Effect of Eminex® on Greenhouse Gas and Ammonia Emissions from Dairy Slurry and Lagoon Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1—Slurry Collection and Experimental Set Up
2.2. Experiment 2—Lagoon Wastewater Collection and Experimental Setup
2.3. Emissions Sampling
2.4. Emissions Calculations
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of Fresh Dairy Slurry
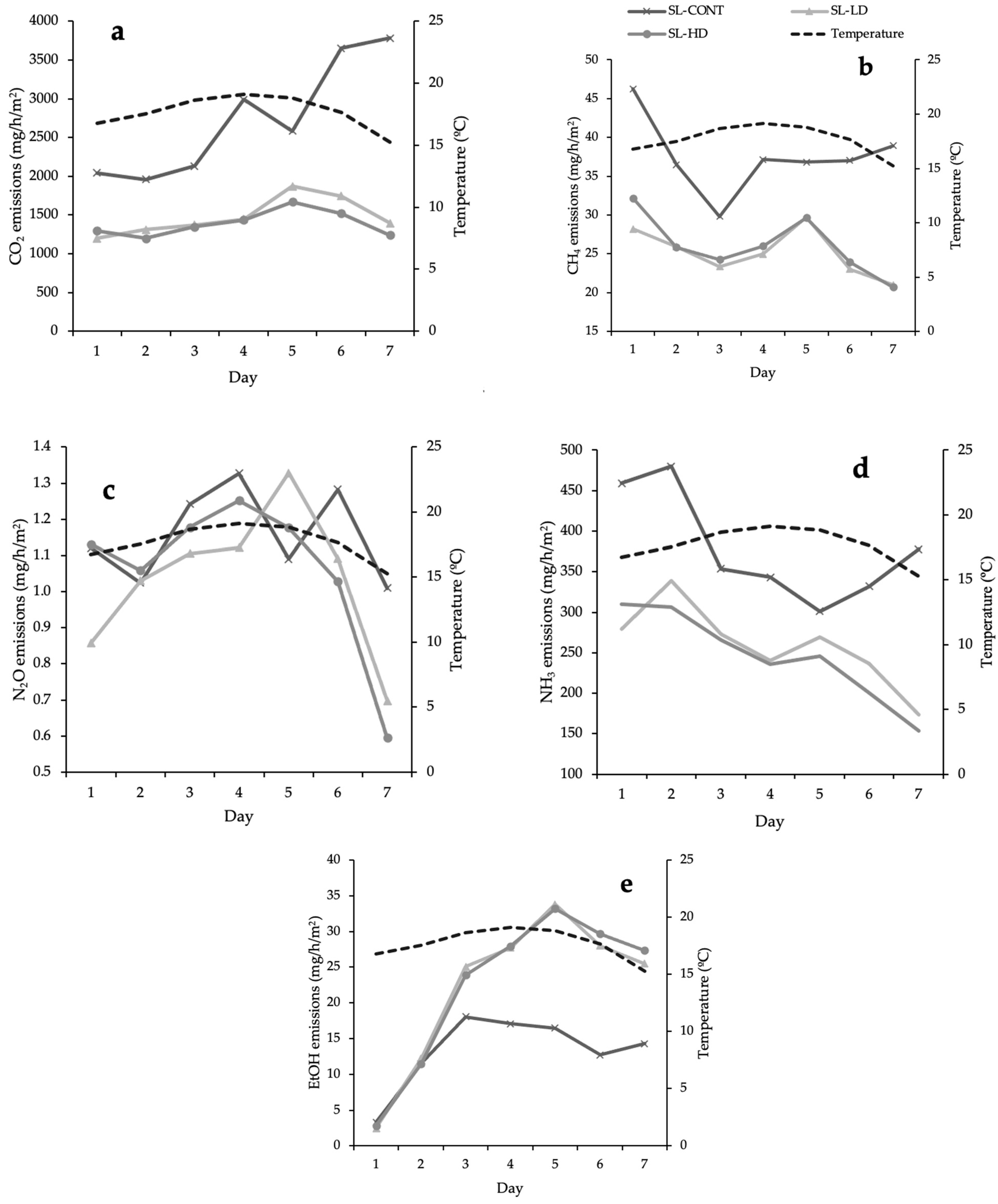
3.2. Fresh Slurry Gaseous Emissions
3.3. Physical Characteristics of Dairy Lagoon Wastewater
