Abstract
Natural substrates used in horticultural practice quickly degrade during plant cultivation. Methods to extend their service life are sought using natural materials, the acquisition and disposal of which do not burden the natural environment. The paper presents a sustainable method for modifying the coconut fiber substrate by impregnation with a wood-based isolate activated for polymerization and the addition of biochar pellets with retention-increasing properties. The modifications applied to the substrates were shown to have an impact on some of their physical properties, which directly impacted their usefulness in the horticultural production of dessert raspberries. It was found that after a year of operation, the modified substrates showed significantly lower levels of degradation markers. The shrinkage of the impregnated substrate was ~50% lower than that of the control sample, while the substrate with the addition of biochar pellets resulted in similar shrinkage and the lowest plant root mass (25.47%). The usefulness of these substrates was also verified by measuring the physiological parameters of the plants, which determined the ability to photosynthesize and build biomass, as well as susceptibility to stress potentially caused by substrate modifications. The recorded values of these parameters indicate, in most cases, that there is no disturbance of the homeostasis of raspberry plants grown using these substrates. However, plant productivity (measured by the yield of harvested raspberry fruit) indicates that the use of impregnated coconut fiber substrate with the addition of biochar pellets allows for obtaining the highest fruit yields (fruit yield—2.43 kg plant−1). The yields obtained in combination with the extended durability of the modified substrates during operation recommend this solution for use in horticultural practice and make the production more sustainable.
1. Introduction
In countries with intensive horticultural production, soilless cultivation plays an increasingly important role. In recent years, the area of such crops has been increasing, and so has the diversity of plant species used for this purpose [1]. Rubus idaeus L. is a widely cultivated plant due to the production of fruits with great taste and health benefits [2]. This species is grown both on the ground and under cover, mainly using soilless methods, that is, production vases filled with substrate with various physical and chemical characteristics [3]. Coconut fiber, also known as coconut peat, is used in soilless cultivation due to its favorable physicochemical properties. Its application in horticultural production gained interest starting in the 1980s [4]. Coconut fiber is a waste material originating from the coconut industry, mainly in the Philippines, Indonesia, Sri Lanka, Malaysia, and Thailand. Currently, it is the third most widely used substrate in horticulture after peat (which accounts for 2/3 of the market) and mineral substrates such as vermiculite, perlite, or mineral wool. Used in horticultural crops, coconut fiber-based substrates have favorable air–water properties. They mineralize slower than peat, are light, and do not shrink when dry. Moreover, phenolic acids produced as a result of the slow decomposition of lignins in the substrate and its favorable air-water properties stimulate the development of the root system of many plant species grown on this substrate [5,6]. As an organic substrate, the coconut fiber-based substrate gradually degrades during the plant production cycle. This significantly shortens its lifecycle and reduces the profitability of plant production [7]. For comparison, under standard soil cultivation conditions, the profitability of raspberry production lasts for many seasons, while in soilless cultivation using coconut fiber, the profitability of raspberry production is limited to only 3 years [8]. This necessitates the search for new material solutions that allow the period of use of this type of substrates to be extended while maintaining high yields, which determine the profitability of horticultural production [9]. Mineral materials, such as expanded clay, mineral wool, and glass fiber, can be added to organic substrates, such as coconut fiber. However, after exploitation of the substrate, these materials generate environmental problems related to their use [10].
The use of soilless substrates requires the use of fertigation and dosing of nutrients in the form of liquid solutions [11,12]. A major environmental and economic problem with these types of solutions is the appearance of leachate, which carries away some of the nutrients not absorbed by the plant [13]. Many research groups are looking for methods to reduce this problem by using additives that increase the retention capacity of this type of substrate. One such additive may be biochar, most often produced from renewable raw materials [14,15]. Because of its well-developed specific surface, biochar has a high adsorption capacity and high water capacity [16]. These characteristics support its significant potential for use also in horticultural production [17]. The available data show that biochar was tested as an independent substrate for growing plants or as a dusty mixture with various components used in horticultural production, such as coconut fiber, peat, or perlite. In all of these cases, a generally positive effect of adding biochar to the substrate was demonstrated. Not only were its physical properties improved, but above all, the resistance of plants to some diseases and pests increased [18]. However, the full use of biochar properties is significantly dependent on the method of application. It is generally applied in loose form (grain diameter < 0.5 mm) to highly porous materials. During plant fertigation, it is quickly washed out of the substrate. Hence, its potentially positive effect on the growth and development of plants is significantly limited [18].
