Response of Maize (Zea mays L.) to Soil Contamination with Diclofenac, Ibuprofen and Ampicillin and Mixtures of These Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Maize Seedling Growth Test
2.3. Photosynthetic Pigments Content Measurement
2.4. Chlorophyll Fluorescence Measurement
2.5. Statistical Analysis
3. Results and Discussion
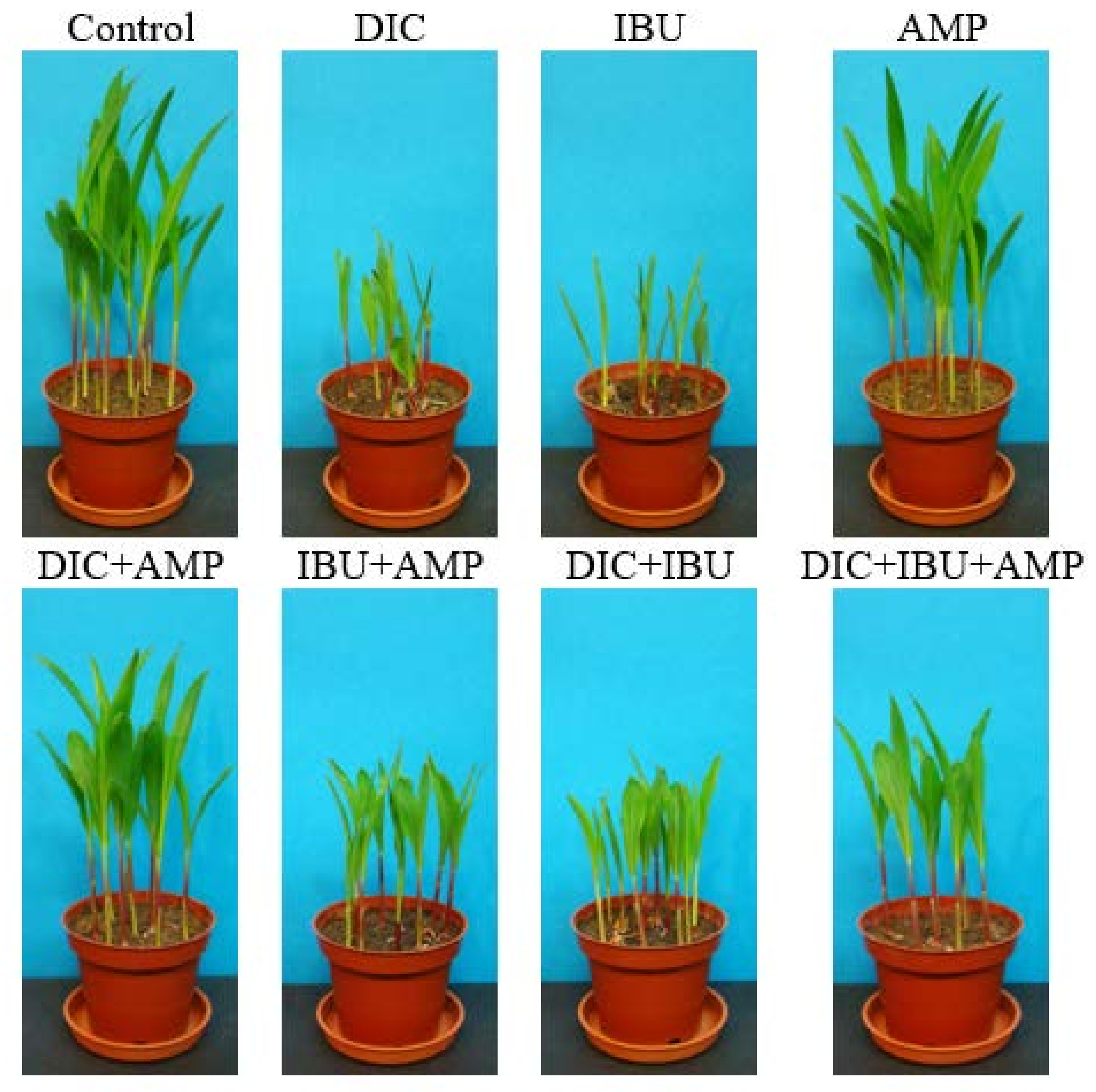
3.1. Phytotoxicity Test Results
3.2. Interaction of DIC, IBU and AMP and Their Mixtures on the Content of Photosynthetic Pigments
3.3. Chlorophyll Fluorescence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pino, M.R.; Muñiz, S.; Val, J.; Navarro, E. Phytotoxicity of 15 common pharmaceuticals on the germination of Lactuca sativa and photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2016, 23, 2253–22541. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Harshkova, D.; Pokora, W.; Baścik-Remisiewicz, A.; Tułodziecki, S.; Aksmann, A. Does diclofenac act like a photosynthetic herbicide on green algae? Chlamydomonas reinhardtii synchronous culture-based study with atrazine as reference. Ecotoxicol. Environ. Saf. 2021, 208, 111630. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Harshkova, D.; Guściora, M.; Aksmann, A. Phytotoxic activity of diclofenac: Evaluation using a model green alga Chlamydomonas reinhardtii with atrazine as a reference substance. Chemosphere 2018, 209, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Redshaw, C.H. Evaluation of biological endpoints in crop plants after exposure to non-steroidal anti-inflammatory drugs (NSAIDs): Implications for phytotoxicological assessment of novel contaminants. Ecotox. Environ. Saf. 2015, 112, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Rai, S.; Kookana, R.S.; Boxall, A.B.A. Fate and uptake of pharmaceuticals in soil-plant systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Di Baccio, D.; Pietrini, F.; Bertolotto, P.; Pérez, S.; Barcelò, D.; Zacchini, M.; Donati, E. Response of Lemna gibba L. to high and environmentally relevant concentrations of ibuprofen: Removal, metabolism and morpho-physiological traits for biomonitoring of emerging contaminants. Sci. Total Environ. 2017, 584–585, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wieczerzak, M.; Kudłak, B.; Namieśnik, J. Impact of selected drugs and their binary mixtures on the germination of Sorghum bicolor (sorgo) seeds. Environ. Sci. Pollut. Res. 2018, 25, 18717–18727. [Google Scholar] [CrossRef] [PubMed]
- Grzesiuk, M.; Spijkerman, E.; Lachmann, S.C.; Wacker, A. Environmental concentrations of pharmaceuticals directly affect phytoplankton and effects propagate through trophic interactions. Ecotoxicol. Environ. Saf. 2018, 156, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Opriş, O.; Ciorîţă, A.; Soran, M.