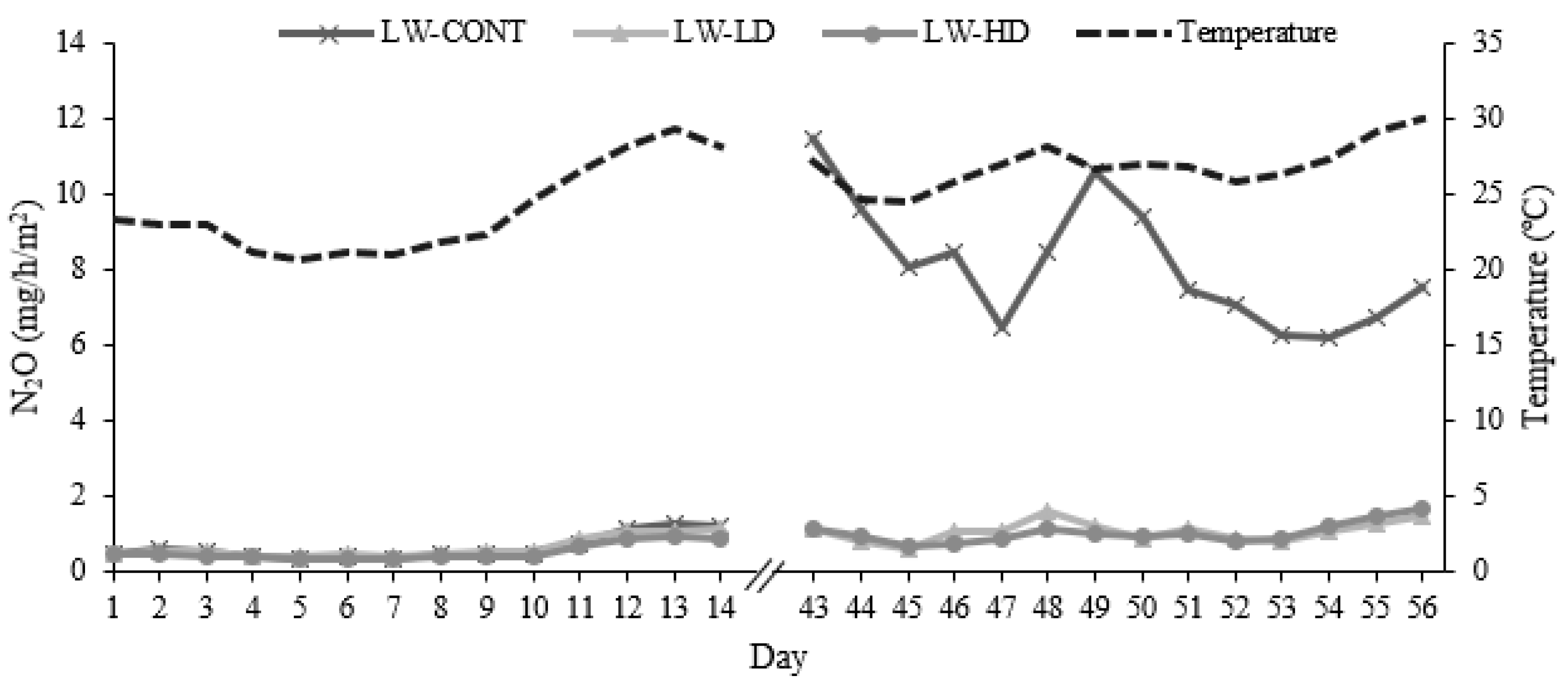
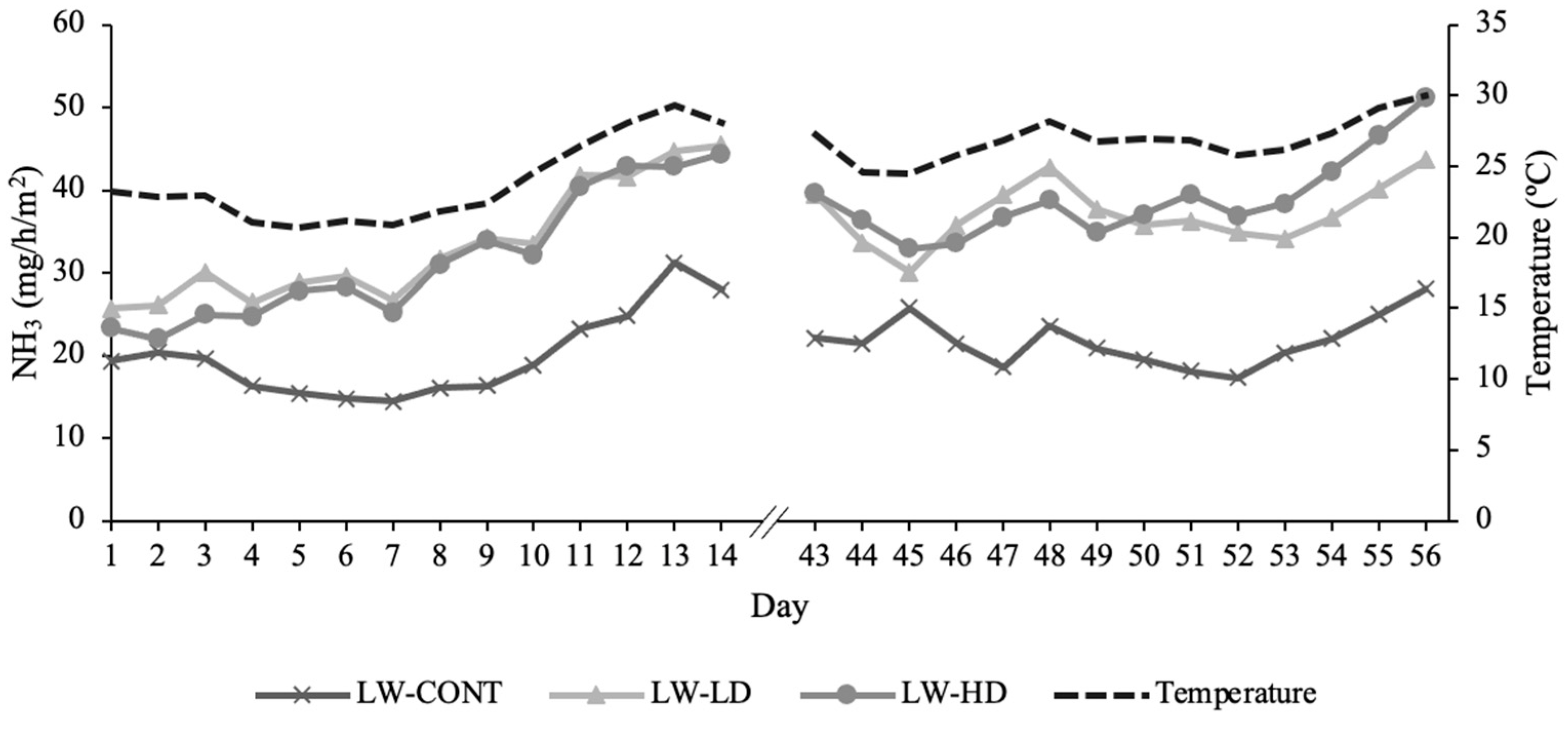
3.4. Lagoon Wastewater Gaseous Emissions
3.5. Future of Eminex®
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Mashad, H.M.; Barzee, T.J.; Franco, R.B.; Zhang, R.; Kaffka, S.; Mitloehner, F. Anaerobic Digestion and Alternative Manure Management Technologies for Methane Emissions Mitigation on Californian Dairies. Atmosphere 2023, 14, 120. [Google Scholar] [CrossRef]
- USDA. Pacific Region Milk Production. 2021; pp. 1–2. Available online: https://www.nass.usda.gov/Statistics_by_State/California/Publications/Livestock_Releases/Milk_Production/2022/202208MILKPROD.pdf (accessed on 10 December 2023).
- Varma, V.S.; Parajuli, R.; Scott, E.; Canter, T.; Lim, T.T.; Popp, J.; Thoma, G. Dairy and swine manure management—Challenges and perspectives for sustainable treatment technology. Sci. Total Environ. 2021, 778, 146319. [Google Scholar] [CrossRef] [PubMed]
- EPA. Annexes to the Inventory of U.S. Greenhouse Gas Emissions and Sinks; EPA: Washington, DC, USA, 2023; pp. 1–531.
