coast (Mediterranean Sea). Estuar. Coast Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Jahan, S.; Strezov, V.; Weldekidan, H.; Kumar, R.; Kan, T.; Sarkodie, S.A.; He, J.; Dastjerdi, B.; Wilson, S.P. Interrelationship of microplastic pollution in sediments and oysters in a seaport environment of the eastern coast of Australia. Sci. Total Environ. 2019, 695, 133924. [Google Scholar] [CrossRef]
- Turra, A.; Manzano, A.B.; Dias, R.J.S.; Mahiques, M.M.; Barbosa, L.; Balthazar-Silva, D.; Moreira, F.T. Three-dimensional distribution of plastic pellets in sandy beaches: Shifting paradigms. Sci. Rep. 2014, 4, 4435. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, L.; Li, R.; Wang, Y.; Guo, J.; Yu, K.; Wang, S. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment. Environ. Sci. Technol. 2020, 54, 2210–2217. [Google Scholar] [CrossRef]
- Cutroneo, L.; Carbone, C.; Consani, S.; Vagge, G.; Canepa, G.; Capello, M. Environmental complexity of a port: Evidence from circulation of the water masses, and composition and contamination of bottom sediments. Mar. Pollut. Bull. 2017, 119, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, E.; de la Cal, A.; Larrazabal, D.; Fabrellas, B.; Rodriguez Fernandez-Alba, A.; Borrull, F.; Marce, R.M.; Barcelo, D. Occurrence of polybrominated diphenylethers, polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls in coastal sediments from Spain. Environ. Pollut. 2005, 136, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Birnbaum, L.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- CCME—Canadian Council of Ministers of the Environment. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Polychlorinated Dioxins and Furans (PCDD/Fs); Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2001. [Google Scholar]
- Rappe, C. Sources of exposure, environmental concentrations and exposure assessment of PCDDs and PCDFs. Chemosphere 1993, 27, 211–225. [Google Scholar] [CrossRef]


| Sampling Point | Location in the Port of Gdynia | Coordinates | Depth of Sampling (m) | Description of Sediment | |
|---|---|---|---|---|---|
| 1 | Basin VI | 54.321591 | 18.313102 | 11.6 | gravelly muddy |
| 2 | Basin VI | 54.320827 | 18.312607 | 11.3 | gravelly muddy |
| 3 | Basin V | 54.320882 | 18.310144 | 15.2 | slightly gravelly |
| 4 | Basin V | 54.320128 | 18.314160 | 12.2 | slightly gravelly muddy |
| 5 | Basin IV | 54.314723 | 18.314536 | 12.9 | slightly gravelly muddy |
| 6 | Basin I | 54.314812 | 18.331066 | 9.6 | slightly gravelly muddy |
| 7 | Basin III | 54.311497 | 18.332384 | 14.5 | slightly gravelly muddy |
| Sampling Point | Location in the Port of Gdynia | Coordinates | Depth of Sampling (m) | Description of Sediment | |
|---|---|---|---|---|---|
| 1 | Basin VI | 54.321559 | 18.313191 | 11.1 | slightly gravelly muddy |
| 2 | Basin VI | 54.320857 | 18.312534 | 10.8 | slightly gravelly muddy |
| 3 | Basin V | 54.320877 | 18.310040 | 14.7 | slightly gravelly muddy |
| 4 | Basin V | 54.320099 | 18.314127 | 11.6 | slightly gravelly muddy |
