On Caprock Seal Integrity of Tuscaloosa Mudstone at Cranfield, MS (USA), CO2 Injection Site
Abstract
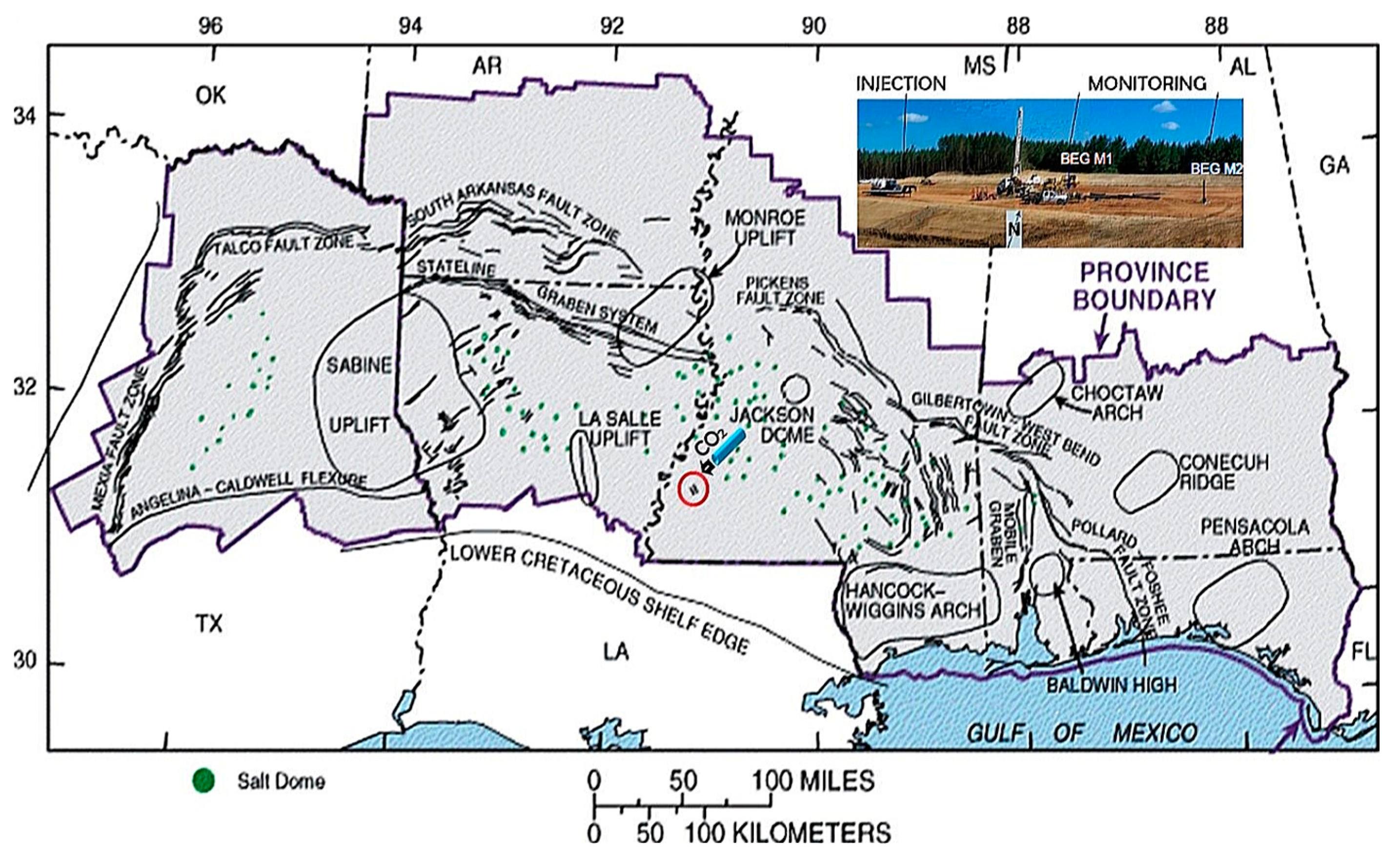
:1. Introduction
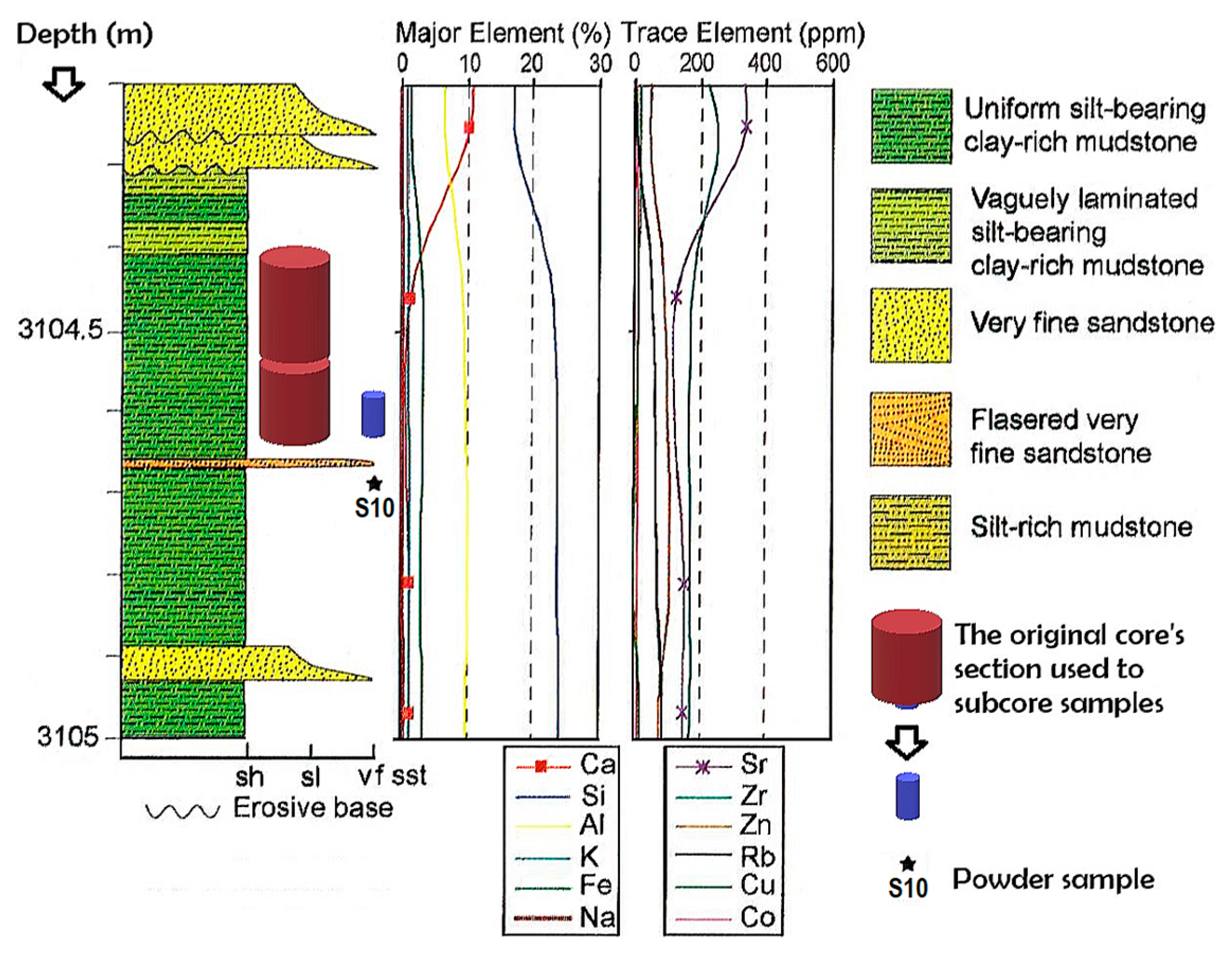
2. Materials and Methods
3. Results
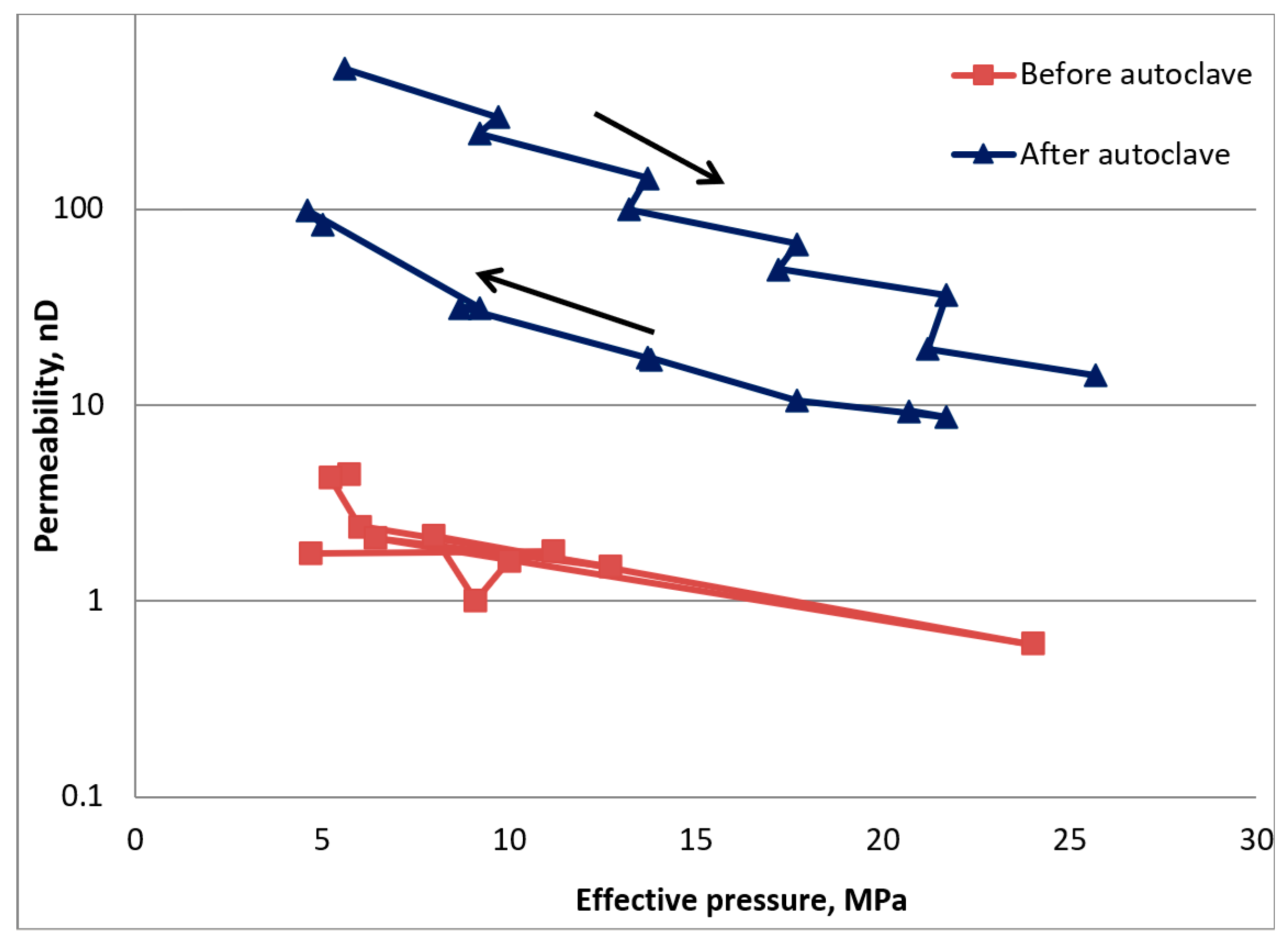
3.1. Porocity and Permeability Measurements before CO2 Exposure
3.1.1. First Subcore Sample (Horizontal Axis)
3.1.2. Second Subcore Sample (Vertical Axis)
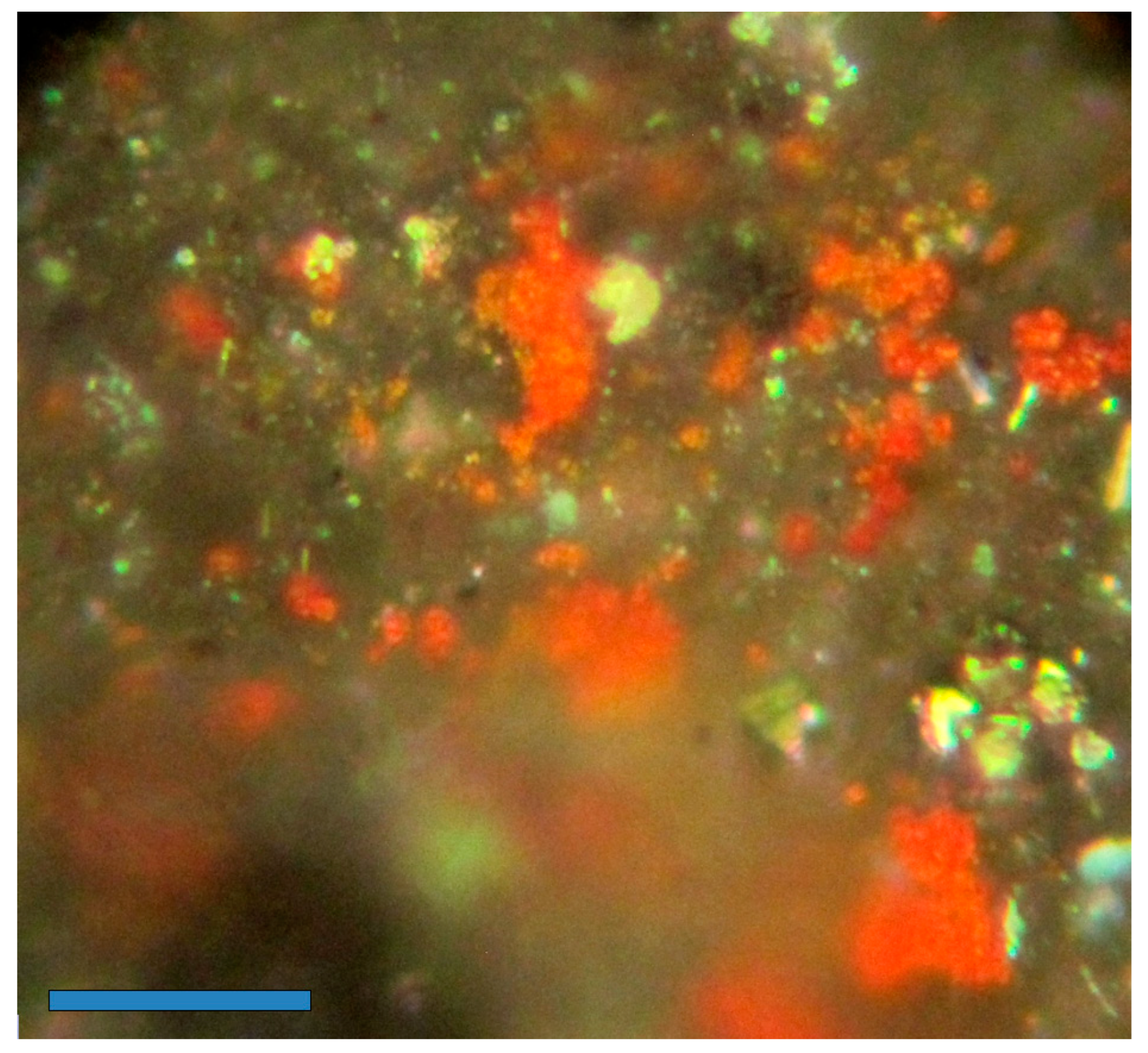
3.2. Geochemical Studies
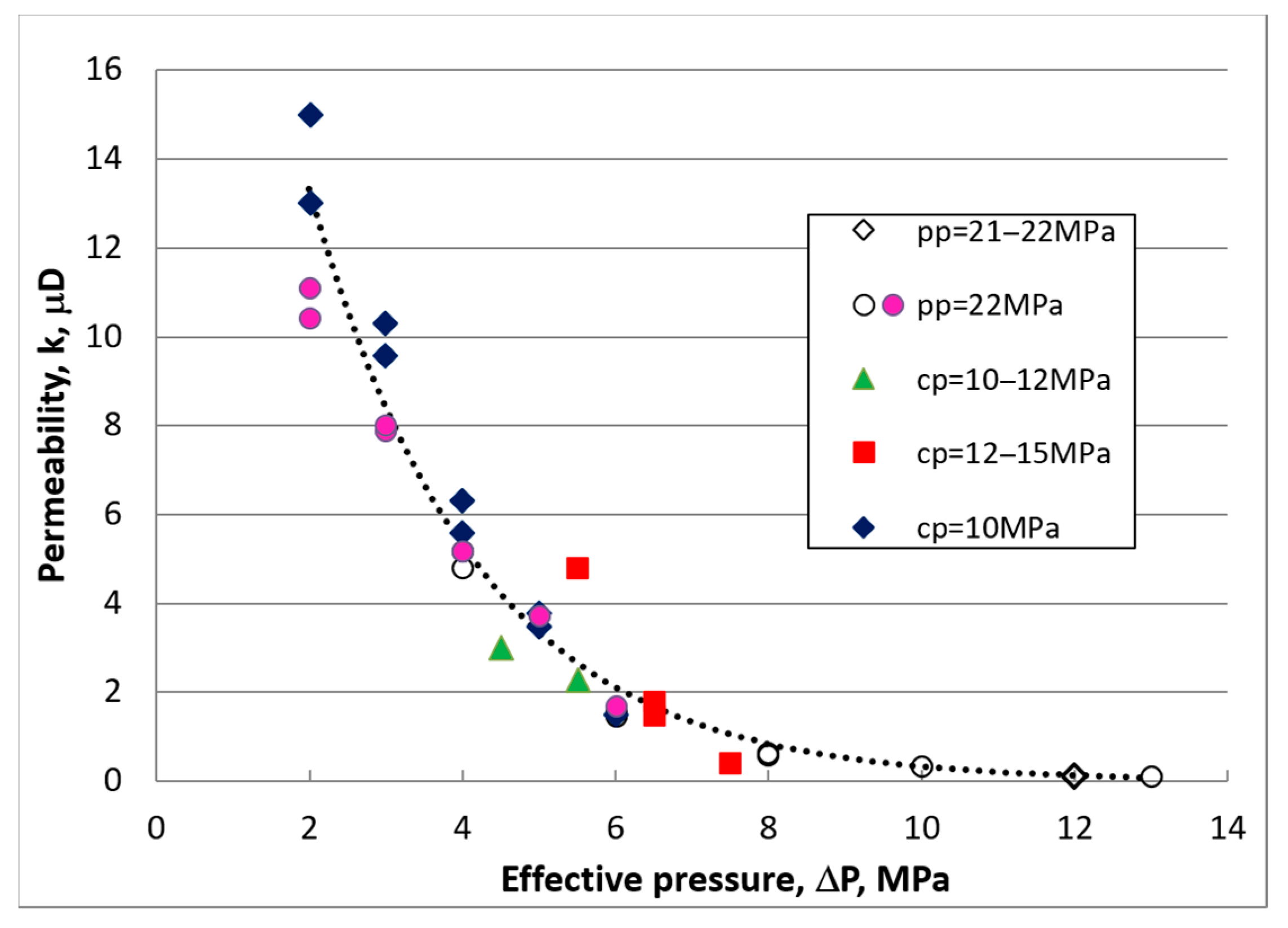
