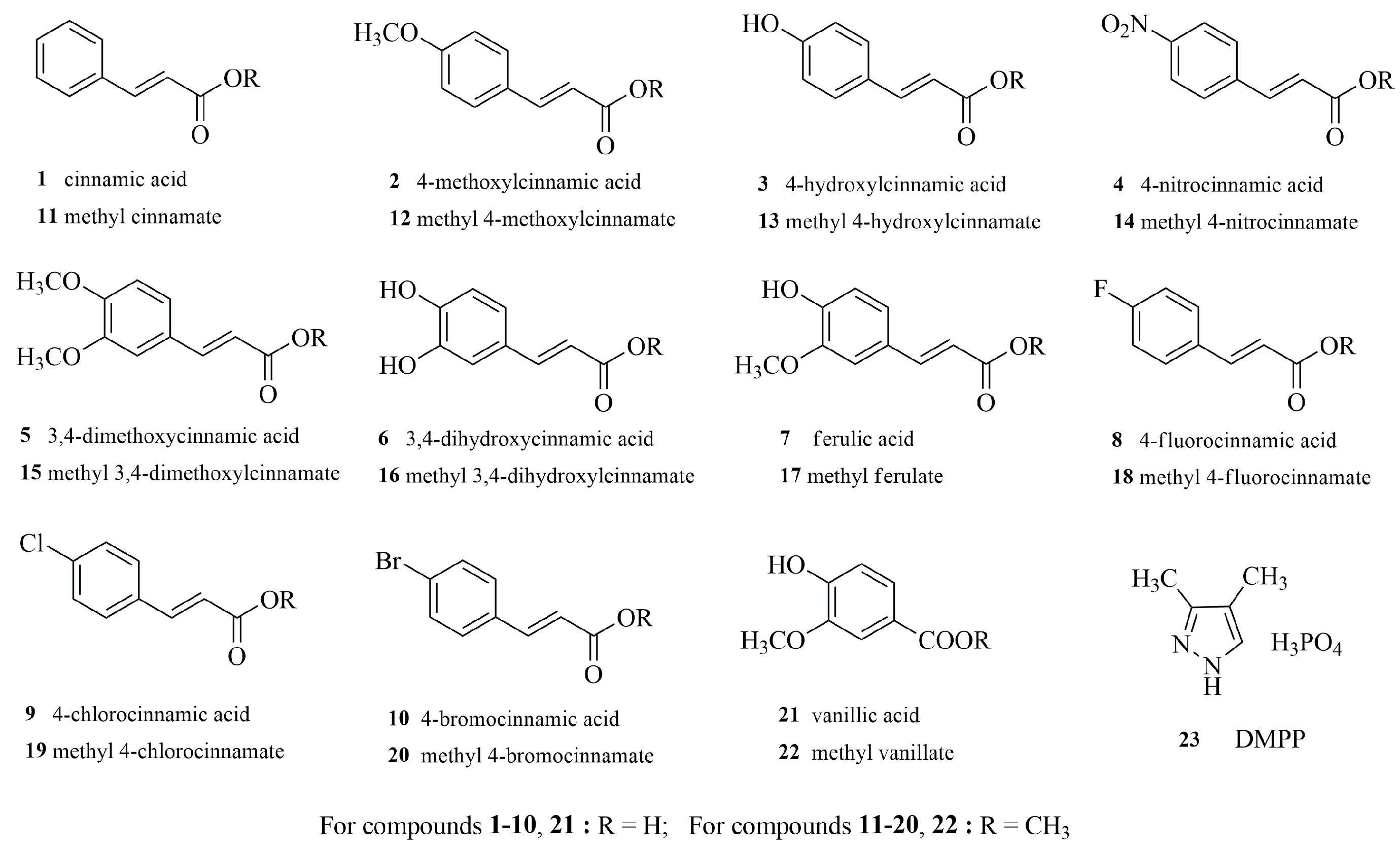
Screening Potential Nitrification Inhibitors through a Structure–Activity Relationship Study—The Case of Cinnamic Acid Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Soil Sampling
2.3. Nitrification Inhibition Rates in Liquid Batch Cultures
2.4. Nitrification Inhibition Test in Soil
2.5. Soil DNA Extraction and Quantitative Polymerase Chain Reaction
2.6. Statistical Analysis
3. Results and Discussion
3.1. SAR Study of Cinnamic Acid Derivatives Inhibiting the Growth of Ammonia-Oxidizing Bacteria
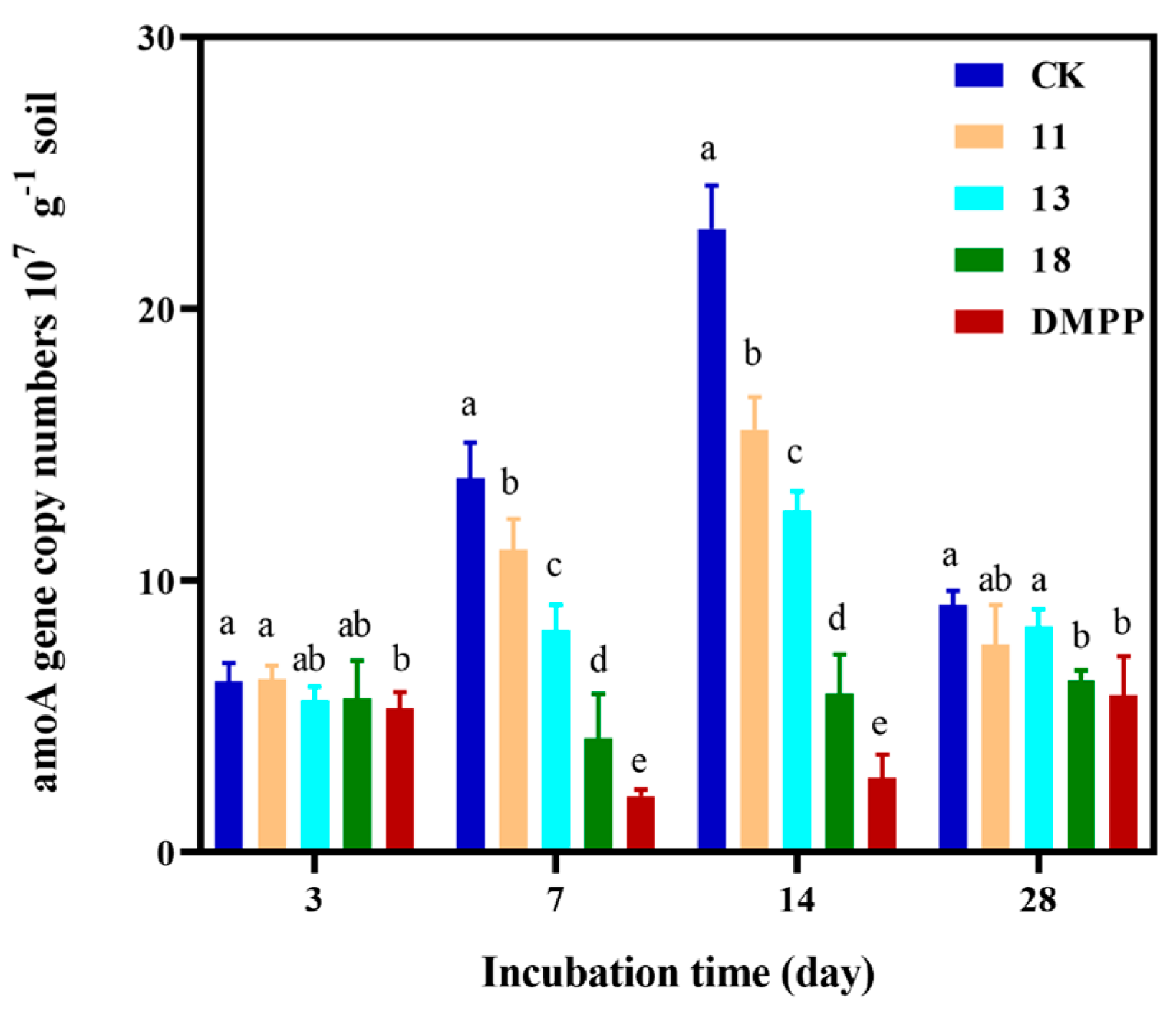
3.2. The Effect of Cinnamic Acid Derivatives on Soil Nitrification
3.3. Implications and Insights of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruber, N.; Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I. Soil nitrifiers and nitrification. In Nitrification; Ward, B.B., Arp, D.J., Klotz, M.G., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 347–383. [Google Scholar]
- Qiao, C.L.; Qiao, C.L.; Liu, L.L.; Hu, S.J.; Compton, J.E.; Greaver, T.L.; Li, Q.L. How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Global Change Biol. 2015, 21, 1249–1257. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Deng, J.; Wang, L.G. Assessing impacts of nitrogen management on nitrous oxide emissions and nitrate leaching from greenhouse vegetable systems using a biogeochemical model. Geoderma 2021, 382, 114701–114715. [Google Scholar] [CrossRef]
- Ni, G.F.; Leung, P.M.; Daebeler, A.; Guo, J.H.; Hu, S.H.; Cook, P.; Nicol, G.W.; Daims, H.; Greening, C. Nitrification in acidic and alkaline environments. Essays Biochem. 2023, 67, 753–768. [Google Scholar] [CrossRef]
- Wright, C.L.; Laura, E.L.M. Nitrification and beyond: Metabolic versatility of ammonia oxidising archaea. ISME J. 2023, 17, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- He, J.Z.; Hu, H.W.; Zhang, L.M. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 2012, 55, 146–154. [Google Scholar] [CrossRef]
- Hu, H.W.; He, J.Z. Comammox—A newly discovered nitrification process in the terrestrial nitrogen cycle. J. Soils Sediments 2017, 17, 2709–2717. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Kou, Y.P.; Wei, K.; Chen, G.X.; Wang, Z.Y.; Xu, H. Effects of 3,4-dimethylpyrazole phosphate and dicyandiamide on nitrous oxide emission in a greenhouse vegetable soil. Plant Soil Environ. 2015, 61, 29–35. [Google Scholar] [CrossRef]