As a natural raw material, coconut fiber undergoes changes that are mainly caused by microorganisms, leading to systematic decomposition. During this process, the fiber loses its physical properties, especially strength, retention, and the ability to retain plant nutrients. One way to counteract this process could be to impregnate its surface with materials resistant to microorganisms. Polymeric materials could be used to protect the surface of the fibers. However, known polymer materials with the required properties (low susceptibility to microorganisms) are usually harmful to the environment or phytotoxic [19]. Often, these are also synthetic materials, which excludes their use in organic farming. There are a number of polymeric materials that can be obtained from natural raw materials, but not all of them are suitable for the impregnation processes of fibrous raw materials. This is related to the rapid gelation process observed after the polymerization is initiated, which makes the impregnation process difficult or impossible. It seems that the material intended for this process should remain liquid for a long time and acquire polymeric properties after being applied to the impregnated surface.
The presented research describes an innovative method of producing modified substrates based on coconut fiber for horticultural production and the raw materials necessary to obtain them. It was assumed that the additives used would influence the properties of the produced substrates, especially their susceptibility to degradation during operation. Additionally, the influence of the tested substrates on general physical properties and degradability during raspberry plant vegetation was determined. Furthermore, the sustainability of the horticultural production of raspberry plants using the modified substrates was confirmed by the results.
2. Materials and Methods
2.1. Substrates Variants
In the production process of dessert raspberries of the Malling™ Bella variety, three variants of substrates were used:
- ▪
- W0—substrate consisting only of coconut fibers (control).
- ▪
- W1—substrate consisting of coconut fibers impregnated with activated for polymerization isolate (100%). The substrate was prepared according to the procedure described in Section 2.2.
- ▪
- W2—substrate consisting of coconut fibers impregnated with activated for polymerization isolate (90% by weight) with an admixture of pelleted biochar from sunflower husks (10% by weight). The substrate was prepared in a ribbon mixer using 9 kg of W1 substrate and 1 kg of pelleted biochar prepared according to the procedure described in Section 2.3. The mixing process was continued until the biochar pellets were evenly distributed in the mass of the impregnated coconut fibers.
2.2. The Process of Producing Substrates and Their Impregnation
To produce modified substrates, coconut fibers were used (Ceres, Pyzdry, Poland). They were placed in a drum mixer with a tight cover equipped with a dust filter (SCI-B22L, Schwartzmann, Poland). During the mixing process, using a pneumatic paint sprayer (VORGel, symbol 81647, Młochów, Poland), a wood-based isolate activated for polymerization was applied to the surface of the coconut fibers (Fibro Lep, Fibris, Przemyśl, Poland). The wood-based isolate (Fibro Lep, Fibris, Przemyśl, Poland) in liquid form (100 g) was placed in a round-bottom flask with a reflux condenser and heated to boiling point. Then 0.5 g of benzoyl peroxide (Pol-Aura, Zawroty, Poland) was added in small portions and heated for an hour. The mixture thus prepared was usable for 2 h, after which the start of gelation was observed. The isolate contains 50% dry matter, which consists of 60% by weight sugars, 2% by weight resinous substances consisting of mono and sesquiterpene hydrocarbons having unsaturated bonds in their structure capable of polymerization, 38% by weight mineral compounds decomposing during roasting in a weight analysis to gaseous substances, and 2.3% by weight ash.
A nozzle with a diameter of 0.4 mm was used for the application, with an isolate flow of 1.6 dm3 min−1. Nine kilograms of coconut fibers were used as a single input into the drum mixer, on which 1 kg of liquid isolate mixture was applied. The mixing process lasted for 15 min at a speed of 25 rpm. After the mixing process, the impregnated coconut fibers were left to stabilize for a period of 48 h. After this time, no liquid fractions were observed (the entire isolate was polymerized).
2.3. Production of a Pelleted Biochar
The ingredients from which the pellet was made were mixed in a ribbon mixer (Protechnika, MU-1, Poland). It contained 10 kg of dusty biochar (pyrolysis temperature 450–550 °C in time 45 min) obtained from sunflower husks and 250 g of wheat flour. During the mixing process, using a pneumatic paint sprayer (VORGEl, symbol 81647, Poland) with a 0.4 mm spray nozzle and a flow of 1.6 dm3 min−1, 1.5 kg of wood-based isolate activated for polymerization was added (Fibro Lep, Fibris, Przemyśl, Poland). The mixing process was carried out for 5 min to obtain a uniform mixture. Immediately before the pelleting process, the resulting mixture was moistened by adding 250 g of water. This procedure produced a mixture with a consistency that allowed the pelleting process to be carried out. Pellet production was carried out using a Pellet mill MGL 200 (Kovonovak, Citonice, Czech Republic) with a 34 mm thick forming matrix and 6 mm diameter forming holes. The pellet was dried at 60 °C for 3 h to obtain a moisture level of ~5%.
