Abstract
This study aimed to evaluate the possibility of using milk thistle endosperm (MTE) extract with a protein content of >2 g/100 mL to obtain a fermented product, an alternative to milk beverages. Directed lactic fermentation with Lacticaseibacillus rhamnosus was carried out. The course of the process was monitored. The changes in pH, the saturation of the medium with dissolved oxygen, and temperature were measured. The values of the main nutritional parameters, silymarin content, amino acid profile/content, and the PDCAAS value (Protein Digestibility-Corrected Amino Acid Score) were determined in the extract before and after fermentation. The lactic acid fermentation of the MTE extract took place in four phases, with the activity of L. rhamnosus being the most intense between 4 and 8 h into the process. As a result of fermentation, there were changes in the total amino acid content in the tested medium, suggesting the catabolism of aspartic acid, arginine, proline, and isoleucine via LAB took place. Particularly significant was the increase in the content of lysine and valine (4.95 and 4.68 g/100 g of total AA, respectively), which are the limiting amino acids in MTE. Although MTE contained approximately 1% silymarin, no presence of this flavonolignan complex was found in the extracts.
1. Introduction
The production of plant-based dairy alternatives (PBDAs) is one of the key sectors supporting a sustainable food system (SFS). According to Mordor Intelligence data, the market size of so-called non-dairy yogurts (PBDY) in Europe is currently valued at USD 1.36 billion. It is estimated that by 2029 this value will increase twice [1]. Consumers’ belief in the good nutritional quality and potentially health-promoting properties of fermented foods is one of the most critical determinants influencing their purchasing decisions. However, it should be taken into account that in the case of alternative products, consumers expect that their taste profile does not differ from the taste profile of dairy products. If this happens, they will not be accepted, despite the marketing expenditure [2]. A similar problem arises in obtaining a consistency of PBDY that would naturally correspond with fermented milk products. Generally, obtaining a plant product with high visual substitutability for yogurt is problematic from a technological point of view. However, this feature is essential for consumers, which forces manufacturers to use a wide range of thickening and stabilizing substances. This procedure makes it easier to modify the consistency of the product and better adapt it to the recipients’ expectations. The most frequently used additives include starches (corn, rice, potato, and tapioca), modified starches, locust bean gum, pectins, carrageenan, agar-agar, and gums (gellan, acacia, xanthan, and guar) [3,4,5].
A simpler product solution in which the amount of food additives can be significantly reduced is the production of plant-based beverages. It enables the use of eco-friendly technologies for sustainable economic development [6]. Such unconventional solutions include ultrasound-assisted technology, ultra-high-pressure homogenization, enzyme-assisted extraction, and fermentation [7]. Lactic acid bacteria (LAB) efficiently ferment plant-based “milk”. This process improves the nutritional value of the final product by increasing the content of amino acids and vitamins while significantly shaping the health-promoting properties of the product (including antimicrobial, anticancer, and immunomodulatory properties) [8,9,10]. The selection of the LAB strain for a specific plant raw material allows for the creation of the odor profile of the fermented product [11] and improves its consistency through exopolysaccharides (EPSs) produced by LAB during fermentation. The ability to generate EPSs is possessed by bacteria from the genera Leuconostoc, Limosilactobacillus, Lactiplantibacillus, and Latilactobacillus [12]. The lactic fermentation process itself, or its combination with other procedures in the production of plant drinks (e.g., thermal processes and sprouting), can significantly reduce the content of anti-nutritional ingredients, including phytates [13,14,15]. Such properties are demonstrated by many strains, including Lactobacillus plantarum MTCC 1325 [16], L. fermentum B4655, L. plantarum B4495, L. casei B1922, L. bulgaricus CFR2028, L. acidophilus B4496 LAB [17], L. reuteri [18], L. plantarum C8 [19], and Lactococcus lactis RQ1066 [20]. This fact is essential from the perspective of positively verifying fermented plant products for safe use in human nutrition.
An auspicious direction for obtaining a fermented plant beverage is the use of potentially probiotic strains, which benefit the balance and structure of the intestinal microbiota and protect the body against pathogenic species [21]. This group of microorganisms includes, among others, Lacticaseibacillus rhamnosus (formerly known as Lactobacillus rhamnosus). According to the available literature data, L. rhamnosus, which is a facultative heterofermentative microorganism, has been successfully used for the fermentation of plant matrices, including soybean, oats, coconuts [22], cowpea-peanut [23], or germinated bean seed beverages [24]. Its effects have been well documented in terms of clinical benefits. Its applications include alleviating antibiotic-associated diarrhea, treating inflammation-associated diseases, and enhancing cholesterol efflux [25]. So far, only one publication describes the use of L. rhamnosus CCTCC M2021577 for the fermentation of extract from milk thistle fruits [26]. Generally, there is a lack of information in the literature on the possibility of processing this plant to obtain alternative protein preparations, fermented and unfermented beverages, and other products from the novel food category, even though milk thistle is a valuable source of nutrients, especially protein. We discussed this topic in detail in our previous publication [27]. Milk thistle (Silybum marianum) is an even more attractive raw material because the waste from its processing into silymarin, i.e., endosperm (MTE), could be successfully used for food purposes. So far, MTE has only been used as feed.
Obtaining a fermented beverage from milk thistle endosperm fits perfectly into the upcycling idea. According to [28], upcycling is defined as the “reuse of discarded materials which increases in value” [29], or “a process of converting materials into new materials of higher quality and increased functionality” [30]. Upcycled food must simultaneously meet three conditions: (I) it must be a product consisting of or containing materials that would otherwise be wasted; (II) the material must be transformed into a product intended for human consumption; and (III) it must be created through a process that is associated with an increase in value [28].
Taking into account the above premises, the aim of this work was to (I) verify the impact of lactic fermentation, using the commercially available probiotic strain L. rhamnosus, on the basic parameters of the nutritional value, silymarin content, and amino acid profile of an upcycled milk thistle endosperm beverage; and (II) to characterize of the dynamics of the lactic fermentation process of milk thistle extract.
2. Materials and Methods
2.1. Characterization of Plant Material
Milk thistle endosperm (S. marianum; MTE) was obtained from the Poznań Herbal Company ‘Herbapol’ S.A. (Poznań, Poland). The nutritional parameters of the MTE are shown in Table 1 and have been previously described by the authors of [31].

Table 1.
Nutritional value and sylimarin content in MTE (mean ± SD).
