Green Energy Production and Integrated Treatment of Pharmaceutical Wastewater Using MnCo2O4 Electrode Performance in Microbial Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Sample and Preliminary Characterization
- -
- Soluble chemical oxygen demand (SCOD)—Soluble form of organics;
- -
- Particulate chemical oxygen demand (PCOD)—Insoluble form of organics.
2.2. Fabrication of MnCo2O4-ACFF Electrode
2.3. Experimental Setup of DMFC
2.4. Operational Parameters
2.5. Experimental Study
- -
- P—power;
- -
- —voltage;
- -
- —current intensity.
2.6. Coulombic Efficiency (CE)
- -
- 8—numerical constant;
- -
- —Faraday constant (96,500 C/mol);
- -
- —Flow of wastewater (m3 /s);
- -
- —Removal rate of COD (g/L).
- -
- measured in this study suggested that methanogenic bacteria may be present in the inoculum. In the steady state of the DMFC, the polarization curve corresponding to each OL was measured by changing the external resistance (16,000–100 Ω).
3. Results and Discussion
3.1. DMFC’s Acclimatization Phase
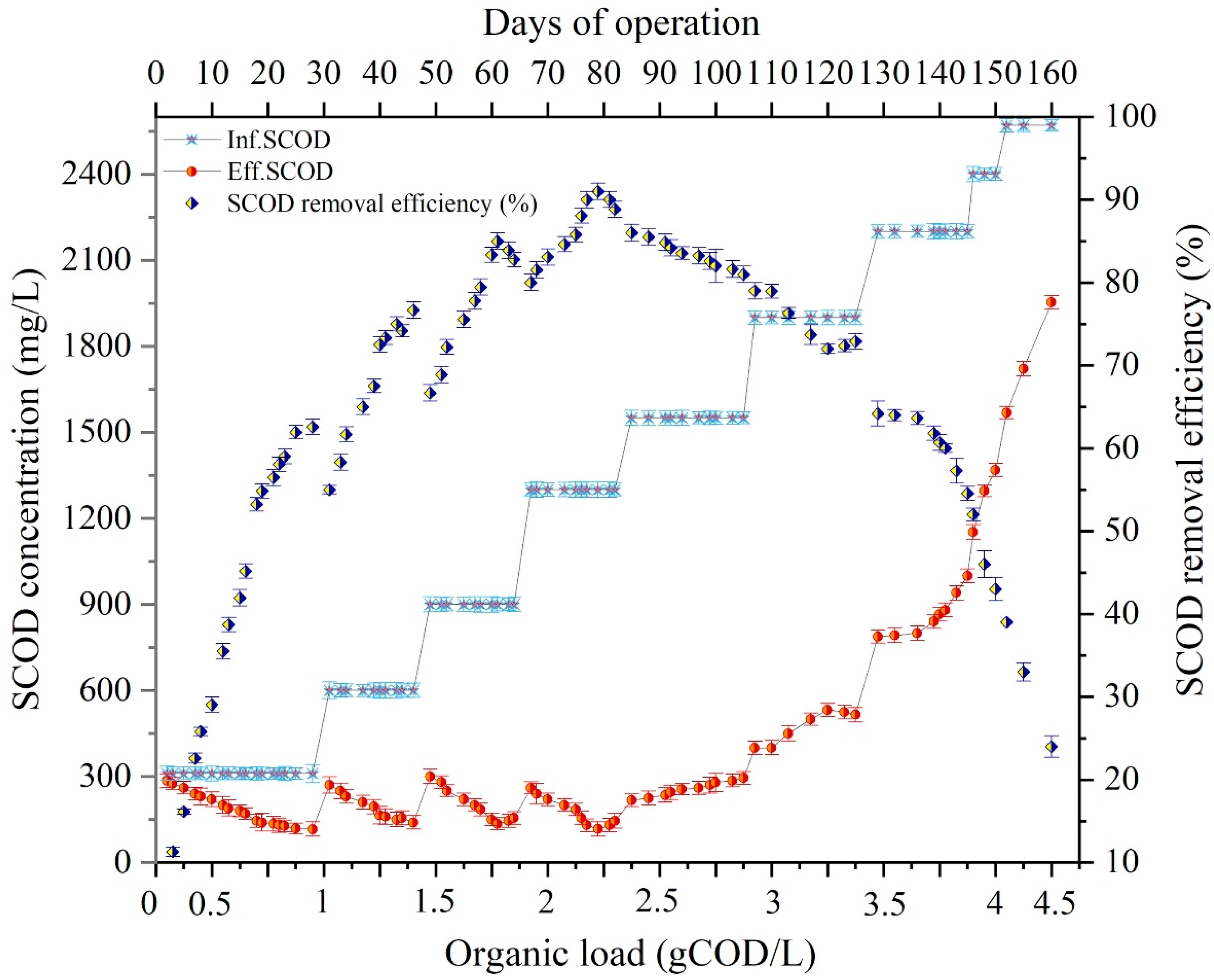
3.2. Effect of OL in DMFC on TCOD and SCOD Removal
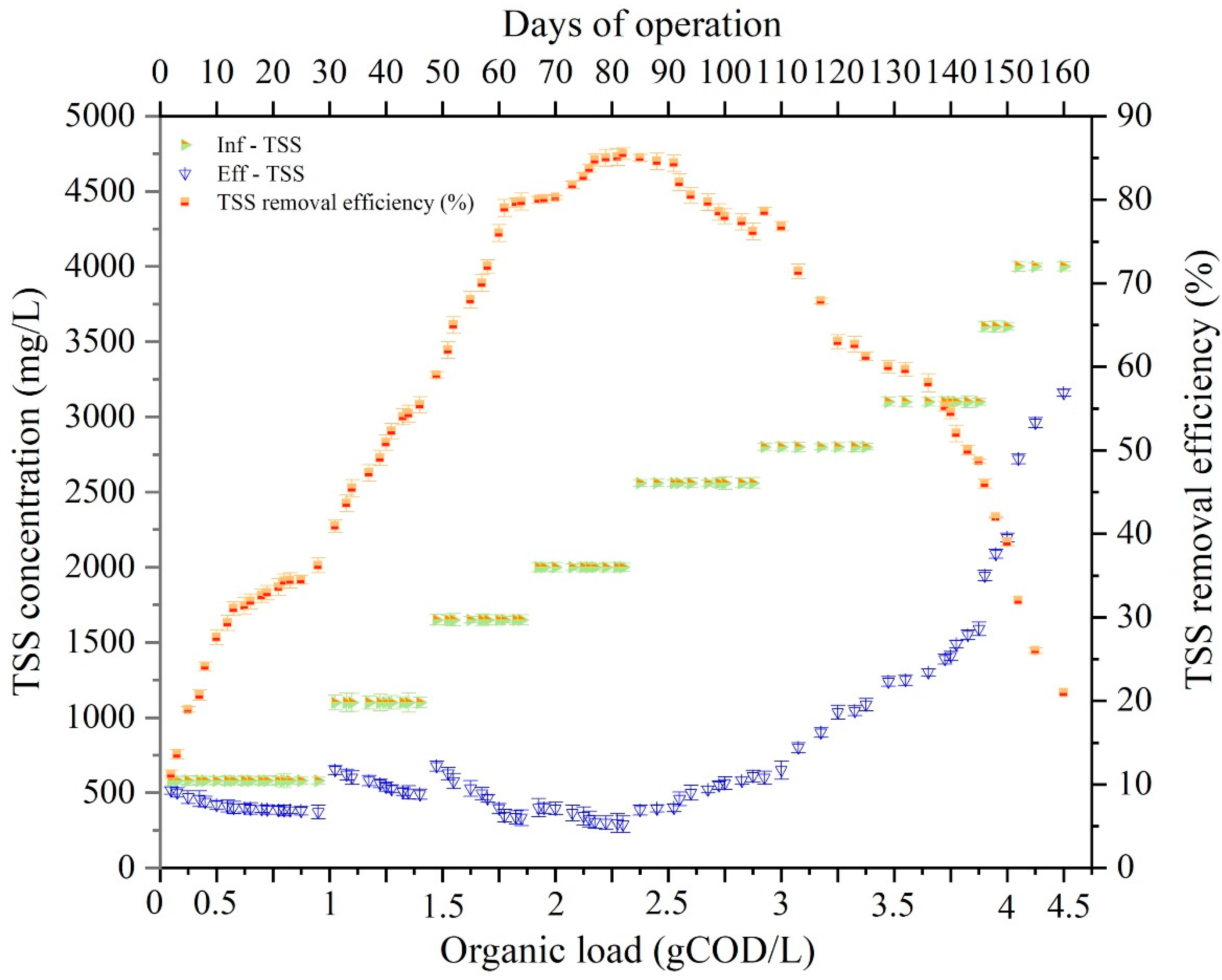
3.3. Impact of OL on TSS Removal Efficiency
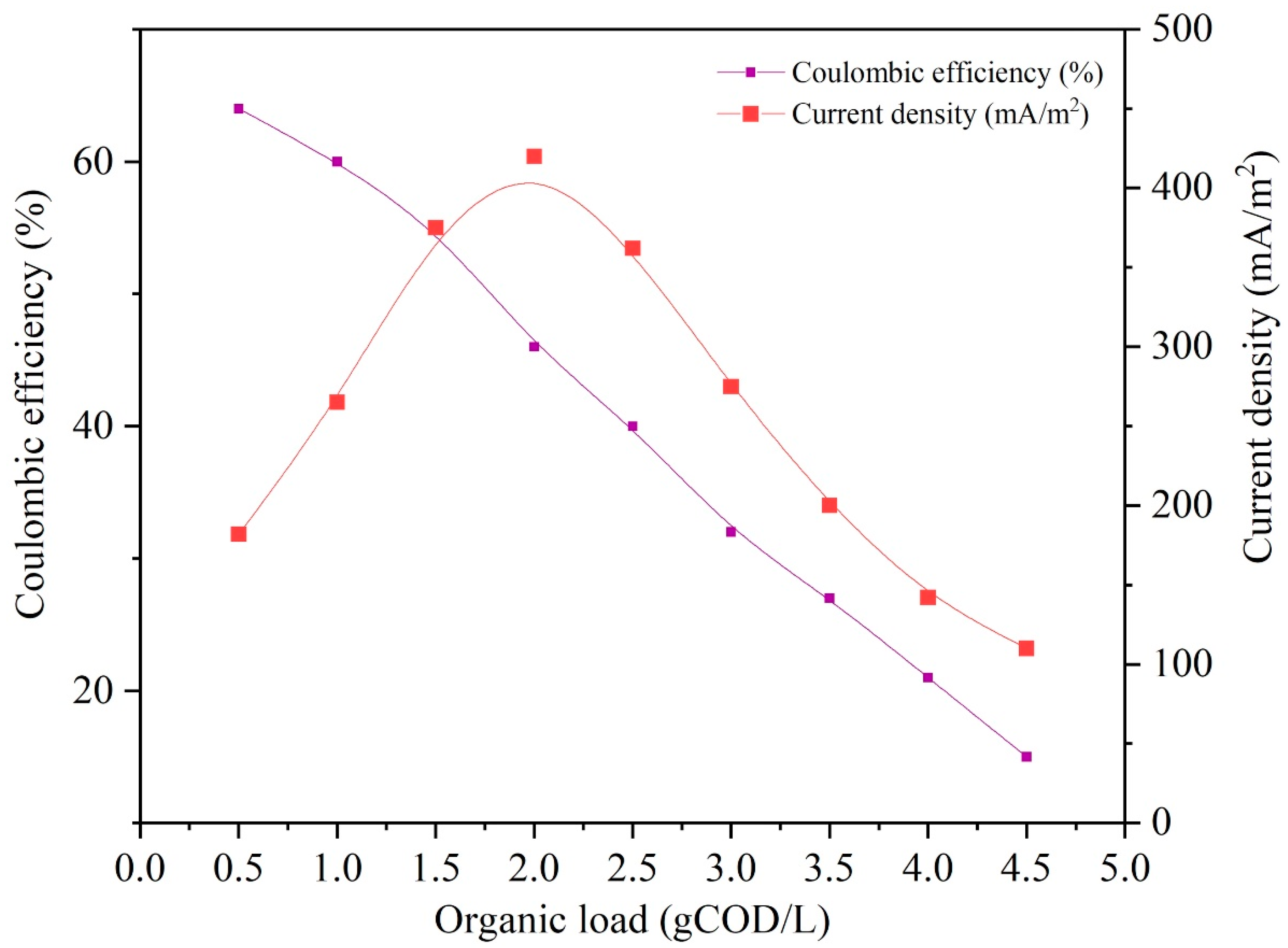
3.4. OL’s Impact on Electricity Production
3.5. Coulombic Efficiency
4. Importance and Future Prospects of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thapa, B.S.; Pandit, S.; Patwardhan, S.B.; Tripathi, S.; Mathuriya, A.S.; Gupta, P.K.; Lal, R.B.; Tusher, T.R. Application of microbial fuel cell (MFC) for pharmaceutical wastewater treatment: An overview and future perspectives. Sustainability 2022, 14, 8379. [Google Scholar] [CrossRef]
- Mamta; Bhushan, S.; Rana, M.S.; Raychaudhuri, S.; Simsek, H.; Prajapati, S.K. Algae- and bacteria-driven technologies for pharmaceutical remediation in wastewater. In Removal of Toxic Pollutants through Microbiological and Tertiary Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–408. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Rabeea, S.A.; Merchant, H.A.; Khan, M.U.; Kow, C.S.; Hasan, S.S. Surging trends in prescriptions and costs of antidepressants in England amid COVID-19. DARU J. Pharm. Sci. 2021, 29, 217–221. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directorate for Public Health—Scientific Committee on Health, E. and E.R. (SCHEER). Statement II on Emerging Health and Environmental Issues; SCHEER: Baden-Württemberg, Germany, 2022. [Google Scholar]
- Anwar, M.; Iqbal, Q.; Saleem, F. Improper disposal of unused antibiotics: An often overlooked driver of antimicrobial resistance. Expert Rev. Anti. Infect. Ther. 2020, 18, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Frangou, S.; Travis-Lumer, Y.; Kodesh, A.; Goldberg, Y.; New, F.; Reichenberg, A.; Levine, S.Z. Increased incident rates of antidepressant use during the COVID-19 pandemic: Interrupted time-series analysis of a nationally representative sample. Psychol. Med. 2023, 53, 4943–4951. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Sheob, M.; Saeed, B.; Khan, S.U.; Shirinkar, M.; Frontistis, Z.; Basheer, F.; Farooqi, I.H. Use of electrocoagulation for treatment of pharmaceutical compounds in water/wastewater: A review exploring opportunities and challenges. Water 2021, 13, 2105. [Google Scholar] [CrossRef]
- Das, S.; Calay, R.K. Experimental study of power generation and COD removal efficiency by air cathode microbial fuel cell using Shewanella baltica 20. Energies 2022, 15, 4152. [Google Scholar] [CrossRef]
- Tamilarasan, K.; Shabarish, S.; Banu, J.R.; Sharmila, V.G. Sustainable power production from petrochemical industrial effluent using dual chambered microbial fuel cell. J. Environ. Manag. 2024, 351, 119777. [Google Scholar] [CrossRef]
- Khalili, H.B.; Mohebbi-Kalhori, D.; Afarani, M.S. Microbial fuel cell (MFC) using commercially available unglazed ceramic wares: Low-cost ceramic separators suitable for scale-up. Int. J. Hydrogen Energy 2017, 42, 8233–8241. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial fuel cell construction features and application for sustainable wastewater treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, A. Sustained energy production from wastewater in microbial fuel cell: Effect of inoculum sources, electrode spacing and working volume. 3 Biotech 2021, 11, 344. [Google Scholar] [CrossRef]