- Sokolov, V.K.; VanderZaag, A.; Habtewold, J.; Dunfield, K.; Wagner-Riddle, C.; Venkiteswaran, J.J.; Crolla, A.; Gordon, R. Dairy manure acidification reduces CH4 emissions over short and long-term. Environ. Technol. 2021, 42, 2797–2804. [Google Scholar] [CrossRef]
- Peterson, C.B.; El Mashad, H.M.; Zhao, Y.; Pan, Y.; Mitloehner, F.M. Effects of SOP Lagoon Additive on Gaseous Emissions from Stored Liquid Dairy Manure. Sustainability 2020, 12, 1393. [Google Scholar] [CrossRef]
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2021. 2023; pp. 1–881. Available online: https://www.epa.gov/system/files/documents/2023-04/US-GHG-Inventory-2023-Main-Text.pdf (accessed on 10 December 2023).
- CARB. Current California GHG Emission Inventory Data. Available online: https://ww2.arb.ca.gov/ghg-inventory-data (accessed on 10 December 2023).
- Wattiaux, M.A.; Uddin, M.E.; Letelier, P.; Jackson, R.D.; Larson, R.A. Invited Review: Emission and mitigation of greenhouse gases from dairy farms: The cow, the manure, and the field. Appl. Anim. Sci. 2019, 35, 238–254. [Google Scholar] [CrossRef]
- EPA. Particulate Matter (PM) Basics. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 25 June 2024).
- Khairunisa, B.H.; Loganathan, U.; Ogejo, J.A.; Mukhopadhyay, B. Nitrogen transformation processes catalyzed by manure microbiomes in earthen pit and concrete storages on commercial dairy farms. Environ. Microbiome 2023, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, F.; Clemens, J.; Trimborn, M. Calcium cyanamide reduces methane and other trace gases during long-term storage of dairy cattle and fattening pig slurry. Waste Manag. 2023, 161, 61–71. [Google Scholar] [CrossRef]
- Shaw, S.L.; Mitloehner, F.M.; Jackson, W.; DePeters, E.J.; Fadel, J.G.; Robinson, P.H.; Holzinger, R.; Goldstein, A.H. Volatile Organic Compound Emissions from Dairy Cows and Their Waste as Measured by Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol 2007, 41, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Filipy, J.; RUmburg, B.; Mount, G.; Westberg, H.; Lamb, B. Identification and quantification of volatile organic compounds from a dairy. Atmos. Environ. 2006, 40, 1480–1494. [Google Scholar] [CrossRef]
- Yuan, B.; Coggon, M.M.; Koss, A.R.; Warneke, C.; Eilerman, S.; Peischl, J.; Aikin, K.C.; Ryerson, T.B.; de Gouw, J.A. Emissions of volatile organic compounds (VOCs) from concentrated animal feeding operations (CAFOs): Chemical compositions and separation of sources. Atmos. Chem. Phys. 2017, 17, 4945–4956. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R.; Rumsey, T.; Hafner, S.; Montes, F.; Rotz, A.C.; Arteaga, V.; Zhao, Y.; Mitloehner, F.M. A Mass Transfer Model of Ethanol Emission from Thin Layers of Corn Silage. J. ASABE Trans. 2010, 53, 1903–1909. [Google Scholar] [CrossRef]
- Lara, S.; Williams, A.; Allen, S.; Gonzalez, A.; Hancock, S.; Hill, S. Senate Bill No. 1383. 2016. Available online: http://www.leginfo.ca.gov/pub/15-16/bill/sen/sb_1351-1400/sb_1383_bill_20160831_amended_asm_v95.pdf (accessed on 10 December 2023).
- CDFA. Alternative Manure Management Program. Available online: https://www.cdfa.ca.gov/oefi/AMMP/ (accessed on 10 December 2023).
- CDFA. Dairy Digester Research & Development Program. Available online: https://www.cdfa.ca.gov/oefi/ddrdp/ (accessed on 5 June 2024).