2.2. Lactic Fermentation (LF) of MTE Extract—Preparation of MTE Fermented Beverage
The method of preparing the raw (unfermented) extract from MTE and the basic parameters of the lactic fermentation process were consistent with that of [31]. Protein extraction from crushed MTE was carried out in an aqueous medium (1:6.66 w/w) in the presence of anhydrous sodium carbonate. The pH of the mixture was ±7.80; the extraction time and temperature were 20 min and 37 °C, respectively (Thermomix TM-6, Vorwerk, Germany). The obtained crude extract was centrifuged (MPW-351, Warsaw, Poland; 5 min, 2000 rpm). Then, the sugar content was corrected by adding beet molasses (Canisius-Hanssen B.V. Stroopfbriek, Stroopfbriek, The Netherlands) containing 65.2 carbohydrates/100 g (dose: 20 g of molasses/L of purified extract). The MTE extract was pasteurized for 20 min at 85 °C (Thermomix TM-6, Vorwerk, Germany) and left to cool (±35 °C).
The process of directed lactic fermentation was carried out using a strain of L. rhamnosus (Lactoferm LCR Pro-Tek®) (Biochem s.r.l., Biochemical Research Center, Roma, Italy). Fermentation was carried out in a BIOFLO 3000 bioreactor (New Brunswick Scientific, Edison, NJ, USA). To the bioreactor with a working capacity of 5 L, four liters of MTE extract and 0.4 g of a freeze-dried preparation of probiotic bacteria L. rhamnosus were added. The amount of probiotics was selected based on the producer’s recommendations for cow’s milk (10 g of the preparation/100 L), which provided 1.4 × 107 CFU of L. rhamnosus/mL of fermented MTE extract. The contents of the bioreactor was gently mixed (50 rpm). The fermentation process was monitored using AFS-Bio Comamnad Multi-Process Management Software version 2.62 (New Brunswick Scientific, Edison, NJ, USA), measuring changes in pH, the saturation of the medium with dissolved oxygen (DO), and the process temperature.
2.3. Nutrition Value of MTE Extracts
Protein, carbohydrates, sugars, fat, fiber, and ash content were determined in unfermented and fermented MTE extracts, according to the methods previously described by the authors of [32].
Sodium and calcium contents were determined using inductively coupled plasma atomic emission spectrometry (ICP-OES). An ICP-OES iCAP 6200 Duo Emission Spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and an UltraWAVE pressure mineralizer (Milestone SRL, Milan, Italy) were used for analysis. Sample preparation was by Polish standards [33,34].
Saturated fatty acid (SFA) content was quantified using a GC (Agilent 7890 A series, Agilent Tech. Inc., St. Clara, CA, USA) coupled with an FID (flame-ionization detector) and fused silica capillary column J&W Scientific HP-88 series (Agilent Tech. Inc., St. Clara, CA, USA). The samples were analogously prepared as described in [27], and chromatographic separation conditions were used as proposed by the authors of [35].
2.4. Sylimarin Content in MTE and MTE Extracts
The content of silymarin (%) in the freeze-dried MTE and MTE extracts (before and after fermentation) was determined by high-performance liquid chromatography (Agilent Technologies Infinity 1260 HPLC, Agilent Tech. Inc., St. Clara, CA, USA) according to the procedure described in detail in [36]. The mobile phase A was a mixture of phosphoric acid, methanol, and water in the ratio 0.5:35:65 (v/v/v); the mobile phase B was a mixture of similar composition in the ratio 0.5:50:50 (v/v/v). Gradient: 0–28 min → 100–0% phase A, 0–100% phase B; 28–35 min → 0% phase A, 100% phase B. Flow rate: 0.8 mL/min; detection: 288 nm; injection: 10 μL. Chromatography column: Merk LiChospher 100 RP-18 (125 mm × 4 mm (5 μm)).
2.5. Determination of Total Amino Acid (AA) Content
The analysis was determined using Agilent 1100 Series HPLC coupled with the UV DAD detector (Agilent, St. Clara, CA, USA). The chromatographic column parameters and conditions for the separation of AAs were described in [37,38]. The tryptophan content was determined after alkaline hydrolysis of the MTE extracts, according to the method in [39]. Sulfur AAs (cysteine and methionine) were determined as methionine sulfone and cysteic acid by performic acid oxidation before its digestion using 6M HCl [40]. The contents of methionine sulfone and cysteic acid were converted to methionine and cysteine, respectively.
2.6. PDCAAS (Protein Digestibility-Corrected Amino Acid Score) Calculation
The following formula was used to calculate the PDCAAS:
where
PDCAAS = AAS (Amino Acid Score) × TD (true fecal digestibility)
AAS = [(g of AAs in 100 g of MTE extract protein/g of AAs in 100 g of requirement pattern)] × 100%
The AA with the lowest AAS value is called the limiting amino acid.
Values for PDCAAS (Protein Digestibility-Corrected Amino Acid Score) were calculated using the requirement patterns recommended by FAO/WHO [41]. The reference scoring pattern (g/100 g protein) for children was the following: His 2.0; Ile 3.2; Leu 6.6; Lys 5.7; sulfuric AAs 2.7; aromatic AAs 5.2; Thr 3.1; Trp 0.85; and Val 4.3. The reference scoring pattern (g/100 g protein) for older children, adolescents, and adults was the following: His 1.6; Ile 3.0; Leu 6.1; Lys 4.8; sulfuric AAs 2.3; aromatic AAs 4.1; Thr 2.5; Trp 0.66; and Val 4.0. According to [42], the TD value was 86.92%.
2.7. Statistical Analysis
The nutrition value parameters, including amino acid content, of unfermented and fermented MTE extracts are presented as the mean ± SD (n = 3).
3. Results and Discussion
3.1. Dynamics of the Lactic Fermentation Process of MTE Extract
The course of the MTE fermentation process is shown in Figure 1.
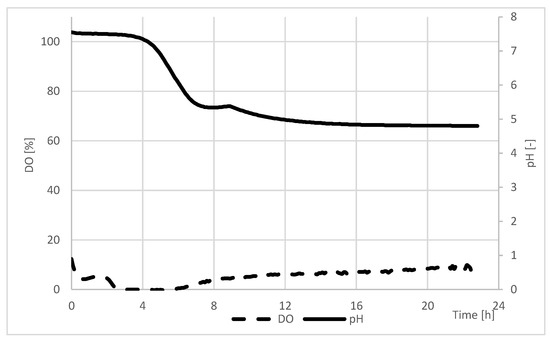
Figure 1.
Time-course of MTE extract fermentation in a batch bioreactor at 36 °C.
