Synthesis and Characterization of Biochars and Activated Carbons Derived from Various Biomasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon (AC) from Okara Powder Waste
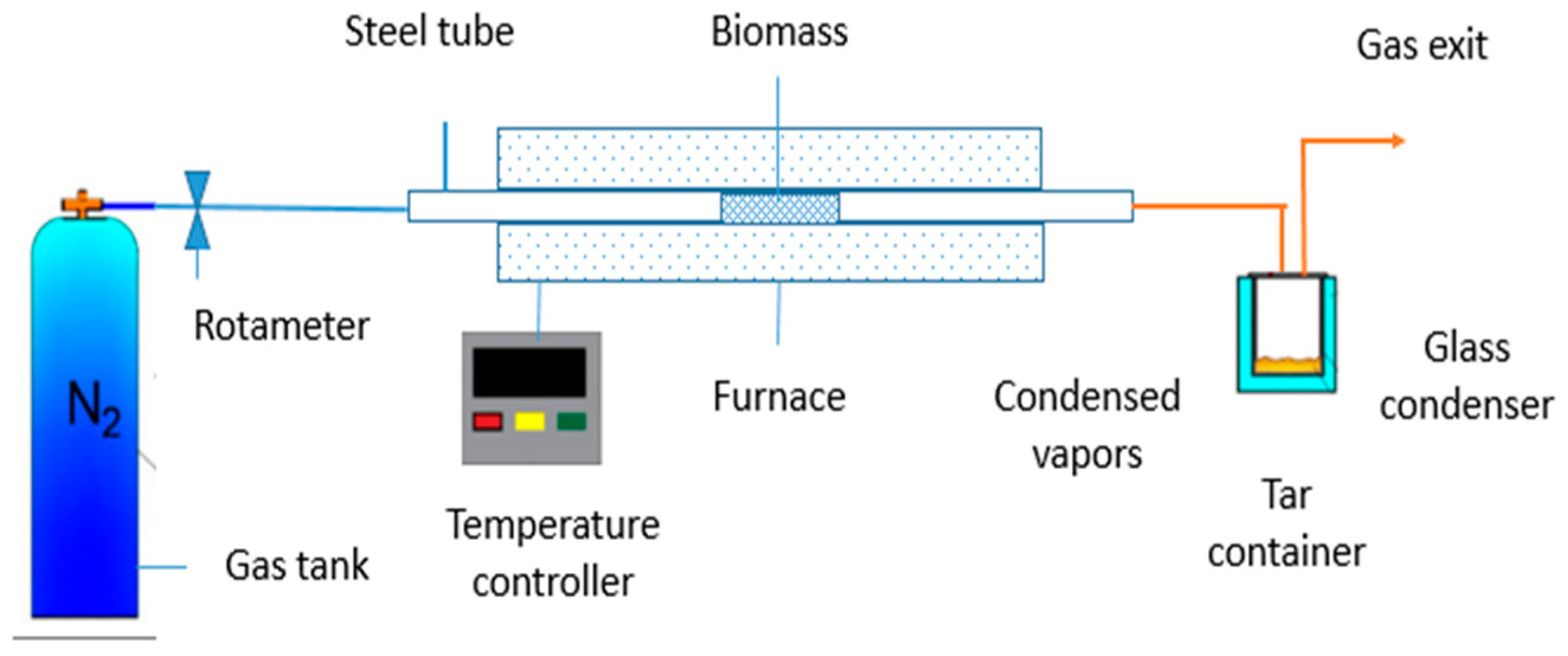
2.1.1. Pyrolysis Experimental Setup
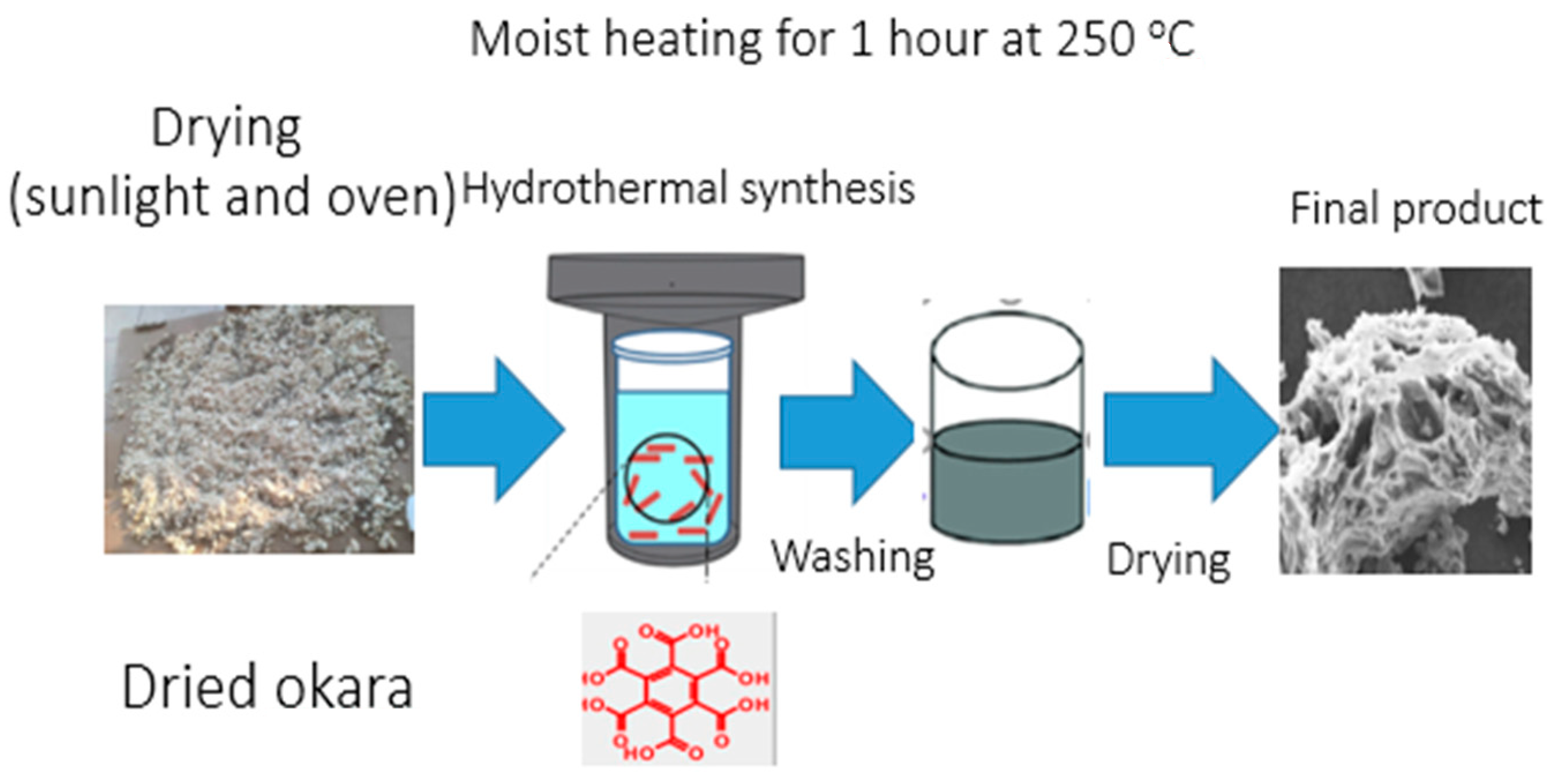
2.1.2. Preparation of Activated Carbon via the Hydrothermal Carbonization Method
2.1.3. Activation of Biochar/Hydrochar
2.2. Characterization of the Materials
2.3. Calculation of the Biomass Conversion
3. Results and Discussion
3.1. Characterizations of the Biomass Materials
3.1.1. EDX Analysis of Various Biomasses
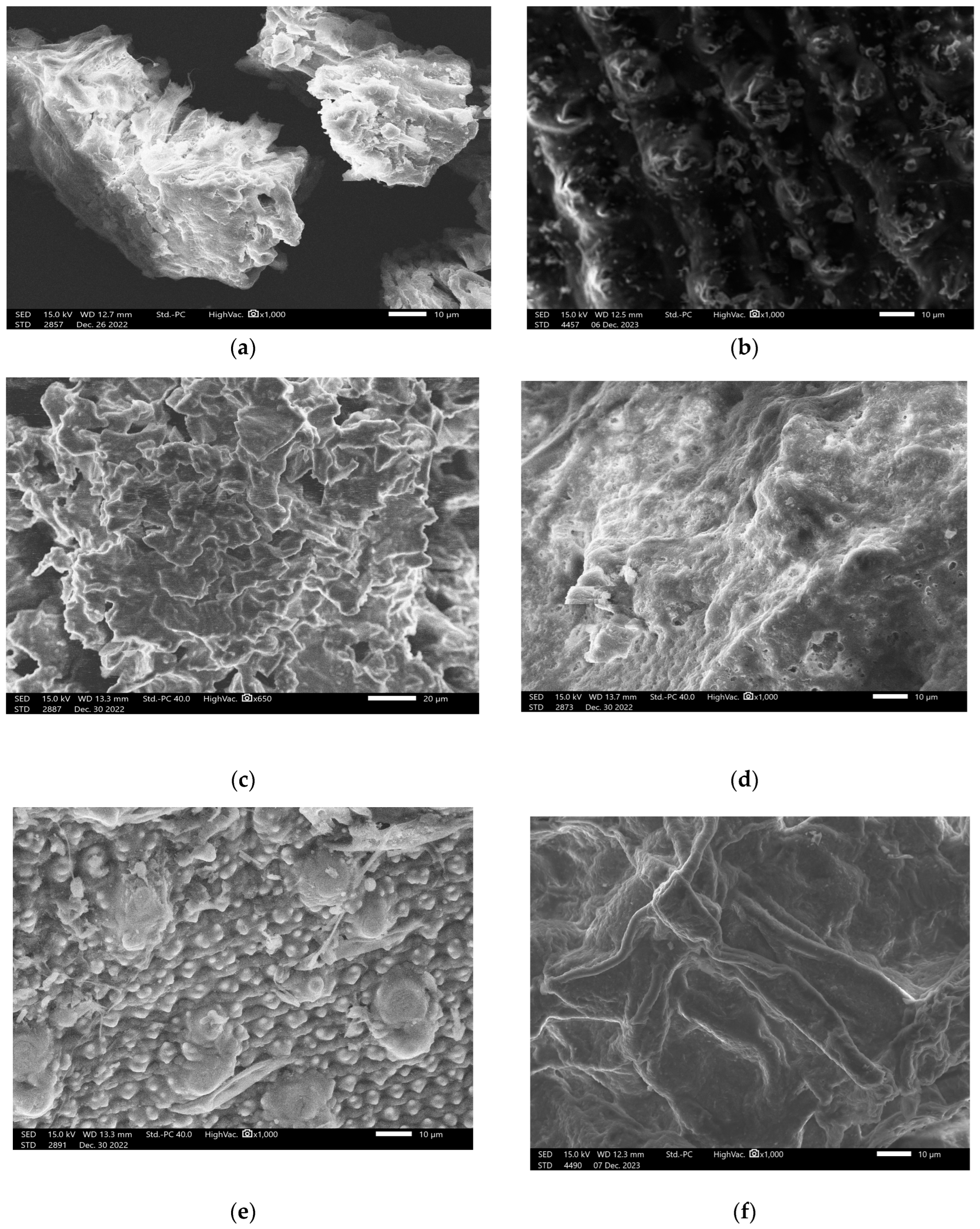
3.1.2. SEM Images of Different Biomasses
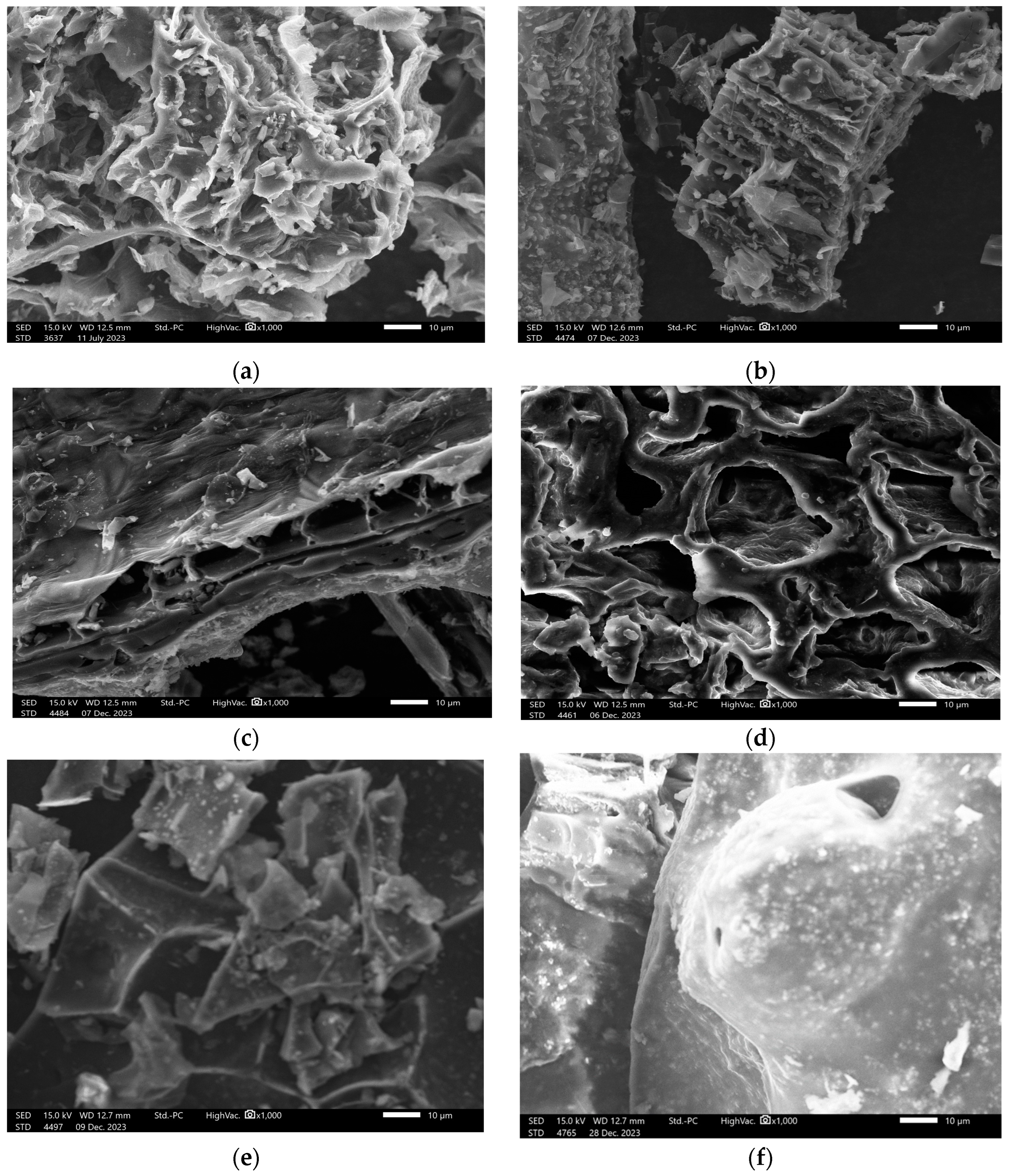
3.1.3. SEM Images of Activated Carbons Synthesized from Different Biomasses
3.1.4. FTIR Analysis
3.1.5. TG-DSC Analysis
3.2. Biomass Pyrolysis Products and Their Properties
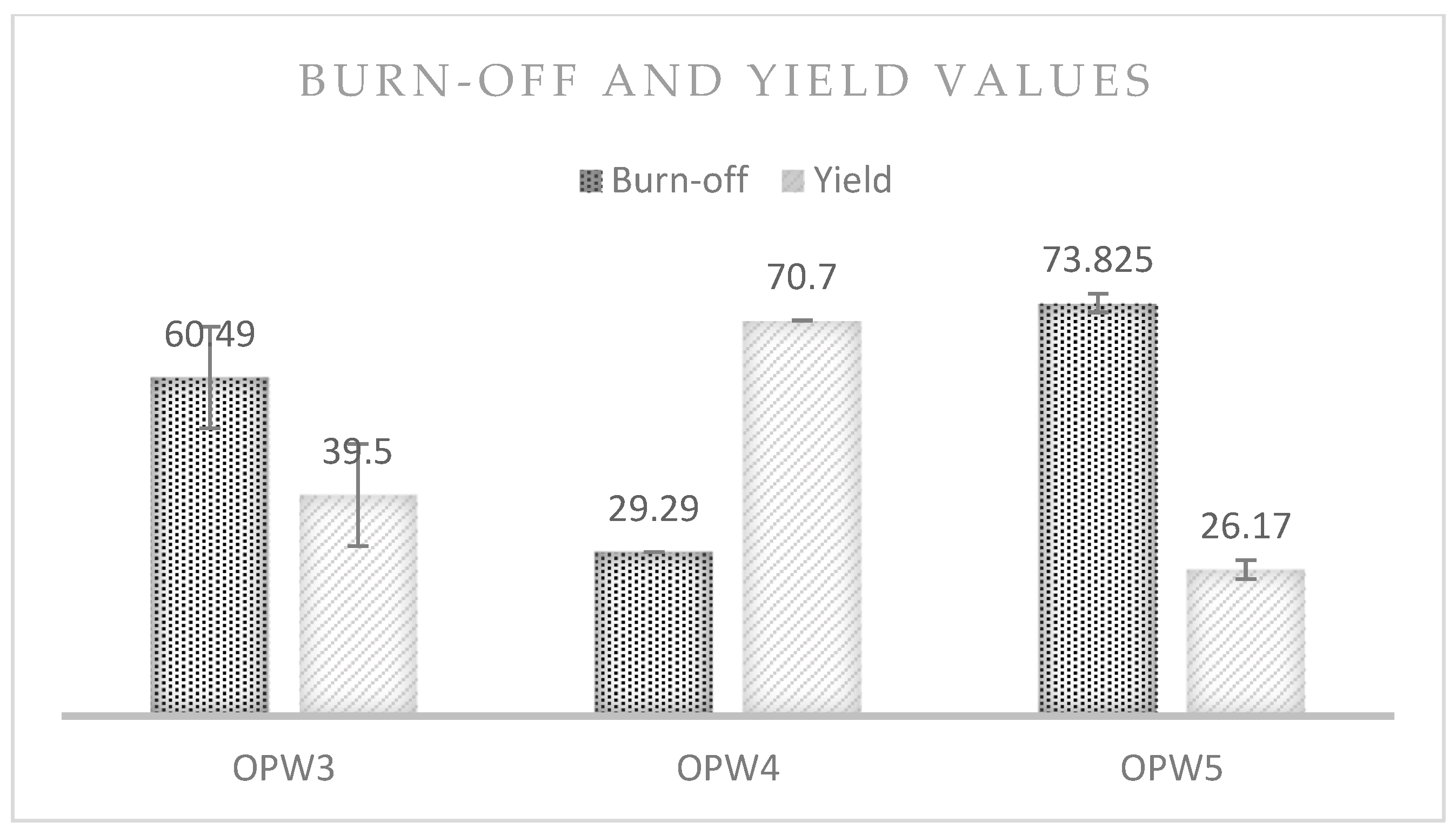
3.2.1. Biochar and Hydrochar Yield and Burn-Off
3.2.2. Surface Areas (SBET) and Pores
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P.; Houghton, R.A.; Marland, G.; Hackler, J.; Boden, T.A.; Conway, T.J.; Canadell, J.G.; Raupach, M.R.; Ciais, P.; Le Quéré, C. Update on CO2 emissions. Nat. Geosci. 2010, 3, 811–812. [Google Scholar] [CrossRef]
- Hoang, T.D.; Bandh, S.A.; Malla, F.A.; Qayoom, I.; Bashir, S.; Peer, S.B.; Halog, A. Carbon-Based Synthesized Materials for CO2 Adsorption and Conversion: Its Potential for Carbon Recycling. Recycling 2023, 8, 53. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture, 1st ed.; Mohammad, R.R., Mohammad, F., Mohammad, A.M., Eds.; Woodhead Publishing: Cambrigde, UK, 2020; pp. 3–28. [Google Scholar]
- Gao, J.; Shiong, S.C.S.; Liu, Y. Reduction of CO2 to chemicals and Fuels: Thermocatalysis versus electrocatalysis. J. Chem. Eng. 2023, 472, 145033. [Google Scholar] [CrossRef]
- Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, J.M.; Morgan, V.I. Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn. J. Geophys. Res. 1996, 101, 4115–4128. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Chen, W.; Xia, M.; Chen, Y.; Chen, H.; Zeng, K.; Yang, H. Biomass pyrolysis mechanism for carbon-based high-value products. Proc. Combust. Inst. 2023, 39, 3157–3181. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, G.; Chen, S. Preparation of a solid amine micro spherical adsorbent with high CO2 adsorption capacity. Langmuir 2020, 36, 7715–7723. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.; Marliza, T.S.; Abu Tahari, N.M.; Wan Isahak, R.W.; Yusop, R.M.; Mohamed Hisham, W.M.; Yarmo, A.M. Studies on CO2 adsorption and desorption properties from various types of iron oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888–7897. