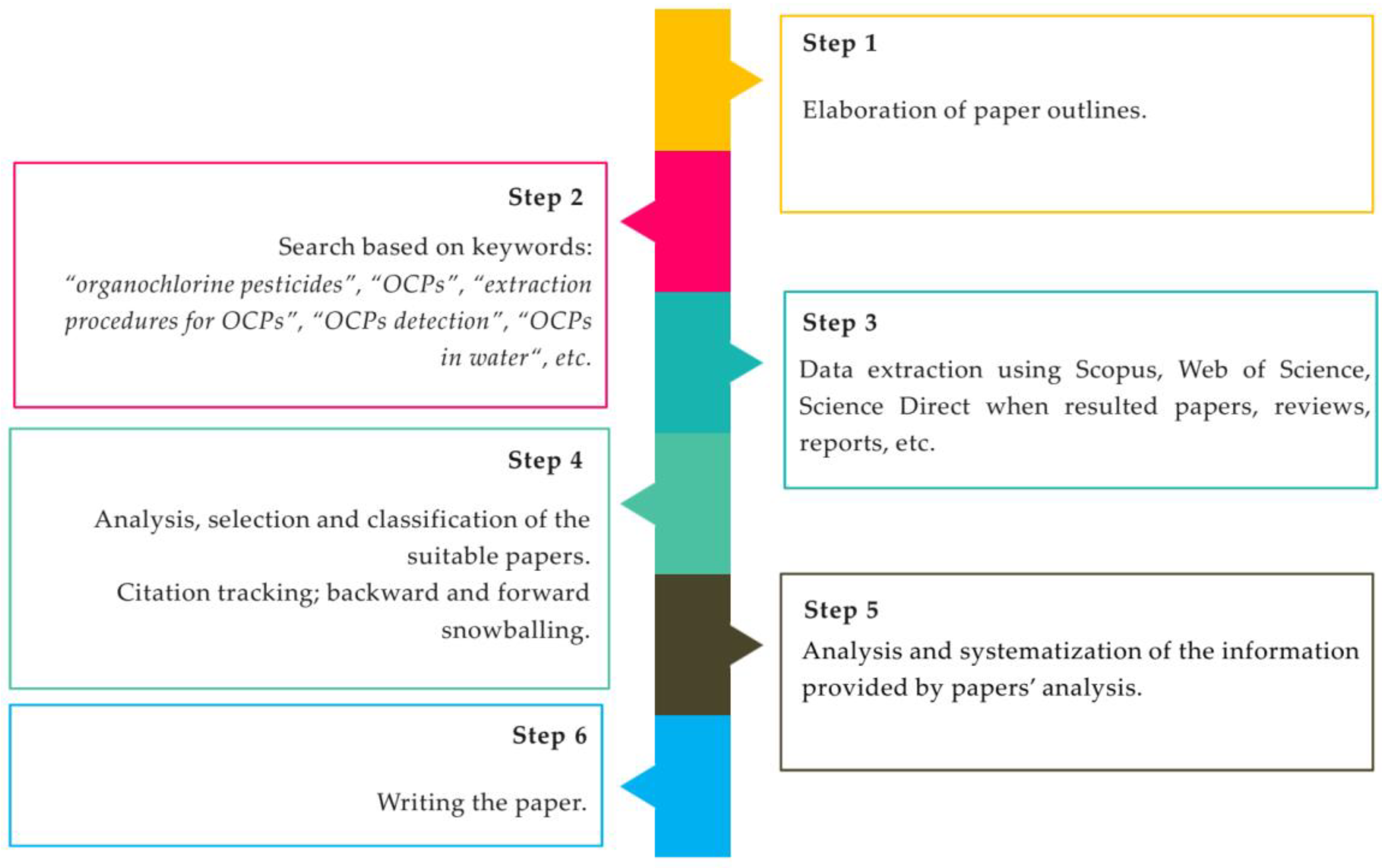
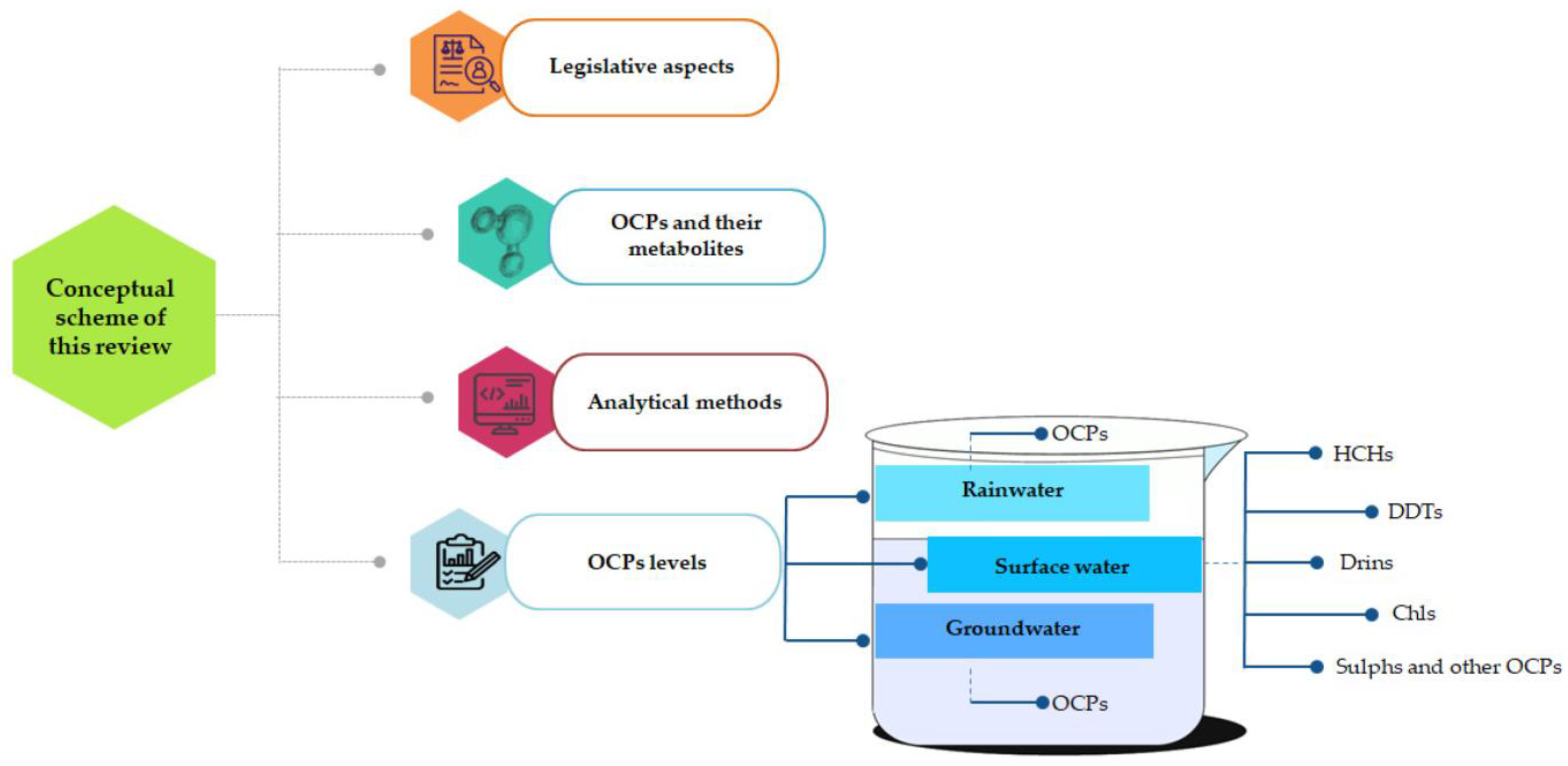
Sustainable Water Monitoring via Analytical Techniques and Protocols Applied in the Assessment of Organochlorine Pesticides
Abstract
1. Introduction
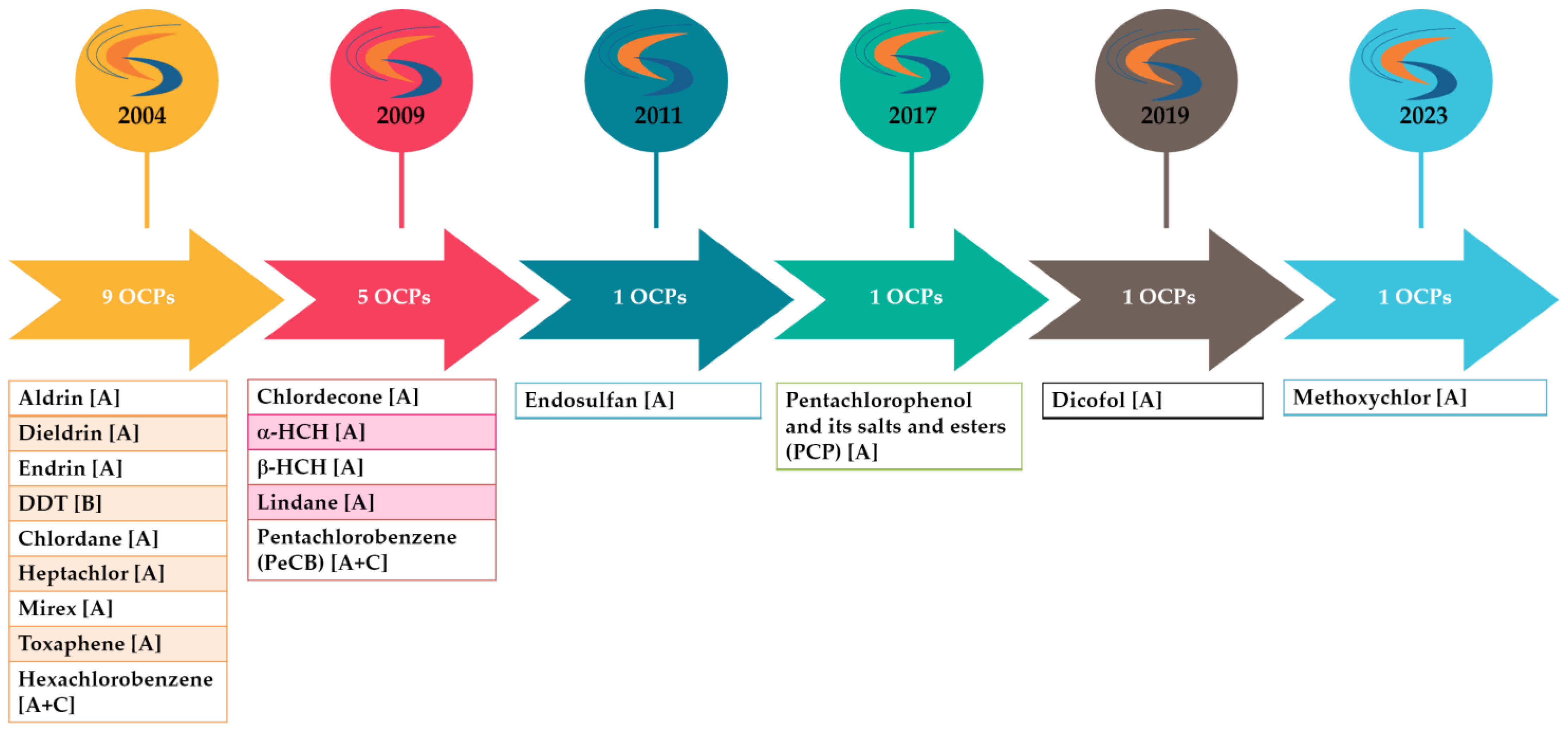
2. Legislative Aspects–Brief Presentation
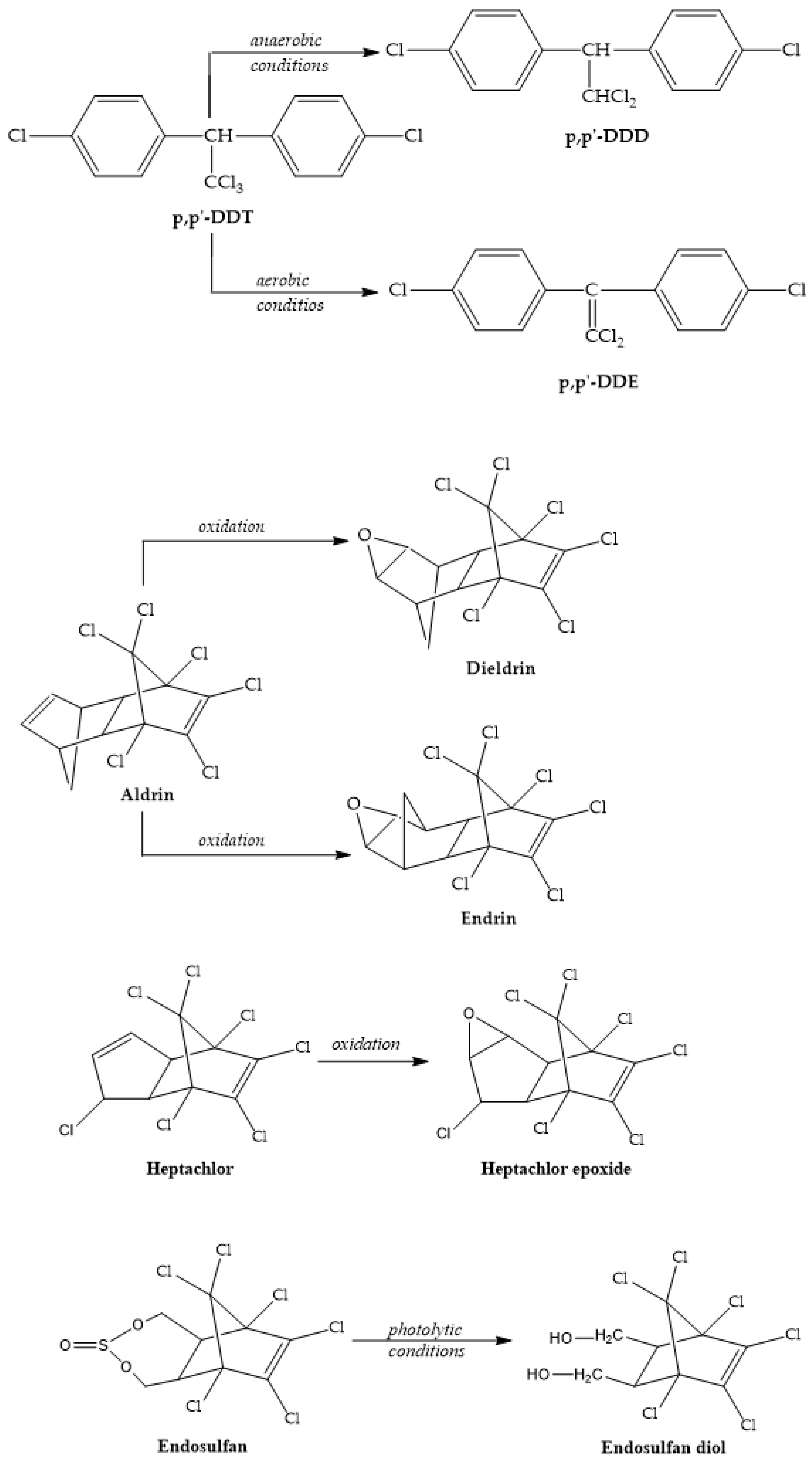
3. Main Characteristics of OCPs and Their Metabolites
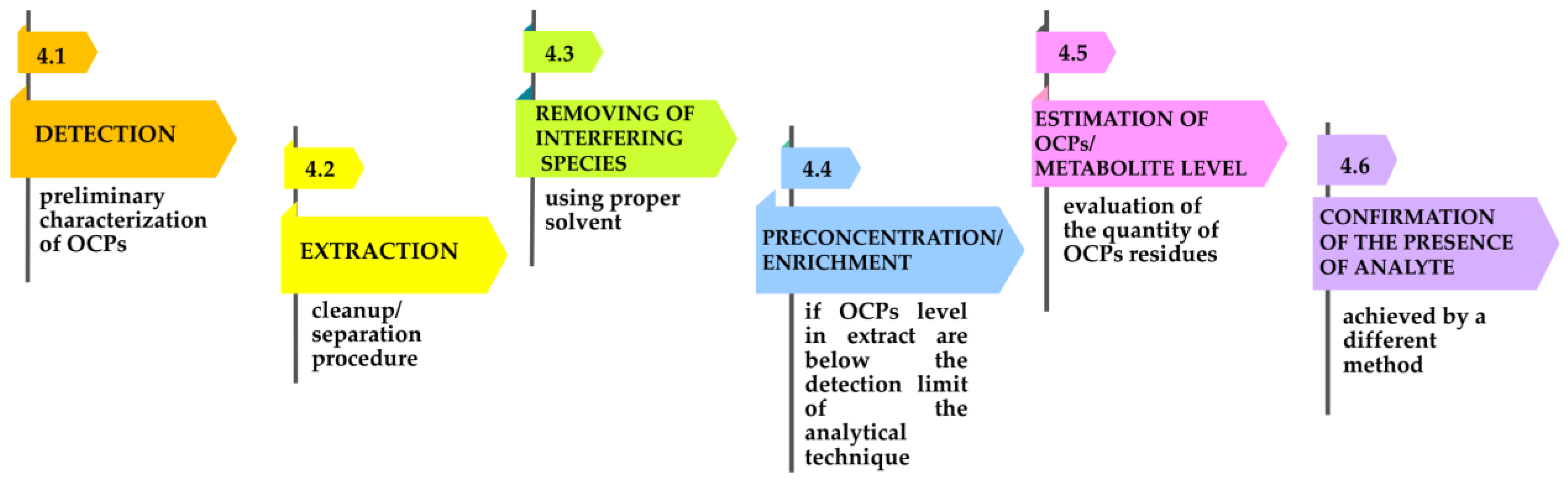
4. Analytical Methods and Protocols Used for OCP Assessment
4.1. Detection
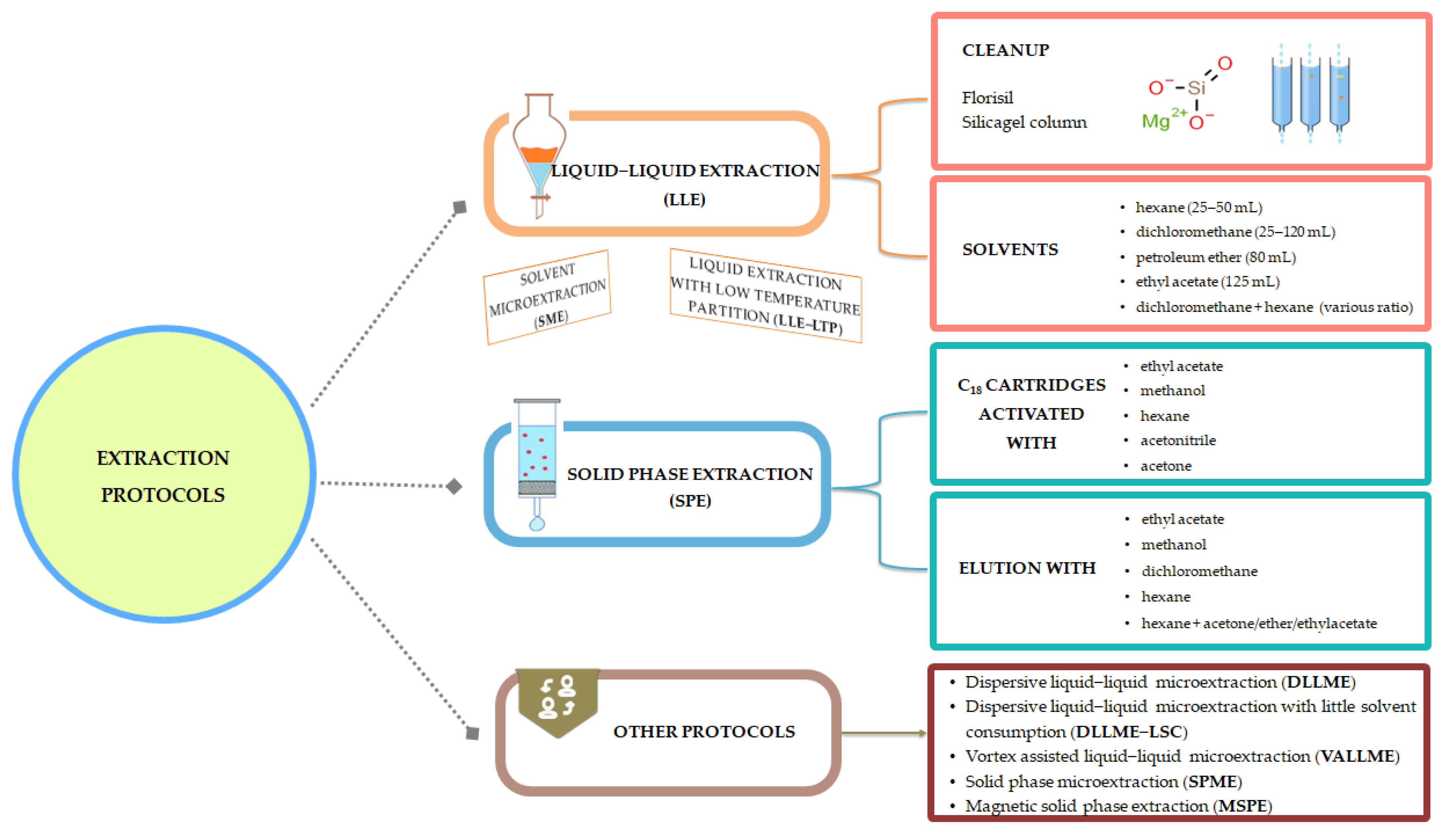
4.2. Extraction