3.3. Permeability Measurements after CO2 Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Appendix A

| Test No. | Method | Conf P (MPa) | Pore P (MPa) | k (nD) |
|---|---|---|---|---|
| 1 | Sine-6 | 10 | 4.4 | 522 |
| 2 | Pressure step | 15 | 5.3 | 296 |
| 3 | Sine-6 | 15 | 5.8 | 233–255 |
| 4 | Pressure step | 20 | 6.3 | 145 |
| 5 | Sine-6 | 20 | 6.8 | 100 |
| 6 | Pressure step | 25 | 7.3 | 66.5 |
| 7 | Sine-6 | 25 | 7.8 | 49.7 |
| 8 | Sine-6 | 30 | 8.3 | 36.3 |
| 9 | Pressure step | 30 | 8.8 | 19.4 |
| 10 | Sine-6 | 35 | 9.3 | 14.1 |
| 11 | Pressure step | 40 | 10.3 | <DL |
| 12 | Sine-6 | 35 | 10.3 | <DL |
| 13 | Pressure step | 30 | 9.3 | 9.35 |
| 14 | Pressure step | 30 | 8.3 | 8.65 |
| 15 | Pressure step | 10 | 4.4 | 522 |
| 16 | Pressure step | 15 | 5.3 | 296 |
| 17 | Pressure step | 15 | 5.8 | 233–255 |
| 18 | Pressure step | 25 | 7.3 | 10.5 |
| 19 | Pressure step | 20 | 6.3 | 16.3–19.1 |
| 20 | Pressure ramp | 20 | 6.2 | 16.4–17.8 |
| 21 | Pressure step | 15 | 6.3 | 31.5 |
| 22 | Sine-6 | 15 | 5.8 | 31.5 |
| 23 | Pressure step | 10 | 5.4 | 99 |
| 24 | Sine-6 | 10 | 5.0 | 83.1 |
References
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Ali, M.; Jha, N.K.; Pal, N.; Kesharz, A.; Hoteit, H.; Sarmadivaleh, M. Recent