Based on the course of changes in the amount of dissolved oxygen and the pH value of the reaction mixture, four phases of the process can be observed. The first one, which can be described as adaptation, was when the bacteria adapted their enzymatic apparatus to the components of the new medium. During this time, the saturation of MTE extract with dissolved oxygen remained at approximately 5%, and the pH of the reaction mixture remained at approximately 7.4. In the next phase, intensive fermentation, the bacteria consumed the sugar component of the medium. They produced lactic acid, as evidenced by a rapid drop in the pH of the reaction mixture (from 7.4 to 5.4). In the next stage, the pH of the reaction mixture remained constant (approximately 5.35), and the saturation of the fermentation medium with dissolved oxygen slowly began to increase. It was in this stage when the bacteria began preparing to use other medium components. The last, and longest, stage of the process was characterized by slow changes in the pH of the mixture and a gradual increase in the amount of dissolved oxygen. At this stage, the microorganisms probably metabolized some amino acids.
When the pH of the fermented MTE extract reached 4.8, the fermentation process was completed. The saturation of the mixture with dissolved oxygen reached 7%.
Fermentation was carried out at a temperature of 36 °C, which is typical for producing this fermented drink [43]. As a result, the fermentation was slow, and the pH of the reaction mixture did not drop to extremely acidic values (pH 2.3–3.6), which is often the case with probiotics [43].
3.2. Nutritional Parameter Changes during MTE Extract Lactic Fermentation
The values of the analyzed nutritional quality parameters changed due to the fermentation of the milk thistle extract. The most significant differences were observed in the content of carbohydrates, including sugars (Table 2), which resulted from the biochemical specificity of lactic fermentation (LF). Lactic acid bacteria (LAB) convert these compounds into lactic acid, which decreases the fermented product’s pH [44]. Preparing the MTE extract for LF requires its prior enrichment with an easily assimilable carbon source for LAB (in this case, beet molasses). According to our previous experience, as a result of directed lactic fermentation of the MTE extract with the participation of L. rhamnosus, without its supplementation with sugars, negative sensory changes occurred, primarily the development of an intense, unpleasant odor, probably accompanying intensified proteolytic processes. This phenomenon was also found when the MTE extract was prepared using a higher proportion of extractant (water) to the weight of the raw material, i.e., 15:1. The sugar content was <0.5 mg/100 mL, and the protein content was <0.8 g/100 mL [31] L. rhamnosus can produce lactic acid from many carbohydrate substrates, including sucrose, arabinose, cellobiose, sorbitol, ribose, esculin [45], and glucose [46], which offers a wide range of possibilities for conducting directed lactic fermentation. However, in the case of sugar deficiency in the medium, LAB can change the direction of metabolic processes and use alternative energy sources contained in the medium, e.g., proteins. The course of lactic fermentation for milk thistle extract is described in detail in Section 3.1.

Table 2.
Nutritional parameters and sylimarin content of MTE extract before and after lactic fermentation (unfermented extract and fermented beverage, respectively) (mean ± SD).
The critical quality parameter is protein content, which is used to assess the nutritional quality of fermented plant beverages intended to be an alternative to dairy products. Fermented milk thistle beverage (MTB) contained 2.20 g of this ingredient/100 mL (Table 2), accounting for 74.83% of the total protein in MTE. According to Regulation No. 1924/2006 of the European Parliament and the Council, food is a source of protein when at least 12% of its energy value comes from protein. In turn, the declaration of a high protein content applies to products in which the share of energy from proteins is at least 20% [47]. In the case of the MTB, the share of energy from proteins was over 19%. This is essential information, considering the values of this indicator in products from the PBDAs sector available on the market, including plant-based yogurts (PBYA). According to [3], only 3 of 32 coconut yogurts, 1 of 12 almond yogurts, and 2 of 8 oat products contained enough protein to be labeled as a protein source. In each of these product groups, only one product was identified that would meet the requirements of food with a high content of this ingredient.
The lactic fermentation process did not cause a significant change in the protein content in the MTB compared to the unfermented extract. Still, differences are apparent when converting these values to [kcal] from protein per 100 kcal (Table 2). In the case of LF, it is possible to decompose proteins present in the plant matrix into simpler forms. According to [48], this process can improve protein digestibility. This is due to the partial hydrolysis of proteins into polypeptides, oligopeptides, and free amino acids, which favors their better availability and easier absorption. Proteases and peptidases catabolize proteins into amino acids. Their further conversion to aldehydes, (thio)esters, alcohols, and acids is particularly crucial. This process occurs during cheeses’ production and ripening, influencing their flavor and texture development, as mentioned by the authors of [49]. As shown in our research, the fermentation of the MTE extract by L. rhamnosus influenced the amino acid profile. This issue is discussed in detail in Section 3.3.
There was a noticeable increase in the SFA and UFA content in the MTB by 18 and 4%, respectively, compared to the unfermented extract (Table 2). According to research [24], changes in the fatty acid profile of plant drinks during their fermentation are possible. The authors verified the effect of LF using ten strains of bacteria of the Lactobacillus genus on the share of SFA (palmitic and stearic) and UFA (oleic, linoleic, and α-linolenic) in a fermented-bean-based drink. These changes were not always statistically significant; however, in the case of Lactobacillus helveticus LH–B01, a significant increase in the share of C18:0 acid was found (from 5.66 to 7.33%). In the beverage fermented by strains such as L. paracasei BGP1, L. acidophilus La3, L. rhamnosus LH32, and L. fermentum ATCC 9338, the share of C18:2 n-6 and C18:3 n-3 acids increased significantly. The transformations of lipids and fatty acids occurring during PBDA lactic fermentation are unknown and have not been documented. However, many publications indicate that LAB have an intracellular system of hydrolyzing enzymes, including lipases and esterases. Esterases are able to hydrolyze the carboxyl ester bond in aqueous solutions. They use water-soluble substrates. They can only hydrolyze triglycerides consisting of short-chain fatty acids [50]. However, lipases can catalyze the hydrolysis of carboxyl ester bonds in triglycerides. Free fatty acids (FFAs) and glycerin are then released at the lipid–water interface in emulsions. Lipases prefer substrates that do not dissolve in water, e.g., triglycerides composed of fatty acids with longer chains [50]. The activity of these enzymes and the transformations they initiate are currently described mainly in the context of dairy products, especially cheese [51,52,53,54].