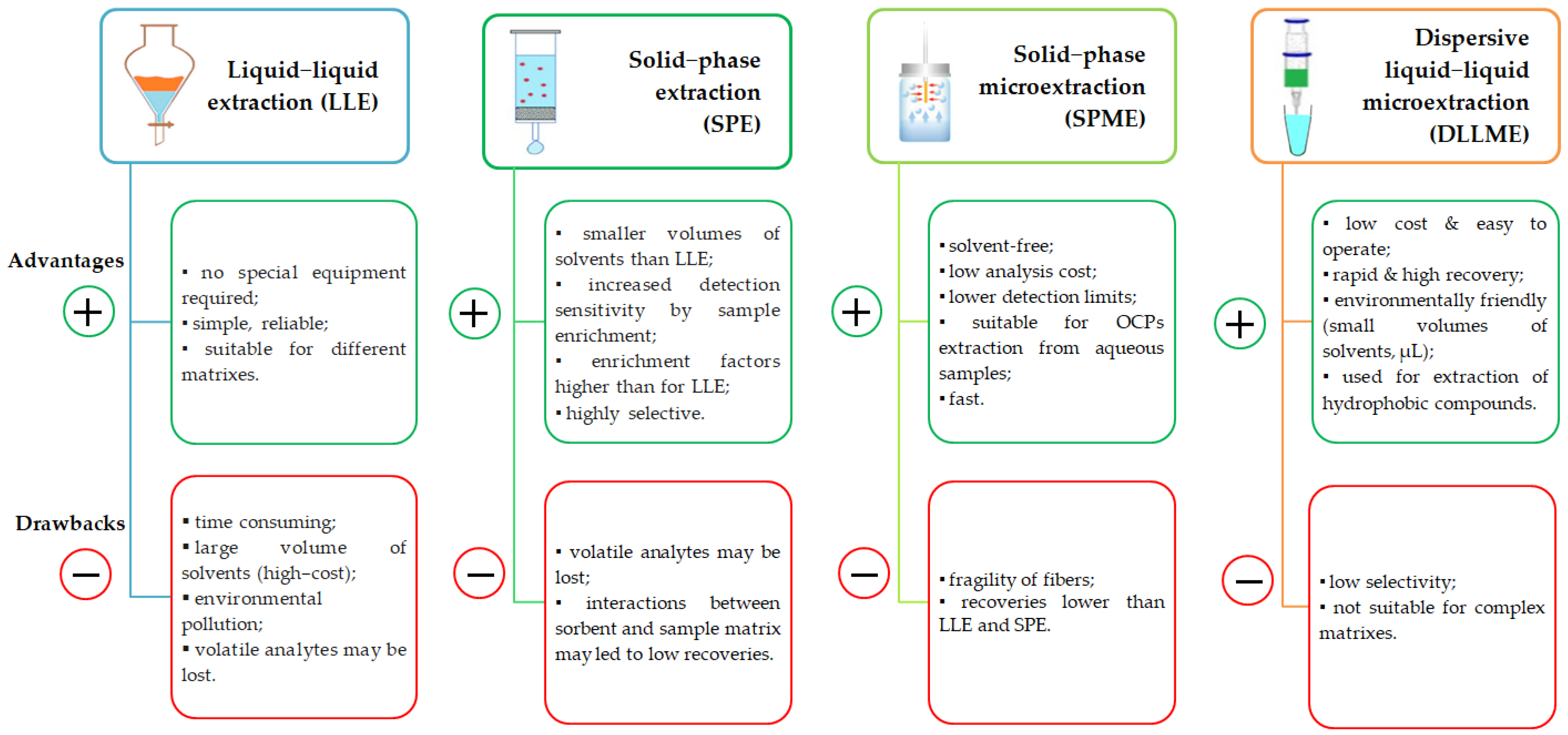
- Liquid–liquid extraction (LLE) is widely used for OCP extraction from aqueous matrices. It is considered as a simple method and it involves the dissolution of analytes in two different immiscible liquids (mainly water and organic solvents, such as ethyl acetate, dichloromethane, and petroleum ether) (Table 2). LLE is time-consuming and it also has other disadvantages; one that it is worth mentioning is the large volume of solvents that are associated with environmental issues and, worse, with carcinogenic effects on humans [35], and low enrichment of the analytes [33].