2.4. Analysis of Selected Physical Properties of the Obtained Substrates
2.4.1. Specific Density
The specific density (SD) of the prepared substrates was determined using a GeoPyc 1360 quasi-fluid pycnometer (Micromeritics Instrument Corp., Norcross, GA, USA). The device measures the volume of a sample placed in a measuring cylinder and filled with powder with a grain size of less than 250 µm. Using powder instead of liquid prevents wetting and seepage into the pores of the tested material, enabling the testing of highly absorbent materials. A glass measuring cylinder partially filled with quasi-liquid powder was placed in the device’s handle. In the first stage, the volume of the powder used in the cylinder was determined based on its diameter and measurement of the piston displacement. Next, an appropriate sample was placed in the measuring cylinder, and the volume of the powder and the tested material was measured. Based on the difference in sample volume and mass, the specific density (SD) of the tested sample was calculated. Measurements were carried out in 12 repetitions for each variant of the tested substrate.
2.4.2. Bulk Density
Bulk density (BD) was determined based on the procedure described in the standard PN-EN ISO 17828_2016-02E [20] and the mass of material in a known volume was determined. A standardized container with V = 0.005 m3 was used for the tests, and the bulk density value in the working condition, BDar, was calculated using the following equation:
where
m2—mass of the container with the material [kg]
m1—mass of empty container [kg]
V—volume of the container [m3]
BDar—bulk density under working conditions [kg m−3]
2.4.3. General Porosity
The general porosity of substrates εz was determined based on the specific density (SD) and the bulk density (BD). The relationship between SD and BD can be written as an equation:
where
SD—specific density of the substrate [kg m−3]
BD—bulk density of the substrate [kg m−3]
εz—general porosity of the substrate [%]
2.4.4. Susceptibility to Compaction
The compactability Y was determined using an Insight 2 testing machine (MTS Systems Corporation, Prairie, MN, USA) with a measuring cylinder and a piston. The material was placed in a 5 dm3 cylinder and compacted with a force of 800 N. This force was maintained for 60 s, after which the load was removed and the material expanded, increasing its volume. After 120 s, the percentage increase in the volume of the material in the cylinder was measured in 12 repetitions for each variant of the tested substrate.
2.4.5. Water Properties of the Obtained Substrates
The prepared substrates were analyzed for their water retention capacity, which is of great importance in horticultural production. For this purpose, for example, the maximum water capacity (MWHC) of the analyzed substrate variants was determined. To determine the maximum water capacity, the samples were placed in 1 dm3 metal cylinders by loosely pouring samples with a humidity corresponding to zero water capacity. The tested substrate was then gradually immersed in water and left for 24 h. After this time, the samples were removed from the water and left for 15 min. After this time, samples of individual substrates, fully saturated with water, were weighed and placed in an electric dryer with forced air circulation and dried at 105 °C until a constant weight was obtained. Water capacity was determined as the ratio of the mass of water absorbed by the substrate to the mass of the dry fraction. Based on the difference between the dry weight of the sample when fully saturated with water and the dry weight the maximum water capacity of the developed substrates was calculated. Measurements were carried out in 10 repetitions for each variant of the tested substrate.
In addition to the maximum water capacity, the substrates were also analyzed in terms of their ability to retain water, from completely dry to fully saturated with water. For this purpose, 1 dm3 of water was poured through 1 dm3 of each substrate variant every day. During these tests, the ability of the prepared substrates to retain water was analyzed. The experiment was carried out until a decrease in the amount of retained water was observed in all analyzed media, which was achieved under the study conditions after 7 weeks. As in the case of the maximum water capacity, measurements were carried out in 10 repetitions for each analyzed substrate variant.
2.5. Production Experiment
The suitability of the produced substrates for horticultural production was verified in a cultivation experiment Rubus idaeus L Malling™ Bella variety. The experiment was carried out in polytunnels according to the methodology presented in the work of Balawejder et al. [8].