- Karuppiah, T.; Uthirakrishnan, U.; Sivakumar, S.V.; Authilingam, S.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. Processing of electroplating industry wastewater through dual chambered microbial fuel cells (MFC) for simultaneous treatment of wastewater and green fuel production. Int. J. Hydrogen Energy 2022, 47, 37569–37576. [Google Scholar] [CrossRef]
- Karuppaiah, T.; Shankaran, S.; Vincent, G.S. Effluent therapy of electroplating industry through HUASB coupled with dual chambered microbial fuel cell. AIP Conf. Proc. 2023, 2766, 020045. [Google Scholar]
- Haavisto, J.M.; Kokko, M.E.; Lay, C.H.; Puhakka, J.A. Effect of hydraulic retention time on continuous electricity production from xylose in up-flow microbial fuel cell. Int. J. Hydrogen Energy 2017, 42, 27494–27501. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater 2017. Available online: https://www.standardmethods.org/doi/book/10.2105/SMWW.2882 (accessed on 19 May 2024).
- Morovati, R.; Hoseini, M.; Azhdarpoor, A.; Dehghani, M.; Baghapour, M.A.; Yousefinejad, S. Removal of Diclofenac Sodium from Wastewater in Microbial Fuel Cell by Anode Modified with MnCo2O4. Sustainability 2022, 14, 13907. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Walter, X.A.; Madrid, E.; Gajda, I.; Greenman, J.; Ieropoulos, I. Microbial fuel cell scale-up options: Performance evaluation of membrane (c-MFC) and membrane-less (s-MFC) systems under different feeding regimes. J. Power Sources 2022, 520, 230875. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, K.; Banu, J.R.; Jayashree, C.; Yogalakshmi, K.N.; Gokulakrishnan, K. Effect of organic loading rate on electricity generating potential of upflow anaerobic microbial fuel cell treating surgical cotton industry wastewater. J. Environ. Chem. Eng. 2017, 5, 1021–1026. [Google Scholar] [CrossRef]