- Dispersive liquid–liquid microextraction (DLLME) requires injecting an appropriate mixture of extraction solvent and disperser solvent rapidly into an aqueous sample, resulting in a cloudy solution. DLLME is a low-cost, rapid, and easy-to-operate procedure with high recovery and it is also environmentally friendly, if we consider that very low volumes of solvents are used. This method is used to extract hydrophobic compounds. In addition to OCP extraction, this method has proven its utility for organophosphorus pesticides, herbicides, and polycyclic aromatic hydrocarbons [42]. For instance, the extraction of 14 OCPs (α-HCH, β-HCH, δ-HCH, γ-HCH, aldrin, dieldrin, endrin, heptachlor, heptachlor oxide, α-chlordane, β-chlordane, p,p’-DDT, p,p’-DDD, and p,p’-DDE) from the water of the Jajrood River in Iran was performed by DLLME using 0.5 mL of ether (disperser solvent) and 13.5 μL of carbon disulfide (extraction solvent), which were injected into the sample solution.
- Vortex-assisted liquid–liquid microextraction (VALLME) was developed by Ozcan [47] for OCP quantification from aqueous samples. This optimized method used bromoform (50 μL) for extraction, followed by vortex extraction for 2 min at 3000 rpm with no NaCl addition for ionic strength adjustment, centrifugation for 5 min at 4000 rpm, and a 5 mL water sample. The mean recoveries for the OCPs assessed were between 71% and 104%. The performance of this extraction procedure was compared with that of LLE, and the conclusion was that recovery values were comparable (75–105%). This extraction method is suitable for the qualitative and quantitative assessment of OCPs from aqueous matrices, and it is easy to use and rapid.
- Solid-phase extraction (SPE) involves the use of disks or columns able to retain analytes, which are then released with small volumes of solvents. This is a great advantage over LLE. The conditioning of cartridges containing octadecyl groups chemically bonded to silica is performed using different solvents, such as methanol [48], hexane [49], acetonitrile [50], ethyl acetate [14], or solvent mixtures [51].
- Solid-phase microextraction (SPME) was developed during the 1990s and presents superiority over the above-mentioned extraction procedures, as it is solvent-free, with lower detection limits, sensitivity, and good reproducibility, and it has proven its efficiency when coupled with GC techniques [33,34,35]. Moreover, SPME proved its utility for OCP extraction from aqueous samples. For instance, Jackson and Andrews [56] reported that the SPME of OCPs, separation (micro-bore 0.1 mm capillary column), and measurement (GC) from river water samples took less than 10 min.
- Magnetic solid-phase extraction (MSPE) is another method based on the adsorption of the analyte of interest on a magnetic adsorbent, the advantage being that magnetic particles with nano size dimensions have a large specific surface area and consequently, a higher extraction capacity.
| Sample Volume, L | Extraction | Cleanup | Instrumentation | Column | Recovery, % | Ref. |
|---|---|---|---|---|---|---|
| 0.5 | LLE procedure - 25 mL n-hexane (extraction); - Dehydration with Na2SO4. | - SupelcleanENVI_Florisil (0.5 g) SPE tubes; - Complete evaporation + re-dissolution in n-hexane. | GC equipped with 63Ni-ECD and HP 3396 integrator | Fused silica capillary column Ultra-2 (50 m × 0.2 mm i.d. × 0.33 μm film thickness) | 82–96 | [61] |
| 0.5 | LLE procedure - 25 mL n-hexane (extracting three times); - Dehydration with 0.5 g Na2SO4; - Evaporation to 1–2 mL at 50 °C. | GC equipped with 63Ni-ECD | 4% SE–30/6% QF column | - | [62] | |
| 0.5 | LLE procedure - Extraction with 50 mL dichloromethane in hexane (trice); - Drying on Na2SO4; - Concentration to 0.5 mL and redissolution in 5 mL hexane; - Concentration to 0.5 mL. | GC equipped with 63Ni-ECD | BP5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [63] | |
| 0.6 | LLE procedure - 80 mL petroleum ether for 10 min; - Organic phase was purified with 10 mL H2SO4 + Na2SO4 solution 2%; - Dehydration with Na2SO4; - Concentration to 1 mL. | GC equipped with μECD | Capillary column HP-5 (30 cm × 0.32 mm i.d. × 0.25 μm film thickness) | 89–97 | [37] | |
| 1.0 | LLE procedure - Extraction with 25 mL hexane (three times); - Dehydration with 0.5 g Na2SO4; - Evaporation to 1–2 mL at 50 °C. | GC equipped with 63Ni-ECD | 4% SSE–30/60% QF capillary column | - | [61] | |
| 1.0 | LLE procedure - 50 mL mixture of 15% dichloromethane and 85% n-hexane (three extractions); - Dehydration with Na2SO4; - Concentration to 2 mL. | Florisil column + elution with 200 mL 6%d iethylether in petroleum ether (5 mL∙min−1 flow rate) | GC | - | - | [64] |
| 1.0 | LLE procedure - 100 mL dichloromethane (extracting two times); - Drying over anhydrous MgSO4; - Concentration to 2 mL. | - Silica cartridges with 2 g of anhydrous Na2SO4 conditioned with 6 mL dichloromethane; - Extracts are loaded onto cartridges; - Extracts are concentrated to dryness and re-dissolved in 1 mL ethyl acetate. | GC equipped with 63Ni-ECD | capillary column coated with VF-5 30 m + 10 m EZ guard column × 0.25 mm i.d. × 0.25 μm film thickness) | 70–119 | [65] |
| 1.0 | LLE procedure - 25–30 mL dichloromethane was used for extraction; - Extract was concentrated to 4 mL. | - Chromatography column (alumina-to-silica gel ratio 1:2); - Elution with 20 mL mixture of dichloromethane and n-hexane (2:3, v/v); - Concentration to 0.5–1.0 mL. | GC-MS/MS | HP-5 MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 69.46 ± 32.81 (for TCmX); 82.14 ± 6.46 (for PCB 65); 79 ± 12.71 (for PCB155) | [6] |
| 1.0 | LLE procedure - 250 mL water sample was treated with 50 mL saturated NaCl solution and 30 mL dichloromethane; - Dehydration with anhydrous Na2SO4; - Extract was dried and re-dissolved in 2 mL hexane. | Purification with H2SO4. | GC equipped with 63Ni-ECD and confirmation by GC/MS | HP-5MS fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 83–110 | [38] |
| 1.0 | LLE procedure - Water sample was treated with 120 mL dichloromethane; - Organic phase was dried on anhydrous Na2SO4; - Aqueous phase was treated twice with dichloromethane (2 × 60 mL) and lower phase was extracted; - Extract was concentrated to 0.5 mL and reconstituted to cyclohexane: acetone (9:1, v/v); - Concentration to 1 mL. | - Silica gel grade 60 (10 g) and alumina with 3% H2O (5 g) packed in a glass column and topped with anhydrous Na2SO4; - Elution with 100 mL mixture of hexane and dichloromethane (1:1, v/v). | HRGC-HRMS | Rtx-Dioxin2 (40 m × 0.18 mm i.d. × 0.18 μm film thickness) | 82–107 | [66] |
| 1.0 | LLE procedure - Dichloromethane for extraction | - Silica gel chromatography; - Drying and dissolving in ethyl acetate. | GC equipped with 63Ni-ECD | SPB-5 [(5% phenyl)-methyl polysiloxane) capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [67] |
| LLE procedure - Extraction with hexane; - Drying on anhydrous Na2SO4. | - Florisil - Evaporation; - Dissolution in 1 mL hexane | GC equipped with μECD | DB 1 capillary column (30 m × 0.32 mm i.d. × 0.50 μm film thickness) | - | [68] | |
| 1.0 | LLE procedure - Extraction with hexane; - Drying on anhydrous Na2SO4; - Concentration. | GC equipped with 63Ni-ECD | DB-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [69] | |
| 1.0 | LLE procedure - Extraction with dichloromethane (thrice); - Concentration and drying on Na2SO4; - Extract is made up to 5 mL with hexane. | GC equipped with 63Ni-ECD | PE-17 fused silica capillary (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 62.14–125.20 | [70] | |
| 1.0 | LLE procedure - Extraction with dichloromethane (3 × 20 mL); - Concentration. | - Silica gel column; - Concentration to 2 mL. | GC equipped with 63Ni-ECD | DB-5 capillary column (5%-phenyl-95%-dimethylpolysiloxane) (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 76–95 | [29] |
| 1.0 | LLE procedure - Extraction with 30 mL dichloromethane; - Drying on anhydrous Na2SO4; - Concentration. | GC equipped with 63Ni-ECD | Elite GC DB-5 (60 m × 0.25 mm i.d.) | - | [71] | |
| 2.0 | LLE procedure - 25 mL dichloromethane for extraction (three times); - Dehydration with Na2SO4; - Concentration to 2–3 mL. | - Neutral alumina/silica gel (1:2, v/v) column; - Elution with 30 mL dichloromethane and n-hexane (2:3, v/v); - Concentration to 0.2 mL. | GC equipped with 63Ni-ECD | HP-5MS capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 70.8 ± 17 (TCmX); 86.6 ± 20.8 (PCB209) | [72] |
| - | LLE procedure - 25 mL water sample + 10% NaCl + 125 mL ethyl acetate; - Organic phase is evaporated until complete dryness; - Sample is reconstituted in 5 mL n-hexane. | GC equipped with 63Ni-ECD | Fused silica capillary column (25 m × 0.53 mm i.d. × 0.15 μm film thickness) | - | [36] | |
| 2.0–3.0 | LLE procedure - 500 mL water sample + 10–15 g NaCl + 3 × 50 mL 15% dichloromethane in hexane; - Dehydration with anhydrous Na2SO4; - Concentration to near-dryness; - Dichloromethane removal is performed by addition of 5 mL of hexane (thrice); - Final volume of 2 mL in hexane | GC equipped with 63Ni-ECD | SPB-5 of 5% diphenyl/95% dimethyl fused silica capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 80–111 | [73] | |
| 2.5 | LLE procedure - 1000 mL water samples mixed with 50 mL hexane; - Resultant organic phase is re-extracted twice with 50 mL hexane; - Drying on anhydrous Na2SO4; - Concentrated. | - Florisil column; - Elution with 10 mL hexane aliquots; - Evaporation to dryness and dissolution in 1 mL ethyl acetate. | GC equipped with 63Ni-ECD | Fused silica gel capillary column VF-5 ms (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 79–96 | [74] |