-L.; Lung, I.; Copolovici, D.; Copolovici, L. Evaluation of the photosynthetic parameters, emission of volatile organic compounds and ultrastructure of common green leafy vegetables after exposure to non-steroidal anti-inflammatory drugs (NSAIDs). Ecotoxicology 2019, 28, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Huang, T.; Wang, S.; Wang, X.; Su, L.; Li, C.; Zhao, Y. Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere 2017, 168, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; den Braver, M.W.; van Gestel, C.A.M.; van Straalen, N.M.; Roelofs, D. Ecotoxicogenomic assessment of diclofenac toxicity in soil. Environ. Pollut. 2015, 199, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Eluk, D.; Nagel, O.G.; Zimmerman, J.; Molina, M.P.; Althaus, R.L. Effect of antibiotics on the germination and root elongation of argentine intensive crops. Int. J. Environ. Res. 2016, 10, 471–480. [Google Scholar]
- Gomes, M.P.; Brito, J.C.M.; Rocha, D.C.; Navarro-Silva, M.A.; Juneau, P. Individual and combined effects of amoxicillin, enrofloxacin, and oxytetracycline on Lemna minor physiology. Ecotoxicol. Environ. Saf. 2020, 203, 111025. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, T.; Liu, Y.; Li, L.; Huang, X.; Xie, J. Effects of antibiotics stress on root development, seedling growth, antioxidant status and abscisic acid level in wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2023, 252, 114621. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Antoniou, C.; Christodoulou, C.; Hapeshi, E.; Stavrou, I.; Michael, C.; Fatta-Kassinos, D.; Fotopoulos, V. Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. Sci. Total Environ. 2016, 557–558, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Opriș, O.; Lung, I.; Soran, M.-L.; Alexandra Ciorîță, A.; Copolovici, L. Investigating the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the composition and ultrastructure of green leafy vegetables with important nutritional values. Plant Physiol. Biochem. 2020, 151, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.R.; Garg, S.; Malan, A. Paracetamol and ibuprofen effect on seed quality attributes of Triticum aestivum (wheat). Int. Res. J. Environ. Sci. 2018, 7, 44–48. [Google Scholar]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef] [PubMed]
- Drzymała, J.; Kalka, J. Ecotoxic interactions between pharmaceuticals in mixtures: Diclofenac and sulfamethoxazole. Chemosphere 2020, 259, 127407. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Timis, D.; Taschina, M.; Copolovici, D.; Cioca, G.; Bungau, S. Diclofenac influence on photosynthetic parameters and volatile organic compounds emission from Phaseolus vulgaris L. plants. Rev. Chim. 2017, 68, 2076–2078. [Google Scholar] [CrossRef]
- Taschina, M.; Moisa, C.; Lupitu, A.; Copolovici, D.M.; Copolovici, L. Influence of nonsteroidal anti-inflammatory drugs (NSAIDs) on photosynthetic parameters and secondary metabolites of plants from Fabaceae family. Appl. Sci. 2022, 12, 6326. [Google Scholar] [CrossRef]
