Integrated Ozonation and Photocatalysis to Remove Pollutants for Reuse of Rainwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples of Rainwater
2.2. Treatment of Rainwater
2.3. Chemical Analysis
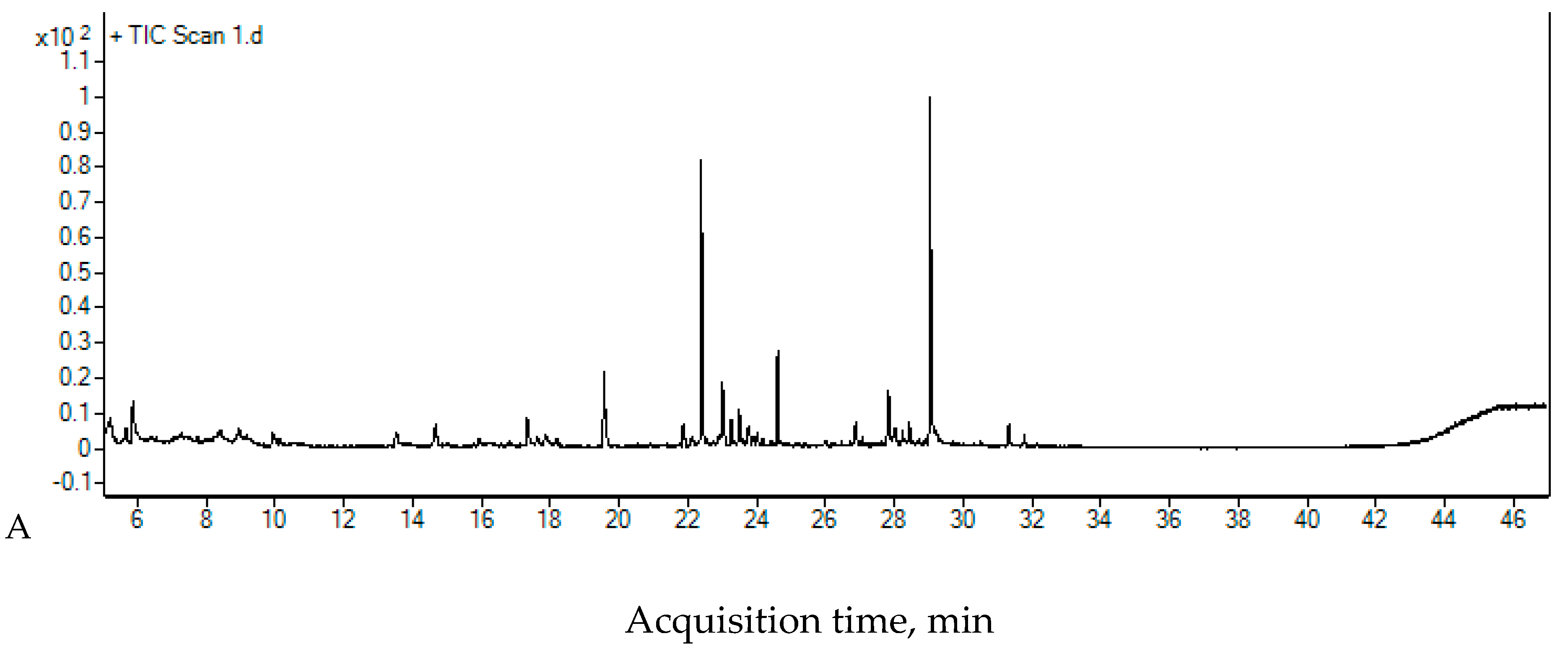
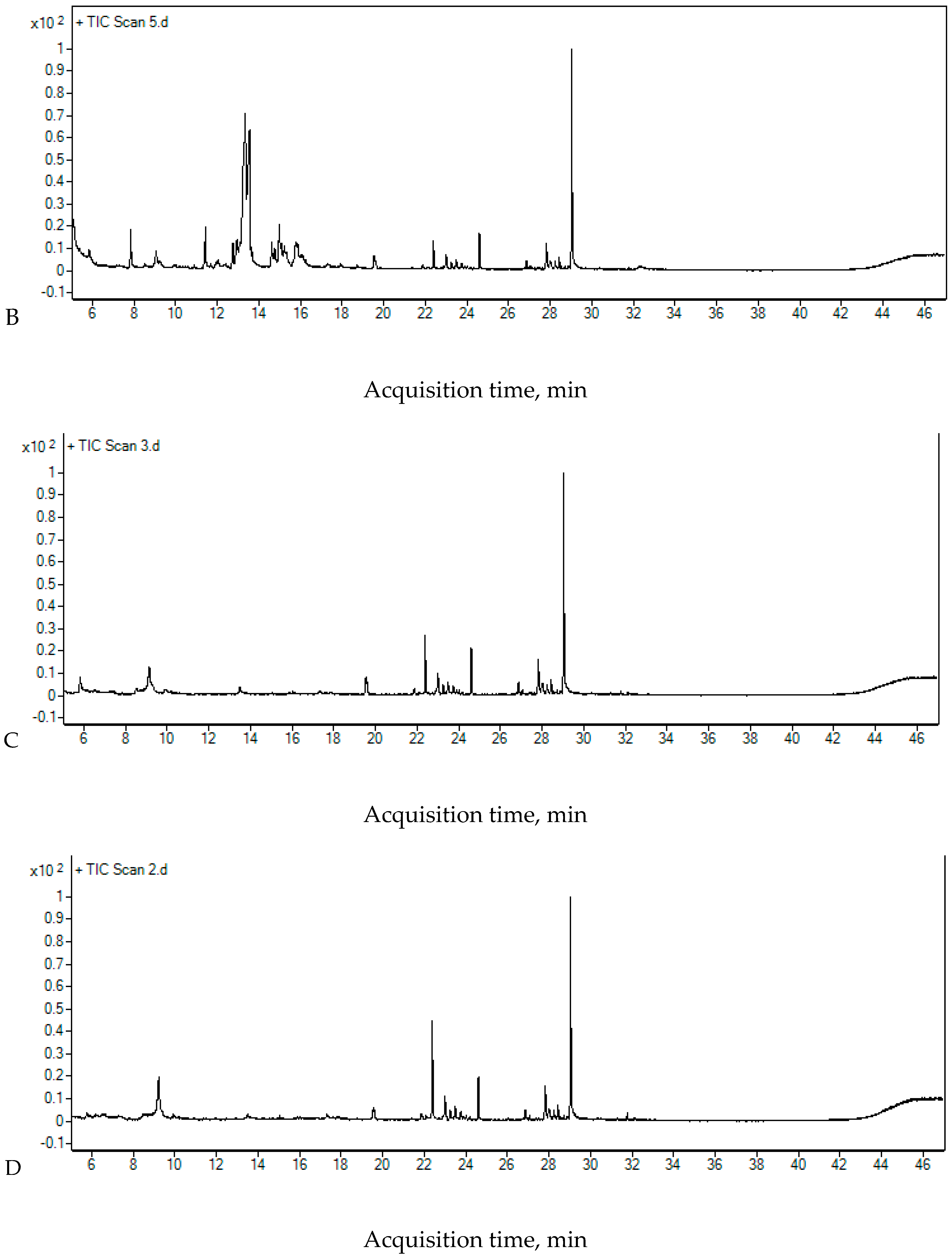
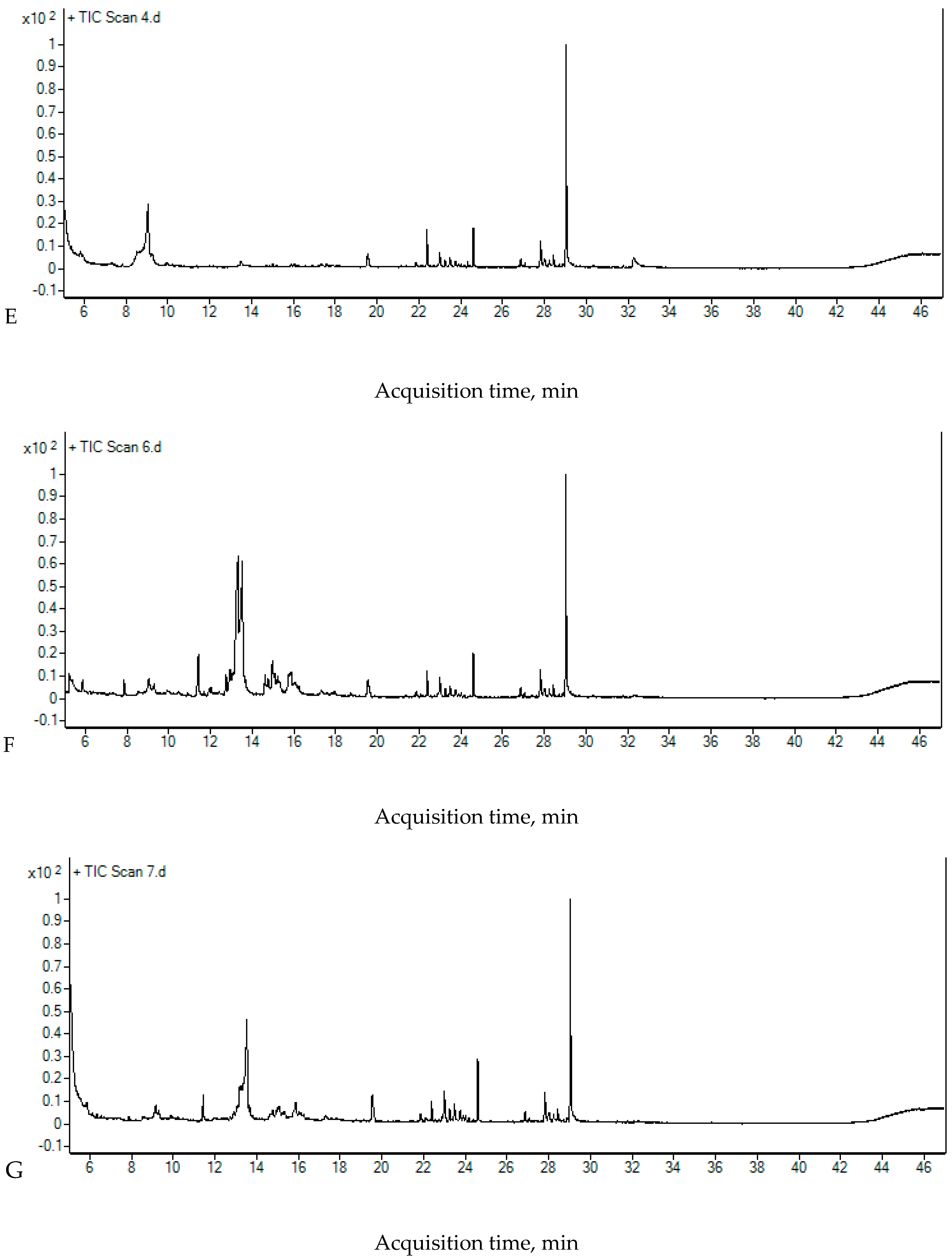
2.4. The Chromatographic Analysis
2.5. Microbiological Analysis
2.6. Ecotoxicity and Genotoxicity Tests
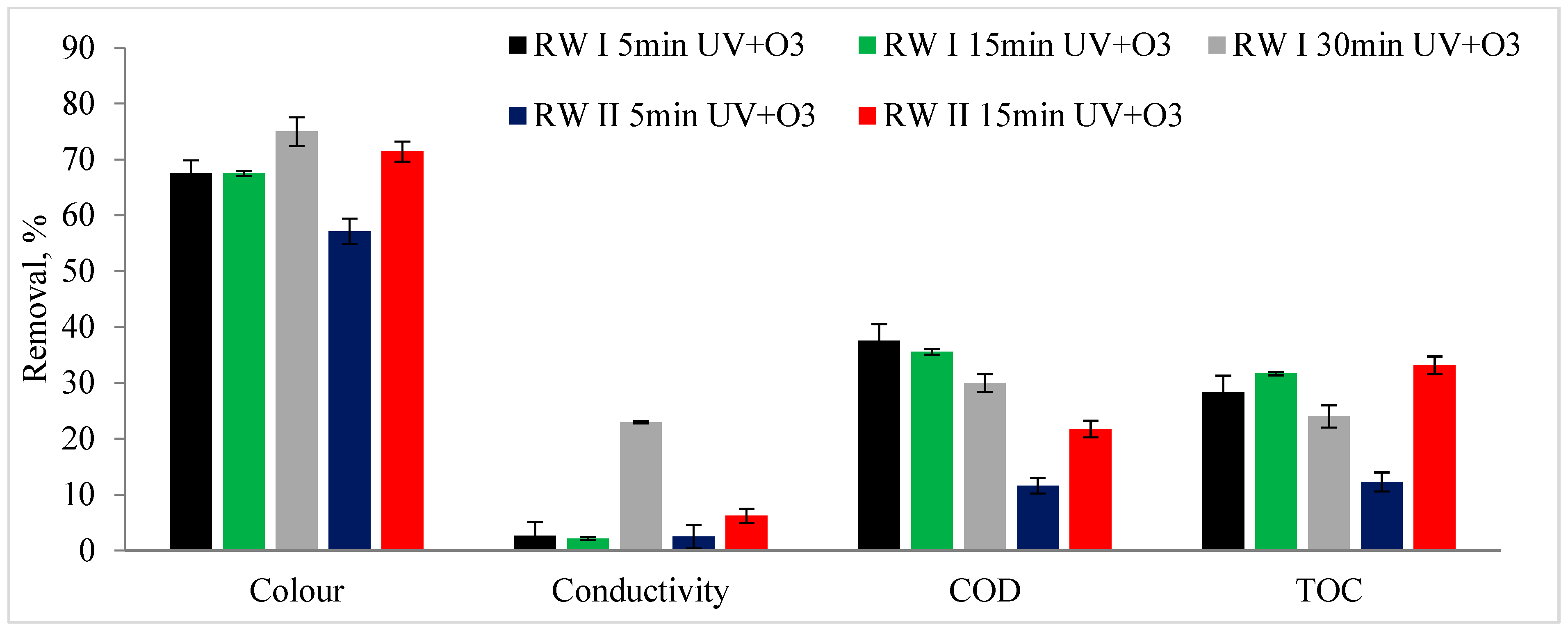
3. Results and Discussion
4. Conclusions
- The analysis of rainwater revealed its complex nature, attributed to the diversity of pollutants, encompassing alkanes, phenolic derivatives, and benzothiazole. The presence of these contaminants highlights the need for research on rainwater ecotoxicity. Investigating the emission and control of pollutants, especially alkanes, emerged as crucial for maintaining a healthy environment.
- The photocatalytic ozonation process demonstrated high efficiency in degrading benzothiazole, confirming its potential for the removal of organic pollutants.
- It is noteworthy that the photocatalytic ozonation process successfully eliminated heavy metals such as lead, nickel, and copper, employing metal oxide as a catalyst. However, the concentration of zinc did not undergo significant changes following the process. This observation may suggest the existence of specific chemical properties of zinc, underscoring the necessity for additional research to comprehend the mechanisms influencing its stability within the process.
- The count of coliforms and E. coli bacteria was ten times higher in rainwater collected from the roof compared to the runoff from the highway. The photocatalytic ozonation process led to the inactivation of E. coli in both instances, irrespective of the duration of the process. Conversely, in the rainwater sourced from the highway, only 62.5% of coliforms were removed.