| - | LLE-LTP procedure - 4 mL water sample and 8 mL acetonitrile are added to a vial and placed in a freezer (−20 °C); - 2 mL organic phase is transferred to a falcon tube with anhydrous Na2SO4; - 1 mL extract is analyzed. | GC-MS in selective ion monitoring mode (SIM) | DB-5MS capillary column with 5% phenyl stationary phase and 95% methylpolysiloxane (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 80–110 | [41] | |
| - | DLLME procedure - 5 mL water sample is transferred to a 10 mL screw-cap glass test tube; - 0.5 mL acetone (dispenser solvent) + 13.5 μL CS2 (extraction solvent) are injected into sample. | GC equipped with 63Ni-ECD | Fused-silica BPX5 column (25 m × 0.25 mm i.d. × 0.25 μm film thickness) | 78.9–101.3 | [45] | |
| 0.5 | DLLME procedure - 5 mL sample is poured into a 10 mL falcon tube; - 10 μL tetrachloroethylene (extraction solvent) + 1000 μL acetone (dispenser solvent). | GC-MS | HP-5 column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [43] | |
| - | DLLME procedure - 10 mL sample is placed in a 15 mL glass test tube and spiked with each pesticide at 10 μg∙L−1; - 10 μL tetrachloroethylene (extraction solvent) and 1000 μL acetone (disperser solvent) are dropped into the sample solution; - 2 μL sample is injected into GC-MS for analysis | GC-MS | VF-5-Ms Factor-four Varian column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 56–113 | [44] | |
| 0.5 | SPE procedure - C18 cartridges are washed with 5 mL ethyl acetate, conditioned with 5 mL methanol, and washed with 2 × 5 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 6 mL ethyl acetate; - Drying on Na2SO4; - Concentration. | GC-μECD | DB-5MS column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 78–93 | [52] | |
| 1.0 | SPE procedure - C18 column is conditioned with 6 mL hexane and dried; - 6 mL water sample is passed through column after previously applying 12 mL methanol; - Eluate is evaporated to dryness and dissolved in 1 mL hexane. | GC equipped with 63Ni-ECD | Fused silica capillary column HP-5 (5%PhMe silicone; 10 m × 0.53 mm i.d. × 2.65 μm film thickness) | 91–93 | [49] | |
| 1.0 | SPE procedure - C18 cartridges are activated with 5 mL ethyl acetate and 10 mL methanol and washed with 10 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 5 mL methanol and 5 mL dichloromethane; - Drying on anhydrous Na2SO4; - Concentration to 0.7 mL. after dichloromethane is added. | GC-MS in EI mode | HP-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [53] | |
| 1.0 | SPE procedure - SPE cartridges are conditioned with 12 mL methanol and 12 mL deionized and purified water; - Water samples are passed through cartridges; - Cartridges are washed with 6.0 mL purified water; - Elution with 1.0 mL dichloromethane. | GC-MS | DB-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 70.25–103.5 88.25–127.5 | [75] | |
| 1.0 | SPE procedure - Water samples are extracted using SPE disks; - 10 mL dichloromethane + ethyl acetate (1:1, v/v) as cleaning solvent; - Elution with 5 mL ethyl acetate (twice); - Dehydration with Na2SO4; - Extract is concentrated to 1 mL. | GC equipped with 63Ni-ECD | Fused silica capillary column coated with 5% diphenyl–95% dimethylsiloxane (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [76] | |
| 1.0 | SPE procedure - C18 column is washed with 20 mL methanol and 10 mL ultrapure water; - Elution with 10 mL hexane; - Concentration to 2 mL. | GC equipped with 63Ni-ECD | Fused silica capillary column HP-608 (30 m × 0.53 mm i.d. × 0.50 μm film thickness) | 28.65–121.57 | [54] | |
| 1.0 | SPE procedure - Water samples are passed through SPE cartridges previously rinsed with 5 mL ethyl acetate, 5 mL methanol, and 10 mL ultrapure water; - Elution twice with 10 mL ethyl acetate; - Dehydration using Na2SO4; - Concentration to 0.5 mL. | GC equipped with 63Ni-ECD | HP-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 72.4–105 | [14] | |
| 1.0 | SPE procedure - C18-bonded silica cartridges are washed with 20 mL methanol and 10 mL ultrapure water; - Water sample is passed through cartridges; - Elution with 10 mL hexane; - Concentration to 0.2 mL | GC equipped with 63Ni-ECD | HP-608 fused capillary column (30 m × 0.53 mm i.d. × 0.50 μm film thickness) | 81.96–104.92 | [77] | |
| 1.0 | SPE procedure - Water sample is passed through C18 cartridge activated with 10 mL methanol and washed with 10 mL double-distilled water; - Elution with dichloromethane (3 × 10 mL); - Concentration to 5 mL | GC equipped with 63Ni-ECD | DB-5 capillary column (5%-phenyl-95%-dimethylpolysiloxane) (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 56–76 | [29] | |
| 1.0 | SPE procedure - C18 cartridge is washed with 6 mL ethyl acetate and 6 mL dichloromethane, and then washed with 10 mL methanol and 10 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 10 mL dichloromethane; - Drying on Na2SO4; - Concentration. | GC equipped with 63Ni-ECD GC-MS in selective ion monitoring mode (SIM) | HP-5 capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 74–113 | [27] | |
| 1.0 | SPE procedure - FlorisilSPE cartridges are conditioned with 5 mL hexane, 5 mL methanol, and 5 mL ultrapure water; - Elution with 10 mL ethyl acetate; - Drying on Na2SO4; - Concentration to 1 mL. | GC-MS | HP-5 capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 76.11–108.32 | [78] | |
| 1.0 | SPE procedure - C18 cartridges are washed with 5 mL ethyl acetate, conditioned with 5 mL methanol, and washed with 2 × 5 mL ultrapure water; - Water samples are percolated through cartridges; - Elution with 6 mL ethyl acetate; - Drying on Na2SO4; - Concentration to 1 mL. | GC equipped with 63Ni-ECD | DB-5 fused capillary column (30 m × 0.32 mm i.d. × 0.25 μm film thickness) | 76–87 | [79] | |
| 2.0 | SPE + SPME procedure - Water samples are passed through C18 columns previously washed with methanol and ultrapure water; - Elution with 10 mL hexane; - Eluate is collected in SPME vials containing 0.10 g NaCl. | GC equipped with μECD | DB-1 capillary column (30 m × 0.32 mm i.d. × 3 μm film thickness) | 36.5–112.2 | [58] | |
| 2.5 | SPE procedure - Water sample is passed through C18 Sep-Pak cartridges conditioned with 5 mL acetonitrile and 5 mL methanol; - Elution with ethyl acetate. | GC equipped with 63Ni-ECD | Glass column (2 m × 2 mm i.d.) with 1:1 mixture of 10% OV-101 and 15% OV-210 on ChromosorbWHP | 42–102 | [50] | |
| 2.5 | SPE procedure - 250 mL water sample is passed through C18 Sep-Pak cartridges conditioned with 10 mL mixture of acetonitrile + dichloromethane (1:1, v/v); - Elution with 3 mL acetone, 3 mL hexane + acetone (1:1, v/v), and 3 mL hexane; - Evaporation to dryness; - Residue is dissolved in 1 mL cyclohexane. | GC-MS/MS | Fused-silica untreated capillary column connected to Factor Four capillary column VF-5 ms (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 70–110 | [51] | |
| 2.5 | SPE procedure - Dichloromethane for extraction. | Celite–activated charcoal column. | GC equipped with 63Ni-ECD | Glass column with 1:1 mixture of 10% OV-101 and 15% OV-210 on ChromosorbWHP | [80] | |
| 5.0 | SPE procedure - Water sample is drawn through C18 column conditioned with 3 × 3 mL hexane:acetone, 3 mL methanol, and 3 mL distilled water; - Retained species are dissolved in hexane:acetone mixture; - Concentration to 2 mL. | Alumina–silicic acid column (2 g alumina and 3 g silicic acid) - Elution with 20 mL dichloromethane; - Concentration to 1 mL. | GC equipped with 63Ni-ECD | DB 1702 column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 74–96 | [16] |
| 5.0 | SPE procedure - Samples are passed through cartridges conditioned with 10 mL methanol + 2 × 5 mL deionized water; - Elution with 6 mL dichloromethane; - Dehydration with Na2SO4; - Concetration to 0.4 mL. | GC equipped with μECD GC coupled to MS detector in selected ion mode | Capillary column HP-5MS (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 86.7–116.4 | [48] | |
| - | SPE procedure - Samples passed through C18 column; - Elution with 5 mL hexane + ether (4.5:0.5, v/v); - Concentration to 100 μL and then dilution to 1 mL (with hexane). | GC equipped with 63Ni-ECD | DB-101 fused-silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | - | [81] | |
| - | SPE procedure - 100 mL sample is passed through C18 SPE laminar disk; - Elution with 10 mL ethyl acetate and 3 mL hexane; - Drying on Na2SO4 followed by concentration. | GC equipped with 63Ni-ECD GC-MS | Methyl–phenyl–cyanopropyl silicone fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) | 96–104 | [82] |
4.3. Removal of Interfering Species-Cleanup
4.4. Preconcentration or Enrichment
4.5. Detection and Quantification Methods of OCPs and Theirmetabolites
4.6. Confirmatory Techniques
5. OCP Levels Detected in Water from Different Sources
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-HCH | China | Shaying River | 2.0 | 0.7–3.3 | [37] |
| Songhua River | 4.18 | 2.27–6.91 | [81] | ||
| Jiuxi Valley | 1.45 | 0.12–5.24 | [14] | ||
| Jiuxi Valley | 7.17 | 4.31–13.0 | [14] | ||
| Surface water | - | 1.01–3.86 | [27] | ||
| Weihe River | 1.10 | 0.04–2.87 | [78] | ||
| Qiantang River | - | <0.08–72.24 | [79] | ||
| Greece | Surface water | - | ND–440 | [54] | |
| India | River Ganges, Kanpur | 190 | - | [70] | |
| Romania | River water | 5.80 | - | [103] | |
| Arieș River | 8.34; 24.10 | - | [58] | ||
| Danube, Jiu River, Olt River | - | <1–5 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–7.0 | [76] | |
| Küçuk Menderes River | - | 20–24 | [63] | ||
| KusadasiDilek National Park | 52 | ND–120 | [16] | ||
| β-HCH | China | Shaying River | 3.1 | 1.0–7.5 | [37] |
| Songhua River | 10.18 | <0.001–18.31 | [81] | ||
| Jiuxi Valley | 0.968 | 0.509–2.98 | [14] | ||
| Jiuxi Valley | 3.84 | 2.25–11.5 | [14] | ||
| Surface water | - | 0.29–3.44 | [27] | ||