- Harshkova, D.; Aksmann, A. Environmental pollution by non-steroidal anti-inflammatory drugs—Diclofenac as an example. Kosmos 2019, 68, 185–194. (In Polish) [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, N.; Chen, G.; Xu, J.; Ouyang, G.; Zhu, F. Stress symptoms and plant hormone-modulated defense response induced by the uptake of carbamazepine and ibuprofen in Malabar spinach (Basella alba L.). Sci. Total Environ. 2021, 793, 148628. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno, A.; Saenz, M.E.; Juárez, A.B.; Moretton, J. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicol. Environ. Saf. 2015, 113, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Manzoor, M.; Iram Gul, I.; Zafar, R.; Jamil, H.I.; Niazi, A.K.; Ali, M.A.; Park, T.J.; Arshad, M. Phytotoxicity of different antibiotics to rice and stress alleviation upon application of organic amendments. Chemosphere 2020, 258, 127353. [Google Scholar] [CrossRef]
- Iori, V.; Zacchini, M.; Pietrini, F. Growth, physiological response and phytoremoval capability of two willow clones exposed to ibuprofen under hydroponic culture. J. Hazard Mater. 2013, 262, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Moro, I.; Matozzo, V.; Piovan, A.; Moschina, E.; Vecchia, F.D. Morpho-physiological effects of ibuprofen on Scenedesmus rubescens. Environ. Toxicol. Pharmacol. 2014, 38, 379–387. [Google Scholar] [CrossRef]
- Matejczyk, M.; Ofman, P.; Dąbrowska, K.; Świsłocka, R.; Lewandowski, W. Synergistic interaction of diclofenac and its metabolites with selected antibiotics and amygdalin in wastewaters. Environ. Res. 2020, 186, 109511. [Google Scholar] [CrossRef]
- Huber, C.; Bartha, B.; Schröder, P. Metabolism of diclofenac in plants—Hydroxylation is followed by glucose conjugation. J. Hazard Mater. 2012, 243, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Zezulka, S.; Kummerová, M.; Babula, P.; Hájková, M.; Oravec, M. Sensitivity of physiological and biochemical endpoints in early ontogenetic stages of crops under diclofenac and paracetamol treatments. Environ. Sci. Pollut. Res. 2019, 26, 3965–3979. [Google Scholar] [CrossRef] [PubMed]
- OECD/OCDE 208, 2006. Guidelines for the Testing of Chemical. Terrestrial Plant: Seedling Test: Seedlings Emergence and Seedling Growth Test. Available online: https://www.oecd-ilibrary.org/environment/test-no-208-terrestrial-plant-test-seedling-emergence-and-seedling-growth-test_9789264070066-en (accessed on 8 January 2024).
- Wang, L.-S.; Wang, L.; Wang, L.; Wang, G.; Li, Z.-H.; Wang, J.-J. Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the wheat (Triticum aestivum L.) seedlings. Environ. Toxicol. 2009, 24, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I. Zawartość wybranych składników szpinaku (Spinacia oleraceae L.) uprawianym przy zróżnicowanej zawartości wapnia. Rocz. AR W Pozn. CCCLX 2004, 38, 105–110. (In Polish) [Google Scholar]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll-nutrient relationships identify nutritionally caused decline in Picea abies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, L.; Xie, H.; Wang, J.; Wang, J.; Sun, F.; Wang, F. Effects of the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate on the growth of wheat seedlings. Environ. Sci. Pollut. Res. 2014, 21, 3936–3945. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Piotrowicz-Cieślak, A.I.; Rydzyński, D.; Adomas, D.; Nałęcz-Jawecki, G. Biomarkers of leguminous plant viability in response to soil contamination with diclofenac. Pol. J. Environ. Stud. 2014, 23, 263–269. [Google Scholar]
- Bellino, A.; Lofrano, G.; Maurizio Carotenuto, M.; Libralato, G.; Baldantoni, D. Antibiotic effects on seed germination and root development of tomato (Solanum lycopersicum L.). Ecotoxicol. Environ. Saf. 2018, 148, 135–141. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Q.; Sun, F.; Zhang, L. Ecotoxicological effects of paracetamol on seed germination and seedling development of wheat (Triticum aestivum L.). J. Hazard Mater. 2009, 169, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, B.; Telesiński, A.; Biczak, R. Effect of diclofenac and naproxen and their mixture on spring barley seedlings and Heterocypris incongruens. Environ. Toxicol. Pharmacol. 2021, 88, 103746. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, B.; Telesiński, A.; Sysa, M.; Godela, A.; Ščurek, R.; Biczak, R. Ibuprofen and ketoprofen—Inert drugs or potential environmental hazard. Sustainability 2023, 15, 1613. [Google Scholar] [CrossRef]
- Svobodníková, L.; Kemmerová, M.; Zezulka, S.; Babula, P.; Sendecká, K. Root response in Pisum sativum under naproxen stress: Morphoanatomical, cytological, and biochemical traits. Chemosphere 2020, 258, 127411. [Google Scholar] [CrossRef]
- Opriş, O.; Copaciu, F.; Soran, M.L.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R.; Pawłowska, B. Reaction of spring barley seedlings and H. incongruens crustaceans to the presence of acetylsalicylic acid in soil. J. Environ. Manag. 2022, 302, 113936. [Google Scholar] [CrossRef] [PubMed]
- Kummerová, M.; Zezulka, S.; Babula, P.; Tříska, J. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Daniel, D.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Li, N.; Cao, B.; Huang, T.; Li, P.; Liu, S.; Zhang, Y.; Xu, K. Effect of ofloxacin levels on growth, photosynthesis and chlorophyll fluorescence kinetics in tomato. Plant Physiol. Biochem. 2023, 194, 374–382. [Google Scholar] [CrossRef]
- Hájková, M.; Kummerová, M.; Zezulka, Š.; Babula, P.; Váczi, P. Diclofenac as an environmental threat: Impact on the photosynthetic processes of Lemna minor chloroplasts. Chemosphere 2019, 224, 892–899. [Google Scholar] [CrossRef] [PubMed]


| Concentration of Drugs [mg∙kg−1 of Soil DW] | DIC | IBU | AMP | DIC + AMP | IBU + AMP | DIC + IBU | DIC + IBU + AMP |
|---|---|---|---|---|---|---|---|
| GP [%] | |||||||
| 0 | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a |
| 1 | 86.70 ± 15.30a | 96.70 ± 5.80a | 90.00 ± 0.00a | 90.00 ± 10.00a | 100.00 ± 0.00a | 96.70 ± 5.80a | 96.70 ± 5.80a |