- McCarty, G.W. Modes of action of nitrification inhibitors. Biol. Fert. Soils 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.M.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Moir, J.L.; Cameron, K.C.; Di, H.J. Effects of the nitrification inhibitor dicyandiamide on soil mineral N, pasture yield, nutrient uptake and pasture quality in a grazed pasture system. Soil Use Manag. 2007, 23, 111–120. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.T.; Sun, D.; Shi, Y.L. Efficiency of two nitrification inhibitors (dicyandiamide and 3,4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 22075. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.M.; Hash, C.T.; George, T.S.; Rao, P.S.; Nardi, P. Biological nitrification inhibition—A novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012, 114, 249–302. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Subbarao, G.V.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.Y.; Berry, W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Ono, H.; Yoshida, M.; Yoshihashi, T.; Zhu, Y.Y.; Zakir, H.A.K.M.; Deshpande, S.P.; Hash, C.T. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 2013, 366, 243–259. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.F.; Yu, F.W.; Kronzucker, H.J.; Shi, W.M. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.T.; Arcos, J.C. Role of structure activity relationship analysis in evaluation of pesticides for potential carcinogenicity. ACS Symp. Ser. 1989, 414, 175–200. [Google Scholar]
- Xu, J.W.; Lv, M.; Xu, H. The Advances in bioactivities, mechanisms of action and structural optimizations of matrine and its derivatives. Mini-Rev. Med. Chem. 2022, 22, 1716–1734. [Google Scholar] [CrossRef]
- Yu, L.J.; Dai, A.L.; Zhang, W.; Liao, A.J.; Guo, S.X.; Wu, J. Spiro derivatives in the discovery of new pesticides: A research review. J. Agric. Food Chem. 2022, 70, 10693–10707. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification Inhibitors from the root tissues of brachiaria humidicola-a tropical grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Lee, H.S.; Lee, S.G.; Park, J.D.; Ahn, Y.J. Insecticidal and Fumigant activities of cinnamomum cassia bark-derived materials against mechoris ursulus (Coleoptera: Attelabidae). J. Agric. Food Chem. 2000, 48, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, L.; Makinen, M.; Lampi, A.; Piiromen, V. Antioxidant activity of steryl ferulate extract from rye and wheat bran. J. Agric. Food Chem. 2005, 53, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Sastry, K.V.H.; Moudgal, R.P.; Mohan, J.; Tyagi, J.S.; Rao, G.S. Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Anal. Biochem. 2002, 306, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Tourna, M.; Stieglmeier, M.; Spang, A.; Könneke, M.; Schintlmeister, A.; Urich, T.; Engel, M.; Schloter, M.; Wagner, M.; Richter, A.; et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. USA 2011, 108, 8420–8425. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.L.; Stieglmeier, M.; Dai, J.L.; Urich, T.; Schleper, C. Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizingbacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol. Lett. 2013, 344, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Dutta, A.; Kumar, S.; Pathak, H.; Jain, M.C. Mitigation of N2O emission from an alluvial soil by application of karanjin. Biol. Fertil. Soils 2001, 33, 438–442. [Google Scholar] [CrossRef]
- Wilson, S.J.; Song, B.K.; Anderson, I.C. Geochemical factors impacting nitrifying communities in sandy sediments. Environ. Microbiol. 2023, 25, 3180–3191. [Google Scholar] [CrossRef]
- Okano, R.; Takazaki, H.; Matsuba, D.; Tokuyama, T.; Sato, Y.; Wakabayashi, K. Nitrosomonas europaea ATCC25978 is the right ammonia-oxidizing bacterium for screening nitrification inhibitors. J. Pestic. Sci. 2004, 29, 50–52. [Google Scholar] [CrossRef]
- Benckiser, G.; Christ, E.; Herbert, T.; Weiske, A.; Blome, J.; Hardt, M. The nitrification inhibitor 3, 4-dimethylpyrazole-phosphat (DMPP)-quantification and effects on soil metabolism. Plant Soil 2013, 371, 257–266. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Zhao, H.B.; Du, Q.J.; Shi, Y.F. Inhibitory effects of tropical medicinal plant extracts on urea hydrolysis and nitrification in soil: A Preliminary Study. Hortscience 2015, 50, 744–749. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Z.W.; Meng, C.S.; Wang, K.L.; Hu, Y.N.; Wang, L.Z.; Wang, Q.M. Discovery and SARs of trans-3-aryl acrylic acids and their analogs as novel anti-tobacco mosaic virus (TMV) agents. PLoS ONE 2013, 8, e56475. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J. Biological and pharmacological activities of 1,3,4-thiadiazole based compounds. Mini-Rev. Med. Chem. 2015, 15, 762–775. [Google Scholar] [CrossRef]
- Lorenzo, P.; Reboredo-Durán, J.; Muñoz, L.; Freitas, H.; González, L. Herbicidal properties of the commercial formulation of methyl cinnamate, a natural compound in the invasive silver wattle (Acacia dealbata). Weed Sci. 2020, 68, 69–78. [Google Scholar] [CrossRef]
- Nuntanakorn, P.; Jiang, B.; Einbond, L.S.; Yang, H.; Kronenberg, F.; Weinstein, I.B.; Kennelly, E.J. Polyphenolic constituents of Actaea racemosa. J. Nat. Prod. 2006, 69, 314–318. [Google Scholar] [CrossRef]

| pH | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) |
|---|---|---|---|---|---|---|
| 7.11 | 20.7 | 2.45 | 1.32 | 17.1 | 16.2 | 27.9 |
| Compounds | EC50 (μM) | Compounds | EC50 (μM) |
|---|---|---|---|
| 1 | 828 | 11 | 25 |
| 2 | 721 | 12 | 230 |
| 3 | 742 | 13 | 20 |
| 4 | >1000 | 14 | 850 |
| 5 | >1000 | 15 | >1000 |
| 6 | 893 | 16 | 260 |
| 7 | >1000 | 17 | 75 |
| 8 | >1000 | 18 | 8 |
| 9 | >1000 | 19 | 100 |
| 10 | >1000 | 20 | 760 |
| 21 | >1000 | 22 | >1000 |
| 23 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, J.; Li, G.; Wu, M. Screening Potential Nitrification Inhibitors through a Structure–Activity Relationship Study—The Case of Cinnamic Acid Derivatives. Sustainability 2024, 16, 5791. https://doi.org/10.3390/su16135791
Zhang J, Liu J, Li G, Wu M. Screening Potential Nitrification Inhibitors through a Structure–Activity Relationship Study—The Case of Cinnamic Acid Derivatives. Sustainability. 2024; 16(13):5791. https://doi.org/10.3390/su16135791
Chicago/Turabian StyleZhang, Jie, Jia Liu, Guilong Li, and Meng Wu. 2024. "Screening Potential Nitrification Inhibitors through a Structure–Activity Relationship Study—The Case of Cinnamic Acid Derivatives" Sustainability 16, no. 13: 5791. https://doi.org/10.3390/su16135791