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Pinto, M.L.; Mafra, L.; Guil, J.M.; Pires, J.; Rocha, J. Adsorption and activation of CO2 by amine-modified nanoporous materials studied by solid-state NMR and 13CO2 adsorption. Chem. Mater. 2011, 23, 1387–1395. [Google Scholar] [CrossRef]
- Chanapattharapol, K.C.; Krachuamram, S.; Youngme, S. Study of CO2 adsorption on iron oxide doped MCM-41. Microporous Mesoporous Mater. 2017, 245, 8–15. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.S.; Jiang, H.R.; Wang, H. Carbonyl-incorporated aromatic hyper-cross-linked polymers with microporous structure and their functional materials for CO2 adsorption. Ind. Eng. Chem. Res. 2020, 59, 15955–15966. [Google Scholar] [CrossRef]
- Furuvik, N.C.; Wang, L.; Jaiswal, R.; Thapa, R.; Eikeland, M.S.; Moldestad, B.M. Experimental study and SEM-EDS analysis of agglomerates from gasification of biomass in fluidized beds. Energy 2022, 252, 124034. [Google Scholar] [CrossRef]
- Hoang, T.D.; Nghiem, N. Recent developments and current status of commercial production of fuel ethanol. Fermentation 2021, 7, 314. [Google Scholar] [CrossRef]
- Sezali, N.A.A.; Ong, H.L.; Villagracia, A.R.; Hoang, T.D. Bio-based nanomaterials for energy application: A review. Vietnam J. Chem. 2024, 62, 1–12. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, S.; He, S.; Wu, C. Direct air capture of CO2 by KOH-activated bamboo biochar. J. Energy Inst. 2022, 105, 399–405. [Google Scholar] [CrossRef]
- Mumtaz, H.; Farhan, M.; Amjad, M.; Riaz, F.; Kazim, A.H.; Sultan, M.; Farooq, M.; Mujtaba, M.A.; Hussain, I.; Imran, M.; et al. Biomass waste utilization for adsorbent preparation in CO2 capture and sustainable environment applications. Sustain. Energy Technol. Assess. 2021, 46, 101288. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, T.; Shih, C.H.; Lo, S.L.; Sun, L.; Zhong, Y.; Qiu, C. Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 2015, 84, 75–82. [Google Scholar] [CrossRef]
- Travis, W.; Gadipelli, S.; Guo, Z. Superior CO2 adsorption from waste coffee ground derived carbons. RSC Adv. 2015, 5, 29558–29562. [Google Scholar] [CrossRef]