| 10 | 86.70 ± 15.30a | 90.00 ± 0.00a | 96.70 ± 5.80a | 96.70 ± 5.80a | 100.00 ± 0.00a | 100.00 ± 0.00a | 86.70 ± 5.80a |
| 100 | 96.70 ± 5.80a | 90.00 ± 10.00a | 90.00 ± 0.00a | 100.00 ± 0.00a | 96.70 ± 5.80a | 80.00 ± 0.00a | 96.70 ± 5.80a |
| 1000 | 70.00 ± 10.00a | 86.70 ± 15.30a | 100.00 ± 0.00a | 100.00 ± 0.00a | 86.70 ± 15.30a | 90.00 ± 10.00a | 86.70 ± 5.80a |
| GR [%] | |||||||
| 0 | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a | 86.70 ± 15.30a |
| 1 | 86.70 ± 15.30a | 96.70 ± 5.80a | 90.00 ± 0.00a | 90.00 ± 10.00a | 100.00 ± 0.00a | 96.70 ± 5.80a | 96.70 ± 5.80a |
| 10 | 86.70 ± 15.30a | 90.00 ± 0.00a | 96.70 ± 5.80a | 96.70 ± 5.80a | 100.00 ± 0.00a | 100.00 ± 0.00a | 86.70 ± 5.80a |
| 100 | 96.70 ± 5.80a | 90.00 ± 10.00a | 90.00 ± 0.00a | 100.00 ± 0.00a | 96.70 ± 5.80a | 80.00 ± 0.00a | 96.70 ± 5.80a |
| 1000 | 70.00 ± 10.00a | 86.70 ± 15.30a | 100.00 ± 0.00a | 100.00 ± 0.00a | 86.70 ± 15.30a | 90.00 ± 10.00a | 86.70 ± 5.80a |
| Concentration of Drugs [mg∙kg−1 of Soil DW] | DIC | IBU | AMP | DIC + AMP | IBU + AMP | DIC + IBU | DIC + IBU + AMP |
|---|---|---|---|---|---|---|---|
| Fresh weight [g pot−1] | |||||||
| 0 | 6.531 ± 0.190a | 6.531 ± 0.190a | 6.531 ± 0.190a | 6.531 ± 0.190b | 6.531 ± 0.190a | 6.531 ± 0.190ab | 6.531 ± 0.190a |
| 1 | 6.596 ± 0.173a | 6.613 ± 0.330a | 6.098 ± 0.080a | 6.370 ± 0.182b | 7.392 ± 0.308a | 6.713 ± 0.686a | 6.130 ± 0.552a |
| 10 | 6.580 ± 0.404a | 6.278 ± 0.081a | 6.432 ± 0.454a | 7.556 ± 0.530a | 7.423 ± 0.487a | 6.704 ± 0.079a | 5.910 ± 0.024a |
| 100 | 6.170 ± 0.669a | 5.478 ± 0.318b | 6.670 ± 0.073a | 6.605 ± 0.262ab | 6.445 ± 0.029a | 5.634 ± 0.485b | 5.867 ± 0.427a |
| 1000 | 1.960 ± 0.025b | 1.846 ± 0.016c | 6.409 ± 0.212a | 5.305 ± 0.510c | 2.991 ± 0.575b | 3.042 ± 0.101c | 4.174 ± 0.386b |
| Dry weight [g g−1 FW] | |||||||
| 0 | 0.0919 ± 0.0015b | 0.0919 ± 0.0015b | 0.0919 ± 0.0015a | 0.0919 ± 0.0015b | 0.0919 ± 0.0015b | 0.0919 ± 0.0015b | 0.0919 ± 0.0015b |
| 1 | 0.0901 ± 0.0013b | 0.0928 ± 0.0026b | 0.0916 ± 0.0008a | 0.0852 ± 0.0033b | 0.0875 ± 0.0013bc | 0.0870 ± 0.0027b | 0.0928 ± 0.0013ab |
| 10 | 0.0880 ± 0.0025b | 0.0910 ± 0.0006b | 0.0899 ± 0.0011a | 0.0865 ± 0.0033b | 0.0878 ± 0.0017bc | 0.0896 ± 0.0034b | 0.0902 ± 0.0016b |
| 100 | 0.0895 ± 0.0027b | 0.0908 ± 0.0029b | 0.0895 ± 0.0027a | 0.0923 ± 0.0034b | 0.0865 ± 0.0021c | 0.0876 ± 0.0022b | 0.0917 ± 0.0014b |
| 1000 | 0.1403 ± 0.0075a | 0.1291 ± 0.0030a | 0.0932 ± 0.0022a | 0.1005 ± 0.0019a | 0.1147 ± 0.0028a | 0.1108 ± 0.0007a | 0.0967 ± 0.0020a |
| Shoot length [cm] | |||||||
| 0 | 24.07 ± 2.05a | 24.07 ± 2.05a | 24.07 ± 2.05a | 24.07 ± 2.05a | 24.07 ± 2.05a | 24.07 ± 2.05a | 24.07 ± 2.05a |
| 1 | 22.80 ± 1.77a | 22.45 ± 1.34ab | 22.65 ± 1.51ab | 22.35 ± 1.47ab | 22.65 ± 1.45a | 23.85 ± 1.45a | 22.60 ± 2.16ab |
| 10 | 22.95 ± 1.99a | 23.35 ± 1.31ab | 21.35 ± 1.56b | 23.90 ± 1.31a | 23.35 ± 1.08a | 23.20 ± 1.86a | 21.75 ± 1.74bc |