- Meyer, D.; Heguy, J.; Karle, B.; Robinson, P. Characterize Physical and Chemical Properties of Manure in California Dairy Systems to Improve Greenhouse Gas Emission Estimates; University of California, Davis: Davis, CA, USA, 2019; pp. 1–70. [Google Scholar]
- Hou, Y.; Velthof, G.L.; Oenema, O. Mitigation of ammonia, nitrous oxide and methane emissions from manure management chains: A meta-analysis and integrated assessment. Glob. Chang. Biol. 2014, 21, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Cowley, C.; Brorsen, B.W. Anaerobic Digester Production and Cost Functions. Ecol. Econ. 2018, 152, 347–357. [Google Scholar] [CrossRef]
- Chiodini, M.E.; Constantini, M.; Zoli, M.; Bacenetti, J.; Aspesi, D.; Poggianella, L.; Acutis, M. Real-Scale Study on Methane and Carbon Dioxide Emission Reduction from Dairy Liquid Manure with the Commerical Additive SOP LAGOON. Sustainability 2023, 15, 1803. [Google Scholar] [CrossRef]
- Dixon, G.R. Calcium Cyanamide—A Synoptic Review of an Environmentally Benign Fertiliser Which Enhances Soil Health. In Proceedings of the XXVIIIth IHC—IS on Environmental, Edaphic & Gen Factors Affecting Plants, Seeds, and Turfgrass, Lisbon, Portugal, 22–27 August 2010; 2012. Available online: https://www.researchgate.net/publication/284135320_Calcium_cyanamide_-_A_synoptic_review_of_an_environmentally_benign_fertiliser_which_enhances_soil_health (accessed on 10 December 2023).
- Hermann, C.; Jost, C.; Theuerl, S.; Mundt, K.; Bock, D. To determine the effect of calcium cyanamide additives on methane emissions during the storage of livestock slurry under anaerobic conditions. 2021; 1–40. [Google Scholar]
- CDFA. Recommendations for Short-Lived Climate Pollutants; California Department of Food and Agriculture: Sacramento, CA, USA, 2015; pp. 1–26.
- Kaffka, S.; Barzee, T.J.; El Mashad, H.; Williams, R.; Zicari, S.; Zhang, R. Evaluation of Dairy Manure Management Practices for Greenhouse Gas Emissions Mitigation in California; UC Davis: Davis, CA, USA, 2016; pp. 1–127. [Google Scholar]
- Hristov, A.N.; Hanigan, M.; Cole, A.; Todd, R.; McAllister, T.A.; Ndegwa, P.M.; Rotz, A. Review: Ammonia emissions from dairy farms and beef feedlots. Can. J. Anim. Sci. 2011, 91, 135. [Google Scholar] [CrossRef]
- Hoskins, B.; Wolf, A.; Wolf, N. Dry Matter Analysis; A3769; University of Wisconsin Extension: Madison, WI, USA, 2003; pp. 14–17. [Google Scholar]
- Watson, M.; Wolf, A.; Wolf, N. Total Nitrogen; A3769; University of Wisconsin Extension: Madison, WI, USA, 2003; pp. 18–24. [Google Scholar]
- Supelco. GC Separation of VFA C2–C5; Bulletin 749B; Supelco Inc.: Bellefonte, PA, USA, 1975. [Google Scholar]
- Supelco. Analyzing Fatty Acids by Packed Column Gas Chromatography; Bulletin 856A; Supelco GC: Bellefonte, PA, USA, 1990. [Google Scholar]
- W.H. Miner Institute. Volatile Fatty Acid SOP; W.H. Miner Institute: Chazy, NY, USA.