advance in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook. Earth-Sci. Rev. 2022, 225, 103895–103922. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyznska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776–127814. [Google Scholar] [CrossRef]
- Mineralogy of Sandstones: Porosity and Permeability, Geological Digressions. Available online: https://www.geological-digressions.com/mineralogy-of-sandstones-porosity-and-permeability (accessed on 14 March 2024).
- Carbon Storage FAQs, National Energy Technology Laboratory. Available online: https://www.netl.doe.gov/carbon-management/carbon-storage/faqs/carbon-storage-faqs (accessed on 14 March 2024).
- Tang, Y.; Yang, R.; Du, Z.; Zeng, F. Experimental Study of Formation Damage Caused by Complete Water Vaporization and Salt Precipitation in Sandstone Reservoirs. Transp. Porous Med. 2015, 107, 205–218. [Google Scholar] [CrossRef]
- Wang, H.; Alvarado, V.; Bagdonas, D.A.; McLaughlin, J.F.; Kaszuba, J.P.; Grana, D.; Campbell, E.; Ng, K. Effect of CO2-brine-rock reactions on pore architecture and permeability in dolostone: Implications for CO2 storage and EOR. Int. J. Greenh. Gas. Control 2021, 107, 103283. [Google Scholar] [CrossRef]
- Cavanagh, A.; Wildgust, N. Pressurization and Brine Displacement Issues for Deep Saline Formation CO2 Storage. Energy Procedia 2011, 4, 4814–4821. [Google Scholar] [CrossRef]
- Merrill, R.K.; Gann, D.E.; Jennings, S.P. Tishomingo County geology and mineral resources [Mississippi]. Miss. Off. Geol. Bull. 1988, 127, 178. [Google Scholar]
- Carbon Storage Atlas, National Energy Technology Laboratory. Available online: https://netl.doe.gov/coal/carbon-storage/atlas/secarb/phase-III/cranfield-project (accessed on 26 March 2024).