Lactic acid fermentation may improve mineral bioavailability and increase their content in plant matrices [55]. The key ingredient in cow’s milk is calcium (approx. 120 mg/100 mL). It is true that some plant raw materials, e.g., almonds, hazelnuts, sesame, or sunflower seeds, have a similar or sometimes even higher content of this ingredient [56]. However, the beverages or yogurt substitutes obtained from them are ultimately low in Ca compared to fermented beverages or yogurts made from cow’s milk. It encourages producers of PBDAs to enrich them. According to [57], four different calcium salts were added to PBYAs. The most commonly used are tricalcium phosphate (TCP) and citrate, followed by lactate and carbonate. In general, milk thistle fruits are a valuable source of calcium. The Ca content in defatted milk thistle flour is 912 mg/100 g [58]. Our research shows that MTE contains 944-954 mg Ca/100 g FM [unpublished data]. As shown in Table 2, unfermented, non-Ca-fortified milk thistle extract contains 14.90 mg Ca/100 mL, accounting for 10.4% of the total calcium in MTE. After the fermentation process, this content increased by 2.75%. It can be assumed that the phytase activity of the LAB strain used in our experiment is responsible for this phenomenon. This is confirmed in [59]. These authors found that the phytase activity of L. plantarum L7 resulted in a significant increase in iron, manganese, magnesium, calcium, and sodium levels in a rice-based fermented drink. However, the authors of [60] indicate that during fermentation, the increase in the content of ingredients may also be caused by the loss of dry matter, which results from the microbiological decomposition of proteins and carbohydrates during this process.
The compound in milk thistle best known and described in the literature is silymarin. It is, in fact, a complex of flavonolignans, among which silybin has the most significant activity [61]. This compound is known for its hepatoprotective properties [62], which are responsible for its antioxidant activity and stabilizing the cell membrane, preventing/inhibiting lipid peroxidation [63]. Milk thistle endosperm contained approximately 1% total silymarin (Table 1). However, its presence was not detected in the MTE extract before or after fermentation. This fact may result from the conditions under which the protein extraction from the MTE was carried out (water as an extractant, the pH of the solution, the short extraction time, and the relatively low temperature) and the specificity of the LAB strain used. As shown in the experiment in [26], the extraction efficiency of silybin in fermented milk from milk thistle seeds was 11.24–12.14 mg/g of seeds, which accounted for 72.6–78.4% of the total silybin in S. marianum seeds, with a soluble protein yield reaching 33.8–39.0% of the total protein in seeds. These authors demonstrated that a NaHCO3 heat treatment was crucial in obtaining silybin. The most favorable extraction parameters in this respect were a 1% addition of NaHCO3, a temperature of 60 °C, and a time of 3 h. However, it should be kept in mind that the therapeutic effectiveness of silybin is limited, on the one hand, by its low solubility in water and, on the other hand, by low intestinal permeability. Therefore, it is poorly absorbed (20–50%) from the gastrointestinal tract and is characterized by a low bioavailability from oral preparations [64].
Table 3 compares commercially available plant-based yogurts [57,65] with the fermented milk thistle beverage regarding their nutritional parameters. The data show that the MTB is richer in protein than PBYAs, obtained from coconut, oats, and cashew nuts. It is characterized by a fat content similar to soy, oat, and pea products. At the same time, it has at least five times less saturated fatty acids than coconut yogurt. In terms of fiber content (0.9 g/150 mL), it is within the ranges given for the analyzed commercial competitive products. A characteristic feature of the MTB is also the low content of carbohydrates, including sugars, it has. However, it should be taken into account that, unlike commercially available products, the milk thistle drink did not contain any sweeteners that could affect the content of this macronutrient. In the case of plant-based products, sweeteners play an important role in shaping their taste and masking the sometimes unpleasant, specific sensory profile of plant proteins [66]. PBYAs may contain added sweeteners, syrups, or fruit purees [67].

Table 3.
Comparison of nutritional values of MTE fermented beverage and commercially available PBYA (150 g portions).
3.3. Changes in Amino Acid Profile and Content during Fermentation
Table 4 and Table 5 present the profile and content of amino acids in the unfermented and fermented MTE extract.

Table 4.
Profile and contents of amino acids (AAs) and the PDCAAS value of milk thistle unfermented extract (mean ± SD).

Table 5.
Profile and contents of amino acids (AAs) and the PDCAAS value of milk thistle fermented extract (mean ± SD).
The content of individual amino acids in the MTE extract changed due to fermentation. The analysis of the direction of these changes and the quantitative relationships between the AA content in the unfermented extract and MTB provided interesting information. The MTB had a lower content of endogenous amino acids—Asp, Arg, Pro, and Ile (exogenous amino acids). The largest, 12%, loss concerned arginine (from 10.38 to 9.12 g/100 g of all AAs in FM). According to [45], heterofermentative lactic acid bacteria can catabolize Arg, an additional energy source. In addition to the ATP synthesized through glycolysis, a further ATP-generating step may involve the breakdown of Arg via the arginine deiminase pathway, whereby carbamate kinase synthesizes ATP from carbamoyl phosphate with the synthesis of ammonia and CO2. This biochemical process is associated with the stage of cheese ripening. Access to fermentable carbohydrates, which are necessary for the growth of LAB, is then limited or missing [45]. Arg and Glu are important for adapting lactic acid bacteria to an acidic environment. Their deaminated metabolites are a key energy substrate for metabolism and cell growth [68]. In their review, the authors of [69] reported that fermentation contributes to the increase in all amino acids, except Met and Cys. However, our research did not confirm this. Other authors also described the reduction in the content of Asp, Arg, Pro, and Ile as a result of the LF of other plant matrices. For example, the authors of [70] found that in soy milk fermented with L. paracasei L9 and a commercial starter (L. bulgaricus and S. thermophilus), the total content of these AAs was significantly reduced, in contrast to the free amino acids. Similarly to the MTE extract, Arg showed the greatest decrease in soy milk due to fermentation (by 13 and 18%, respectively). In turn, the fermentation of peanut–soy milk with LAB strains such as L. acidophilus, L. rhamnosus, and L. delbrueckii subsp. delbrueckii contributed to the reduction in Arg concentration [71]. As shown by [72], the fermentation of spray-dried protein extract from sweet lupine (Lupinus angustifolius) seeds with bacterial strains L. plantarum L1047 and Pediococcus pentosaceus P113 without added sugar resulted not only in a decrease in the content of Arg, but also in Asp and Pro. These authors linked the sperm-like odor of the tested lupine extracts with the presence of 1-pyrroline. This Schiff base is known as a Strecker degradation product of proline.
The 7% decrease in Ile content observed in our research due to fermentation of the MTE extract by L. rhamnosus may indicate the ability of this strain to catabolize isoleucine. The mechanism of catabolizing BCCAs (branched-chain amino acids), i.e., Leu, Ile, and Val in lactic acid bacteria, is known. BCCAs are precursors of volatile compounds (e.g., aldehydes, esters, acids, and alcohols). In fermented foods and beverages, they can develop flavor profiles and generate off-flavors [73]. According to [74], the degradation of BCAAs starts by the action of an α-oxoglutarate-dependent aminotransferase (BcaT; EC.2.6.1.42), which catalyzes the hydrolysis of Leu, Ile, and Val to α-oxoisocaproate, α-oxo-γ -methyl valerate, and α-oxoisovalerate, respectively. Conversely, BcaTs also catalyze the final step in the bio-synthesis of BCAAs.