| 5 | Basin IV | 54.314682 | 18.314412 | 12.3 | gravelly muddy |
| 6 | Basin I | 54.311568 | 18.332266 | 9.0 | slightly gravelly muddy |
| 7 | Basin III | 54.314802 | 18.331187 | 13.7 | slightly gravelly muddy |
| Sampling Point | Location in the Port of Gdynia | Coordinates | Depth of Sampling (m) | Description of Sediment | |
|---|---|---|---|---|---|
| 1 | Basin VI | 54.321563 | 18.313227 | 11.2 | slightly gravelly muddy |
| 2 | Basin VI | 54.320888 | 18.312582 | 11.1 | slightly gravelly muddy |
| 3 | Basin V | 54.320880 | 18.310066 | 14.6 | slightly gravelly muddy |
| 4 | Basin V | 54.320106 | 18.314179 | 11.7 | slightly gravelly |
| 5 | Basin IV | 54.314675 | 18.314408 | 12.3 | gravelly muddy |
| 6 | Basin I | 54.311586 | 18.332268 | 9.0 | slightly gravelly muddy |
| 7 | Basin III | 54.314802 | 18.331265 | 13.7 | gravelly muddy |
| Sampling Point | Location in the Port of Gdynia | Coordinates | Depth of Sampling (m) | Description of Sediment | |
|---|---|---|---|---|---|
| 1 | Basin VI | 54.321596 | 18.313061 | 11.0 | slightly gravelly muddy |
| 2 | Basin VI | 54.320861 | 18.312545 | 10.7 | slightly gravelly muddy |
| 3 | Basin V | 54.320883 | 18.310072 | 14.8 | slightly gravelly |
| 4 | Basin V | 54.320082 | 18.314132 | 11.7 | gravelly muddy |
| 5 | Basin IV | 54.314699 | 18.314527 | 12.8 | slightly gravelly muddy |
| 6 | Basin I | 54.311550 | 18.332299 | 9.1 | slightly gravelly muddy |
| 7 | Basin III | 54.314815 | 18.331348 | 13.6 | gravelly muddy |
| Congener/Abbreviation | Toxic Equivalency Factor (TEF) |
|---|---|
| Dioxins | |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) | 1 |
| 1,2,3,7,8-Pentachlorodibenzo-p-dioxin (1,2,3,7,8-PeCDD) | 1 |
| 1,2,3,4,7,8-Hexachlorodibenzo-p-dioxin (1,2,3,4,7,8-HxCDD) | 0.1 |
| 1,2,3,6,7,8-Hexachlorodibenzo-p-dioxin (1,2,3,6,7,8-HxCDD) | 0.1 |
| 1,2,3,7,8,9-Hexachlorodibenzo-p-dioxin (1,2,3,7,8,9-HxCDD) | 0.1 |
| 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin (1,2,3,4,6,7,8-HpCDD) | 0.01 |
| Octachlorodibenzo-p-dioxin (OCDD) | 0.003 |
| Furans | |
| 2,3,7,8-Tetrachlorodibenzofuran (2,3,7,8-TCDF) | 0.01 |
| 1,2,3,7,8-Pentachlorodibenzofuran (1,2,3,7,8-PeCDF) | 0.03 |
| 2,3,4,7,8-Pentachlorodibenzofuran (2,3,4,7,8-PeCDF) | 0.3 |
| 1,2,3,4,7,8-Hexachlorodibenzofuran (1,2,3,4,7,8-HxCDF) | 0.1 |
| 1,2,3,6,7,8-Hexachlorodibenzofuran (1,2,3,6,7,8-HxCDF) | 0.1 |
| 1,2,3,7,8,9-Hexachlorodibenzofuran (1,2,3,7,8,9-HxCDF) | 0.1 |
| 2,3,4,6,7,8-Hexachlorodibenzofuran (2,3,4,6,7,8-HxCDF) | 0.1 |
| 1,2,3,4,6,7,8-Heptachlorodibenzofuran (1,2,3,4,6,7,8-HpCDF) | 0.01 |
| 1,2,3,4,7,8,9-Heptachlorodibenzofuran (1,2,3,4,7,8,9-HpCDF) | 0.01 |
| Octachlorodibenzofuran (OCDF) | 0.003 |
| Sampling Points | Basins of the Port of Gdynia | Concentration [μg MPs/kg d.w.] | Spectroscopy Identification of Polymer Type * |
|---|---|---|---|
| 1 | Basin VI | 13,734.24 | PP, PS, PS/PVC |
| 2 | 9591.06 | PS, PS/PVC, PA6 | |
| 3 | Basin V | 9191.34 | LDPE, PE |
| 4 | 8812.0 | PP, PE, oxidised PS | |