2.6. Assessment of Selected Physiological Properties of Raspberry Plants Grown on the Developed Substrates
The impact of substrates on the growth and development of raspberry plants was determined by measuring the chlorophyll content in the leaves, selected parameters of chlorophyll fluorescence, gas exchange, and photosynthesis. The relative chlorophyll content in raspberry leaves was measured using a Chlorophyll Content Meter CCM-200plus (Opti-Sciences, Hudson, NH, USA). Measurements were carried out in 24 repetitions for each variant of the test experiment. The influence of the substrates used on the maximum quantum yield of PSII photochemistry (Fv/Fm) and the maximum quantum yield of primary photochemistry (Fv/F0) was measured using an analyzer fluorimeter (Pocket PEA, Hansatech Instruments, King’s Lynn, Norfolk, UK). Using the LCpro-SD portable photosynthesis measurement system (ADC BioScientific Ltd., Hoddesdon, UK), the following parameters were determined: net photosynthetic rate (PN), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci). The selected chlorophyll fluorescence and gas exchange parameters were measured in 24 repetitions for each experimental variant according to the methodologies described in the work of Matłok et al. [21].
2.7. The Degree of Substrate Degradation during Plant Production
After the first year of cultivation, the degree of degradation of the prepared substrates was compared to the control sample by determining the growth of the root mass and the shrinkage of the substrate.
2.7.1. Substrate Shrinkage
The substrate shrinkage (S) is the percentage loss of substrate volume in relation to its initial volume. The shrinkage of the developed substrates was determined after 1 year of cultivation (using samples of the substrates in which the raspberries were grown). To determine the shrinkage, the volume loss of substrate Vu was determined after the first year of use. The space in the pot above the level of the substrate used for a year was filled with calcined quartz sand of known bulk density BDs to the level that the substrate was reached at the beginning of the season. Then, the mass of the added sand (ms) was determined. Based on a mass of sand, ms, and the known bulk density of the sand, BDs, the volume of the lost substrate was calculated according to the following equation:
where
ms—mass of sand [kg]
BDs—bulk density of sand [kg m−3]
Vu—volume of the lost substrate [m−3]
Knowing the initial volume of the substrate Vp and the volume of substrate lost after a year of cultivation Vu, the contraction S was determined from the equation:
where
S—substrate shrinkage [%]
Vp—initial volume of the substrate [m3]
2.7.2. Root Mass Fraction
As in the case of shrinkage, the mass of roots mr produced in the tested substrate by a single raspberry bush was determined after the end of the first vegetation period. The mass of roots mr was determined based on the difference between the weight of the pot with the substrate, the root system developed in it mpdI, and the weight of the pot with the substrate before planting the plant, mpd, using dry samples.
where
mr—root mass after the first year of cultivation [g]
mpdI—mass of pot with substrate and roots [g]
mpd—mass of pot with substrate [g]
Then, the percentage of root mass Ur was determined in the mass of the substrate according to the relation:
where
Ur—share of the root mass in the mass of the substrate [%]
mpd—mass of the pot with substrate [g]
md—mass of the pot [g].
2.8. Fruit Yield Siz
In the production experiment, long-shoot seedlings of long cane plants were used, the first full fruiting of which was recorded in the second year after planting. The impact of the substrates produced on the amount of raspberry fruit yield was estimated in the second year of cultivation by determining the number of fruits per plant and the average weight of a single fruit (g).
2.9. Statistical Analysis of the Results
All statistical analyzes were performed with STATISTICA 13.3 software (StatSoft, Tulsa, OK, USA). To show the existence of uniform groups of objects (p < 0.05), the multiple comparison Tukey’s HSD test for multiple comparisons was performed following a one-dimensional analysis of variance (ANOVA).
3. Results and Discussion
3.1. Physical Properties of the Obtained Substrates
Coconut fiber is a natural material that undergoes degradation due to various factors when used as a horticultural substrate. In most cases, this significantly limits the time of using these substrates in horticultural production and thus affects the profitability of production. Modification of the surface of the coconut fibers by applying a polymeric layer obtained from activated isolate for the polymerization process should increase its strength and susceptibility to degradation during the operation during plant cultivation. The initial properties of the impregnated substrate (W1) were determined by measuring the specific density (SD), the bulk density (BD), the total porosity, and the compactibility (Y) (Table 1). When the obtained results were analyzed, a slight increase in specific density (SD) was found. This is due to the amount of remaining dry polymer mass of the coconut fiber. It should be noted that the activated polymerization isolate contained only 1% of the substances capable of polymerization and 50% of the other substances, and the remainder was water, which was removed during the drying process. However, changes in the bulk density of the W1 substrate indicate a significant stiffening of the coconut fiber structure, which tended to create a compact structure of the piled material during the formation of the bulk cone compared to the W0 control (substrate containing only coconut fibers). The overall porosity of the impregnated material (W1) also increased, which is a typical phenomenon caused by the reinforcement of the structure of the coconut fiber with polymeric material. Moreover, the W2 substrate was supplemented with pellets made from biochar. This additive was used to improve the physical properties of the substrate. It was shown that the specific density (SD) and bulk density (BD) increased significantly compared to the control substrate (W0). The recorded results are not surprising because biochar pellets have a compact structure with a higher density and modified porosity compared to dusty biochar, with additives that act as a binder.