- R Core Team. R: A Language and Environmental for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Hilgert, J.E.; Amon, B.; Amon, T.; Belik, V.; Dragoni, F.; Ammon, C.; Cárdenas, A.; Petersen, S.O.; Herrmann, C. Methane Emissions from Livestock Slurry: Effects of Storage Temperature and Changes in Chemical Composition. Sustainability 2022, 14, 9934. [Google Scholar] [CrossRef]
- Cárdenas, A.; Ammon, C.; Schumacher, B.; Stinner, W.; Herrmann, C.; Schneider, M.; Weinrich, S.; Fischer, P.; Amon, T.; Amon, B. Methane emissions from the storage of liquid dairy manure: Influences of season, temperature and storage duration. Waste Manag. 2021, 121, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E. Urease Activity in Bovine Feces. J. Dairy Sci. 1981, 65, 2157–2163. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Castro-Ramos, J.J.; Solís-Oba, A.; Solís-Oba, M.; Calderón-Vázquez, C.L.; Higuera-Rubio, J.M.; Castro-Rivera, R. Effect of the initial pH on the anaerobic digestion process of dairy cattle manure. AMB Express 2022, 12, 162. [Google Scholar] [CrossRef]
- Kamusoko, R.; Jingura, R.M.; Chikwambi, Z.; Parawira, W. Chapter 25—Biogas: Microbiological research to enhance efficiency and regulation. In Handbook of Biofuels; Academic Press: Cambridge, MA, USA, 2022; pp. 485–497. [Google Scholar]
- Chen, B.; Azman, S.; Dewil, R.; Appels, L. Alkaline anaerobic digestion of livestock manure: Unveiling mechanisms, applications, and perspective. Chem. Eng. J. 2023, 477, 146852. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; González-Mntez de Goñi, J.; Esteban-Gutiérrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Gagnon, N.; Topp, E.; Masse, E.; Massé, D.I.; Talbot, G. Physico-chemical characteristics and methanogen communities in swine and dairy manure storage tanks: Spatio-temporal variations and impact on methanogenic activity. Water Res. 2013, 47, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Huang, G.; Wagner, T.; Ermler, U. Structural Basis of Hydrogenotrophic Methanogenesis. Annu. Rev. Microbiol. 2020, 74, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 24, 120–137. [Google Scholar] [CrossRef]
- D’Silva, T.C.; Isha, A.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kumar, R.; Chaudhary, V.P.; Singh, H.; Khan, A.A.; Tyagi, V.K.; et al. Enhancing methane production in anaerobic digestion through hydrogen assisted pathways—A state-of-the-art review. Renew. Sust. Energ. Rev. 2021, 151, 111536. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, K. Effects of pH, Total Solids, Temperature and Storage Duration on Gas Emissions from Slurry Storage: A Systematic Review. Atmosphere 2021, 12, 1156. [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and greenhouse gas emissions from slurry storage—A review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, B.R.; Kim, T.H. Urease and nitrification inhibitors with pig slurry effects on ammonia and nitrous oxide emissions, nitrate leaching, and nitrogen use efficiency in perennial ryegrass sward. Anim. Biosci. 2021, 34, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Ai, S.; Cui, L. Dicyandiamide has more inhibitory activities on nitrification than thiosulfate. PLoS ONE 2018, 13, e0200598. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.H.; Boehm, M.T. Manure Ammonia and Hydrogen Sulfide Emissions from a Western Dairy Storage Basin. J. Environ. Qual. 2015, 44, 127–136. [Google Scholar] [CrossRef]
- Ro, K.S.; Dietenberger, M.A.; Libra, J.A.; Proeschel, R.; Atiyeh, H.K.; Sahoo, K.; Park, W.J. Production of Ethanol from Livestock, Agricultural, and Forest Residuals: An Economic Feasibility Study. Environments 2019, 6, 97. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.; Li, Q.; Zeng, X.; Liu, Y.; Li, Y. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Raghavarao, K.S.M.S.; Madhusudhan, M.C.; Hrishikesk Tavanandi, A.; Niranjan, K. Chapter 12—Athermal Membrane Processes for the Concentration of Liquid Foods and Natural Colors. In Emerging Technologies for Food Processing, 2nd ed.; Sun, D., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 213–237. [Google Scholar]
- Page, L.H.; Ni, J.; Zhang, H.; Heber, A.J.; Mosier, N.S.; Liu, X.; Joo, H.; Ndegwa, P.M.; Harrison, J.H. Reduction of volatile fatty acids and odor offensiveness by anaerobic digestion and solid separation of dairy manure during manure storage. J. Environ. Manag. 2015, 152, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.O. Symposium review: Greenhouse gas emissions from liquid dairy manure: Prediction and mitigation. J. Dairy Sci. 2018, 101, 6642–6654. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure. J. Environ. Qual. 2004, 33, 27–36. [Google Scholar] [CrossRef]
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 2011, 166, 514–531. [Google Scholar] [CrossRef]
- Alzchem Group. Available online: https://www.alzchem.com/en/brands/eminex/ (accessed on 4 June 2022).