The direction and dynamics of amino acid metabolism, and consequently also the modifications of the profiles of extracellular and intracellular metabolites by LAB, are, therefore, an individual issue, depending on the specificity of the strain, the type of medium in which the fermentation process is carried out, and the type/availability of substrates. This has been proven, among others, in a study [75] in which L. rhamnosus GG was cultured in a modified MRS medium supplemented with various carbon sources, including galacto-oligosaccharides and β-mannase. Subsequently, combined transcriptomics and metabolomics analysis revealed alterations in pathways associated with membrane transport, amino acid metabolism, and carbohydrate metabolism. Valuable information on the variability of the metabolite profile and growth characteristics of L. rhamnosus was provided by the authors of [76]. These authors dealt with the L. rhamnosus Probio-M9 strain and its mutant L. rhamnosus R7970. They proved that R7970 was superior to Probio-M9 in its acid-producing ability. It also showed better growth in both agar and liquid media. Based on the transcriptomic and metabolomic analyses, the authors showed that R7970 had increased the metabolism of carbohydrates and amino acids, which resulted in improved acid production rate, tolerance, and efficiency in obtaining and using nutrients and energy [76]. Except for Asp, Arg, Pro, and Ile, whose content in the MTE extract decreased due to fermentation, and Hyp (absent in the unfermented extract and MTB), the share of the remaining amino acids increased. The highest increase, exceeding 8%, was observed in the case of His (from 2.58 to 2.80 g/100 g of total AAs). Histidine is an amino acid necessary for building proteins in the body; it is a structural element of enzymes, including serine proteases. In human muscles and the brain, His is a precursor of carnosine, which is believed to have an antioxidant and buffering role. By enzymatic decarboxylation, His can be converted to histamine. This reaction takes place in the stomach (enterochromaffin-like cells), the immune system (mast cells), and in various areas of the brain, where histamine can serve as a neurotransmitter [77]. The phenomenon of histamine intolerance is known. Both an increased availability of histamine and an impaired degradation of this biochemical amine may contribute to its development. The basic conditions for increased availability may be the endogenous overproduction of histamine resulting from an allergy, mastocytosis, bacteria, gastrointestinal bleeding, or increased exogenous intake of His or histamine with food or alcohol [78].
In addition to determining the amino acid profile, changes in protein quality in the milk thistle extracts were also monitored by determining the PDCAAS index of essential amino acids (Protein Digestibility-Corrected Amino Acid Score; Section 2.7) and comparing its values for individual AAs with the reference protein. The results are presented in Table 4 and Table 5. The fermentation of the MTE extract positively affected the PDCAAS of all amino acids considered in this context, except Ile. Assuming that the true fecal digestibility of milk thistle protein is almost 87% [42], it can be concluded that the MTB covers the needs of the considered age groups for His, Thr, Ile, Trp, sulfur AAs (Met+Cys), and aromatic AAs (Phe+Tyr). This confirms our previous research on milk thistle endosperm [27]. In milk thistle protein, the limiting amino acids are Lys and Val. It should be emphasized, however, that the value of the PDCAAS coefficient for both of these amino acids increased not only as a result of extract fermentation, but was also much higher in milk thistle extracts than in the endosperm of S. marianum [31]. In the fermented beverage (MTB), the PDCAAS value for Lys was higher by 18% (reference group: children 6 months to 3 years old) and 21% (older children, adolescents, and adults) compared to MTE and compared to the unfermented extract by 2 and 3%, respectively. The PDCAAS for Val recorded approximately a 10% increase in the fermented beverage for milk thistle endosperm. Therefore, this amino acid is no longer the second limiting amino acid in the MTB in the context of the requirements set by FAO/WHO for older children, adolescents, and adults. The demand for Val in this age group is met by over 100%.
Lys and Met are the most important limiting amino acids in legumes and cereals, which makes these raw materials inferior in terms of nutritional quality [79]. In turn, MTE and MTE extracts are excellent sources of sulfur AAs (Table 4 and Table 5), but, like other plant raw materials, they are poorer in lysine. L-Lys performs many important functions in the body. It is necessary to form structural, catalytic proteins and hormones and to support the immune system’s functioning [80]. Fermentation is an effective way to increase the content of this amino acid in plant products. As pointed out by the authors of [80], the key issue is the selection of appropriate microorganisms for a specific plant medium. In their research, these authors focused on fermentation in chickpea milk, using 31 strains, 9 of which significantly increased the production of Lys. Bacteria from the genera Bacillus, Lacticaseibacillus, Limosilactobacillus, and Levilactobacillus possessed these properties. B. amyloliquefaciens NCC 156 was highly effective. This microorganism underwent several distinct phases of clearly changed metabolism. The initial phase of fermentation (0–16 h) was the main phase of its growth, and, at the same time, Lys production (increased by 42.8%). For comparison, L. paracasei subsp. paracasei NCC 2511, despite the lower metabolic activity and completely different behavior during fermentation, contributed to an increase in total lysine by over 45% in the first 24 h of the process [80].
4. Conclusions
Milk thistle endosperm is a waste product that is produced during one of the stages of the industrial production of silymarin. After dehulling S. marianum fruits, only the husk is sent for further processing, while the endosperm is sold for feed purposes. Paradoxically, MTE is one of the most promising raw materials for the sustainable food industry. The rapidly growing market of alternative proteins and plant-based foods, including yogurt substitutes and dairy beverages, needs new, nutritionally valuable, cheap, and available protein sources. The results presented in this work contribute to popularizing research on processing this plant “waste” into a new product intended for human consumption. It has been shown that a fermented, upcycled milk thistle endosperm beverage, in terms of nutritional value, would compete with non-dairy yogurts, which are gaining an increasingly stronger position in the PBDAs market, especially in terms of protein content. The fact that using MTE extract for lactic fermentation is possible creates broad prospects for developing the milk thistle food range, including creating probiotic or synbiotic formulations. An additional advantage of this beverage is its interesting and valuable amino acid profile. The MTB is a valuable source of sulfur amino acids (Met+Cys), aromatic amino acids (Phe+Tyr), tryptophan, threonine, and histidine. Importantly, due to fermentation, the value of the PDCAAS index for Lys, the limiting amino acid in the MTE protein, increased by 18 and 21%, adequate in relation to the FAO standard, in the two age groups considered. However, issues related to the course of the lactic fermentation process in the MTE extract require continued investigation and detailed knowledge, including transcriptomic and metabolomic analyses, the selection of an optimal carbohydrate substrate, and selection of LAB strains that would not only be able to effectively carry out the fermentation process in this medium but also ensure an attractive sensory profile in the final product.