- Ecotoxicological studies revealed that rainwater, before and after photocatalytic ozonation, showed no toxicity to crustaceans. Highway stormwater runoff displayed a slight decrease in bioluminescence of A. fischeri and a slight reduction in total leaf area of L. minor, both before and after the process. Rainwater from the roof, at the highest concentrations, slightly stimulated algal growth and bioluminescence of A. fischeri, but post-treatment samples showed slight toxicity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukarram, M.M.T.; Kafy, A.A.; Rukiya, Q.U.; Almulhim, A.I.; Das, A.; Fattah, A.; Rahman, M.T.; Chowdhury, M.A. Perception of coastal citizens on the prospect of community-based rainwater harvesting system for sustainable water resource management. Resour. Conserv. Recycl. 2023, 198, 107196. [Google Scholar] [CrossRef]
- Deng, Y. Pollution in rainwater harvesting: A challenge for sustainability and resilience of urban agriculture. J. Hazard. Mater. Lett. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Mazurkiewicz, K.; Jeż-Walkowiak, J.; Michałkiewicz, M. Physicochemical and microbiological quality of rainwater harvested in underground retention tanks. Sci. Total Environ. 2022, 814, 152701. [Google Scholar] [CrossRef]
- Vlastos, D.; Antonopoulou, M.; Lavranou, A.; Efthimiou, I.; Dailianis, S.; Hela, D.; Lambropoulou, D.; Paschalidou, A.K.; Kassomenos, P. Assessment of the toxic potential of rainwater precipitation: First evidence from a case study in three Greek cities. Sci. Total Environ. 2019, 648, 1323–1332. [Google Scholar] [CrossRef]
- Villafañe, A.B.; Ronda, A.C.; Pirani, L.S.R.; Picone, A.L.; Lucchi, L.D.; Romano, R.M.; Pereyra, M.T.; Arias, A.H. Microplastics and anthropogenic debris in rainwater from Bahia Blanca, Argentina. Heliyon 2023, 9, e17028. [Google Scholar] [CrossRef]
- FAO; UN-Water. Progress on Level of Water Stress. Global Status and Acceleration Needs for SDG Indicator 6.4.2. Rome 2021. Available online: https://www.unwater.org/publications/progress-level-water-stress-2021-update (accessed on 18 May 2024).
- Singh, S.; Yadav, R.; Kathi, S.; Singh, A.N. Treatment of harvested rainwater and reuse: Practices, prospects, and challenges. In Advances in Environmental Pollution Research, Cost Effective Technologies for Solid Waste and Wastewater Treatment; Kathi, S., Devipriya, S., Thamaraiselvi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 14; pp. 161–178. [Google Scholar]
- Deksissa, T.; Trobman, H.; Zendehdel, K.; Azam, H. Integrating urban agriculture and stormwater management in a circular economy to enhance ecosystem services: Connecting the dots. Sustainability 2021, 13, 8293. [Google Scholar] [CrossRef]
- Keresztesi, Á.; Nita, I.-A.; Birsan, M.-V.; Bodor, Z.; Pernyeszi, T.; Micheu, M.M.; Szép, R. Assessing the variations in the chemical composition of rainwater and air masses using the zonal and meridional index. Atmos. Res. 2020, 237, 104846. [Google Scholar] [CrossRef]
- Wei, L.; Yue, Q.; Chen, G.; Wang, J. Microplastics in rainwater/stormwater environments: Influencing factors, sources, transport, fate, and removal techniques. Trends Analyt. Chem. 2023, 165, 117147. [Google Scholar] [CrossRef]
- Niloy, N.M.; Haque, M.; Tareq, S.M. Characterization of dissolved organic matter at urban and industrial rainwater of Bangladesh by fluorescence spectroscopy and EEM-PARAFAC modeling. Environ. Chall. 2021, 5, 100250. [Google Scholar] [CrossRef]
- Santos, P.V.S.; Libânio, M.; Teixeira, M.C. Chitosan in the treatment of mine spoil rainwater—An approach to protect the aquatic biota. Sci. Total Environ. 2024, 912, 168900. [Google Scholar] [CrossRef]
- Baú, S.R.C.; Bevegnu, M.; Giubel, G.; Gamba, V.; Cadore, J.S.; Brião, V.B.; Shaheed, M.H. Development and economic viability analysis of photovoltaic (PV) energy powered decentralized ultrafiltration of rainwater for potable use. J. Water Process Eng. 2022, 50, 103228. [Google Scholar] [CrossRef]
- Anchan, S.S.; Prasad, H.S. Feasibility of roof top rainwater harvesting potential—A case study of South Indian University. Clean. Eng. Technol. 2021, 4, 100206. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Liu, C.; Wang, S.; Wang, X.; Hou, H.; Wang, J.; Li, H. Purification of harvested rainwater using slow sand filters with low-cost materials: Bacterial community structure and purifying effect. Sci. Total Environ. 2019, 674, 344–354. [Google Scholar] [CrossRef]
- Marszałek, A. Adsorption of copper and lead from rainwater using adsorbents based on diatomite and calcium alginate. Desalin. Water Treat. 2022, 275, 81–91. [Google Scholar] [CrossRef]
- Marszałek, A.; Kamińska, G.; Salam, N.F.A. Simultaneous adsorption of organic and inorganic micropollutants from rainwater by bentonite and bentonite-carbon nanotubes composites. J. Water Process Eng. 2022, 46, 102550. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Z.; Ngo, H.H.; He, X.; Desmond, P.; Ding, A. Membrane technology for rainwater treatment and reuse: A mini review. Water Cycle 2021, 2, 51–63. [Google Scholar] [CrossRef]
- Marszałek, A.; Dudziak, M. Application of the Ultrafiltration and Photooxidation Process for the Treatment of Rainwater. Water Air Soil Pollut. 2021, 232, 504. [Google Scholar] [CrossRef]
- Qumar, U.; Hassan, J.Z.; Bhatti, R.A.; Raza, A.; Nazir, G.; Nabgan, W.; Ikram, M. Photocatalysis vs adsorption by metal oxide nanoparticles. J. Mater. Sci. Technol. 2022, 131, 122–166. [Google Scholar] [CrossRef]
- Affek, K.; Muszynski, A.; Doskocz, N.; Zaleska-Radziwill, M. Ecotoxicological effects of disinfection of treated wastewater. Desalination Water Treat. 2021, 233, 190–198. [Google Scholar] [CrossRef]
- Mohan, H.; Vadivel, S.; Rajendran, S. Removal of harmful algae in natural water by semiconductor photocatalysis—A critical review. Chemosphere 2022, 302, 134827. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. Int. 2021, 28, 62572–62582. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef] [PubMed]
- Kudlek, E. Transformation of contaminants of emerging concern (CECs) during UV-catalyzed processes assisted by chlorine. Catalysts 2020, 10, 1432. [Google Scholar] [CrossRef]
- Kudlek, E. Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes. Water 2018, 10, 955. [Google Scholar] [CrossRef]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent advances in the elimination of persistent organic pollutants by photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Gao, X.; Meng, X. Photocatalysis for heavy metal treatment: A review. Processes 2021, 9, 1729. [Google Scholar] [CrossRef]
- Available online: https://powietrze.gios.gov.pl/ (accessed on 5 May 2024).