| 100 | 22.20 ± 1.25a | 21.65 ± 1.18b | 22.75 ± 1.60ab | 22.55 ± 1.42a | 23.40 ± 1.15a | 23.20 ± 1.27a | 21.40 ± 1.02bc |
| 1000 | 6.57 ± 1.81b | 9.00 ± 1.94c | 22.55 ± 1.07ab | 20.35 ± 1.38b | 14.90 ± 1.15b | 14.30 ± 1.03b | 19.75 ± 1.32c |
| Root length [cm] | |||||||
| 0 | 18.25 ± 3.00a | 18.25 ± 3.00a | 18.25 ± 3.00ab | 18.25 ± 3.00a | 18.25 ± 3.00a | 18.25 ± 3.00a | 18.25 ± 3.00a |
| 1 | 18.20 ± 1.78a | 17.77 ± 1.16ab | 18.19 ± 1.18ab | 18.20 ± 1.49a | 18.84 ± 1.71a | 17.88 ± 1.74a | 18.93 ± 1.70a |
| 10 | 16.21 ± 1.60b | 16.27 ± 1.64b | 19.15 ± 1.20a | 19.56 ± 1.64a | 17.40 ± 1.58a | 17.82 ± 1.31a | 18.43 ± 1.60a |
| 100 | 16.07 ± 1.50b | 13.38 ± 1.73c | 16.98 ± 1.63bc | 17.64 ± 1.44a | 17.31 ± 1.54a | 15.36 ± 1.59b | 16.93 ± 1.16a |
| 1000 | 3.70 ± 0.74c | 2.90 ± 1.09d | 15.13 ± 1.57c | 11.14 ± 1.80b | 4.62 ± 1.21b | 4.61 ± 1.20c | 8.26 ± 1.06b |
| Concentration of Drugs [mg kg−1 of Soil DW] | DIC | IBU | AMP | DIC + AMP | IBU + AMP | DIC + IBU | DIC + IBU + AMP |
|---|---|---|---|---|---|---|---|
| Chlorophyll a [mg g−1 DW] | |||||||
| 0 | 11.246 ± 0.026a | 11.246 ± 0.026a | 11.246 ± 0.026a | 11.246 ± 0.026a | 11.246 ± 0.026a | 11.246 ± 0.026a | 11.246 ± 0.026a |
| 1 | 8.679 ± 0.029b | 9.387 ± 0.023b | 10.952 ± 0.035b | 10.863 ± 0.029b | 10.429 ± 0.018b | 10.628 ± 0.016b | 8.386 ± 0.072b |
| 10 | 7.456 ± 0.034c | 7.502 ± 0.091d | 10.065 ± 0.035c | 9.585 ± 0.039c | 10.418 ± 0.142b | 8.609 ± 0.044c | 7.749 ± 0.025c |
| 100 | 5.570 ± 0.051d | 7.705 ± 0.037c | 7.438 ± 0.158d | 8.681 ± 0.017d | 7.342 ± 0.043c | 7.258 ± 0.021d | 6.966 ± 0.021d |
| 1000 | 4.932 ± 0.024e | 5.767 ± 0.028d | 6.847 ± 0.022e | 8.469 ± 0.003e | 4.784 ± 0.013d | 5.522 ± 0.156e | 6.151 ± 0.030e |
| Chlorophyll b [mg g−1 DW] | |||||||
| 0 | 6.310 ± 0.017a | 6.310 ± 0.017a | 6.310 ± 0.017b | 6.310 ± 0.017b | 6.310 ± 0.017a | 6.310 ± 0.017b | 6.310 ± 0.017a |
| 1 | 5.430 ± 0.021b | 5.098 ± 0.014b | 6.683 ± 0.018a | 6.143 ± 0.066b | 6.110 ± 0.024b | 6.564 ± 0.034a | 5.486 ± 0.049b |
| 10 | 4.552 ± 0.028c | 4.687 ± 0.034c | 5.903 ± 0.031c | 5.164 ± 0.020d | 5.731 ± 0.022c | 5.262 ± 0.026c | 5.074 ± 0.026c |
| 100 | 3.377 ± 0.017d | 4.469 ± 0.021d | 4.163 ± 0.100d | 5.389 ± 0.012c | 4.209 ± 0.055d | 4.396 ± 0.038d | 3.883 ± 0.013d |
| 1000 | 2.376 ± 0.014e | 2.807 ± 0.008e | 3.944 ± 0.024e | 5.149 ± 0.026d | 2.244 ± 0.043e | 2.780 ± 0.076e | 3.584 ± 0.042e |
| Chlorophyll a/chlorophyll b | |||||||
| 0 | 1.783 ± 0.006b | 1.783 ± 0.006c | 1.783 ± 0.006a | 1.783 ± 0.006b | 1.783 ± 0.006bc | 1.783 ± 0.006b | 1.783 ± 0.006a |
| 1 | 1.598 ± 0.008d | 1.841 ± 0.007b | 1.639 ± 0.008d | 1.768 ± 0.021b | 1.705 ± 0.012d | 1.621 ± 0.009d | 1.529 ± 0.003c |
| 10 | 1.638 ± 0.013c | 1.601 ± 0.008e | 1.705 ± 0.006c | 1.856 ± 0.006a | 1.818 ± 0.014b | 1.636 ± 0.004cd | 1.527 ± 0.006c |
| 100 | 1.649 ± 0.007c | 1.724 ± 0.007d | 1.787 ± 0.006a | 1.611 ± 0.004d | 1.744 ± 0.017cd | 1.651 ± 0.012c | 1.794 ± 0.010a |