| Weihe River | 4.50 | 0.28–21.10 | [78] | ||
| Qiantang River | - | <0.16–19.99 | [79] | ||
| Greece | Surface water | - | ND–401 | [54] | |
| Romania | Arieș River | 9.61; 46.90 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1–6 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–17.4 | [76] | |
| Küçuk Menderes River | - | 101–121 | [63] | ||
| Kusadasi Dilek National Park | 11 | ND–68 | [16] | ||
| γ-HCH | China | Shaying River | 5.2 | 1.8–9.3 | [37] |
| Songhua River | 4.89 | 0.001–5.94 | [81] | ||
| Jiuxi Valley | 1.08 | 0.844–1.84 | [14] | ||
| Jiuxi Valley | 3.16 | 2.42–5.02 | [14] | ||
| Surface water | - | 1.92–6.99 | [27] | ||
| Weihe River | 3.85 | 0.13–24.55 | [78] | ||
| Qiantang River | - | <0.08–173.11 | [79] | ||
| Ghana | Densu River | - | 20–100 | [74] | |
| Greece | Surface waters | ND–81 | [54] | ||
| Iliki Lake | 15 | - | [50] | ||
| Pinios River | - | 2–12 | [80] | ||
| India | River Ganges of Kanpur | 260 | - | [70] | |
| Philippines | Pampanga River | 29 | - | [67] | |
| Romania | Arieș River | 13.8; 125.1 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Moara Domneasca pond | 11.5 | 8–18 | [69] | ||
| Turkey | Mid-Black Sea region | - | ND–4.2 | [76] | |
| Küçuk Menderes River | - | 159–198 | [63] | ||
| KusadasiDilek National Park | 20 | ND–92 | [16] | ||
| δ-HCH | China | Shaying River | 6.2 | 1.8–12.5 | [37] |
| Songhua River | 8.78 | <0.001–16.53 | [81] | ||
| Jiuxi Valley | 0.198 | 0.142–0.312 | [14] | ||
| Jiuxi Valley | 0.598 | 0.485–0.756 | [14] | ||
| Weihe River | 14.32 | 0.14–157.89 | [78] | ||
| Qiantang River | - | <0.08–46.26 | [79] | ||
| Ghana | Densu River | - | 10–1070 | [74] | |
| Greece | Surface water | - | ND–189 | [54] | |
| Romania | Arieș River | 2.07; 3.60 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–250.5 | [76] | |
| Küçuk Menderes River | - | ND–16 | [63] | ||
| KusadasiDilek National Park | 44 | ND–54 | [16] | ||
| ΣHCH | China | Yangtze River | 0.44 | 0.17–1.14 | [104] |
| Beiluo River | 0.34 | 0.090–0.61 | [6] | ||
| Jiuxi Valley | 3.69 | 1.71–8.12 | [14] | ||
| Jiuxi Valley | 14.8 | 9.94–23.5 | [14] | ||
| Pearl River Delta | - | 0.84–12.23 | [105] | ||
| Weihe River | 19.85 | 2.41–178.18 | [78] | ||
| Qiantang River | - | 0.74–202.8 | [79] | ||
| Greece | Surface water | - | ND–421 | [54] | |
| Romania | Danube, Jiu River, Olt River | - | 1–9 | [68] | |
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| p,p’-DDT | Bangladesh | Ditch and pond water | - | 190–1540 | [38] |
| China | Songhua River | 11.78 | <0.001–14.120 | [81] | |
| Yangtze River | 13.4 | - | [48] | ||
| Huaihe River | 41.3 | - | [48] | ||
| Pearl River | 10.1 | - | [48] | ||
| Jiuxi Valley | 1.11 | 0.715–1.84 | [14] | ||
| Jiuxi Valley | 1.48 | 0.849–2.12 | [14] | ||
| Surface water | - | ND–3.17 | [27] | ||
| Weihe River | 0.77 | 0.10–4.50 | [78] | ||
| Qiantang River | - | <0.08–24.70 | [79] | ||
| Ghana | Densu River | - | 10–20 | [74] | |
| Greece | Surface water | - | ND–35 | [54] | |
| Philippines | Pampanga River | - | 37–40 | [67] | |
| Romania | Arieș River | 35.70; 42.60 | - | [58] | |
| Danube, Jiu River, Olt River | - | <2 | [68] | ||
| Turkey | Küçuk Menderes River | - | 21–50 | [63] | |
| Kusadasi Dilek National Park | 6 | ND–18 | [16] | ||
| o,p’-DDT | China | Songhua River | 7.47 | <0.001–10.81 | [81] |
| Jiuxi Valley | 0.904 | 0.247–1.860 | [14] | ||
| Jiuxi Valley | 1.45 | 0.708–4.13 | [14] | ||
| Weihe River | 4.68 | 0.04–115.53 | [78] | ||
| Qiantang River | - | <0.08–10.88 | [79] | ||
| Romania | Arieș River | 1.84; 4.25 | - | [58] | |
| Danube, Jiu River, Olt River | - | <9 | [68] | ||
| p,p’-DDE | Bangladesh | Ditch and pond water | - | 200–650 | [38] |
| China | Songhua River | 13.11 | <0.001–17.45 | [81] | |
| Jiuxi Valley | 0.161 | <0.05–0.246 | [14] | ||
| Jiuxi Valley | 0.283 | 0.0724–0.622 | [14] | ||
| Surface water | - | ND–0.65 | [27] | ||
| Weihe River | 0.43 | 0.03–2.17 | [78] | ||
| Qiantang River | - | <0.08–93.40 | [79] | ||
| Ghana | Densu River | - | ND–20 | [74] | |
| Greece | Surface waters | - | ND–64 | [54] | |
| Romania | Arieș River | 10.80; 15.00 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Uluabat Lake | 0.113 | 0.022–0.237 | [61] | |
| Kusadasi Dilek National Park | 3 | ND–44 | [16] | ||
| o,p’-DDE | China | Weihe River | 0.37 | 0.03–1.05 | [78] |
| p,p’-DDD | Bangladesh | Ditch and pond water | - | 210–830 | [38] |
| China | Songhua River | - | <0.001–0.014 | [81] | |
| Jiuxi Valley | 0.0612 | 0.04–0.128 | [14] | ||
| Jiuxi Valley | 0.12 | <0.04–0.316 | [14] | ||
| Surface water | - | ND–2.41 | [27] | ||
| Weihe River | 0.98 | 0.04–4.32 | [78] | ||
| Qiantang River | - | <0.08–13.56 | [79] | ||
| Greece | Surface waters | - | ND–112 | [54] | |
| Romania | Arieș River | 9.07; 11.06 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Mid-Black Sea region | - | ND–8.9 | [76] | |
| Küçuk Menderes River | - | 23–52 | [63] | ||
| Kusadasi Dilek National Park | 5 | ND–43 | [16] | ||
| o,p’-DDD | China | Weihe River | 0.15 | 0.01–1.00 | [78] |
| Romania | Arieș River | 6.41; 8.07 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| ΣDDT | China | Yangtze River | 0.52 | 0–0.98 | [104] |
| Beiluo River | 1.39 | 0.50–4.48 | [6] | ||
| Jiuxi Valley | 2.23 | 1.19–3.31 | [14] | ||
| Jiuxi Valley | 3.34 | 1.90–6.23 | [14] | ||
| Weihe River | 7.37 | 0.94–116.83 | [78] | ||
| Qiantang River | - | 0.40–97.54 | [79] | ||
| Romania | Danube, Jiu River, Olt River | - | <1–4 | [68] | |
| South Africa | Jukskei River | - | 1200–3250 | [29] | |
| Turkey | Meriç Delta | - | ND–1010 | [62] | |
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| Aldrin | China | Shaying River | 2.3 | 1.0–3.8 | [37] |
| Jiuxi Valley | 0.736 | <0.05–1.62 | [14] | ||
| Jiuxi Valley | 1.28 | 0.213–2.45 | [14] | ||
| surface water | 1.81 | 0.78–4.74 | [27] | ||
| Weihe River | 0.54 | 0.11–2.00 | [78] | ||
| Qiantang River | - | <0.08–103.9 | [79] | ||
| Ghana | Densu River | - | ND–20 | [74] | |
| Greece | Surface waters | - | ND–104 | [54] | |
| Philippines | Pampanga River | 29 | - | [67] | |
| Romania | Arieș River | 9.75; 12.60 | - | [58] | |
| Danube, Jiu River, Olt River | - | <2–2 | [68] | ||
| Turkey | Meriç Delta | - | ND–40 | [62] | |
| Küçuk Menderes River | - | 17–1790 | [63] | ||
| Kusadasi Dilek National Park | 3 | ND–2180 | [16] | ||
| Dieldrin | China | Shaying River | 4.6 | - | [37] |
| Qiantang River | - | <0.15–42.06 | [79] | ||
| Ghana | Densu River | - | ND–20 | [74] | |
| Greece | Surface waters | - | ND–39 | [54] | |
| India | River Ganges of Kanpur | 1671 | - | [70] | |
| Philippines | Pampanga River | - | 28–29 | [67] | |
| Romania | Arieș River | 0.103; 0.421 | - | [58] | |
| Danube, Jiu River, Olt River | - | <1 | [68] | ||
| Turkey | Meriç Delta | - | ND–10 | [62] | |
| Mid-Black Sea region | - | ND–1.8 | [76] | ||
| Küçuk Menderes River | - | 8–5117 | [63] | ||
| Kusadasi Dilek National Park | 8 | ND–30 | [16] | ||
| Endrin | China | Shaying River | 6.2 | 1.0–11.3 | [37] |
| Jiuxi Valley | 0.063 | <0.04–0.110 | [14] | ||
| Jiuxi Valley | 0.126 | <0.04–0.196 | [14] | ||
| Surface waters | 2.22 | 0.53–4.39 | [27] | ||
| Weihe River | 4.74 | 0.37–26.28 | [78] | ||
| Qiantang River | - | <0.10–28.46 | [79] | ||
| Ghana | Densu River | - | 10–30 | [74] | |
| Turkey | Küçuk Menderes River | - | 14–191 | [63] | |
| Kusadasi Dilek National Park | 8 | ND–32 | [16] | ||
| Surface waters | 5.37 | 0.45–11.75 | [27] | ||
| Ghana | Densu River | - | 30–150 | [74] | |
| Greece | Surface waters | - | ND-80 | [54] | |
| Philippines | Pampanga River | - | 417–1341 | [67] | |
| Turkey | Küçuk Menderes River | - | 43–138 | [63] | |
| Kusadasi Dilek National Park | 9 | ND–9 | [16] | ||
| Endrin ketone | Ghana | Densu River | - | ND–20 | [74] |
| Turkey | Küçuk Menderes River | - | 41–56 | [63] | |
| ΣEndrin | China | Beiluo River | 2.31 | 0.15–20.91 | [6] |
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| Heptachlor | China | Shaying River | 8.3 | 1.3–17.9 | [37] |
| Jiuxi Valley | 0.426 | <0.04–1.75 | [14] | ||
| Jiuxi Valley | 0.316 | 0.0634–1.23 | [14] | ||
| Surface waters | - | 1.12–2.32 | [27] | ||
| Weihe River | 0.89 | 0.05–3.22 | [78] | ||
| Qiantang River | - | <0.15–88.34 | [79] | ||
| Ghana | Densu River | - | ND–40 | [74] | |
| Greece | Surface waters | - | ND–20 | [54] | |
| Philippines | Pampanga River | 28 | - | [67] | |
| Romania | Arieș River | 2.44; 3.16 | - | [58] | |
| Turkey | Küçuk Menderes River | - | 46–181 | [63] | |
| Heptachlor epoxide | China | Shaying River | 1.4 | 0.6–1.9 | [37] |
| China | Qiantang River | - | <0.08–111.8 | [79] | |
| Turkey | Mid-Black Sea region | - | ND–21.4 | [76] | |
| Küçuk Menderes River | - | 110–297 | [63] | ||
| Philippines | Pampanga River | - | 19–70 | [67] | |
| Turkey | Meriç Delta | - | ND–200 | [62] | |
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-Endosulfan | China | Shaying River | 8.5 | 2.1–40.7 | [37] |
| Surface waters | - | ND–1.62 | [27] | ||
| Ghana | Densu River | - | ND–40 | [74] | |
| Romania | Danube, Jiu River, Olt River | - | <1 | [68] | |
| Turkey | Küçuk Menderes River | - | 3–7 | [63] | |
| Kusadasi Dilek National Park | 11 | ND–30 | [16] | ||
| Surface waters | - | ND–1.85 | [27] | ||
| Philippines | Pampanga River | - | 40–64 | [67] | |
| Romania | Danube, Jiu River, Olt River | - | <1 | [68] | |
| Turkey | Küçuk Menderes River | - | 23–25 | [63] | |
| Kusadasi Dilek National Park | 3 | ND–39 | [16] | ||
| Endosulfan sulfate | Greece | Surface waters | - | ND–58 | [54] |
| Turkey | Kusadasi Dilek National Park | 5 | ND–7 | [16] | |
| Küçuk Menderes River | - | 19–121 | [63] | ||
| ΣEndosulfan | China | Surface waters | 1.37 | 0.067–9.25 | [6] |