Table 1.
Selected physical properties of the substrates produced based on coconut fiber (Mean ± SD, n = 10).
The use of various types of additives significantly affects some of the physical properties of the substrates used in horticultural production. Various additives are currently used in horticultural crop substrates to reduce their susceptibility to degradation [9,10]. The horticularea substrates were modified by adding pelleted biochar, whose usefulness results primarily from its significant density, hardness, and high adsorption capacity. As Xing et al. (2018) [22] point out, pyrolytic biochar pellets differ in terms of properties depending on the conditions used in the production process. This provides a wide range of possibilities for predicting the properties of horticultural substrates prepared with its addition. In the W2 substrates used in the experiments, a 10% addition of pelleted biochar was used. This significantly modified the physical properties of the substrate (Table 1).
The substrates were exploited during the experimental cultivation of raspberry plants in production pots, using long cane seedlings capable of high-level yielding for 5 years. However, observations made so far in horticultural practice when using unmodified substrates (W0) indicate that the period of cultivation of raspberry plants is reduced to 3 years due to progressive degradation of the substrate. The greatest changes in the physical parameters of the substrates (degradation) are observed after the first year of vegetation. This is due to the intensive growth and development of plants, mainly the root system. The prepared substrates were analyzed after one year of horticultural use for raspberry cultivation. Typical parameters indicating the susceptibility of substrates to degradation were determined, i.e., the share of root mass in the substrate mass and substrate shrinkage (Table 2).

Table 2.
Selected parameters that determine the degree of wear (degradation) of the substrate after one year of raspberry cultivation (n = 10).
The study has shown that the use of the impregnation process of coconut fibers that are the base of the substrate significantly hindered the growth of the root system of raspberry plants. In most cases, excessive root system growth occurs when there is a deficit or lack of availability of water or nutrients. Raspberry fruit production using soilless substrates usually takes place in production pots, and water and nutrients are supplied through a fertigation system [23]. The observation of limited growth of the root ball could indicate easier access to water and fertilizing ingredients for plants or a negative impact on the growth and development of plants. These effects were verified by physiological measurements of plants. The weight of individual fruits and their yield per single plant were also determined, as described in Section 3.2. However, these results clearly eliminate the negative impact of the prepared substrate on both the condition of plants and their yield.
The degradation of the coconut fiber substrate is manifested by shrinkage. This shrinkage is the result of the progressive mineralization of coconut fibers following microbiological activity and enzymes secreted through the root system of the plant [24]. The impregnation of coconut fibers (W1) reduced the shrinkage of the substrate by more than 50% after the first year of horticultural use in raspberry production. This effect is extremely beneficial and clearly indicates that the degradation of this substrate has been limited. In light of the observed hindered growth of the root ball, this predicts a longer period of use of this substrate in horticultural cultivation. In the case of the substrate with the addition of biochar pellets (W2), a similar trend of reduced substrate shrinkage compared to the control (W0) was observed. However, it was higher than the shrinkage recorded for the W1 substrate. Most likely, this is due to the decomposition of the biochar pellets that constitute its additive.
3.2. The Retention of Water Ability of the Obtained Substrates
The maximum water holding capacity (MWHC) of the prepared substrates in the dry state ranged from 2.43 to 4.53 kg H2O kg−1 (Figure 1). The W2 substrate had the highest maximum water holding capacity value, as its ability to retain water was almost 36% higher compared to the control (W0). In turn, in the case of the W1 substrate, the average maximum water holding capacity value obtained was lower compared to both the control substrate (W0) and the W2 substrate by 27% and 46%, respectively (Figure 1).
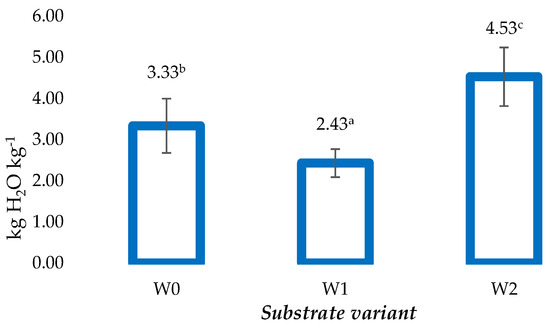
Figure 1.
Maximum water holding capacity (MWHC) of the substrates produced based on coconut fiber (Mean ± SD) (n = 10). Identical superscripts (a, b, c) denote non-significant differences between means values according to the post-hoc Tukey’s HSD test.