| 1000 | 2.076 ± 0.019a | 2.055 ± 0.0015a | 1.736 ± 0.014b | 1.645 ± 0.008c | 2.132 ± 0.037a | 1.986 ± 0.008a | 1.716 ± 0.015b |
| Carotenoids [mg g−1 DW] | |||||||
| 0 | 3.486 ± 0.012a | 3.486 ± 0.012a | 3.486 ± 0.012a | 3.486 ± 0.012a | 3.486 ± 0.012a | 3.486 ± 0.012a | 3.486 ± 0.012a |
| 1 | 2.716 ± 0.005b | 2.873 ± 0.010b | 3.519 ± 0.004a | 3.315 ± 0.009b | 3.091 ± 0.018b | 3.365 ± 0.015b | 2.708 ± 0.016b |
| 10 | 2.392 ± 0.012c | 2.379 ± 0.024c | 3.158 ± 0.005b | 2.947 ± 0.017c | 3.118 ± 0.074b | 2.717 ± 0.009c | 2.634 ± 0.173b |
| 100 | 1.735 ± 0.008d | 2.360 ± 0.007c | 2.312 ± 0.041c | 2.759 ± 0.013d | 2.286 ± 0.014c | 2.291 ± 0.012d | 2.105 ± 0.014c |
| 1000 | 1.356 ± 0.006e | 1.588 ± 0.008d | 2.119 ± 0.008d | 2.622 ± 0.006e | 1.383 ± 0.015d | 1.624 ± 0.043e | 1.952 ± 0.014c |
| Chlorophyll (a + b)/carotenoids | |||||||
| 0 | 5.037 ± 0.013c | 5.037 ± 0.013d | 5.037 ± 0.013b | 5.037 ± 0.013c | 5.037 ± 0.013b | 5.037 ± 0.013a | 5.037 ± 0.013bc |
| 1 | 5.194 ± 0.020b | 5.042 ± 0.010d | 5.012 ± 0.010b | 5.130 ± 0.013b | 5.194 ± 0.021a | 5.110 ± 0.035a | 5.123 ± 0.019ab |
| 10 | 5.019 ± 0.026c | 5.123 ± 0.006c | 5.057 ± 0.022ab | 5.005 ± 0.014c | 5.147 ± 0.017ab | 5.105 ± 0.021a | 5.060 ± 0.028bc |
| 100 | 5.156 ± 0.016b | 5.159 ± 0.012b | 5.017 ± 0.024b | 5.102 ± 0.017b | 5.052 ± 0.064b | 5.089 ± 0.049a | 5.020 ± 0.055c |
| 1000 | 5.388 ± 0.020a | 5.401 ± 0.018a | 5.092 ± 0.020a | 5.351 ± 0.031a | 5.082 ± 0.090ab | 5.112 ± 0.013a | 5.154 ± 0.031a |
| Concentration of Drugs [mg kg−1 of Soil DW] | DIC | IBU | AMP | DIC + AMP | IBU + AMP | DIC + IBU | DIC + IBU + AMP |
|---|---|---|---|---|---|---|---|
| F0 | |||||||
| 0 | 198.80 ± 6.46ab | 198.80 ± 6.46b | 198.80 ± 6.46a | 198.80 ± 6.46a | 198.80 ± 6.46a | 198.80 ± 6.46a | 198.80 ± 6.46a |
| 1 | 198.00 ± 2.58ab | 191.50 ± 5.26b | 180.75 ± 10.72a | 195.25 ± 7.04a | 176.50 ± 4.43c | 190.50 ± 8.06b | 194.00 ± 14.58a |
| 10 | 195.50 ± 2.38b | 192.25 ± 4.27b | 190.00 ± 12.03a | 197.25 ± 5.74a | 180.00 ± 6.83bc | 192.50 ± 3.11ab | 192.25 ± 7.04a |
| 100 | 192.25 ± 3.86b | 199.00 ± 7.44b | 193.00 ± 23,65a | 191.00 ± 8.04a | 193.25 ± 6.60ab | 187.50 ± 8.43b | 185.00 ± 11.22a |
| 1000 | 207.00 ± 3.56a | 213.00 ± 7.53a | 207.00 ± 17.34a | 188.00 ± 8.60a | 191.75 ± 2.09abc | 209.75 ± 14.61a | 191.75 ± 4.19a |
| Fm | |||||||
| 0 | 955.0 ± 25.9a | 955.0 ± 25.9ab | 955.0 ± 25.9a | 955.0 ± 25.9a | 955.0 ± 25.9a | 955.0 ± 25.9a | 955.0 ± 25.9a |
| 1 | 949.5 ± 7.8ab | 907.5 ± 44.7b | 865.3 ± 56.2a | 956.3 ± 39.2a | 835.3 ± 42.1c | 904.0 ± 29.8a | 934.5 ± 37.6ab |
| 10 | 895.0 ± 28.7b | 925.0 ± 17.8ab | 870.8 ± 92.1a | 954.3 ± 6.1a | 861.3 ± 34.5bc | 911.0 ± 80.2a | 952.5 ± 18.5ab |
| 100 | 932.8 ± 32.8ab | 969.0 ± 20.0a | 903.0 ± 66.5a | 890.0 ± 46.9a | 930.3 ± 23.4ab | 882.0 ± 40.5a | 881.5 ± 51.2b |
| 1000 | 910.8 ± 26.4ab | 930.0 ± 27.2ab | 958.8 ± 63.9a | 888.5 ± 59.8a | 871.5 ± 64.9bc | 902.8 ± 75.0a | 914.8 ± 28.6ab |
| Fv | |||||||
| 0 | 765.2 ± 24.8a | 765.2 ± 24.8a | 765.2 ± 24.8a | 765.2 ± 24.8a | 765.2 ± 24.8a | 765.2 ± 24.8a | 765.2 ± 24.8a |