- ISO 9308; Water Quality—Quantification of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Testing Waters with Low Associated Microflora. International Organization of Standardization: Geneva, Switzerland, 2014.
- ISO 8692; Water Quality—Freshwater Algal Growth Inhibition Test with Unicellular Green Algae, Polish Committee for Standardization. International Organization of Standardization: Geneva, Switzerland, 2012.
- ISO 20079; Water Quality—Determination of Toxic Effects of Water Components and Wastewater on Duckweed (Lemna minor)—Duckweed Growth Inhibition Test. International Organization of Standardization: Geneva, Switzerland, 2006.
- ISO 6341; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test, Polish Committee for Standardization. International Organization of Standardization: Geneva, Switzerland, 2013.
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Environmental Bio Detection Products Inc. (EBPI). Instructions for Use, The SOS-Chromotest Kit Version 6.5, Environmental Bio Detection Products Inc. (EBPI): Burlington, ON, Canada, 1992.
- Jolibois, B.; Guerbet, M.; Vassal, S. Detection of hospital wastewater genotoxicity with the SOS chromotest and Ames fluctuation test. Chemosphere 2003, 51, 539–543. [Google Scholar] [CrossRef]
- Jiang, Y.; He, B.; Liu, A. Understanding the influence of additives on methyl orange degradation using adsorption/photocatalysis functioned materials. J. Water Process Eng. 2023, 53, 103621. [Google Scholar] [CrossRef]
- Perera, A.S.; Melia, P.M.; Bristow, R.M.; McGettrick, J.D.; Singer, R.J.; Bear, J.C.; Busquets, R. A non-doped microporous titanosilicate for bimodal adsorption-photocatalysis based removal of organic water pollutants. Microporous Mesoporous Mater. 2022, 345, 112276. [Google Scholar] [CrossRef]
- Nabizadeh, R.; Amrollahi, R.; Ghafary, B.; Alam, S.N. Influence of ozone supply mode and aeration on photocatalytic ozonation of organic pollutants in wastewater using TiO2 and ZnO nanoparticles. Heliyon 2023, 9, e22854. [Google Scholar] [CrossRef] [PubMed]
- Fatima, B.; Siddiqui, S.I.; Rajor, H.K.; Malik, M.A.; Narasimharao, K.; Ahmad, R.; Vikrant, K.; Kim, T.; Kim, K.-H. Photocatalytic removal of organic dye using green synthesized zinc oxide coupled cadmium tungstate nanocomposite under natural solar light irradiation. Environ. Res. 2023, 216, 114534. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, K.; El-Din, M.G.; Snyder, S.A. Ozonation and Advanced Oxidation Treatment of Emerging Organic Pollutants in Water and Wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Reports EPA (Environmental Protection Agency) on Rainwater Quality and Its Impact on the Environment. National Water Quality Inventory Report to Congress. Available online: https://www.epa.gov/waterdata/national-water-quality-inventory-report-congress (accessed on 18 May 2024).
- Fatima, B.; Siddiqui, S.I.; Ahmed, R.; Chaudhry, S.A. Green synthesis of f-CdWO4 for photocatalytic degradation and adsorptive removal of Bismarck Brown R dye from water. Water Resour. Ind. 2019, 22, 100119. [Google Scholar] [CrossRef]
- Figueredo, M.; Rodríguez, E.M.; Rivas, J.; Beltrán, F.J. Photocatalytic ozonation in water treatment: Is there really a synergy between systems? Water Res. 2021, 206, 117727. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gheni, A.; ElGawady, M.A. Reduced zinc leaching from scrap tire during pavement applications. Waste Manag. 2018, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Councell, T.B.; Duckenfield, K.U.; Landa, E.R.; Callender, E. Tire-wear particles as a source of zinc to the environment. Environ. Sci. Technol. 2004, 38, 4206–4214. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment-a review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef]
- Gromaire, M.; Chebbo, G.; Constant, A. Impact of zinc roofing on urban runoff pollutant loads: The case of Paris. Water Sci. Technol. 2002, 45, 113–122. [Google Scholar] [CrossRef]
- Chizoruo, I.F.; Onyekachi, I.B. Roof runoff water as source of pollution: A case study of some selected roofs in Orlmetropolis, Imo State, Nigeria. Int. Lett. Nat. Sci. 2016, 50, 53–61. [Google Scholar]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Cui, H. Synergistic effect of adsorptive photocatalytic oxidation and degradation mechanism of cyanides and Cu/Zn complexes over TiO2/ZSM-5 in real wastewater. J. Hazard. Mater. 2021, 416, 125802. [Google Scholar] [CrossRef] [PubMed]