As shown by this investigation, the analyzed substrates were characterized by different water retention capacities (Figure 2). In the control substrate (W0), the average values of retained water in individual weeks of the experiment ranged between 56–89.1%. This substrate had the lowest average amount of water retained in the first week of the experiment and the highest in the fourth week. In the case of the W1 substrate, the average amount of retained water ranged from 60.9% to 88.8%. In the case of this variant, the smallest amount of retained water was found in the seventh (last) week of the experiment and the largest in the fourth week. Slightly different relationships were observed for the W2 substrate. In comparison to the other experimental variants, it was also characterized by the highest maximum water holding capacity. In the case of W2, the range of retained water in individual weeks of the experiment was 47.7–91.8%. Similarly to the control, the smallest amount of retained water was recorded in the first week of the experiment, while the largest was recorded in the sixth week.
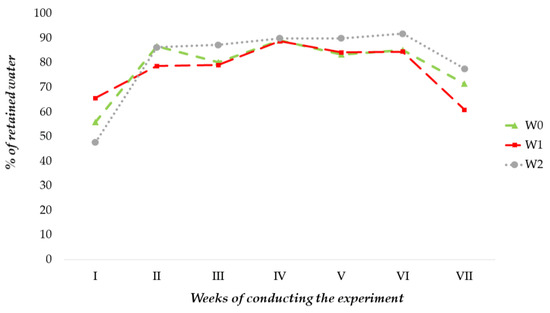
Figure 2.
Dynamics of water retention by coconut fiber-based substrates in individual weeks of the experiment (n = 10).
The ability of the substrate used in horticultural crops to retain water is of fundamental importance. Peat, which is commonly used in cultivation, especially with a significant degree of humification, is highly hydrophobic after drying. For this reason, it is very difficult to rehydrate because of the small angle of adhesion of the drop surface to the dried peat [25]. The analyzed substrates based on coconut fiber, in a fully dry state, absorb approximately 50% of the water supplied in the fertigation process (Figure 2). These substrates become fully capable of retaining water after about week 4, while after about week 7, the amount of retained water gradually decreases. The absorbability of substrates after drying is increased by adding various materials to the base material, such as perlite, vermiculite, or hydrogel [25]. Biochar is also of great importance in this respect due to its large specific surface area, which allows it to retain significant amounts of water [17]. The research also showed a positive effect of the pelleted biochar additive, which significantly increased the water holding capacity of the W2 substrate. According to the available data, biochar for gardening substrates is generally applied in a dusty form. This causes its rapid washing out during plant fertigation and lowers its retention capacity [18]. Biochar in the form of pellets can be assumed to not be washed out in the fertigation process. This is evidenced by the fact that in the W2 substrate, after week VII of the experiment, a higher water retention capacity was observed compared to the other experimental variants (an average of 78%) (Figure 2).
3.3. Evaluating the Suitability of the Produced Substrates for Plant Production: A Case Study of Raspberries
3.3.1. Chlorophyll Content in Raspberry Leaves and Selected Chlorophyll Fluorescence Parameters
The addition of a substance of natural origin (an isolate activated for polymerization) can affect many parameters of a plant. If negatively affected by this addition, these plants can potentially show symptoms of stress. The level of plant stress caused by various factors can be determined by measuring selected markers. For plants, such markers could be relative chlorophyll content in leaves (SPAD), maximum photochemical efficiency of PSII (Fv/Fm), and maximum quantum yield of primary photochemistry (Fv/Fo) [26]. These parameters can be measured at every stage of plant growth and development without their destruction and loss of plant tissues, which could cause secondary stresses that disrupt future plant growth [27].
A measure of the ability of plants to assimilate carbon dioxide is the chlorophyll content in parts of green plants. The content of this pigment can be modified by biotic and abiotic stresses. These can be macroscopically observed in the form of necrosis and chlorosis [28]. Small changes in the relative chlorophyll content must be instrumentally measured because they do not cause observable changes. The relative chlorophyll content was measured using the SPAD method in the first and second years of raspberry cultivation using the developed (modified) substrates in relation to the control sample. The recorded values indicate that there is no significant effect of the substrates used and the additives they contain on the relative chlorophyll content in plants (Figure 3A). Subtle differences in the level of potential stress resulting from the modified substrate applied can be measured by selected chlorophyll fluorescence parameters (Figure 3B,C). The maximum photochemical efficiency of PSII [Fv/Fm] is a recognized indicator of the efficiency of photosynthesis, as well as the degree of plant health [29]. The values of this parameter recorded in both years of the experiment clearly indicate the lack of significant differences between the research variants. This is confirmed by the lack of additional stress factors in the case of plants grown on modified substrates (W1 and W2) compared to the standard coconut fiber substrate (W0). An indicator with even greater sensitivity to stress factors for plants is the measurement of the maximum quantum yield of primary photochemistry [Fv/Fo]. As pointed out by Baker [30], this parameter is more sensitive and indicates changes in the ability of plants to assimilate carbon under the influence of various stress factors. The values recorded for this parameter over the years of research indicate typical relationships that show differences in the state of plant development. However, the lack of differences between individual research variants in particular years indicates the lack of a negative impact of applied substrate modifications on the condition of raspberry plants.