- Ou, S.; Kashima, H.; Aaron, D.S.; Regan, J.M.; Mench, M.M. Full cell simulation and the evaluation of the buffer system on air-cathode microbial fuel cell. J. Power Sources 2017, 347, 159–169. [Google Scholar] [CrossRef]
- Jayashree, C.; Tamilarasan, K.; Rajkumar, M.; Arulazhagan, P.; Yogalakshmi, K.N.; Srikanth, M.; Banu, J.R. Treatment of seafood processing wastewater using upflow microbial fuel cell for power generation and identification of bacterial community in anodic biofilm. J. Environ. Manag. 2016, 180, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Poureshghi Oskouei, F.; Dong, N.P.; Das, S.; Petrich, C.; Calay, R.K. Enhanced Performance of Microbial Fuel Cells Using PVDF Activated Carbon Air Cathode and Electrochemically and Chemically Treated Carbon Felt Anode. In Electrochemical Society Meeting Abstracts 242; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2022; p. 2037. [Google Scholar]
- Oon, Y.L.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Dahalan, F.A.; Oon, Y.S.; Lehl, H.K.; Thung, W.E. Synergistic effect of up-flow constructed wetland and microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2016, 203, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Mise, S.R.; Saware, S. Electricity generation using textile wastewater by single chambered microbial fuel cell. Int. Res. J. Eng. Technol. 2016, 3, 710–716. [Google Scholar]
- Lee, C.Y.; Lin, Y.H. The electricity generation in microbial fuel cells using reaeration mechanism for cathodic oxygen reduction. J. Civ. Env. Eng. 2015, 5, 6. [Google Scholar]
- Sawasdee, V.; Pisutpaisal, N. Simultaneous pollution treatment and electricity generation of tannery wastewater in air-cathode single chamber MFC. Int. J. Hydrogen Energy 2016, 41, 15632–15637. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Kim, T.G.; Cho, K.S. Characterization of the COD removal, electricity generation, and bacterial communities in microbial fuel cells treating molasses wastewater. J. Environ. Sci. Health—Part. A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Naina Mohamed, S.; Thomas, N.; Tamilmani, J.; Boobalan, T.; Matheswaran, M.; Kalaichelvi, P.; Alagarsamy, A.; Pugazhen dhi, A. Bioelectricity generation using iron (II) molybdate nanocatalyst coated anode during treatment of sugar wastewater in microbial fuel cell. Fuel 2020, 277, 118119. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Ajit Hiraman, P.; Muthukumar, K.; Jayabalan, T. Bioelectricity production from kitchen wastewater using microbial fuel cell with photosynthetic algal cathode. Bioresour. Technol. 2020, 295, 122226. [Google Scholar] [CrossRef]
- Mateo-Ramírez, F.; Addi, H.; Hernandez-Fernandez, F.J.; Godínez, C.; de los Ríos, A.P.; Lotfi, E.M.; El Mahi, M.; Blanco, L.J.L. Air breathing cathode-microbial fuel cell with separator based on ionic liquid applied to slaughterhouse wastewater treatment and bio-energy production. J. Chem. Technol. Biotechnol. 2017, 92, 642–648. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, S.; Luo, Z.; Ma, J.; Zhu, Q.; Zhang, S. A novel MBBR–MFC integrated system for high-strength pulp/paper wastewater treatment and bioelectricity generation. Sep. Sci. Technol. 2020, 55, 2490–2499. [Google Scholar] [CrossRef]
- Ghadge, A.N.; Ghangrekar, M.M. Performance of low cost scalable air-cathode microbial fuel cell made from clayware separator using multiple electrodes. Bioresour. Technol. 2015, 182, 373–377. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Gao, B. The tubular MFC with carbon tube air-cathode for power generation and N, N-dimethylacetamide treatment. Environ. Technol. 2016, 37, 762–767. [Google Scholar] [CrossRef]
- Prasertsung, N.; Ratanatamskul, C. Effects of organic loading rate and operating temperature on power generation from cassava wastewater by a single-chamber microbial fuel cell. Desalin. Water Treat. 2014, 52, 937–946. [Google Scholar] [CrossRef]