| Parameters | Day 0 | Treatment | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| SL-CONT | SL-LD | SL-HD | T | D | T × D | |||
| Total Solids, %DM | 13.2 | 14.0 | 11.5 | 11.7 | 1.61 | NS | 0.001 | NS |
| Total N, %DM | 0.57 | 0.54 | 0.55 | 0.56 | 0.012 | 0.01 | 0.02 | 0.005 |
| Total C, %DM | 44.6 | 45.1 | 44.9 | 44.7 | 0.103 | NS | 0.01 | NS |
| pH | 8.03 | 7.79 | 7.88 | 7.93 | 0.117 | NS | NS | NS |
| Acetic acid, ppm | 2863 | 4887 a* | 5319 * | 5361 b | 145 | 0.08 | <0.001 | 0.02 |
| Propionic acid, ppm | 661.4 | 800 | 820 | 832 | 22.9 | NS | <0.001 | NS |
| Butyric acid, ppm | 402.7 | 509 | 509 | 515 | 34 | NS | 0.001 | <0.001 |
| Gas Emissions 1 | Treatment 2 | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|
| SL-CONT | SL-LD | SL-HD | T | D | T × D | ||
| CO2 (mg/h/m2) | 2733 a | 1519 b | 1387 b | 345 | 0.06 | 0.02 | NS |
| CH4 (mg/h/m2) | 37.5 a | 26.2 b | 0.56 | 3.2 | 0.06 | 0.01 | NS |
| N2O (mg/h/m2) | 1.2 | 1.1 | 1.1 | 0.4 | NS | NS | NS |
| NH3 (mg/h/m2) | 378 a | 269 b | 246 b | 41.3 | 0.04 | <0.001 | NS |
| EtOH (mg/h/m2) | 13.2 a | 22.3 b | 22.2 b | 3.5 | <0.001 | <0.001 | NS |
| Parameters | Day 0 | Treatment | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| SL-CONT | SL-LD | SL-HD | T | D | T × D | |||
| Total Solids, %DM | 0.42 | 0.42 | 0.52 | 0.56 | 0.06 | NS | 0.01 | NS |
| Total N, %DM | 0.03 | 0.03 a | 0.04 b | 0.04 b | 0.004 | 0.02 | <0.001 | 0.005 |
| Total C, %DM | 0.2 | 0.22 | 0.25 | 0.26 | 0.01 | NS | <0.001 | NS |
| pH | 7.38 | 7.66 | 7.77 | 7.79 | 0.06 | NS | <0.001 | NS |
| Acetic acid, ppm | ND 1 | 2.9 a | 21.1 b | 18.6 b | 5.2 | 0.07 | <0.001 | 0.02 |
| Gas Emissions 1 | Treatment 2 | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|
| SL-CONT | SL-LD | SL-HD | T | D | T × D | ||
| CO2 (mg/h/m2) | 507 | 492 | 446 | 82.2 | NS | <0.001 | NS |
| CH4 (mg/h/m2) | 13.7 a | 2.6 b | 2.0 b | 3.3 | 0.06 | <0.001 | <0.001 |
| N2O (mg/h/m2) | 4.4 | 0.84 | 0.77 | 1.1 | NS | <0.001 | <0.001 |
| NH3 (mg/h/m2) | 21.3 | 35.2 | 35.3 | 6.9 | 0.04 | <0.001 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, A.S.; Morales, B.; El Mashad, H.M.; Pan, Y.; Zhao, Y.; Mitloehner, F.M. Effect of Eminex® on Greenhouse Gas and Ammonia Emissions from Dairy Slurry and Lagoon Wastewater. Sustainability 2024, 16, 5778. https://doi.org/10.3390/su16135778
Rocha AS, Morales B, El Mashad HM, Pan Y, Zhao Y, Mitloehner FM. Effect of Eminex® on Greenhouse Gas and Ammonia Emissions from Dairy Slurry and Lagoon Wastewater. Sustainability. 2024; 16(13):5778. https://doi.org/10.3390/su16135778
Chicago/Turabian StyleRocha, Alice S., Briana Morales, Hamed M. El Mashad, Yuee Pan, Yongjing Zhao, and Frank M. Mitloehner. 2024. "Effect of Eminex® on Greenhouse Gas and Ammonia Emissions from Dairy Slurry and Lagoon Wastewater" Sustainability 16, no. 13: 5778. https://doi.org/10.3390/su16135778
APA StyleRocha, A. S., Morales, B., El Mashad, H. M., Pan, Y., Zhao, Y., & Mitloehner, F. M. (2024). Effect of Eminex® on Greenhouse Gas and Ammonia Emissions from Dairy Slurry and Lagoon Wastewater. Sustainability, 16(13), 5778. https://doi.org/10.3390/su16135778