- Lu, J.; Milliken, K.; Reed, R.M.; Hovorka, S. Diagenesis and sealing capacity of the middle Tuscaloosa mudstone at the Cranfield carbon dioxide injection site, Mississippi, U.S.A. Environ. Geosci. 2011, 18, 35–53. [Google Scholar] [CrossRef]
- Soong, Y.; Howard, B.H.; Dilmore, R.M.; Haljasmaa, I.; Crandall, D.M.; Zhang, L.; Zhang, W.; Lin, R.; Irdi, G.A.; Romanov, V.N.; et al. CO2/brine/rock interactions in Lower Tuscaloosa formation. Greenh. Gas. Sci. Technol. 2016, 6, 824–837. [Google Scholar] [CrossRef]
- Soong, Y.; Howard, B.H.; Hedges, S.W.; Haljasmaa, I.; Warzinski, R.P.; Irdi, G.; McLendon, T.R. CO2 sequestration in Saline Formation. Aerosol Air Qual. Res. 2014, 14, 522–532. [Google Scholar] [CrossRef]
- Zhang, J.J. Abnormal pore pressure mechanisms. In Applied Petroleum Geomechanics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–280. [Google Scholar]
- Biot, M.A. General theory of three-dimensional consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]
- Soong, Y.; Hedges, S.W.; Howard, B.H.; Dilmore, R.M.; Allen, D.E. Effect of contaminants from flue gas on CO2 sequestration in saline formation. Int. J. Energy Res. 2014, 38, 1224–1232. [Google Scholar] [CrossRef]
- Dai, X.; Wei, C.; Wang, M.; Song, Y.; Chen, R.; Wang, X.; Shi, X.; Vandeginste, V. Mineralization mechanism of carbon dioxide with illite interlayer cations using molecular dynamics simulation and experiments. J. CO2 Util. 2022, 64, 102161. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Luhmann, A.J.; Kong, X.-Z.; Saar, M.O.; Seyfried, W.E. CO2 sequestration in feldspar-rich sandstone: Coupled evolution of fluid chemistry, mineral reaction rates, and hydrogeochemical properties. Geochim. Cosmochim. Acta 2015, 160, 132–154. [Google Scholar] [CrossRef]
| Sample No. | Illite | Kaolinite | Quartz | K-Feldspar | Pyrite | Calcite | Albite | Anatase |
|---|---|---|---|---|---|---|---|---|
| S10 | 35.5 | 30.7 | 16.2 | 7.5 | 4.7 | 1.9 | 1.9 | 1.5 |
| Test No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Conf P (MPa) | 10 | 15 | 24 | 28 | 32 | 23 | 23 |
| Pore P (MPa) | 6 | 6.3 | 10.4 | 18.5 | 15 | 13 | 12 |
| k (nD) | 17–21 | 14–18 | 7.5–9.5 | 8.5–10.5 | 2.5–3.1 | 1.8–2.2 | 1.4–1.6 |
| Content | Batch 1 | Mudstone 1 | Batch 2 | Mudstone 2 |
|---|---|---|---|---|
| Na (cat.) | 43,743 | 48,388 | 45,007 | 47,491 |
| Ca (cat.) | 11,798 | 13,202 | 11,397 | 11,586 |
| Mg (cat.) | 1035 | 1240 | 1097 | 1268 |
| Sr (cat.) | 696 | 775 | 734 | 731 |
| K (cat.) | 412 | 530 | 423 | 487 |
| S (an.) | 166 | 332 | 161 | 290 |
| Fe (cat.) | 124 | 210 | 129 | 318 |
| Chloride | 92,223 | 93,005 | 92,952 | 94,412 |
| Bromide | 432 | 432 | 481 | 481 |
| Sulfate | 238 | 271 | 244 | 230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanov, V.; Haljasmaa, I.; Soong, Y. On Caprock Seal Integrity of Tuscaloosa Mudstone at Cranfield, MS (USA), CO2 Injection Site. Sustainability 2024, 16, 5758. https://doi.org/10.3390/su16135758
Romanov V, Haljasmaa I, Soong Y. On Caprock Seal Integrity of Tuscaloosa Mudstone at Cranfield, MS (USA), CO2 Injection Site. Sustainability. 2024; 16(13):5758. https://doi.org/10.3390/su16135758
Chicago/Turabian StyleRomanov, Vyacheslav, Igor Haljasmaa, and Yee Soong. 2024. "On Caprock Seal Integrity of Tuscaloosa Mudstone at Cranfield, MS (USA), CO2 Injection Site" Sustainability 16, no. 13: 5758. https://doi.org/10.3390/su16135758