| 5 | Basin IV | 12,564.27 | PS, PE, oxidised PE |
| 6 | Basin I | 15,571.55 | PU, PE, oxidised PE |
| 7 | Basin III | 21,103.77 | PS, PS/PVC, LDPE, PE-PP |
| Congener | Basins of the Port of Gdynia/Sampling Points | ||||||
|---|---|---|---|---|---|---|---|
| VI | V | IV | I | III | |||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 2,3,7,8-TCDD | 0.235 | - | - | - | 0.274 | 0.321 | - |
| 1,2,3,7,8-PeCDD | 0.981 | 0.687 | - | - | 0.968 | 1.11 | - |
| 1,2,3,4,7,8-HxCDD | 1.65 | 1.26 | - | 0.447 | 1.81 | 1.65 | - |
| 1,2,3,6,7,8-HxCDD | 8.56 | 6.05 | 0.895 | 1.31 | 8.50 | 9.37 | 0.453 |
| 1,2,3,7,8,9-HxCDD | 3.32 | 3.38 | 0.523 | 1.04 | 2.85 | 4.56 | - |
| 1,2,3,4,6,7,8-HpCDD | 191 | 152 | 35.2 | 39.5 | 199 | 185 | 9.78 |
| OCDD | 902 | 839 | 164 | 167 | 962 | 882 | 58.0 |
| 2,3,7,8-TpCDF | 8.47 | 4.05 | 1.68 | 2.02 | 9.11 | 7.21 | 0.523 |
| 1,2,3,7,8-PeCDF | 3.07 | 1.22 | - | - | 3.00 | 2.78 | - |
| 2,3,4,7,8-PeCDF | 6.40 | 3.52 | 1.02 | 1.14 | 6.91 | 5.95 | 0.497 |
| 1,2,3,4,7,8-HxCDF | 9.25 | 7.24 | 1.19 | 1.08 | 9.89 | 13.3 | 0.571 |
| 1,2,3,6,7,8-HxCDF | 4.91 | 2.00 | 0.476 | 0.512 | 3.05 | 5.21 | - |
| 1,2,3,7,8,9-HxCDF | - | - | - | - | - | - | - |
| 2,3,4,6,7,8-HxCDF | 2.13 | 2.01 | - | - | 2.05 | 2.87 | - |
| 1,2,3,4,6,7,8-HpCDF | 31.7 | 24.6 | 4.29 | 4.10 | 32.1 | 39.2 | 2.19 |
| 1,2,3,4,7,8,9-HpCDF | 2.73 | 1.98 | 0.520 | 0.494 | 2.39 | 2.24 | - |
| OCDF | 33.1 | 26.7 | 3.49 | 3.36 | 30.4 | 23.8 | - |
| Congener | Basins of the Port of Gdynia | ||||
|---|---|---|---|---|---|
| VI | V | IV | I | III | |
| TCDD | 0.01 | 0.00 | 0.02 | 0.00 | 0.00 |
| PeCDD | 0.08 | 0.00 | 0.08 | 0.00 | 0.00 |
| ΣHxCDD | 1.08 | 0.73 | 1.03 | 0.82 | 0.00 |
| HpCDD | 15.66 | 17.97 | 15.62 | 16.13 | 14.69 |
| OCDD | 74.85 | 75.11 | 75.49 | 76.88 | 81.32 |
| TpCDF | 0.66 | 0.90 | 0.71 | 0.63 | 1.13 |
| ΣPeCDF | 0.73 | 0.53 | 0.78 | 0.52 | 0.00 |
| ΣHxCDF | 1.34 | 0.79 | 1.18 | 1.61 | 0.00 |
| ΣHpCDF | 2.81 | 2.29 | 2.71 | 3.42 | 2.87 |
| OCDF | 2.76 | 1.67 | 2.39 | 0.00 | 0.00 |
| Σ | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dereszewska, A.; Krasowska, K.; Popek, M. Determination of Selected Organic Contaminants in the Port of Gdynia Sediments: Towards Cleaner Baltic Ports. Sustainability 2024, 16, 5285. https://doi.org/10.3390/su16135285
Dereszewska A, Krasowska K, Popek M. Determination of Selected Organic Contaminants in the Port of Gdynia Sediments: Towards Cleaner Baltic Ports. Sustainability. 2024; 16(13):5285. https://doi.org/10.3390/su16135285
Chicago/Turabian StyleDereszewska, Alina, Katarzyna Krasowska, and Marzenna Popek. 2024. "Determination of Selected Organic Contaminants in the Port of Gdynia Sediments: Towards Cleaner Baltic Ports" Sustainability 16, no. 13: 5285. https://doi.org/10.3390/su16135285
APA StyleDereszewska, A., Krasowska, K., & Popek, M. (2024). Determination of Selected Organic Contaminants in the Port of Gdynia Sediments: Towards Cleaner Baltic Ports. Sustainability, 16(13), 5285. https://doi.org/10.3390/su16135285