- Wang, S.; Lee, Y.R.; Won, Y.; Kim, H.; Jeong, S.E.; Hwang, B.W.; Cho, A.R.; Kim, J.Y.; Park, Y.C.; Nam, H.; et al. Development of high-performance adsorbent using KOH-impregnated rice husk-based activated carbon for indoor CO2 adsorption. J. Chem. Eng. 2022, 437, 135378. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.; Swallah, M.S.; Wang, S.; Zhang, J.; Fang, J.; Lu, J.; Yu, H. Structural characteristics of insoluble dietary fiber from okara with different particle sizes and their prebiotic effects in rats fed high-fat diet. Foods 2022, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Food use of whole soybeans. In Soybeans, 1st ed; Lawrence, A., Johnson, J.W., Richard, G., Eds.; AOCS Press: Champaign, IL, USA, 2008; pp. 441–481. [Google Scholar]
- Xiao, C.W. Functional soy products. In Functional Foods, 2nd ed.; Maria, S., Ed.; Woodhead Publishing: Cambrigde, UK, 2011; pp. 534–556. [Google Scholar]
- Rahman, M.M.; Mat, K.; Ishigaki, G.; Akashi, R. A review of okara (soybean curd residue) utilization as animal feed: Nutritive value and animal performance aspects. Anim. Sci. J. 2021, 92, e13594. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiao, M.; Lu, F. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Abi Bianasari, A.; Khaled, M.S.; Hoang, T.D.; Reza, M.S.; Bakar, M.S.A.; Azad, A.K. Influence of combined catalysts on the catalytic pyrolysis process of biomass: A systematic literature review. Energy Convers. Manag. 2024, 309, 118437. [Google Scholar] [CrossRef]
- Tran, T.K.; Huynh, L.; Nguyen, H.L.; Nguyen, M.K.; Lin, C.; Hoang, T.D.; Hung, N.T.Q.; Nguyen, X.H.; Chang, S.W.; Nguyen, D.D. Applications of engineered biochar in remediation of heavy metal (loid) s pollution from wastewater: Current perspectives toward sustainable development goals. Sci. Total Environ. 2024, 926, 171859. [Google Scholar] [CrossRef] [PubMed]
- Komkiene, J.; Baltrenaite, E. Biochar as adsorbent for removal of heavy metal ions [Cadmium (II), Copper (II), Lead (II), Zinc (II)] from aqueous phase. Int. J. Environ. Sci. Technol. 2016, 13, 471–482. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Miranda, N.T.; Motta, I.L.; Maciel Filho, R.; Maciel, M.R.W. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Sikarwar, V.S.; Ahmad, E.; Pant, K.K.; Murugavelh, S.; Pandey, A.; Bhattacharya, S.; Sarmah, A.; Leu, S.Y. A critical review on biomass pyrolysis: Reaction mechanisms, process modeling and potential challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.T.; Show, L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A comprehensive review on hydrothermal carbonization of biomass and its applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Yoganandham, S.T.; Sathyamoorthy, G.; Renuka, R.R. Emerging extraction techniques: Hydrothermal processing. In Sustainable Seaweed Technologies, 1st ed.; Maria, D.T., Stefan, K., Herminia, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–205. [Google Scholar]