- Karuppiah, T.; Pugazhendi, A.; Subramanian, S.; Jamal, M.T.; Jeyakumar, R.B. Deriving electricity from dye processing wastewater using single chamber microbial fuel cell with carbon brush anode and platinum nano coated air cathode. 3 Biotech 2018, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.J.A.; Molenaar, S.D.; Heijne, A.T.; Buisman, C.J.N. Low substrate loading limits methanogenesis and leads to high coulombic efficiency in bioelectrochemical systems. Microorganisms 2016, 4, 7. [Google Scholar] [CrossRef]
- Naveenkumar, M.; Senthilkumar, K.; Sampathkumar, V.; Anandakumar, S.; Thazeem, B. Bio-energy generation and treatment of tannery effluent using microbial fuel cell. Chemosphere 2022, 287, 132090. [Google Scholar] [CrossRef]
- Subha, C.; Kavitha, S.; Abisheka, S.; Tamilarasan, K.; Arulazhagan, P.; Banu, J.R. Bioelectricity generation and effect studies from organic rich chocolaterie wastewater using continuous upflow anaerobic microbial fuel cell. Fuel 2019, 251, 224–232. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Zheng, Y.; Liu, L.; Zhao, F. Efficient degradation of sulfamethoxazole and the response of microbial communities in microbial fuel cells. Rsc. Adv. 2015, 5, 56430–56437. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Fact. 2019, 18, 39. [Google Scholar] [CrossRef]
- Shen, L.; Ma, J.; Song, P.; Lu, Z.; Yin, Y.; Liu, Y.; Cai, L.; Zhang, L. Anodic concentration loss and impedance characteristics in rotating disk electrode microbial fuel cells. Bioprocess Biosyst. Eng. 2016, 39, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Obileke, K.C.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yang, W.; Logan, B.E. Impact of electrode configurations on retention time and domestic wastewater treatment efficiency using microbial fuel cells. Water Res. 2015, 80, 41–46. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Value |
|---|---|
| pH | 8.3 |
| Total Solids (TSs) | 5800 (mg/L) |
| Total Dissolved Solids (TDSs) | 1800 (mg/L) |
| Total Suspended Solids (TSSs) | 4000 (mg/L) |
| Total Chemical Oxygen Demand (TCOD) | 4500 (mg/L) |
| Biochemical Oxygen Demand (BOD) | 1300 (mg/L) |