| Surface waters | 3.05 | ND–9.23 | [27] | ||
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-HCH | China | Taihu Lake region, shallow groundwater | 5.85 | 0.92–41.77 | [52] |
| India | Kanpur, groundwater | 189 | - | [70] | |
| Delhi, tap water | 4 | 0–12 | [71] | ||
| Romania | Fountain | 6 | - | [103] | |
| β-HCH | China | Taihu Lake region, shallow groundwater | 14.53 | 0.94–107.6 | [52] |
| India | Delhi, tap water | 30 | 22–52 | [71] | |
| γ-HCH | China | Taihu Lake region, shallow groundwater | 8.77 | 1.38–56.05 | [52] |
| Ghana | Well water | 30 | - | [65] | |
| India | Kanpur, groundwater | 303; 471 | - | [70] | |
| Hyderabad, well water | - | 680–1380 | [77] | ||
| Delhi, tap water | 650 | 0–1690 | [71] | ||
| Philippines | Groundwater | 30 | - | [67] | |
| Romania | Tap water | 4 | - | [103] | |
| δ-HCH | China | Taihu Lake region, shallow groundwater | 14.56 | 3.69–80.58 | [52] |
| p,p’-DDT | China | Taihu Lake region, shallow groundwater | 4.64 | ND–60.28 | [52] |
| Ghana | Well water | 40 | - | [65] | |
| India | Hyderabad, well water | - | 150–190 | [77] | |
| Delhi, tap water | 79 | 62–142 | [71] | ||
| o,p’-DDT | India | Delhi, tap water | 820 | 520–1440 | [71] |
| p,p’-DDE | China | Taihu Lake region, shallow groundwater | 46.77 | 0.72–453.5 | [52] |
| India | Delhi, tap water | 78 | 48–126 | [71] | |
| o,p’-DDE | India | Delhi, tap water | 4 | 0–87 | [71] |
| p,p’-DDD | China | Taihu Lake region, shallow groundwater | 1.04 | ND–6.95 | [52] |
| India | Delhi, tap water | 3 | 0–20 | [71] | |
| Aldrin | China | Taihu Lake region, shallow groundwater | 27.59 | ND–356.5 | [52] |
| Dieldrin | Ghana | Well water | 30 | - | [65] |
| Philippines | Groundwater | - | 28–29 | [67] | |
| Endrin | China | Taihu Lake region, shallow groundwater | 11.53 | ND–129.2 | [52] |
| Endrin aldehyde | Philippines | Groundwater | - | 504–998 | [67] |
| China | Taihu Lake region, shallow groundwater | 6.15 | ND–78.79 | [52] | |
| α-Endosulfan | Ghana | Well water | 30 | - | [65] |
| India | Hyderabad, well water | - | 1340–2140 | [77] | |
| β-Endosulfan | India | Hyderabad, well water | - | 210–870 | [77] |
| Endosulfan sulfate | Ghana | Well water | 30 | - | [65] |
| Heptachlor | China | Taihu Lake region, shallow groundwater | 21.98 | 4.97–189.2 | [52] |
| Ghana | Well water | 20 | - | [65] | |
| Philippines | groundwater | 20 | - | [67] | |
| Heptachlor epoxide | China | Taihu Lake region, shallow groundwater | 12.31 | ND–47.12 | [52] |
| Philippines | Groundwater | 22 | - | [67] | |
| OCP | Location | Mean Value | Range | References | |
|---|---|---|---|---|---|
| α-HCH | India | Delhi | - | ND–0.062 | [105] |
| Hisar | - | ND–0.2 | [73] | ||
| Tanzania | Kibaha Coast Region | - | 0.001–100 | [66] | |
| β-HCH | India | Hisar | - | ND–0.1 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.001–99 | [66] | |
| γ-HCH | India | Delhi | - | ND–0.2 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.01–3.1 | [66] | |
| δ-HCH | India | Hisar | - | ND–0.1 | [73] |
| Tanzania | Kibaha Coast Region | - | ND–5.4 | [66] | |
| ΣHCH | India | Hisar | ND–0.4 | [73] | |
| Tanzania | Kibaha Coast Region | - | 0.01–170 | [66] | |
| p,p’-DDT | India | Hisar | - | ND–7 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.003–3000 | [66] | |
| Tanzania | Kibaha Coast Region | - | 0.001–230 | [66] | |
| Tanzania | Kibaha Coast Region | - | 0.0001–95 | [66] | |
| o,p’-DDE | India | Hisar | - | ND–3 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.0001–7 | [66] | |
| Hisar | - | ND–2 | [73] | ||
| o,p’-DDD | Tanzania | Kibaha Coast Region | - | ND–5 | [66] |
| ΣDDT | India | Hisar | - | ND–7.06 | [73] |
| Tanzania | Kibaha Coast Region | - | 0.01–3200 | [66] | |
| Aldrin | Tanzania | Kibaha Coast Region | - | ND–0.044 | [66] |
| Dieldrin | Tanzania | Kibaha Coast Region | - | ND–1.3 | [66] |
| Endrin | Tanzania | Kibaha Coast Region | - | ND–0.2 | [66] |
| α-Endosulfan | Tanzania | Kibaha Coast Region | - | ND–0.2 | [66] |
| β-Endosulfan | Tanzania | Kibaha Coast Region | - | ND–0.03 | [66] |
| Endosulfan sulphate | India | Hisar | - | ND–0.4 | [73] |
| ΣEndosulfan | India | Hisar | - | ND–3.02 | [73] |
| Heptachlor | India | Hisar | - | ND–0.02 | [73] |
| Tanzania | Kibaha Coast Region | - | ND–0.2 | [66] | |
| Methoxychlor | Tanzania | Kibaha Coast Region | - | ND–0.1 | [66] |
6. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Activated carbon extraction |
| DDE | Dichlorodiphenyldichloroethylene |
| DDT | Dichlorodiphenyltrichloroethane |
| DLLME | Droplet liquid–liquid microextraction |
| DLLME-LSC | Dispersive liquid–liquid microextraction with little solvent consumption |
| DLLME-SFO | Dispersive liquid–liquid microextraction based on a solidification of floating organic drop |
| ECD | Electron capture detector |
| EI | Electron impact |
| GC | Gas chromatography |
| GC-MS | Gas chromatography–mass spectrometry |
| GC-QMS | Gas chromatography operating with quadrupole mass detector |
| HCB | Hexachlorobenzene |
| HCH | Hexachlorocyclohexane |
| HRGC-HRMS | High-resolution gas chromatography coupled with high-resolution mass spectrometry |
| HS-SPME | Headspace solid-phase microextraction |
| LLE | Liquid–liquid extraction |
| LLE-LTP | Liquid–liquid extraction with low-temperature partition |
| MDLs | Method detection limits |
| MSPE | Magnetic solid phase extraction |
| OCPs | Organochlorine pesticides |
| PCP | Pentachlorophenol |
| PeCB | Pentachlorobenzene |
| POPs | Persistent organic pollutants |
| SERS | Surface-enhanced Raman spectroscopy |
| SFC | Supercritical fluid chromatography |
| SME | Solvent microextraction |
| SPME | Solid-phase microextraction |
| SPE | Solid-phase extraction |
| TCmX | 2,4,5,6-tetrachloro-m-xylene |
| TLC | Thin-layer chromatography |
| VALLME | Vortex-assisted liquid–liquid microextraction |
| μECD | Micro-cell electron capture detector |
References
- Grung, M.; Lin, Y.; Zhang, H.; Steen, A.O.; Huang, J.; Zhang, G. Pesticide levels and environmental risk in aquatic environments in China—A review. Environ. Int. 2015, 81, 87–97. [Google Scholar] [CrossRef]
- Nayak, P.; Solanki, H. Pesticides and Indian agriculture—A review. Int. J. Res. Granthaalayah 2021, 9, 250–263. [Google Scholar] [CrossRef]
- Rusiecki, J.A.; McAdam, J.; Denic-Roberts, H.; Sjodin, A.; Davis, M.; Jones, R.; Hoang, T.D.; Ward, M.H.; Ma, S.; Zhang, Y. Organochlorine pesticides and risk of papillary thyroid cancer in U.S. military personnel: A nested case-control study. Environ. Health 2024, 23, 28. [Google Scholar] [CrossRef]
- Akoto, O.; Oppong-Otoo, J.; Osie-Fosu, P. Carcinogenic and non-carcinogenic risk of organochlorine pesticide residues in processed cereal-based complementary foods for infants and young children in Ghana. Chemosphere 2015, 132, 193–199. [Google Scholar] [CrossRef]
- Attaullah, M.; Yousuf, M.; Shaukat, S.; Anjum, S.I.; Ansari, M.J.; Buneri, I.D.; Tahir, M.; Amin, M.; Ahmad, N.; Khan, S.U. Serum organochlorine pesticides residues and risk cancer: A case-control study. Saudi J. Biol. Sci. 2018, 25, 1284–1290. [Google Scholar] [CrossRef]
- Guo, J.; Chen, W.; Wu, M.; Qu, C.; Sun, H.; Guo, J. Distribution, sources, and risk assessment of organochlorine pesticides in water from Beiluo River, Loess Plateau, China. Toxics 2023, 11, 496. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on Persistent Organic Pollutants. Available online: https://eur-lex.europa.eu/eli/reg/2019/1021/oj (accessed on 2 May 2024).
- Stockholm Convention. All POPs Listed in the Stockholm Convention. Available online: https://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 2 May 2024).
- The 12 Initial POPs under the Stockholm Convention. Available online: http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx (accessed on 2 May 2024).
- United Nations Environment Programme (UNEP). Why Do Persistent Organic Pollutants Matter? UNEP—UN Environment Programme. Available online: https://www.unep.org/topics/chemicals-and-pollution-action/pollution-and-health/persistent-organic-pollutants-pops/why (accessed on 2 May 2024).
- Bigot, M.; Curran, M.A.J.; Moy, A.D.; Muir, D.C.G.; Hawker, D.W.; Cropp, R.; Dachs, J.; Teixeira, C.F.; Nash Bengtson, S.M. Brief communication: Organochlorine pesticides in an archived firm core from Law Dome, East Antarctica. Cryosphere 2016, 10, 2533–2539. [Google Scholar]
- Directive 2008/105/EC of the European Parliament and the Council of 16 December 2008 on the Environmental Quality Standards in the Field of Water Policy. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 2 May 2024).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32020L2184 (accessed on 2 May 2024).
- Syed, J.H.; Malik, R.N.; Liu, D.; Xu, Y.; Wang, Y.; Li, J.; Zhang, G.; Jones, K.C. Organochlorine pesticides in air and soil and estimated air-soil exchange in Punjab, Pakistan. Sci. Total Environ. 2013, 444, 491–497. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, G.; Liu, Z. Organochlorine pesticides in surface water of Jiuxi Valley, China: Distribution, source analysis, and risk evaluation. J. Chem. 2020, 2020, 5101936. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Turgut, C.; Atatanir, L.; Cutright, T. Evaluation of pesticide contamination in Dilek National Park, Turkey. Environ. Monit. Assess. 2010, 170, 671–679. [Google Scholar] [CrossRef]
- PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1642.htm (accessed on 2 May 2024).
- ChemicalBook. Available online: https://www.chemicalbook.com/ (accessed on 2 May 2024).
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 May 2024).
- Stockholm Convention. Chemical Listed in Annex A. Available online: https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/tabid/5837/Default.aspx (accessed on 2 May 2024).