- Orlović-Leko, P.; Vidović, K.; Ciglenečki, I.; Omanović, D.; Sikirić, M.D.; Šimunić, I. Physico-chemical characterization of an urban rainwater (Zagreb, Croatia). Atmosphere 2020, 11, 144. [Google Scholar] [CrossRef]
- Tapparo, A.; Di Marco, V.; Badocco, D.; D’aronco, S.; Soldà, L.; Pastore, P.; Mahon, B.M.; Kalberer, M.; Giorio, C. Formation of metal-organic ligand complexes affects solubility of metals in airborne particles at an urban site in the Po valley. Chemosphere 2020, 241, 125025. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.; Liggio, J.; Zhang, X.; Saini, A.; Harner, T. Composition and transformation chemistry of tire-wear derived organic chemicals and implications for air pollution. Atmos. Pollut. Res. 2022, 13, 101533. [Google Scholar] [CrossRef]
- Vistnes, H.; Sossalla, N.A.; Asimakopoulos, A.G.; Meyn, T. Occurrence of traffic related trace elements and organic micropollutants in tunnel wash water. J. Hazard. Mater. 2024, 465, 133498. [Google Scholar] [CrossRef]
- Schummer, C.; Groff, C.; Al Chami, J.; Jaber, F.; Millet, M. Analysis of phenols and nitrophenols in rainwater collected simultaneously on an urban and rural site in east of France. Sci. Total Environ. 2009, 407, 5637–5643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, S.; Liu, X.; Tian, L.; Mo, Y.; Yi, X.; Liu, S.; Liu, J.; Li, J.; Zhang, G. Aquatic environmental fates and risks of benzotriazoles, benzothiazoles, and p-phenylenediamines in a catchment providing water to a megacity of China. Environ. Res. 2023, 216, 114721. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-X.; Cheng, Y.-X.; Wu, D.; Fan, L.; Zhao, J.-H.; Xiong, Q.; Chen, Q.-L.; Liu, Y.-S.; Ying, G.-G. Continuous input of organic ultraviolet filters and benzothiazoles threatens the surface water and sediment of two major rivers in the Pearl River Basin. Sci. Total Environ. 2021, 798, 149299. [Google Scholar] [CrossRef]
- Khwaja, H.A. Atmospheric concentrations of carboxylic acids and related compounds at a semiurban site. Atmos. Environ. 1995, 29, 127–139. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A.; Gimeno, O. The role of catalytic ozonation processes on the elimination of DBPs and their precursors in drinking water treatment. Catalysts 2021, 11, 521. [Google Scholar] [CrossRef]
- Wu, J.; Kim, Y.H.; Nam, S.N. Degradation kinetics and mechanisms of volatile organic compounds by ozonation in aqueous solution: A review. J. Chem. Eng. 2018, 334, 1176–1189. [Google Scholar]
- Marfur, N.A.; Jaafar, N.F.; Khairuddean, M.; Nordin, N. A Review on Recent Progression of Modifications on Titania Morphology and its Photocatalytic Performance. Acta Chim. Slov. 2020, 67, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Salimi, F.; Golmohammadi, F. Removal of cadmium from aqueous solution by nano composites of bentonite/TiO2 and bentonite/zno using photocatalysis adsorption process. Silicon 2020, 12, 2721–2731. [Google Scholar] [CrossRef]
- Malankowska, A.; Borowska, E.; Martins, R.C.; Gmurek, M. Editorial Catalysts: Special Issue on Recent Advances in TiO2 Photocatalysts. Catalysts 2021, 11, 790. [Google Scholar] [CrossRef]
- D’auria, M.; Emanuele, L.; Racioppi, R.; Velluzzi, V. Photochemical degradation of crude oil: Comparison between direct irradiation, photocatalysis, and photocatalysis on zeolite. J. Hazard. Mater. 2009, 164, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Affek, K.; Muszyński, A.; Załęska-Radziwiłł, M.; Doskocz, N.; Ziętkowska, A.; Widomski, M. Evaluation of ecotoxicity and inactivation of bacteria during ozonation of treated wastewater. Desalin. Water Treat. 2020, 192, 176–184. [Google Scholar] [CrossRef]
- Hamilton, K.; Reyneke, B.; Waso, M.; Clements, T.; Ndlovu, T.; Khan, W.; DiGiovanni, K.; Rakestraw, E.; Montalto, F.; Haas, C.N.; et al. A global review of the microbiological quality and potential health risks associated with roof-harvested rainwater tanks. npj Clean Water 2019, 2, 7. [Google Scholar] [CrossRef]
- Ahmed, W.; Toze, S. Microbiological quality and associated health risks with the use of roof-captured rainwater. In Rainwater Tank Systems for Urban Water Supply: Design, Yield, Energy, Health Risks, Economics and Social Perceptions; Sharma, A., Begbie, D., Gardner, T., Eds.; IWA, 2015; Chapter 10; pp. 229–252. Available online: https://www.researchgate.net/publication/281333262_Microbiological_quality_and_associated_health_risks_with_the_use_of_roof-captured_rainwater (accessed on 18 May 2024).