- Marzbali, M.H.; Kundu, S.; Halder, P.; Patel, S.; Hakeem, I.G.; Paz-Ferreiro, J.; Madapusi, S.; Surapaneni, A.; Shah, K. Wet organic waste treatment via hydrothermal processing: A critical review. Chemosphere 2021, 279, 130557. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M.I. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 18851. [Google Scholar] [CrossRef]
- Ullah, H.; Lun, L.; Riaz, L.; Naseem, F.; Shahab, A.; Rashid, A. Physicochemical characteristics and thermal degradation behavior of dry and wet torrefied orange peel obtained by dry/wet torrefaction. Biomass Convers. Biorefin. 2021, 13, 7993–8009. [Google Scholar] [CrossRef]
- Rodríguez Correa, C.; Ngamying, C.; Klank, D.; Kruse, A. Investigation of the textural and adsorption properties of activated carbon from HTC and pyrolysis carbonizates. Biomass Convers. Biorefin. 2018, 8, 317–328. [Google Scholar] [CrossRef]
- Mumme, J.; Titirici, M.M.; Pfeiffer, A.; Luder, U.; Reza, M.T.; Masek, O. Hydrothermal carbonization of digestate in the presence of zeolite: Process efficiency and composite properties. ACS Sustain. Chem. Eng. 2015, 3, 2967–2974. [Google Scholar] [CrossRef]
- Yang, P.; Rao, L.; Zhu, W.; Wang, L.; Ma, R.; Chen, F.; Lin, G.; Hu, X. Porous carbons derived from sustainable biomass via a facile one-step synthesis strategy as efficient CO2 adsorbents. Ind. Eng. Chem. Res. 2020, 59, 6194–6201. [Google Scholar] [CrossRef]
- Hoang, T.D.; Ky, N.M.; Thuong, N.T.N.; Nhan, H.Q.; Ngan, N.V.C. Artificial intelligence in pollution control and management: Status and future prospects. In Artificial Intelligence and Environmental Sustainability. Algorithms for Intelligent Systems, 1st ed.; Ong, H.L., Doong, R., Naguib, R., Lim, C.P., Nagar, A.K., Eds.; Springer: Singapore, 2022; pp. 23–43. [Google Scholar]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Taweekun, J.; Ja’afar, F.; Saifullah Abu Bakar, M.; Azad, A.K.; Roy, H.; et al. Ex situ catalytic pyrolysis of invasive Pennisetum purpureum grass with activated carbon for upgrading bio-oil. Sustainability 2023, 15, 7628. [Google Scholar] [CrossRef]
- Lee, S.M.; Roh, J.S. Pore development process according to burn-off of activated carbon black with CO2 gas. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 808–814. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A review on the synthesis and characterization of biomass-derived carbons for adsorption of emerging contaminants from water. C 2018, 4, 63. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, W.; Storz, H.; Lubwama, M.; Kiros, Y. Synthesis and evaluation of activated carbon from rice husks for removal of humic acid from water. Biomass Convers. Biorefin. 2020, 12, 3229–3248. [Google Scholar] [CrossRef]