- Popek, E. Environmental Chemical Pollutants. In Sampling and Analysis of Environmental Chemical Pollutants, 2nd ed.; Popek, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–69. [Google Scholar] [CrossRef]
- EL-Saeid, M.H.; BaQais, A.; Alshabanat, M. Study of the Photocatalytic Degradation of Highly Abundant Pesticides in Agricultural Soils. Molecules 2022, 27, 634. [Google Scholar] [CrossRef]
- Raina, V.; Hauser, A.; Buser, H.R.; Rentsch, D.; Sharma, P.; Lal, R.; Holliger, C.; Poiger, T.; Müller, M.; Kohler, H.-P. Hydroxylated metabolites of β- and δ-hexachlorocyclohexane: Bacterial formation, stereochemical configuration, and occurrence in groundwater at a former production site. Environ. Sci. Technol. 2007, 41, 4292–4298. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Z.; Pang, S.; Bhatt, P.; Chen, S. Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system. Front. Microbiol. 2020, 11, 522. [Google Scholar] [CrossRef]
- Sparling, D. Organochlorine Pesticides. In Ecotoxicology Essentials. Environmental Contaminants and Their Biological Effects on Animals and Plants; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–107. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Li, Q.; Wang, J. Composition, distribution, and risk assessment of organochlorine pesticides in drinking water sources in South China. Water Qual. Expo. Health 2015, 7, 8–97. [Google Scholar] [CrossRef]
- Wolfe, L.; Zepp, R.; Paris, D.; Baughman, G.; Hollis, R. Methoxychlor and DDT degradation in water: Rates and products. Environ. Sci. Technol. 1977, 11, 1077–1081. [Google Scholar] [CrossRef]
- Sibali, L.; Okonkwo, J.; Zvinowanda, C. Determination of DDT and metabolites in surface water and sediment using LLE, SPE, ACE and SE. Bull. Environ. Contam. Toxicol. 2009, 83, 885–891. [Google Scholar] [CrossRef]
- Zacharia, J. Degradation Pathways of Persistent Organic Pollutants (POPs) in the Environment. In Persistent Organic Pollutants; Donyinah, S.K., Ed.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Rathore, H.S. Methods of and problems in analyzing pesticide residues in the environment. In Handbook of Pesticides. Methods of Pesticide Residue Analysis; Nollet, L., Rathore, H., Eds.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 8–44. [Google Scholar]
- Lee, C.-C.; Liu, Y.-S.; Tseng, C.-H.; Chen, J.-Y.; Juang, F.-S.; Chang, Y.-Y.; Hsu, H.-C. Pesticide Residue Testing System for Fruits and Vegetables by Color Identification Technology. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics—Taiwan (ICCE-TW), Yilan, Taiwan, 20–22 May 2019; pp. 1–2. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC 2020, 123, 115772. [Google Scholar] [CrossRef]
- Ohoro, C.R.; Wepener, V. Review of scientific literature on available methods of assessing organochlorine pesticides in the environment. Heliyon 2023, 9, e22142. [Google Scholar] [CrossRef]
- Campanale, C.; Massareli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC 2021, 144, 116423. [Google Scholar] [CrossRef]
- Jan, R.; Shah, J.; Khawaja, M.; Gul, K. DDT residue in soil and water in and around abandoned DDT manufacturing factory. Environ. Monit. Assess. 2009, 155, 31–38. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; van der Hoek, J.P. Residues of organochlorine pesticides (OCPs) in aquatic environment and risk assessment along Shaying River, China. Environ. Geochem. Health 2018, 40, 2525–2538. [Google Scholar] [CrossRef]
- Al Mahmud, M.N.U.; Khalil, F.; Rahman, M.; Mamun, M.I.R.; Shoeb, M.; El-Atu, A.M.A.; Park, J.-H.; Shin, H.-C.; Nahar, N.; Shim, J.-H. Analysis of DDT and its metabolites in soil and water samples obtained in the vicinity of a closed-down factory in Bangladesh using various extraction methods. Environ. Monit. Assess. 2015, 187, 743. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Awofolu, R.O. Methods for selective determination of persistent organochlorine pesticide residues in water and sediments by capillary gas chromatography and electron-capture detection. J. Chromatogr. A 2003, 983, 225–236. [Google Scholar] [CrossRef]
- De Jager, L.; Andrews, A. Development of a rapid screening technique for organochlorine pesticides using solvent microextraction (SME) and fast gas chromatography. Analyst 2000, 125, 1943–1948. [Google Scholar] [CrossRef]
- Mesquita, T.; Santos, R.; Cacique, A.; De Sa, L.; Silveiro, F.; Pinho, G. Easy and fast extraction methods to determine organochlorine pesticides in sewage sludge, soil, and water samples based at low temperature. J. Environ. Sci. Health B 2018, 53, 199–206. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Jorfi, S.; Poormohammadi, A.; Maraghi, E.; Almasi, H. Monitoring and health risk assessment of organochlorine pesticides in Karun River and drinking water Ahvaz city, South West of Iran. Toxin Rev. 2022, 41, 361–369. [Google Scholar] [CrossRef]
- Cortada, C.; Vidal, L.; Pastor, R.; Santiago, N.; Canals, A. Determination of organochlorine pesticides in water samples by dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry. Anal. Chim. Acta 2009, 649, 218–221. [Google Scholar] [CrossRef]
- Faraji, H.; Helalizadeh, M. Determination of organochlorine pesticides in river water using dispersive liquid-liquid microextraction and gas chromatography-electron capture detection. Intern. J. Environ. Anal. Chem. 2010, 90, 869–879. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Huang, S.-D. Dispersive liquid-liquid microextraction with little solvent consumption combined with gas chromatography-mass spectrometry for the pre-treatment of organochlorine pesticides in aqueous samples. J. Chromatogr. A 2009, 1216, 5171–5175. [Google Scholar] [CrossRef]
- Ozcan, S. Viable and rapid determination of organochlorine pesticides in water. Clean-Soil Air Water 2010, 38, 457–465. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Liu, X.; Lu, J.; Zhou, H.; Huang, S. Occurrence and distribution of organochlorine pesticides—Lindane, p,p’-DDT and heptachlor epoxide—In surface water of China. Environ. Int. 2008, 34, 1097–1103. [Google Scholar] [CrossRef]
- Badach, H.; Nazimek, T.; Kaminski, R.; Turski, W. Organochlorine pesticides concentration in the drinking water from regions of extensive agriculture in Poland. Ann. Agric. Environ. Med. 2000, 7, 25–28. [Google Scholar]
- Miliadis, G.E. Determination of pesticide residues in natural waters of Greece by solid phase extraction and gas chromatography. Bull. Environ. Contam. Toxicol. 1994, 52, 25–30. [Google Scholar] [CrossRef]
- Ruiz-Gil, L.; Romero-Gonzalez, R.; Garrido-Frenich, A.; Martinez Vidal, J. Determination of pesticides in water samples by solid phase extraction and gas chromatography tandem mass spectrometry. J. Sep. Sci. 2008, 31, 151–161. [Google Scholar] [CrossRef]
- Wu, C.; Luo, Y.; Gui, T.; Huang, Y. Concentrations and potential health hazards of organochlorine pesticides in shallow groundwater of Taihu Lake region, China. Sci. Total Environ. 2014, 470–471, 1047–1055. [Google Scholar] [CrossRef]
- Chen, C.; Li, T.; Zou, W.; Chen, S.; Zhang, K.; Ma, L. Spatial distribution and sources of organochlorine pesticides in surface waters of Shanghai, China. SN Appl. Sci. 2020, 2, 1739. [Google Scholar] [CrossRef]
- Golfinopoulos, S.; Nikolaou, A.; Kostopoulou, M.; Xilourgidis, N.; Vagi, M.; Lekkas, D. Organochlorine pesticides in the surface waters of Northern Greece. Chemosphere 2003, 50, 507–516. [Google Scholar] [CrossRef]
- Ozcan, S.; Tor, A.; Aydin, M.E. Application of magnetic nanoparticles to residue analysis of organochlorine pesticides in water samples by GC/MS. J. AOAC Int. 2012, 95, 1343–1349. [Google Scholar] [CrossRef]
- Jackson, G.; Andrews, R.J. New fast screening method for organochlorine pesticides in water by using solid-phase microextraction with fast gas chromatography and a pulsed-discharge electron capture detector. Analyst 1998, 123, 1085–1090. [Google Scholar] [CrossRef]
- Mendes, L.D.; Bernardi, G.; Elias, W.C.; de Oliveira, D.; Domingos, J.B.; Carasek, E. A green approach to DDT degradation and metabolite monitoring in water comparing the hydrodechlorimation efficiency of Pd, Au-on-Pd and Cu-on-Pd nanoparticle catalysis. Sci. Total Environ. 2021, 760, 143403. [Google Scholar] [CrossRef]
- Miclean, M.; Senila, L.; Cadar, O.; Roman, M.; Kovacs, M.H. Determination of organochlorine pesticides in Aries River water near industrial dump using SPE/HS-SPME/GC-ECD method. Agric.-Sci. Pract. 2015, 1–2, 123–127. [Google Scholar]
- Nodeh, H.R.; Ibrahim, W.A.W.; Kamboh, M.A.; Sanagi, M.M. Dispersive graphene-based silica coated magnetic nanoparticles as a new adsorbent for preconcentration of chlorination pesticides from environmental water. RSC Adv. 2015, 5, 76424–76434. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, Y.; Sun, Y.; Sheng, X.; Tong, Y.; Guo, J.; Zhou, B.; Zhao, J. Magnetic polyamidoamine dendrimers for magnetic separation and sensitive determination of organochlorine pesticides from water samples by high-performance liquid chromatography. J. Environ. Sci. 2021, 102, 64–73. [Google Scholar] [CrossRef]
- Barlas, N.; Çok, I.; Akbulut, N. The contamination levels of organochlorine pesticides in water and sediment samples in Uluabat Lake, Turkey. Environ. Monit. Assess. 2006, 118, 383–391. [Google Scholar] [CrossRef]
- Erkmen, B.; Kolankaya, D. Determination of organochlorine pesticide residues in water, sediment, and fish samples from the Meriç Delta, Turkey. Int. J. Environ. Anal. Chem. 2006, 86, 161–169. [Google Scholar] [CrossRef]
- Turgut, C. The contamination with organochlorine pesticides and heavy metals in surface water in Küçük Menderes River in Turkey, 2000–2002. Environ. Int. 2003, 29, 29–32. [Google Scholar] [CrossRef]
- El-Alfy, M.; Hasballah, A.; El-Hamid, H.T.A.; El-Zeiny, A.M. Toxicity assessment of heavy metals and organochlorine pesticides in freshwater and marine environments, Rosetta area, Egypt using multiple approaches. Sustain. Environ. Res. 2019, 29, 19. [Google Scholar] [CrossRef]
- Fosu-Mensah, B.; Okoffo, E.; Darko, G.; Gordon, C. Assessment of organochlorine pesticide residues in soils and drinking water sources from cocoa farms in Ghana. SpringerPlus 2016, 5, 869. [Google Scholar] [CrossRef]
- Mahugija, J.A.M.; Henkelmann, B.; Schramm, K.-W. Levels and patterns of organochlorine pesticides and their degradation products in rainwater in Kibaha Coast Region, Tanzania. Chemosphere 2015, 118, 12–19. [Google Scholar]
- Navarrete, I.; Tee, K.A.; Unson, J.R.; Hallare, A. Organochlorine pesticide residues in surface water and groundwater along Pampanga River, Philippines. Environ. Monit. Assess. 2018, 190, 289. [Google Scholar] [CrossRef]
- Roman, C.; Miclean, M.; Roman, M.; Senila, L.; Cadar, O.; Levei, E. Pollution indices for assessment of organochlorine pesticides contamination in Danube water and sediments, Calafat-Turnu Magurele sector, Romania. Stud. UBB Ambient. 2014, LXI, 129–137. [Google Scholar]
- Sandu, M.A.; Madjar, R.M.; Preda, M.; Vîrsta, A.; Stavrescu-Bedivan, M.-M.; Vasile Scăețeanu, G. Assessment of water quality and parasitofauna, and a biometric analysis of the prussian carp of the Romanian lentic ecosystem in Moara Domnească, Ilfov County. Water 2023, 15, 3978. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Sharma, A.K.; Sanghi, R. Organochlorine and organophosphorus pesticide residues in ground water and surface waters of Kanpur, Uttar Pradesh, India. Environ. Int. 2005, 31, 113–120. [Google Scholar] [CrossRef]