| Type of Reactor | Anode Material | Type of Wastewater | Maximum COD Removal Efficiency (%) | Reference |
|---|---|---|---|---|
| Dual-chambered MFC | Carbon cloth | Septic tank wastewater | 50 | Khalili et al. (2017) [13] |
| Air-cathode MFC | Carbon cloth | Tannery wastewater | 88 | Sawasdee et al. (2016) [30] |
| Dual-chambered MFC | Preheated carbon felts | Molasses wastewater | 50 | Lee et al. (2016) [31] |
| Dual-chambered MFC | Plain graphite plate | Sugar industrywastewater | 79.8 ± 1.5 | Naina et al. (2020) [32] |
| Algal cathode MFC | Graphite plate | Kitchen wastewater | 73.5 | Naina et al. (2020) [33] |
| Air-cathode MFC (fed-batch mode) | Graphite rod | Slaughterhouse wastewater | 72 | Mateo-Ramírez et al. (2017) [34] |
| Moving bed biofilm reactor-MFC | Carbon fiber paper | Pulp/paper wastewater | 65.6 | Chen et al. (2020) [35] |
| Air-cathode | Carbon felt | Synthetic wastewater | 78 | Ghadge et al. (2015) [36] |
| Dual-chambered MFC | Activated carbon fiber felt | Seafood processing wastewater | 83 | Jayashree et al. (2016) [25] |
| Dual-chambered MFC | (MnCo2O4—Activated carbon fiber felt) | Pharmaceutical wastewater | 89 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettiyan, A.D.; Karuppiah, T.; Shankaran, S.; Di Fraia, S. Green Energy Production and Integrated Treatment of Pharmaceutical Wastewater Using MnCo2O4 Electrode Performance in Microbial Fuel Cell. Sustainability 2024, 16, 5654. https://doi.org/10.3390/su16135654
Ettiyan AD, Karuppiah T, Shankaran S, Di Fraia S. Green Energy Production and Integrated Treatment of Pharmaceutical Wastewater Using MnCo2O4 Electrode Performance in Microbial Fuel Cell. Sustainability. 2024; 16(13):5654. https://doi.org/10.3390/su16135654
Chicago/Turabian StyleEttiyan, Arul Devi, Tamilarasan Karuppiah, Shabarish Shankaran, and Simona Di Fraia. 2024. "Green Energy Production and Integrated Treatment of Pharmaceutical Wastewater Using MnCo2O4 Electrode Performance in Microbial Fuel Cell" Sustainability 16, no. 13: 5654. https://doi.org/10.3390/su16135654