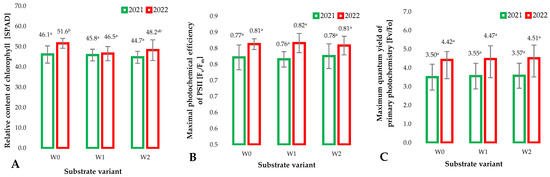
Figure 3.
Relative chlorophyll content (SPAD) (A), maximum photochemical efficiency of PSII (Fv/Fm) (B), average values of chlorophyll fluorescence parameters (maximum quantum yield of primary photochemistry (Fv/Fo) (C), in raspberry leaves depending on the type of substrate (Mean ± SD) (n = 20). Identical superscripts (a, b) denote non-significant differences between means values according to the post-hoc Tukey’s HSD test.
3.3.2. Parameters of Plant Gas Exchange and Photosynthesis Intensity
During the growth and development of plants, the root system has long-term contact with the substrate and the additives used to modify it. The substrates used for raspberry production include terpene compounds derived from the applied isolate activated for polymerization. These compounds could have allelopathic effects that can be directly translated into the physiological parameters of plants, including selected gas exchange parameters. The intensity of this exchange was determined by the net photosynthesis intensity (PN) [mmol(CO2)m−2p−1] (Figure 4A) measured as the equivalent of the amount of carbon dioxide absorbed by plants per unit of leaf area. The significant differences observed between the growing seasons (years 1 and 2 of the study) result from the stage of plant development. However, no significant differences were observed between the tested substrate variants in individual years. This indicates that modifications to coconut fiber-based substrates did not affect carbon dioxide assimilation by raspberry plants grown on them. The intensity of photosynthesis depends on factors that influence the availability of light and carbon dioxide for plants and, to a significant extent, on the abundance and structure of the substrate. Based on the above, it can be concluded that the changes in the structure and chemical composition of the substrate caused by the modifications did not significantly affect the availability of nutrients for plants supplied by fertigation. The ability of plants to assimilate carbon dioxide at the cellular level can be assessed by measuring the intercellular CO2 concentration (Ci) (Figure 4B). It is clearly visible that plants at an earlier stage of development (1st year of research) show a significantly higher intercellular CO2 concentration than in the second year of research. This is related to the need for rapid growth of plant biomass caused by frequent cell divisions, which require the construction of new tissues based on assimilated CO2. However, when the impact of the modified substrates was analyzed, no significant impact on this parameter was observed in the individual years of the study. The lack of significant differences confirms the suitability of the prepared substrates for horticultural production, especially raspberry plants. An important parameter determining the condition of cultivated plants is the measurement of plant transpiration (Figure 4C). When plants are in good condition, as evidenced by the lack of visible plant damage, leaf necrosis, loss of plant turgor, etc.), the measurement of plant transpiration can also indicate the availability of water in the substrate for plants. The coconut fiber additives used (mainly biochar pellets) influenced the availability of water for plants, as evidenced by the increased transpiration of plants grown on W2 substrates compared to other substrates. This effect was especially observed in the second year of the experiment.
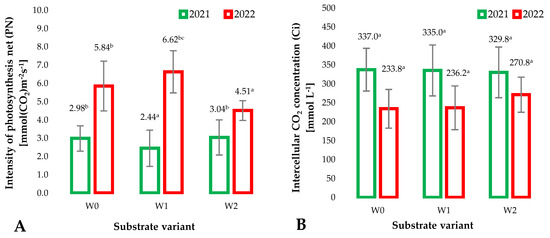
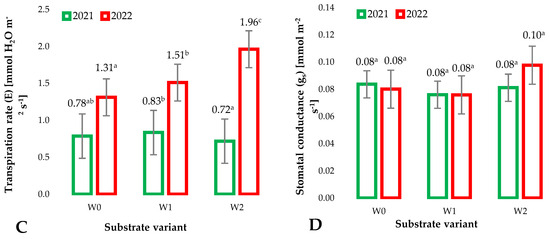
Figure 4.