5. Patents
Teleszko, M., Zając, A., and Krzos, G. (2023) [31]. Fermented protein extract from milk thistle and method of obtaining it (in Polish), P.444467, Patent Office of the Republic of Poland; date of submitting an application for a patent for the invention: 18 April 2023; reporting entity: Wroclaw University of Economics and Business.
Author Contributions
Conceptualization, M.T. and A.Z.; methodology, M.T., A.Z., G.H., Z.G., K.G. and A.H.; software, M.T., G.H. and Z.G.; validation, M.T., G.H., A.Z. and A.H.; formal analysis, M.T.; investigation, M.T., G.H., A.Z., G.K., Z.G., K.G. and A.H.; resources, M.T., G.H., Z.G. and P.K.; data curation, M.T.; writing—original draft preparation, M.T., Z.G. and K.G.; writing—review and editing, A.Z., G.H., G.K. and P.K.; visualization, M.T. and G.H.; supervision, M.T.; project administration, M.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financed by the Ministry of Science and Higher Education in Poland under the program “Regional Initiative of Excellence”, 2019–2022, project number 015/RID/2018/19 and total funding amount 10,721,040.00 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Weronika Marcinkowska for the laboratory technical support.
Conflicts of Interest
Authors Agnieszka Hałaburda and Paweł Kotecki were employed by the company Poznan Herbal Company ‘Herbapol’ S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mordor Intelligence Europe Non-Dairy Yogurt Market Size & Share Analysis—Growth Trends & Forecasts Up to 2029. Available online: https://www.mordorintelligence.com/industry-reports/europe-non-dairy-yogurt-market (accessed on 4 April 2024).
- Jaeger, S.R.; Giacalone, D.; Jin, D.; Ryan, G.S.; Cardello, A.V. Information about Health and Environmental Benefits Has Minimal Impact on Consumer Responses to Commercial Plant-Based Yoghurts. Food Qual. Prefer. 2023, 106, 104820. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional Properties and Health Aspects of Pulses and Their Use in Plant-based Yogurt Alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef] [PubMed]
- Greis, M.; Sainio, T.; Katina, K.; Nolden, A.A.; Kinchla, A.J.; Seppä, L.; Partanen, R. Physicochemical Properties and Mouthfeel in Commercial Plant-Based Yogurts. Foods 2022, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.E.; Kinchla, A.J.; Nolden, A.A. A Comparison of the Nutritional Profile and Nutrient Density of Commercially Available Plant-Based and Dairy Yogurts in the United States. Front. Nutr. 2023, 10, 1195045. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Tixier, A.; Bily, A.; Chemat, F. Green Extraction Processes of Natural Products as Tools for Biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Penha, C.B.; Santos, V.D.P.; Speranza, P.; Kurozawa, L.E. Plant-Based Beverages: Ecofriendly Technologies in the Production Process. Innov. Food Sci. Emerg. Technol. 2021, 72, 102760. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Grom, L.C.; Rocha, R.S.; Balthazar, C.F.; Guimarães, J.T.; Coutinho, N.M.; Barros, C.P.; Pimentel, T.C.; Venâncio, E.L.; Collopy Junior, I.; Maciel, P.M.C.; et al. Postprandial Glycemia in Healthy Subjects: Which Probiotic Dairy Food Is More Adequate? J. Dairy Sci. 2020, 103, 1110–1119. [Google Scholar] [CrossRef]
- da Costa, G.M.; de Paula, M.M.; Costa, G.N.; Esmerino, E.A.; Silva, R.; de Freitas, M.Q.; Barão, C.E.; Cruz, A.G.; Pimentel, T.C. Preferred Attribute Elicitation Methodology Compared to Conventional Descriptive Analysis: A Study Using Probiotic Yogurt Sweetened with Xylitol and Added with Prebiotic Components. J. Sens. Stud. 2020, 35, e12602. [Google Scholar] [CrossRef]
- Tangyu, M.; Fritz, M.; Tan, J.P.; Ye, L.; Bolten, C.J.; Bogicevic, B.; Wittmann, C. Flavour by Design: Food-Grade Lactic Acid Bacteria Improve the Volatile Aroma Spectrum of Oat Milk, Sunflower Seed Milk, Pea Milk, and Faba Milk towards Improved Flavour and Sensory Perception. Microb. Cell Fact. 2023, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Varga, L. Exopolysaccharides from Lactic Acid Bacteria: Techno-Functional Application in the Food Industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Onyesom, I.; Enaholo, A.; Mordi, J. Effect of Processing Techniques on the Contents of Flatulence Factors and Emulsion Properties of Cowpea (Vigna unguiculata). J. Appl. Sci. Environ. Manag. 2005, 9, 65–72. [Google Scholar] [CrossRef][Green Version]
- Soetan, K.; Oyewole, O. The Need for Adequate Processing to Reduce the Anti- Nutritional Factors in Plants Used as Human Foods and Animal Feeds: A Review. Afr. J. Food Sci. 2009, 3, 223–232. [Google Scholar]
- Wang, Y.-C.; Yu, R.-C.; Yang, H.-Y.; Chou, C.-C. Sugar and Acid Contents in Soymilk Fermented with Lactic Acid Bacteria Alone or Simultaneously with Bifidobacteria. Food Microbiol. 2003, 20, 333–338. [Google Scholar] [CrossRef]
- Amritha, G.K.; Venkateswaran, G. Use of Lactobacilli in Cereal-Legume Fermentation and as Potential Probiotics towards Phytate Hydrolysis. Probiotics Antimicrob. Proteins 2018, 10, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of Isoflavone Glycosides to Aglycones, Mineral Bioavailability and Vitamin B Complex in Fermented Soymilk by Probiotic Bacteria and Yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, Sensory and Physicochemical Properties of Fermented Soy Protein Isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S. α-Galactosidase Activity and Oligosaccharides Reduction Pattern of Indigenous Lactobacilli during Fermentation of Soy Milk. Food Biosci. 2018, 22, 32–37. [Google Scholar] [CrossRef]