| 1 | 751.5 ± 7.9ab | 708.5 ± 53.6a | 664.5 ± 82.2a | 761.0 ± 32.4a | 661.3 ± 33.8b | 713.5 ± 24.2a | 735.5 ± 50.4a |
| 10 | 704.3 ± 24.2b | 732.8 ± 15.2a | 685.8 ± 73.7a | 757.0 ± 2.45a | 681.3 ± 28.6b | 764.0 ± 30.0a | 760.3 ± 11.8a |
| 100 | 718.0 ± 30.8ab | 770.0 ± 12.7a | 710.0 ± 43.2a | 699.0 ± 39.9a | 737.0 ± 17.2ab | 694.5 ± 32.4a | 696.5 ± 40.0a |
| 1000 | 703.8 ± 27.4b | 717.0 ± 19.9a | 751.8 ± 76.9a | 700.5 ± 51.4a | 674.8 ± 61.2a | 695.5 ± 57.0a | 723.0 ± 24.6a |
| Fv/Fm | |||||||
| 0 | 0.791 ± 0.007a | 0.791 ± 0.007a | 0.791 ± 0.007a | 0.791 ± 0.007a | 0.791 ± 0.007a | 0.791 ± 0.007a | 0.791 ± 0.007a |
| 1 | 0.791 ± 0.003a | 0.791 ± 0.006a | 0.790 ± 0.006a | 0.796 ± 0.002a | 0.791 ± 0.008a | 0.789 ± 0.006a | 0.761 ± 0.049a |
| 10 | 0.787 ± 0.008ab | 0.792 ± 0.003a | 0.787 ± 0.009a | 0.793 ± 0.005a | 0.790 ± 0.004a | 0.798 ± 0.007a | 0.798 ± 0.004a |
| 100 | 0.791 ± 0.005a | 0.795 ± 0.004a | 0.787 ± 0.011a | 0.785 ± 0.005a | 0.792 ± 0.002a | 0.787 ± 0.002a | 0.790 ± 0.001a |
| 1000 | 0.772 ± 0.008b | 0.771 ± 0.002b | 0.782 ± 0.032a | 0.786 ± 0.005a | 0.778 ± 0.008b | 0.770 ± 0.006b | 0.786 ± 0.002a |
| Fv/F0 | |||||||
| 0 | 3.806 ± 0.169a | 3.806 ± 0.169a | 3.806 ± 0.169a | 3.806 ± 0.169a | 3.806 ± 0.169a | 3.806 ± 0.169a | 3.806 ± 0.169a |
| 1 | 3.796 ± 0.070a | 3.802 ± 0.138a | 3.774 ± 0.138a | 3.897 ± 0.052a | 3.800 ± 0.015a | 3.747 ± 0.130a | 3.750 ± 0.589a |
| 10 | 3.696 ± 0.171ab | 3.812 ± 0.080a | 3.711 ± 0.193a | 3.840 ± 0.113a | 3.785 ± 0.086a | 3.970 ± 0.178a | 3.957 ± 0.092a |
| 100 | 3.785 ± 0.117a | 3.871 ± 0.085a | 3.699 ± 0.235a | 3.659 ± 0.112a | 3.815 ± 0.059a | 3.703 ± 0.047a | 3.765 ± 0.017a |
| 1000 | 3.401 ± 0.162b | 3.367 ± 0.039b | 3.667 ± 0.596a | 3.723 ± 0.115a | 3.516 ± 0.160b | 3.359 ± 0.112b | 3.769 ± 0.053a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biczak, R.; Kierasińska, J.; Jamrozik, W.; Pawłowska, B. Response of Maize (Zea mays L.) to Soil Contamination with Diclofenac, Ibuprofen and Ampicillin and Mixtures of These Drugs. Sustainability 2024, 16, 5698. https://doi.org/10.3390/su16135698
Biczak R, Kierasińska J, Jamrozik W, Pawłowska B. Response of Maize (Zea mays L.) to Soil Contamination with Diclofenac, Ibuprofen and Ampicillin and Mixtures of These Drugs. Sustainability. 2024; 16(13):5698. https://doi.org/10.3390/su16135698
Chicago/Turabian StyleBiczak, Robert, Julia Kierasińska, Wiktoria Jamrozik, and Barbara Pawłowska. 2024. "Response of Maize (Zea mays L.) to Soil Contamination with Diclofenac, Ibuprofen and Ampicillin and Mixtures of These Drugs" Sustainability 16, no. 13: 5698. https://doi.org/10.3390/su16135698
APA StyleBiczak, R., Kierasińska, J., Jamrozik, W., & Pawłowska, B. (2024). Response of Maize (Zea mays L.) to Soil Contamination with Diclofenac, Ibuprofen and Ampicillin and Mixtures of These Drugs. Sustainability, 16(13), 5698. https://doi.org/10.3390/su16135698