- Evans, C.; Coombes, P.; Dunstan, R. Wind, rain and bacteria: The effect of weather on the microbial composition of roof-harvested rainwater. Water Res. 2006, 40, 37–44. [Google Scholar] [CrossRef]
- Huston, R.; Chan, Y.; Gardner, T.; Shaw, G.; Chapman, H. Characterisation of atmospheric deposition as a source of contaminants in urban rainwater tanks. Water Res. 2009, 43, 1630–1640. [Google Scholar] [CrossRef]
- Lye, D.J. Rooftop runoff as a source of contamination: A review. Sci. Total. Environ. 2009, 407, 5429–5434. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Goonetilleke, A.; Gardner, T. Implications of faecal indicator bacteria for the microbiological assessment of roof-harvested rainwater quality in Southeast Queensland, Australia. Can. J. Microbiol. 2010, 56, 471–479. [Google Scholar] [CrossRef]
- Lye, D.J. Health risks associated with consumption of untreated water from household roof catchment systems. J. Am. Water Resour. Assoc. 2002, 38, 1301–1306. [Google Scholar] [CrossRef]
- Simmons, G.; Jury, S.; Thornley, C.; Harte, D.; Mohiuddin, J.; Taylor, M. A Legionnaires’ disease outbreak: A water blaster and roof-collected rainwater systems. Water Res. 2008, 42, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Dhodpakar, R.; Kapley, A. Disinfection by-products and their effect on aquatic and agriculture ecosystem. In Disinfection By-Products in Drinking Water. Detection and Treatment; Narasimha, M., Prasad, V., Eds.; Butterworth Heinemann, 2020; Chapter 9; pp. 205–233. Available online: https://www.sciencedirect.com/science/article/abs/pii/B978008102977000010X?via%3Dihub (accessed on 18 May 2024).
- Huber, M.M.; Göbel, A.; Joss, A.; Hermann, N.; Löffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.; Ternes, T.A.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef] [PubMed]
- Nasuhoglu, D.; Isazadeh, S.; Westlund, P.; Neamatallah, S.; Yargeau, V. Chemical, microbial and toxicological assessment of wastewater treatment plant effluents during disinfection by ozonation. J. Chem. Eng. 2018, 346, 466–476. [Google Scholar] [CrossRef]
- Li, X.-F.; Mitch, W.A. Drinking water disinfection byproducts (DBPs) and human health effects: Multidisciplinary challenges and opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef]
- Latif, M.T.; Othman, M.; Hassan, H. Composition of rainwater and influences over different regions of the world. In Precipitation. Earth Surface Responses and Processes; Rodrigo-Comino, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 4; pp. 63–83. [Google Scholar]

| Parameter | Unit | RW I | RW II |
|---|---|---|---|
| pH | - | 7.65 | 6.8 |
| Conductivity | µS/cm | 605.5 | 56.1 |
| COD | mg/L | 104 | 69 |
| Nickel | mg/L | 0.24 | 0 |
| Copper | mg/L | 0.14 | 0 |
| Zinc | mg/L | 3.95 | 1.7 |
| Lead | mg/L | 0.19 | 0 |
| TOC | mg/L | 17.7 | 7.2 |
| Color | mgPt/L | 157 | 7 |
| N-NO3 | mg/L | 0.9 | 0.9 |
| P-PO4 | mg/L | 1.2 | 0 |
| N-NH4 | mg/L | 0.09 | 0.41 |
| TU | Toxicity Class | Toxicity Assessment |
|---|---|---|
| < 0.4 | I | No toxicity |
| 0.4 ≤ TU < 1 | II | Slight toxicity |
| 1 ≤ TU < 10 | III | Toxicity |
| 10 ≤ TU < 100 | IV | High toxicity |
| > 100 | V | Very high toxicity |
| SOSIF < 1.5 | No genotoxicity | − |
| 1.5 ≤ SOSIF < 2.0 | Slight genotoxicity | + |
| 2.0 ≤ SOSIF < 5.0 | Moderate genotoxicity | ++ |
| SOSIF ≥ 5.0 | Strong genotoxicity | +++ |
| Concentration of Metals [mg/L] | Sample | ||||||
|---|---|---|---|---|---|---|---|
| RW I | 5 min UV+O3 | 15 min UV+O3 | 30 min UV+O3 | RW II | 5 min UV+O3 | 15 min UV+O3 | |
| Pb | 0.19 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ni | 0.24 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cu | 0.14 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zn | 3.95 | 3.95 | 3.95 | 3.95 | 1.70 | 1.70 | 1.70 |
| Compounds | Rt [min] | Area [Count × min] | |||
| RW I | 5 min UV+O3 | 15 min UV+O3 | 30 min UV+O3 | ||
| Benzothiazole | 14.67 | 129,847 | n.d. | n.d. | n.d. |
| 2,3,5,8-Tetramethyldecane | 17.329 | 162,516 | n.d. | n.d. | n.d. |
| 3,8-Dimethylundecane | 17.86 | 101,402 | n.d. | n.d. | n.d. |
| Tetradecane | 19.568 | 310,095 | 259,019 | 138,334 | 137,688 |
| 2,4-Di-tert-butylphenol | 22.395 | 850,713 | 580,112 | 418,153 | 418,870 |
| 2,6,11-Trimethyldodecane | 23.008 | 238,819 | 218,883 | 211,695 | 211,485 |
| Hexadecane | 24.61 | 270,283 | 258,075 | 271,572 | 286,831 |
| Heptadecane | 27.838 | 211,021 | 254,683 | 231,720 | 235,855 |