- Tyagi, S.; Siddarth, M.; Mishra, B.K.; Banerjee, B.D.; Urfi, A.J.; Madhu, S.V. High levels of organochlorine pesticides in drinking water as a risk factor for type 2 diabetes: A study in north India. Environ. Pollut. 2021, 271, 116287. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Xiong, S.; Zeng, F.; Bu, J.; Zhang, B.; Liu, W.; Zhou, H.; Qi, S.; Xu, L.; et al. Rapid transport of organochlorine pesticides (OCPs) in multimedia environment from karst area. Sci. Total Environ. 2021, 775, 145698. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Kathpal, T.S. Pesticide residues in rain water from Hisar, India. Environ. Monit. Assess. 2007, 133, 467–471. [Google Scholar] [CrossRef]
- Kuranchie-Mensah, H.; Atiemo, M.S.; Palm, L.M.N.-D.; Blankson-Arthur, S.; Tutum, A.O.; Fosu, P. Determination of organochlorine pesticide residue in sediment and water from Densu river basin, Ghana. Chemosphere 2012, 86, 286–292. [Google Scholar] [CrossRef]
- De Figueiredo, L.; Chiavelli, L.; Da Costa, W. Determination of concentration levels of organochlorine pesticides in water from Mandacaru Stream in Maringa-Parana-Brazil employing gas-chromatography-mass spectrometry. Anal. Lett. 2013, 46, 1597–1606. [Google Scholar] [CrossRef]
- Geyikçi, F.; Büyükgüngör, H. Monitoring of organochlorine pesticides in the surface waters from Mid-Black Sea region, Turkey. Environ. Monit. Assess. 2011, 173, 127–137. [Google Scholar] [CrossRef]
- Shukla, G.; Kumar, A.; Bhanti, M.; Joseph, P.E.; Taneja, A. Organochlorine pesticide contamination of ground water in the city of Hyderabad. Environ. Int. 2006, 32, 244–247. [Google Scholar] [CrossRef]
- Wang, D.; Yang, S.; Wang, G.; Gao, L.; Wang, Y.; Jiang, Q.; Chen, Y. Residues and distributions of organochlorine pesticides in China’s Weihe River. Pol. J. Environ. Stud. 2016, 25, 1285–1292. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L.; Yang, K.; Chen, Y. Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. J. Hazard. Mat. A 2006, 137, 68–75. [Google Scholar] [CrossRef]
- Miliadis, G.E. Organochlorine and organophosphorus pesticide residues in the water of the Pinios River, Greece. Bull. Environ. Contam. Toxicol. 1995, 54, 837–840. [Google Scholar] [CrossRef]
- Cai, S.R.; Sun, K.; Dong, S.Y.; Wang, Y.M.; Wang, S.; Jia, L. Assessment of organochlorine pesticide residues in water, sediment, and fish of the Songhua River, China. Environ. Forensics 2014, 15, 352–357. [Google Scholar] [CrossRef]
- Concha-Grana, E.; Turnes-Carou, M.I.; Muniategui-Lorenzo, S.; Lopez-Mahia, P.; Prada-Rodriguez, D.; Fernandez-Fernandez, E. Evaluation of HCH isomers and metabolites in soils, leachates, river water and sediments of a highly contaminated area. Chemosphere 2006, 64, 588–595. [Google Scholar] [CrossRef]
- Mikherjee, I.; Gopal, M. Chromatographic techniques in the analysis of organochlorine pesticide residues. J. Chromatogr. A 1996, 754, 33–42. [Google Scholar] [CrossRef]
- Mirzaei, M.; Rakh, M. Preconcentration of organochlorine pesticides in aqueous samples by dispersive liquid-liquid microextraction based on solidification of floating organic drop after SPE with multiwalled carbon nanotubes. J. Sep. Sci. 2014, 37, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mirmigkou, S.; de Boer, J. DDT and metabolites. In Dioxin and Related Compounds. The Handbook of Environmental Chemistry; Alaee, M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 49, pp. 355–378. [Google Scholar] [CrossRef]
- El-Gawad, H.A. Validation method of organochlorine pesticides residues in water using gas chromatography-quadruple mass. Water Sci. 2016, 30, 96–107. [Google Scholar] [CrossRef]
- Ali, I.; Singh, P.; Rawat, M.S.M.; Badoni, A. Analysis of organochlorine pesticides in the Hindon River water, India. J. Environ. Prot. Sci. 2008, 2, 47–53. [Google Scholar]
- Alnahdi, H.; Mousa, R.M.A.; El-Said, W. Development of organochlorine pesticide electrochemical sensor based on Fe3O4 nanoparticles@indium tin oxide electrode. Electroanalysis 2022, 35, 2202100659. [Google Scholar] [CrossRef]
- Goel, P.; Arora, M. Fabrication of chemical sensor for organochlorine pesticide detection using colloidal gold nanoparticles. MRS Commun. 2018, 8, 1000–1007. [Google Scholar] [CrossRef]
- DiScenza, D.; Lynch, J.; Miller, J.; Verderame, M.; Levine, M. Detection of organochlorine pesticides in contaminated marine environments via cyclodextrin-promoted fluorescence modulation. ACS Omega 2017, 2, 8591–8599. [Google Scholar] [CrossRef]
- Moldovan, R.; Iacob, B.-C.; Farcău, C.; Bodoki, E.; Oprean, R. Strategies for SERS detection of organochlorine pesticides. Nanomaterials 2021, 11, 304. [Google Scholar] [CrossRef]
- Li, C.; Qi, X.; Wang, Y.; Meng, Q.; Li, W.; Liu, L.; Zheng, Y.; Cui, H. Organochlorine pesticides in soil–groundwater–plant system in a famous agricultural production area in China: Spatial distribution, source identification and migration prediction. Water 2023, 15, 4147. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Rapid and sensitive quantification of the pesticide lindane by polymer modified electrochemical sensor. Sensors 2021, 21, 393. [Google Scholar] [CrossRef] [PubMed]
- Tzanetou, E.N.; Karasali, H. A comprehensive review of organochlorine pPesticide monitoring in agricultural soils: The silent threat of a conventional agricultural past. Agriculture 2022, 12, 728. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, F.; Liu, W.; Bu, J.; Hu, G.; Xie, S.; Yao, H.; Zhou, H.; Qi, S.; Huang, H. Organochlorine Pesticides in Karst Soil: Levels, Distribution, and Source Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 11589. [Google Scholar] [CrossRef]
- Pănescu, V.-A.; Bocoș-Bințințan, V.; Herghelegiu, M.-C.; Coman, R.-T.; Berg, V.; Lyche, J.L.; Beldean-Galea, M.S. Pollution Assessment with Persistent Organic Pollutants in Upper Soil of a Series of Rural Roma Communities in Transylvania, Romania, Its Sources Apportionment, and the Associated Risk on Human Health. Sustainability 2024, 16, 232. [Google Scholar] [CrossRef]
- Galani, Y.J.H.; Houbraken, M.; Wumbei, A.; Djeugap, J.F.; Fotio, D.; Gong, Y.Y.; Spanoghe, P. Contamination of foods from Cameroon with residues of 20 halogenated pesticides, and health risk of adult human dietary exposure. Int. J. Environ. Res. Public Health 2021, 18, 5043. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cui, Z.; Wang, Y.; Zhang, J. Characteristics and residual health risk of organochlorine pesticides in fresh Vegetables in the suburb of Changchun, Northeast China. Int. J. Environ. Res. Public Health 2022, 19, 12547. [Google Scholar] [CrossRef] [PubMed]
- Kartalović, B.; Mastanjević, K.; Novakov, N.; Vranešević, J.; LjubojevićPelić, D.; Puljić, L.; Habschied, K. Organochlorine Pesticides and PCBs in Traditionally and Industrially Smoked Pork Meat Products from Bosnia and Herzegovina. Foods 2020, 9, 97. [Google Scholar] [CrossRef]
- Đokić, M.; Nekić, T.; Varenina, I.; Varga, I.; SolomunKolanović, B.; Sedak, M.; Čalopek, B.; Kmetič, I.; Murati, T.; Vratarić, D. Distribution of pesticides and polychlorinated biphenyls in food of animal origin in Croatia. Foods 2024, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Đokić, M.; Nekić, T.; Varenina, I.; Varga, I.; SolomunKolanović, B.; Sedak, M.; Čalopek, B.; Vratarić, D.; Bilandžić, N. Pesticides and polychlorinated biphenyls in milk and dairy products in Croatia: A health risk assessment. Foods 2024, 13, 1155. [Google Scholar] [CrossRef] [PubMed]
- Quiralte, D.; Zarzo, I.; Fernandez-Zamudio, M.-A.; Barco, H.; Soriano, J.M. Urban Honey: A review of its physical, chemical, and biological parameters that connect it to the environment. Sustainability 2023, 15, 2764. [Google Scholar] [CrossRef]
- Ferencz, L.; Balog, A. A pesticide survey in soil, water and foodstuffs from Central Romania. Carpathian J. Earth Environ. Sci. 2010, 5, 111–118. [Google Scholar]
- Jin, X.; Liu, Y.; Qiao, X.; Guo, R.; Liu, C.; Wang, X.; Zhao, X. Risk assessment of organochlorine pesticides in drinking water source of the Yangtze River. Ecotoxicol. Environ. Saf. 2019, 182, 109390. [Google Scholar] [CrossRef]
- Malik, A.; Singh, V.; Singh, K. Occurrence and distribution of persistent trace organics in rainwater in an urban region (India). Bull. Environ. Contam. Toxicol. 2007, 7, 639–645. [Google Scholar] [CrossRef]

| OCP | Molecular Formula (Molecular Weight, g∙mol−1) | Water Solubility (mgL−1) at 20–25 °C | Persistence in Environment | Half-Life | Listed by Stockholm Convention | ||
|---|---|---|---|---|---|---|---|
| Water | Soil | ||||||
| HCHs | α-HCH | C6H6Cl6 (290.82) | 2.00 | High | 126 days | 26 years | Annex A * |
| β-HCH | 0.24 | 240 days | 12 years | Annex A * | |||
| γ-HCH | 8.52 | 692 days | 15 years | Annex A * | |||
| δ-HCH | 7.30 | 240 days | 10 years | - | |||
| DDTs | o,p’-DDT | C14H9Cl5 (354.48) | - | High | 100 years | 35 years | Annex B |
| p,p’-DDT | 0.025 | ||||||
| o,p’-DDD | C14H10Cl4 (320.04) | - | High | >30 years | >20 years | - | |
| p,p’-DDD | 0.09 | - | |||||
| o,p’-DDE | C14H8Cl4 (318.02) | - | High | >30 years | >20 years | - | |
| p,p’-DDE | 0.12 | - | |||||
| HCB | C6Cl6 (284.78) | 0.0047 | High | - | 3–6 years | Annex A * Annex C | |
| Drins | Aldrin | C12H8Cl6 (364.91) | 0.027 | Moderate | 32 days | 20–100 days | Annex A * |
| Dieldrin | C12H8Cl6O (380.91) | 0.14 | High | 4 months | 1.5–5.2 years | Annex A * | |
| Endrin | C12H8Cl6O (380.91) | 0.24 | Moderate | 7 days | 1–14 years | Annex A * | |
| Endrin aldehyde | C12H8Cl6O (380.91) | 0.024 | - | - | - | - | |
| Endrin ketone | C12H8Cl6O (380.91) | - | - | - | - | - | |
| Sulphs | α-endosulfan | C9H6Cl6O3S (406.93) | 0.32 | Moderate | 28 days | 35 days | Annex A ** |
| β-endosulfan | C9H6Cl6O3S (406.93) | 0.45 | Moderate | 28 days | 150 days | Annex A ** | |
| Endosulfan sulfate | C9H6Cl6O4S (422.92) | 0.48 | - | - | - | ||
| Chls | Chlordane | C10H6Cl8 (409.78) | 0.1 | High | - | - | Annex A * |
| Heptachlor | C10H5Cl7 (373.32) | 0.056 | High | 2–10 days | 3.5 years | Annex A * | |
| Others | Methoxychlor | C16H15Cl3O2 (345.65) | 0.1 | High | - | - | Annex A * |
| Mirex | C10Cl12 (545.54) | 0.0001 | High | - | - | Annex A * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madjar, R.M.; Vasile Scăețeanu, G.; Sandu, M.A. Sustainable Water Monitoring via Analytical Techniques and Protocols Applied in the Assessment of Organochlorine Pesticides. Sustainability 2024, 16, 5293. https://doi.org/10.3390/su16135293
Madjar RM, Vasile Scăețeanu G, Sandu MA. Sustainable Water Monitoring via Analytical Techniques and Protocols Applied in the Assessment of Organochlorine Pesticides. Sustainability. 2024; 16(13):5293. https://doi.org/10.3390/su16135293
Chicago/Turabian StyleMadjar, Roxana Maria, Gina Vasile Scăețeanu, and Mirela Alina Sandu. 2024. "Sustainable Water Monitoring via Analytical Techniques and Protocols Applied in the Assessment of Organochlorine Pesticides" Sustainability 16, no. 13: 5293. https://doi.org/10.3390/su16135293
APA StyleMadjar, R. M., Vasile Scăețeanu, G., & Sandu, M. A. (2024). Sustainable Water Monitoring via Analytical Techniques and Protocols Applied in the Assessment of Organochlorine Pesticides. Sustainability, 16(13), 5293. https://doi.org/10.3390/su16135293