- Giri, B.S.; Goswami, M.; Kumar, P.; Yadav, R.; Sharma, N.; Sonwani, R.K.; Yadav, S.; Singh, R.P.; Rene, E.R.; Chaturvedi, P.; et al. Adsorption of patent blue V from textile industry wastewater using Sterculia alata fruit shell biochar: Evaluation of efficiency and mechanisms. Water 2020, 12, 2017. [Google Scholar] [CrossRef]
- Reza, M.S.; Taweekun, J.; Afroze, S.; Siddique, S.A.; Islam, M.S.; Wang, C.; Azad, A.K. Investigation of thermochemical properties and pyrolysis of barley waste as a source for renewable energy. Sustainability 2023, 15, 1643. [Google Scholar] [CrossRef]
- Lazzari, E.; Schena, T.; Marcelo, M.C.A.; Primaz, C.T.; Silva, A.N.; Ferrao, M.F.; Bjerk, T.; Caramao, E.B. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Ind. Crops Prod. 2018, 111, 856–864. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Production of biochar from elephant grass (Pernisetum purpureum) using an updraft biomass gasifier with retort heating. Biofuels 2019, 12, 1283–1290. [Google Scholar] [CrossRef]
- Téllez, G.L.; Vigueras-Santiago, E.; Hernández-López, S. Characterization of linseed oil epoxidized at different percentages. Superf. Vacío 2009, 22, 5–10. [Google Scholar]
- Wu, M.; Feng, Q.; Sun, X.; Wang, H.; Gielen, G.; Wu, W. Rice (Oryza sativa L.) plantation affects the stability of biochar in paddy soil. Sci. Rep. 2015, 5, 10001. [Google Scholar] [CrossRef] [PubMed]
- Suratman, A.; Astuti, D.N.; Kusumastuti, P.; Sudiono, S. Okara biochar immobilized calcium-alginate beads as eosin yellow dye adsorbent. Results Chem. 2024, 7, 101268. [Google Scholar] [CrossRef]
- Anukam, A.I.; Berghel, J.; Frodeson, S.; Famewo, E.B.; Nyamukamba, P. Characterization of pure and blended pellets made from Norway spruce and pea starch: A comparative study of bonding mechanism relevant to quality. Energies 2019, 12, 4415. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Le, A.; Phung, V.B.T. Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: Synthesis and investigation. Biomass Convers. Biorefin. 2022, 12, 2407–2416. [Google Scholar] [CrossRef]
- Xie, L.; Sun, G.; Su, F.; Guo, X.; Kong, Q.; Li, X.; Huang, X.; Wan, L.; Li, K.; Lv, C.; et al. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646. [Google Scholar] [CrossRef]
- Wang, K.; Xu, S. Preparation of High Specific Surface Area Activated Carbon from Petroleum Coke by KOH Activation in a Rotary Kiln. Processes 2024, 12, 241. [Google Scholar] [CrossRef]