- Liang, Z.; Sun, J.; Yang, S.; Wen, R.; Liu, L.; Du, P.; Li, C.; Zhang, G. Fermentation of Mung Bean Milk by Lactococcus Lactis: Focus on the Physicochemical Properties, Antioxidant Capacities and Sensory Evaluation. Food Biosci. 2022, 48, 101798. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential Non-Dairy Probiotic Products—A Healthy Approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2021, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Chawafambira, A.; Jombo, T.Z.; Mkungunugwa, T. Effect of Lacticaseibacillus rhamnosus Yoba Fermentation on Physicochemical Properties, Amino Acids, and Antioxidant Activity of Cowpea-Peanut Milk. J. Food Qual. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Ziarno, M.; Bryś, J.; Parzyszek, M.; Veber, A. Effect of Lactic Acid Bacteria on the Lipid Profile of Bean-Based Plant Substitute of Fermented Milk. Microorganisms 2020, 8, 1348. [Google Scholar] [CrossRef] [PubMed]
- Mathipa-Mdakane, M.G.; Thantsha, M.S. Lacticaseibacillus rhamnosus: A Suitable Candidate for the Construction of Novel Bioengineered Probiotic Strains for Targeted Pathogen Control. Foods 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, M.; Ren, M.; Bao, H.; Wang, Q.; Wang, N.; Sun, S.; Xu, J.; Yang, X.; Zhao, X.; et al. From Medical Herb to Functional Food: Development of a Fermented Milk Containing Silybin and Protein from Milk Thistle. Foods 2023, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Haraf, G.; Zając, A.; Krzos, G. Milk Thistle (Silybum marianum (L.) Gaertner) Endosperm as an Alternative Protein Source for a Sustainable Food System (SFS)—Pilot Studies. Sustainability 2023, 15, 14411. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, V. Defining Upcycled Food: The Dual Role of Upcycling in Reducing Food Loss and Waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]
- Bridgens, B.; Powell, M.; Farmer, G.; Walsh, C.; Reed, E.; Royapoor, M.; Gosling, P.; Hall, J.; Heidrich, O. Creative Upcycling: Reconnecting People, Materials and Place through Making. J. Clean. Prod. 2018, 189, 145–154. [Google Scholar] [CrossRef]
- Ellen McArthur Foundation Circularity Indicators: An Approach to Measuring Circularity: METHODOLOGY. Available online: https://emf.thirdlight.com/link/3jtevhlkbukz-9of4s4/@/preview/1?o (accessed on 4 April 2024).
- Teleszko, M.; Zając, A.; Krzos, G.P. 444467 Fermented Protein Extract from Milk Thistle and Method of Obtaining It; Polish Patent Offic: Warszawa, Poland, 2023. (In Polish)
- Teleszko, M.; Zając, A.; Rusak, T. Hemp Seeds of the Polish ‘Bialobrzeskie’ and ‘Henola’ Varieties (Cannabis sativa L. Var. Sativa) as Prospective Plant Sources for Food Production. Molecules 2022, 27, 1448. [Google Scholar] [CrossRef] [PubMed]
- PN-EN 13805:2014-11; Food Products—Determination of Trace Elements—Pressure Mineralization. European Standard Commimitee: Bruxelles, Belgium, 2014.
- PN-EN 13804:2013-06; Food Products—Determination of Trace Elements and Their Chemical Forms—General Remarks and Specific Requirements. European Standard Commimitee: Bruxelles, Belgium, 2014.
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty Acid Profiles and Health Lipid Indices in the Breast Muscles of Local Polish Goose Varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Consiglio d’Europa. Direzione europea per la qualità dei farmaci e cura della salute, E.P.C. In European Pharmacopoeia: 11. Edition: Supplement 11.3: Published in Accordance with the Convention on the Elaboration of a European Pharmacopoeia (European Treaty Series N. 50); European Treaty Series; Council of Europe: London, UK, 2022; ISBN 9789287192592. [Google Scholar]
- Henderson, J.W.; Robert, D.R.; Brian, A.B.; Cliff, W. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids. In Amino Acid Analysis Using Zorbax Eclipse-AAA Columns and the Agilent 1100 HPLC; Agilent Technologies, Application Note 2000; Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.agilent.com/cs/library/chromatograms/59801193.pdf&ved=2ahUKEwih6ISzzvCGAxUWsFYBHfxnAmwQFnoECBAQAQ&usg=AOvVaw30_QZx-0o0Whkcg5ZD1Ste (accessed on 4 April 2024).
- Haraf, G.; Wołoszyn, J.; Okruszek, A.; Orkusz, A.; Wereńska, M. Nutritional Value of Proteins and Lipids in Breast Muscle of Geese from Four Different Polish Genotypes. Eur. Poult. Sci. 2018, 82, 224. [Google Scholar] [CrossRef]
- Çevikkalp, S.A.; Löker, G.B.; Yaman, M.; Amoutzopoulos, B. A Simplified HPLC Method for Determination of Tryptophan in Some Cereals and Legumes. Food Chem. 2016, 193, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Untea, A.E.; Margareta, O.; Panaite, T. Development and Validation of an RP-HPLC Method for Methionine, Cystine and Lysine Separation and Determination in Corn Samples. Rev. Chim. 2013, 64, 673–679. [Google Scholar]
- Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAQ Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 92.
- Zhu, S.; Dong, Y.; Tu, J.; Zhou, Y.; Dai, C. Amino Acid Composition and in Vitro Digestibility of Protein Isolates from Silybum Marianum. J. Food, Agric. Environ. 2013, 11, 136–140. [Google Scholar]
- Bernal-Castro, C.A.; Díaz-Moreno, C.; Gutiérrez-Cortés, C. Inclusion of Prebiotics on the Viability of a Commercial Lactobacillus casei Subsp. Rhamnosus Culture in a Tropical Fruit Beverage. J. Food Sci. Technol. 2019, 56, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Montet, D.; Ray, R.; Zakhia-Rozis, N. Lactic Acid Fermentation of Vegetables and Fruits. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014; pp. 108–140. [Google Scholar]
- Ibrahim, S. Lactic Acid Bacteria: Lactobacillus Spp.: Other Species. In Reference Module in Food Science; 2016; ISBN 9780081005965. Available online: https://www.sciencedirect.com/science/article/abs/pii/B978008100596500857X?via%3Dihub (accessed on 4 April 2024).
- Senedese, A.L.C.; Maciel Filho, R.; Maciel, M.R.W. L-Lactic Acid Production by Lactobacillus Rhamnosus ATCC 10863. Sci. World J. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation (EC). No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; Official Journal of the European Union. 2006; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1924-20141213 (accessed on 4 April 2024).