| Octadecane | 29.045 | 1,062,978 | 1,244,628 | 1,290,282 | 1,225,732 |
| Compounds | Rt [min] | Area [Count × min] | |||
| RW II | 5 min UV+O3 | 15 min UV+O3 | |||
| 2-Ethyl-2,3,3-trimethylbutanoic acid | 13.363 | 8,009,709 | 3,624,809 | 3,534,111 | |
| Neodecanoic acid | 15.777 | 841,463 | 1,257,517 | 493,017 | |
| Tetradecane | 19.543 | 236,610 | 451,864 | 301,737 | |
| 2,4-Di-tert-butylphenol | 22.395 | 244,942 | 216,845 | 182,525 | |
| 2-Bromo dodecane | 23.002 | 206,580 | 265,642 | 435,534 | |
| 2,3,5,8-Tetramethyldecane | 23.484 | 127,281 | 152,386 | 238,923 | |
| Hexadecane | 24.604 | 322,253 | 379,533 | 384,395 | |
| Nonadecane | 27.831 | 288,146 | 266,772 | 268,307 | |
| Octadecane | 29.039 | 1,813,004 | 1,710,724 | 1,729,465 | |
| Compounds | RW I | RW II | |||
|---|---|---|---|---|---|
| 5 min UV+O3 | 15 min UV+O3 | 30 min UV+O3 | 5 min UV+O3 | 15 min UV+O3 | |
| Removal, % | |||||
| Benzothiazole | 100 | 100 | 100 | n.d. | n.d. |
| 2-Ethyl-2,3,3-trimethylbutanoic acid | n.d. | n.d. | n.d. | 54.7 | 55.8 |
| Neodecanoic acid | n.d. | n.d. | n.d. | 0 | 41.4 |
| Tetradecane | 16.4 | 55.4 | 55.6 | 0 | 0 |
| 2,4-Di-tert-butylphenol | 31.8 | 50.8 | 50.7 | 11.4 | 25.4 |
| Hexadecane | 4.5 | 0 | 0 | 0 | 0 |
| Octadecane | 0 | 0 | 0 | 0 | 0 |
| Sample | Bacteria [cfu/mL] | |
|---|---|---|
| Coliforms | Escherichia coli | |
| RW I | 0.5 | 0.01 |
| 5 min UV+O3 | n.d. | n.d. |
| 15 min UV+O3 | n.d. | n.d. |
| 30 min UV+O3 | n.d. | n.d. |
| RW II | 8.0 | 0.15 |
| 5 min UV+O3 | 3.0 (62.5; 0.4) | n.d. |
| 15 min UV+O3 | 3.0 (62.5; 0.4) | n.d. |
| Sample | Parameter | Bioindicator | ||||
|---|---|---|---|---|---|---|
| Desmodesmus quadricauda | Daphnia magna | Allivibrio fischeri | Lemna minor | |||
| Frond Number | Total Frond Area | |||||
| RW I | TU score | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 | 0.52 1 |
| Toxicity class | II | |||||
| Toxicity classification | Slight toxicity | |||||
| CWS (CWS%) | 0.2 (20%) | |||||
| 5 min UV+O3 | TU score | <0.4 0 | <0.4 0 | <0.4 0 | 0.65 1 | 0.70 1 |
| Toxicity class | II | |||||
| Toxicity classification | Slight toxicity | |||||
| CWS (CWS%) | 0.4 (40%) | |||||
| 15 min UV+O3 | TU score | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 |
| Toxicity class | I | |||||
| Toxicity classification | No toxicity | |||||
| CWS (CWS%) | 0 (100%) | |||||
| 30 min UV+O3 | TU score | <0.4 0 | <0.4 0 | 0.84 1 | <0.4 0 | 0.48 1 |
| Toxicity class | II | |||||
| Toxicity classification | Slight toxicity | |||||
| CWS (CWS%) | 0.4 (40%) | |||||
| RW II | TU score | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 |
| Toxicity class | I | |||||
| Toxicity classification | No toxicity (slight stimulation) | |||||
| CWS (CWS%) | 0 (100%) | |||||
| 5 min UV+O3 | TU score | 0.76 1 | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 |
| Toxicity class | II | |||||
| Toxicity classification | Slight toxicity | |||||
| CWS (CWS%) | 0.2 (20%) | |||||
| 15 min UV+O3 | TU score | 0.4 1 | <0.4 0 | <0.4 0 | <0.4 0 | <0.4 0 |
| Toxicity class | II | |||||
| Toxicity classification | Slight toxicity | |||||
| CWS (CWS%) | 0.2 (20%) | |||||
| Sample | SOSIF ± SD | Genotoxicity Assessment | |
|---|---|---|---|
| RW I | Without S9 | 1.14 ± 0.14 | (-) no genotoxicity |
| With S9 | 0.98 ± 0.12 | ||
| 5 min UV+O3 | Without S9 | 1.07 ± 0.02 | |
| With S9 | 0.96 ± 0.03 | ||
| 15 min UV+O3 | Without S9 | 1.15 ± 0.03 | |
| With S9 | 1.00 ± 0.06 | ||
| 30 min UV+O3 | Without S9 | 1.07 ± 0.06 | |
| With S9 | 0.89 ± 0.16 | ||
| RW II | Without S9 | 1.04 ± 0.04 | |
| With S9 | 0.97 ± 0.02 | ||
| 5 min UV+O3 | Without S9 | 1.11 ± 0.07 | |
| With S9 | 1.04 ± 0.02 | ||
| 15 min UV+O3 | Without S9 | 1.26 ± 0.25 | |
| With S9 | 1.02 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszałek, A.; Affek, K.; Załęska-Radziwiłł, M.; Dudziak, M. Integrated Ozonation and Photocatalysis to Remove Pollutants for Reuse of Rainwater. Sustainability 2024, 16, 5352. https://doi.org/10.3390/su16135352
Marszałek A, Affek K, Załęska-Radziwiłł M, Dudziak M. Integrated Ozonation and Photocatalysis to Remove Pollutants for Reuse of Rainwater. Sustainability. 2024; 16(13):5352. https://doi.org/10.3390/su16135352
Chicago/Turabian StyleMarszałek, Anna, Katarzyna Affek, Monika Załęska-Radziwiłł, and Mariusz Dudziak. 2024. "Integrated Ozonation and Photocatalysis to Remove Pollutants for Reuse of Rainwater" Sustainability 16, no. 13: 5352. https://doi.org/10.3390/su16135352