- Malla, F.A.; Dung, T.; Bandh, S.A.; Malla, A.A.; Wani, S.A. Circular Economy to Decarbonize Electricity. In Renewable Energy in Circular Economy, 1st ed.; Bandh, S.A., Malla, F.A., Hoang, A.T., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 71–87. [Google Scholar]
- Hoang, T.D.; Ha, T.; Halog, A.B.; Malla, F.A.; Bandh, S.A. Biofuel Projects and Current Environmental Policies: Vietnam’s Case and Neighboring Asian Countries. In Biofuels in Circular Economy, 1st ed.; Bandh, S.A., Malla, F.A., Eds.; Springer Nature: Singapore, 2023; pp. 73–88. [Google Scholar]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. J. Chem. Eng. 2013, 230, 456–465. [Google Scholar] [CrossRef]
- Karimi, M.; Rodrigues, A.E.; Silva, J.A. Biomass as a source of adsorbents for CO2 capture. In Advances in Bioenergy and Microfluidic Applications, 1st ed.; Mohammad, R.R., Reza, K., Mohammad, A.M., Mohammad, K.D.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–274. [Google Scholar]
- Bandh, S.A.; Malla, F.A.; Hoang, T.D.; Qayoom, I.; Mohi-Ud-Din, H.; Bashir, S.; Betts, R.; Le, T.T.; Nguyen Le, D.T.; Linh Le, N.V.; et al. Track to reach net-zero: Progress and pitfalls. Energy Environ. 2024. [Google Scholar] [CrossRef]


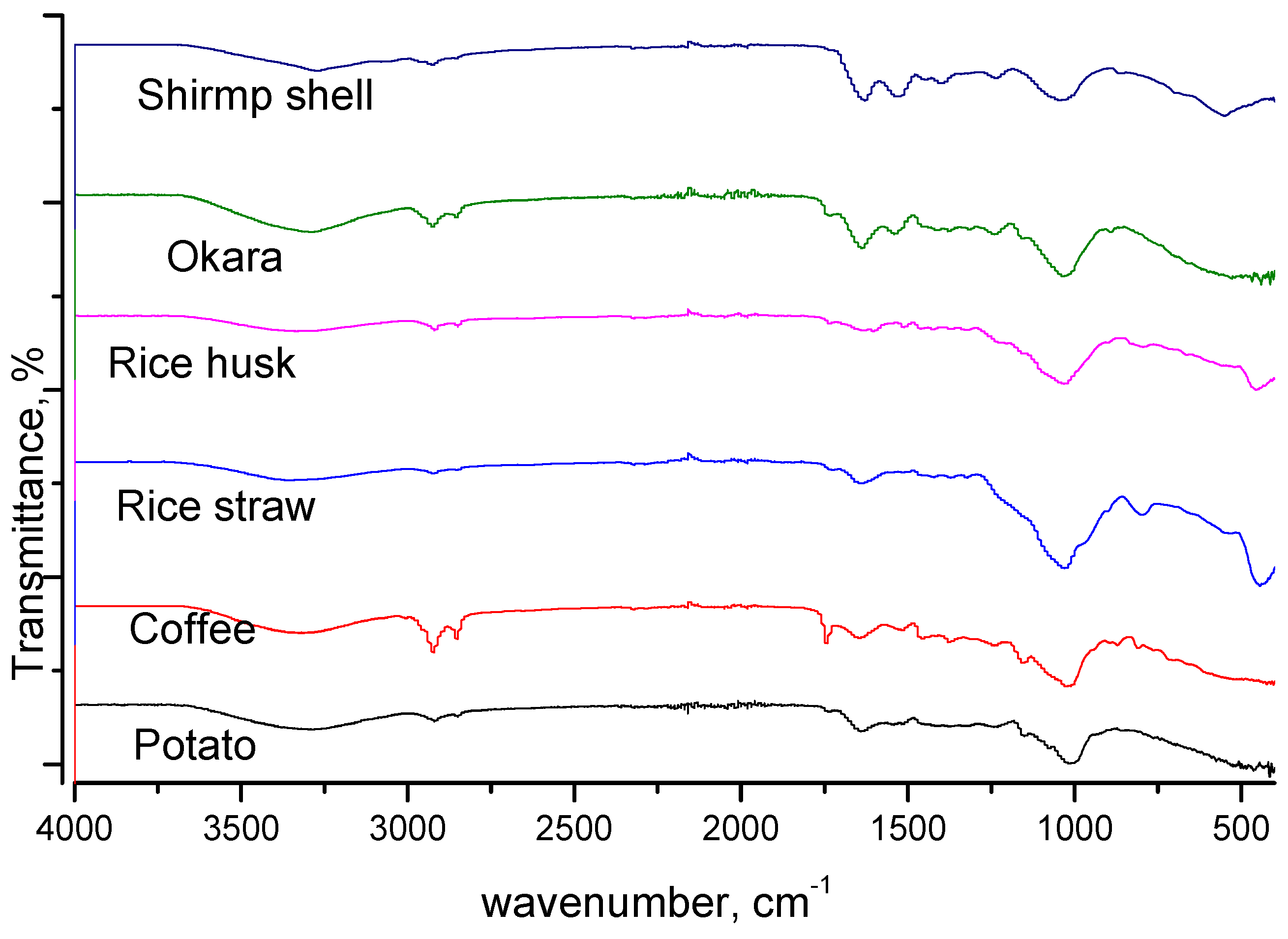
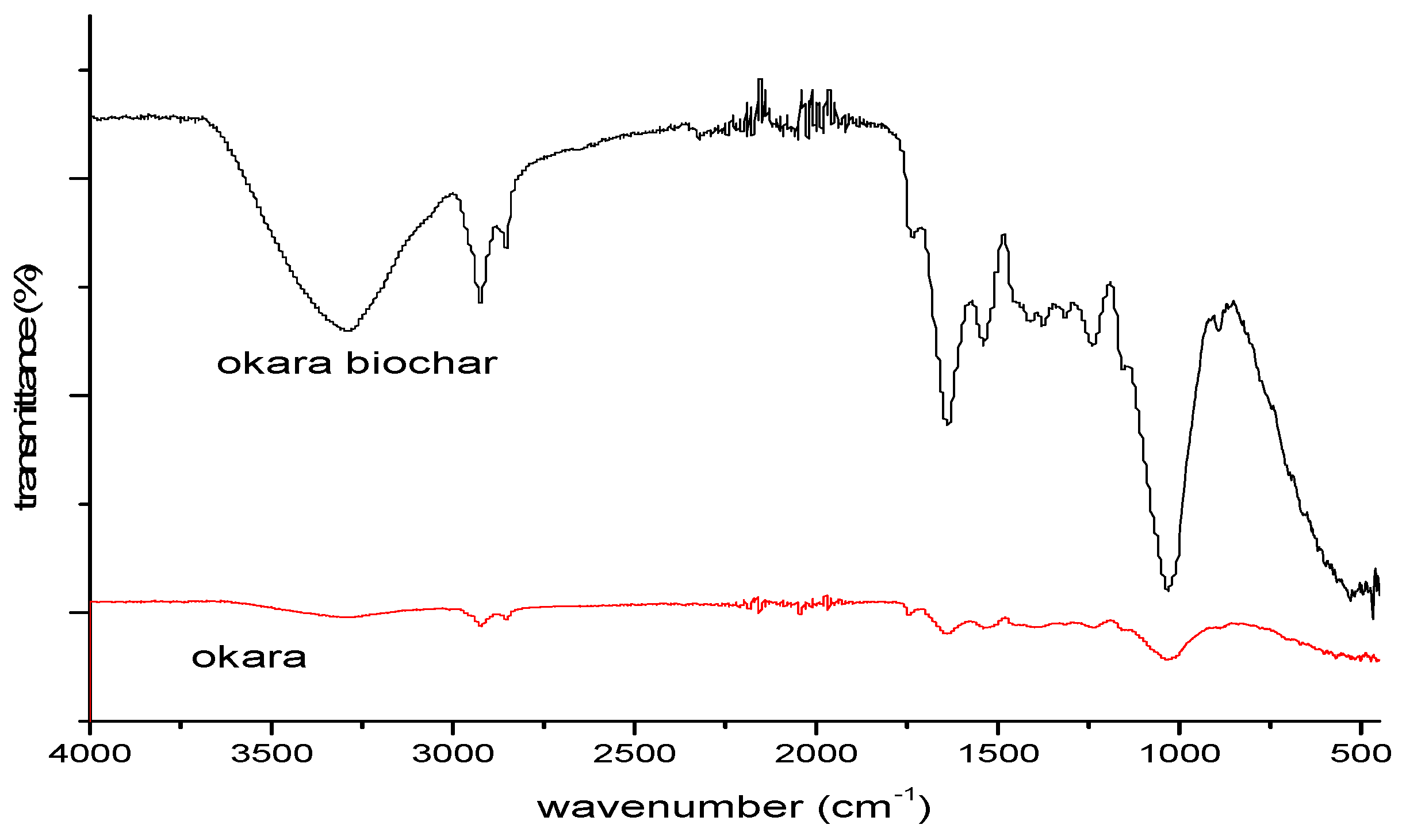
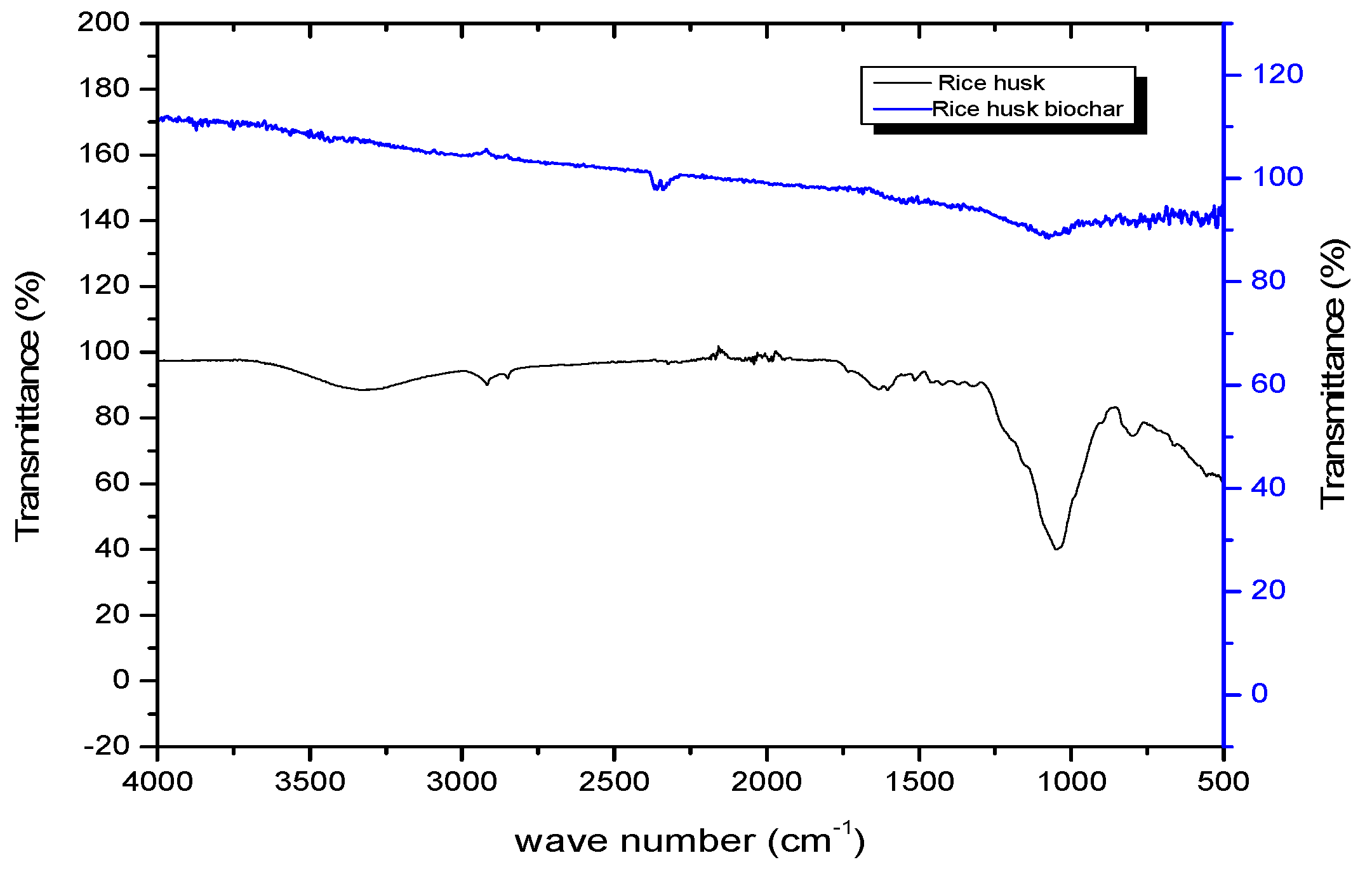
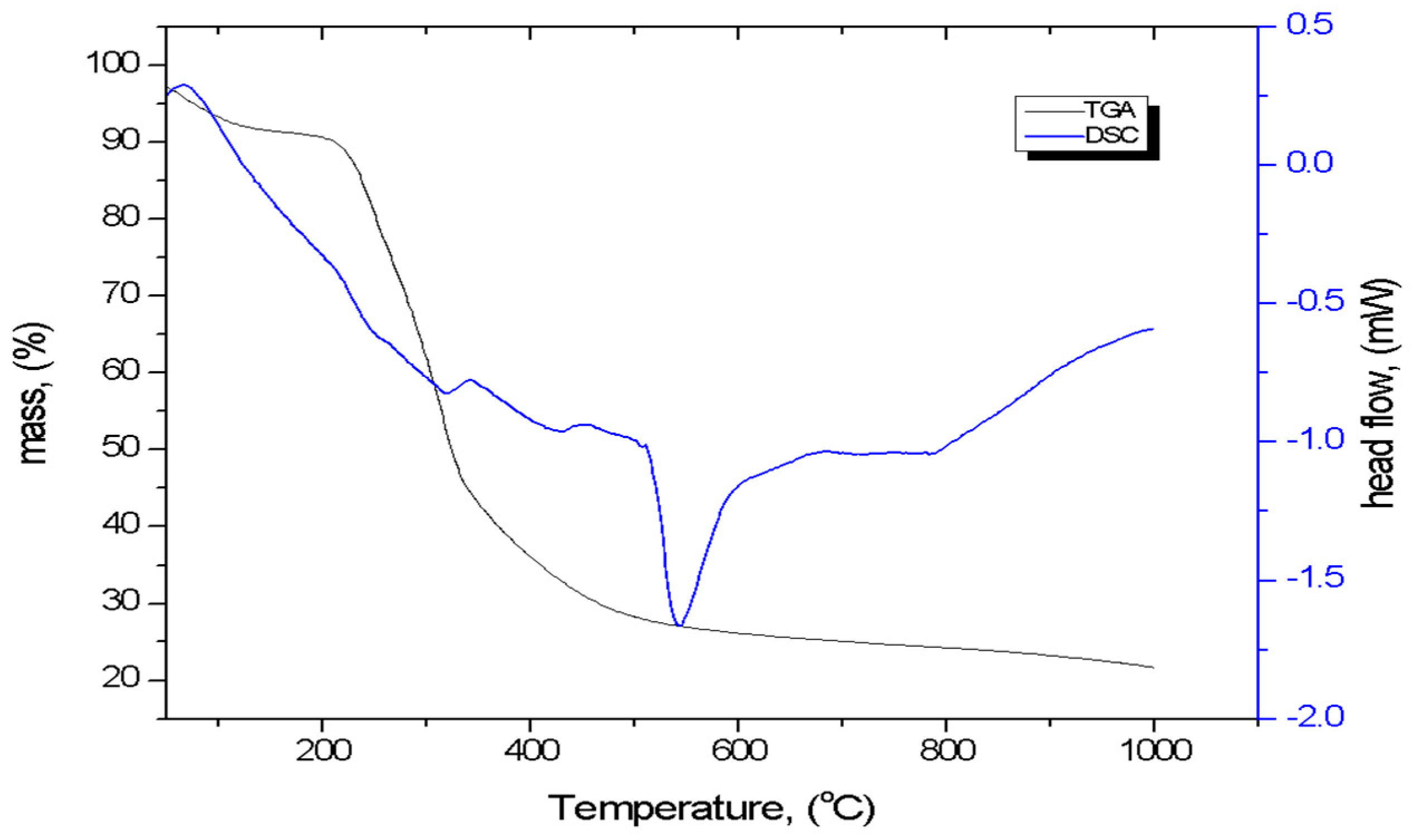


| Biomass Precursor | Sample Code | Pretreatment Methods | Activating Agent |
|---|---|---|---|
| Okara powder waste | OPW1 | Carbonization at 800 °C in an Ar gas flow for 1 h | No activating agent |
| Okara powder waste | OPW2 | Carbonization at 650 °C in a N2 gas flow for 1 h | No activating agent |
| Okara powder waste | OPW3 | Carbonization at 600 °C in a N2 gas flow for 1 h | No activating agent |
| Okara powder waste | OPW4 | Hydrothermal carbonization at 200 °C; the heating rate was 60 °C/h; a temperature of 200 °C was maintained for 4 h; the weight ratio of OPW/water was 12.8 g: 40 mL | No activating agent |
| Okara powder waste | OPW5 | Hydrothermal carbonization at 200 °C; the weight ratio was 12.8 g OPW: 6.4 g zeolite (4 mm in size): 40 mL water; a temperature of 200 °C was maintained for 4 h; the heating rate was 60 °C/h | No activating agent |
| Okara powder waste | OPW6 | Hydrothermal carbonization at 200 °C, with a heating rate of 60 °C/hour and the temperature kept at 200 °C for 4 h; then, the sample was mixed with KOH and melamine with a mass ratio of hydrochar/KOH/melamine = 1:4:2, and pyrolysis (a two-step process) was performed at 600 °C in a N2 flow for 1 h with a N2 flow rate of 1 L/min | KOH and melamine (weight ratio of KOH/melamine = 2:1) |