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Engels, W.; Siu, J.; van Schalkwijk, S.; Wesselink, W.; Jacobs, S.; Bachmann, H. Metabolic Conversions by Lactic Acid Bacteria during Plant Protein Fermentations. Foods 2022, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K. Microbial Lipases Form Versatile Tools for Biotechnology. Trends Biotechnol. 1998, 16, 396–403. [Google Scholar] [CrossRef]
- Katz, M.; Medina, R.; Gonzalez, S.; Oliver, G. Esterolytic and Lipolytic Activities of Lactic Acid Bacteria Isolated from Ewe’s Milk and Cheese. J. Food Prot. 2002, 65, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Mancheño, J.M.; de las Rivas, B.; Muñoz, R. Production and Characterization of a Tributyrin Esterase from Lactobacillus Plantarum Suitable for Cheese Lipolysis. J. Dairy Sci. 2014, 97, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Abeijón Mukdsi, M.C.; Medina, R.B.; Katz, M.B.; Pivotto, R.; Gatti, P.; González, S.N. Contribution of Lactic Acid Bacteria Esterases to the Release of Fatty Acids in Miniature Ewe’s Milk Cheese Models. J. Agric. Food Chem. 2009, 57, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Câmara, S.; Dapkevicius, A.; Riquelme, C.; Elias, R.; Silva, C.; Malcata, F.; Dapkevicius, M. Potential of Lactic Acid Bacteria from Pico Cheese for Starter Culture Development. Food Sci. Technol. Int. 2019, 25, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Puniya, A.K.; Dhewa, T. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health Issues and Technological Aspects of Plant-Based Alternative Milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Brothers, C.J. Nutritional Content and Health Profile of Non-Dairy Plant-Based Yogurt Alternatives. Nutrients 2021, 13, 4069. [Google Scholar] [CrossRef]
- Apostol, L.; Iorga, C.S.; Moşoiu, C.E.; Mustățea, G.; Cucu, Ș.E. Nutrient Composition of Partially Defatted Milk Thistle Seeds. In Proceedings of the Scientific Bulletin; Series, F., Ed.; Apostol 2017 NUTRIENTCO; Biotechnologies: Delhi, India, 2017. [Google Scholar]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a Potential Probiotic, Lactobacillus Plantarum L7, for the Preparation of a Rice-Based Fermented Beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Day, C.N.; Morawicki, R.O. Effects of Fermentation by Yeast and Amylolytic Lactic Acid Bacteria on Grain Sorghum Protein Content and Digestibility. J. Food Qual. 2018, 2018, 3964392. [Google Scholar] [CrossRef]
- Fallah, M.; Davoodvandi, A.; Nikmanzar, S.; Aghili, S.; Mirazimi, S.M.A.; Aschner, M.; Rashidian, A.; Hamblin, M.R.; Chamanara, M.; Naghsh, N.; et al. Silymarin (Milk Thistle Extract) as a Therapeutic Agent in Gastrointestinal Cancer. Biomed. Pharmacother. 2021, 142, 112024. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Ashtary-Larky, D.; Asbaghi, O.; Farrokhi, V.; Jadidi, Y.; Mofidi, F.; Mohammadian, M.; Afrisham, R. Effects of Silymarin Supplementation on Liver and Kidney Functions: A Systematic Review and Dose–Response Meta-analysis. Phyther. Res. 2024, 38, 2572–2593. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, V.A.; Ryzhov, V.M.; Biryukova, O.V.; Mel’nikova, N.B.; Selekhov, V.V. Interaction of Milk-Thistle-Fruit Flavanonols with Langmuir Monolayers of Lecithin and Bilayers of Liposomes. Pharm. Chem. J. 2009, 43, 101–109. [Google Scholar] [CrossRef]
- Wu, J.-W.; Lin, L.-C.; Hung, S.-C.; Chi, C.-W.; Tsai, T.-H. Analysis of Silibinin in Rat Plasma and Bile for Hepatobiliary Excretion and Oral Bioavailability Application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Medici, E.; Craig, W.J.; Rowland, I. A Comprehensive Analysis of the Nutritional Composition of Plant-Based Drinks and Yogurt Alternatives in Europe. Nutrients 2023, 15, 3415. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Alrosan, M.; Tan, T.-C.; Koh, W.Y.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H. Overview of Fermentation Process: Structure-Function Relationship on Protein Quality and Non-Nutritive Compounds of Plant-Based Proteins and Carbohydrates. Crit. Rev. Food Sci. Nutr. 2023, 63, 7677–7691. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, Y.; Yu, J.; Wang, F.; Li, X.; Liu, Y.; Ma, X. Changes of Proteins and Amino Acids in Soymilk during Lactic Acid Fermentation and Subsequent Storage. J. Food Meas. Charact. 2022, 16, 4728–4737. [Google Scholar] [CrossRef]
- do Amaral Santos, C.C.A.; da Silva Libeck, B.; Schwan, R.F. Co-Culture Fermentation of Peanut-Soy Milk for the Development of a Novel Functional Beverage. Int. J. Food Microbiol. 2014, 186, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.; Wittig, M.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Lactic Fermentation to Improve the Aroma of Protein Extracts of Sweet Lupin (Lupinus angustifolius). Food Chem. 2011, 128, 330–337. [Google Scholar] [CrossRef]
- Fernández, M.; Zúñiga, M. Amino Acid Catabolic Pathways of Lactic Acid Bacteria. Crit. Rev. Microbiol. 2006, 32, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Hutson, S. Structure and Function of Branched Chain Aminotransferases; Elsevier: Amsterdam, The Netherlands, 2001; pp. 175–206. [Google Scholar]
- Li, E.; Zhu, Q.; Pang, D.; Liu, F.; Liao, S.; Zou, Y. Analysis of Lactobacillus Rhamnosus GG in Mulberry Galacto-Oligosaccharide Medium by Comparative Transcriptomics and Metabolomics. Front. Nutr. 2022, 9, 853271. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Sun, Y.; Wu, T.; Kwok, L.-Y.; Wang, J.; Zhang, H. Metabolic Profiling and Growth Characteristics of a Spaceflight-Induced Mutant of Lacticaseibacillus rhamnosus: Unveiling Enhanced Carbohydrate and Amino Acid Metabolism for Improved Probiotic Potential. Food Biosci. 2024, 58, 103758. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Histidine Metabolism and Function. J. Nutr. 2020, 150, 2570S–2575S. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Novak, N. Histamine and Histamine Intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R. Fortifying Plants with the Essential Amino Acids Lysine and Methionine to Improve Nutritional Quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Fritz, M.; Aragao-Börner, R.; Ye, L.; Bogicevic, B.; Bolten, C.J.; Wittmann, C. Genome-Based Selection and Application of Food-Grade Microbes for Chickpea Milk Fermentation towards Increased l-Lysine Content, Elimination of Indigestible Sugars, and Improved Flavour. Microb. Cell Fact. 2021, 20, 109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).