| Okara powder waste | OPW7 | Carbonization was performed at 600 °C in N2 for 1 h to make biochar; after that, the biochar was mixed with KOH and water; then, the sample was kept under stirring for 48 h with a biochar/KOH/water weight ratio of 1:4:5, after which it was filtered and dried, then calcined at 600 °C in N2 for 1 h | KOH |
| Rice husk | RH | Carbonization at 600 °C in N2 for 1 h | No activating agent |
| Okara powder waste | OPW8 | Carbonization at 600 °C in N2 for 1 h, followed by a dry mixing process with KOH (ratio of biochar/KOH = 1.3), then drying at 120 °C and carbonization at 600 °C | KOH |
| Okara powder waste | OPW9 | Carbonization at 600 °C in N2 for 1 h, followed by a dry mixing process with KOH (ratio of biochar/KOH = 1.4), then drying at 120 °C and carbonization at 600 °C | KOH |
| Okara powder waste | OPW10 | Carbonization at 600 °C in N2 for 1 h, followed by mixing with a biochar/KOH/water ratio of 1:5:10; then, the sample was kept for 5 days, filtered, dried, and calcined at 600 °C | KOH |
| Element | Coffee Waste | Okara Powder Waste | Rice Straw | Shrimp Shells | Rice Husks | Potato Peels | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | |
| C | 57.35 | 64.17 | 45.73 | 53.36 | 37.88 | 56.56 | 44.38 | 53.4 | 18.91 | 26.44 | 48.12 | 56.79 |
| O | 42.65 | 35.83 | 52.53 | 46.02 | 9.97 | 11.18 | 48.9 | 44.17 | 55.48 | 58.24 | 46.62 | 41.31 |
| K | 1.74 | 0.62 | 5.77 | 2.65 | 5.26 | 1.91 | ||||||
| Ca | 6.72 | 2.42 | ||||||||||
| Si | 46.37 | 29.61 | 55.61 | 15.31 | ||||||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Biomass Precursor | Sample Code | Pretreatment Methods | Activating Agent | SBET (m2/g) | References |
|---|---|---|---|---|---|
| Okara powder waste | OPW2 | Carbonization at 650 °C in a N2 gas flow for 1 h | No activating agent | 1.06 | This work |
| Okara powder waste | OPW1 | Carbonization at 800 °C in an Ar flow for 1 h | No activating agent | 1.16 | This work |
| Lotus leaves | - | Carbonization at 500 °C in N2 flow for 1 h | No activating agent | 3.82 | [17] |
| Lotus leaves | - | Carbonization at 500 °C in N2 flow for 1 h | Melamine | 4.32 | [17] |
| Okara powder waste | OPW4 | Hydrothermal carbonization at 200 °C; heating rate of 60 °C/h; temperature of 200 °C, maintained for 4 h; weight ratio of OPW/water of 12.8 g: 40 mL | No activating agent | 7.01 | This work |
| Okara powder waste | OPW5 | Hydrothermal carbonization at 200 °C; weight ratio of 12.8 g OPW: 6.4 g zeolite 4 mm: 40 mL water; temperature of 200 °C, maintained for 4 h; heating rate of 60 °C/h | Zeolite (4 mm in size) | 14.0 | This work |
| Okara powder waste | OPW3 | Carbonization at 600 °C in a N2 gas flow for 1 h | No activating agent | 20.73 | This work |
| Okara powder waste | OPW6 | Hydrothermal carbonization at 200 °C and then pyrolysis (two-step process) at 600 °C, with a low N2 flow for 1 h and a N2 flow rate of 1 L/m | KOH and melamine | 22.04 | This work |
| Okara powder waste | OPW7 | Carbonization at 600 °C in N2 for 1 h to make biochar; after that, biochar was mixed with KOH and water; then, the sample was kept for 48 h, dried at 120 °C, and calcined at 600 °C in a N2 flow (1 L/min) | KOH (weight ratio of biochar/KOH/water was 1:4:5) | 104.32 | This work |
| Okara powder waste | OPW9 | Carbonization at 600 °C in N2 for 1 h; dry mixing process with KOH | KOH (mass ratio of OPW:KOH = 1/4) | 147.85 | This work |
| Rice husk | RH | Carbonization at 600 °C in N2 for 1 h | No activating agent | 175.48 | This work |
| Okara powder waste | OPW8 | Carbonization at 600 °C in N2 for 1 h; dry mixing process with KOH | KOH (mass ratio of OPW: KOH = 1/3) | 212.60 | This work |
| Banana peels | - | Hydrothermal treatment at 200 °C for 24 h | No activating agent | 294.6 | [60] |
| Elephant grass, | - | Carbonization at 600 °C in a N2 flow for 1 h | KOH | 407 | [47] |
| Okara powder waste | OPW10 | Carbonization at 600 °C in N2 for 1 h, then mixed with KOH, with a biochar/KOH/water mass ratio of 1:5:10, and the sample kept for 5 days | KOH (with a biochar/KOH/water ratio of 1:5:10, with the sample kept for 5 days) | 594.08 | This work |
| Lotus leaf | - | Carbonization at 500 °C in N2 for 1 h | Melamine and KOH | 687 | [17] |
| Firewood | - | Carbonization at 850 °C | Na2CO3/K2CO3 | 818 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.-D.; Liu, Y.; Le, M.T. Synthesis and Characterization of Biochars and Activated Carbons Derived from Various Biomasses. Sustainability 2024, 16, 5495. https://doi.org/10.3390/su16135495
Hoang T-D, Liu Y, Le MT. Synthesis and Characterization of Biochars and Activated Carbons Derived from Various Biomasses. Sustainability. 2024; 16(13):5495. https://doi.org/10.3390/su16135495
Chicago/Turabian StyleHoang, Tuan-Dung, Yan Liu, and Minh Thang Le. 2024. "Synthesis and Characterization of Biochars and Activated Carbons Derived from Various Biomasses" Sustainability 16, no. 13: 5495. https://doi.org/10.3390/su16135495
APA StyleHoang, T.-D., Liu, Y., & Le, M. T. (2024). Synthesis and Characterization of Biochars and Activated Carbons Derived from Various Biomasses. Sustainability, 16(13), 5495. https://doi.org/10.3390/su16135495