Mean values of gas exchange parameters: net photosynthesis intensity (PN) (A), intercellular CO2 concentration (Ci) (B), transpiration rate (E) (C), stomatal conductance (gs) (D), in raspberry leaves depending on the type of substrate (n = 20). Differences in results between the substrate; difference at significant level p < 0.05. Identical superscripts (a, b, c) denote non-significant differences between means values according to the post-hoc Tukey’s HSD test.
Observations made regarding the impact of modified substrates used for the growth of raspberries on net photosynthesis intensity (PN) (A), intercellular CO2 concentration (Ci) (B), and transpiration rate (E) were confirmed by measurement of stomatal conductance (gs) (Figure 4D). This parameter indicates the mechanisms that regulate the opening and closing of the stomata, which are also responsible for gas exchange and the transpiration process. Several biotic and abiotic factors, including those that occur on the substrate, can influence the activity of stomata. This affects the intensity of photosynthesis and, consequently, shapes the growth and development of plants [21]. When analyzing the results obtained regarding the stomatal conductance (gs) (Figure 4D), the authors found that the process of impregnating coconut fibers with the activated isolate for polymerization did not significantly change this parameter. However, this effect was recorded for the W2 substrate containing biochar pellets. The increase in stomatal prominence of plants grown on this substrate compared to the control was 21%. This effect was most likely due to the addition of biochar, which influenced the physical parameters of this substrate, mainly shrinkage (Table 2).
3.4. Productivity of Raspberry Plants Grown on Developed Substrates
Plant yield is not only an indicator of the profitability of cultivation but also a marker of proper growth and development of plants. It is determined, among others, by the substrate used for production [31]. Modifications introduced to the coconut fiber substrate influenced the average weight of the individual raspberry fruits (Figure 5A). This is an extremely important parameter in the harvesting of dessert fruits. Fruits harvested from plants grown on the W2 substrate showed a 10% reduction in individual fruit weight compared to the control sample (W0) and the W1 substrate. However, the determinant of plant productivity is the total yield per plant or unit of crop area. The amount of fruit yield obtained from plants grown on the W2 substrate was the highest at 2.43 kg−1 per plant. This indicates that through both the addition of biochar pellets and the use of impregnation of coconut fibers with polymerization-activated isolate, the modified W2 substrate provided the best conditions for the growth and development of raspberry plants and their yield.
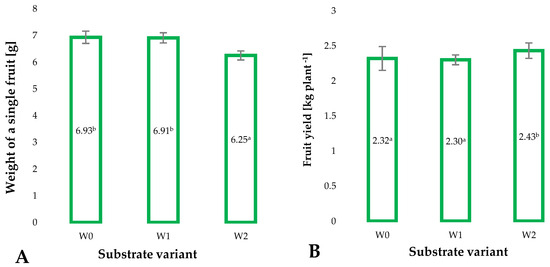
Figure 5.
Average weight of a single raspberry fruit [g] (A) and average fruit yield from a single plant [kg] (B) in the second year of cultivation, depending on the substrate used (n = 100). Identical superscripts (a, b) denote non-significant differences between means values according to the post-hoc Tukey’s HSD test.
4. Conclusions
The production of dessert raspberries is a very demanding process. One of the key factors influencing their cultivation in a soilless system has been shown to be the type of substrate used. Modifications of the coconut fiber-based substrate in the form of impregnation with a polymerization-activated isolate significantly increase its durability. This was confirmed by measurements of root ball expansion and substrate shrinkage after one year of operation. These parameters were much lower in the modified substrates than in the control sample. The modified substrates were also supplemented with biochar pellets using a proprietary method, which significantly improved the retention properties. It was shown that the shrinkage of the impregnated substrate was ~50% lower than the control sample, while the substrate with the addition of biochar pellets resulted in similar shrinkage and the lowest mass of the plant roots. The durability of this type of substrate is not the only determinant of its usefulness in horticultural production. The condition and productivity of plants grown using the developed substrates are equally important. The recorded values of these physical parameters related to the effectiveness of photosynthesis and gas exchange indicate, in most cases, that they do not alter the homeostasis of raspberry plants grown using these substrates. However, plant productivity (measured by the yield of harvested raspberry fruit) indicates that using impregnated coconut fiber substrate with biochar pellets allows the highest fruit yields to be obtained. The yields obtained in combination with the extended durability of the modified substrates during operation recommend this solution for use in horticultural practice and make the production more sustainable.
Author Contributions
Conceptualization, methodology, investigation, M.K.; conceptualization, methodology, investigation and writing—original draft, N.M. and M.B.; investigation, writing—original draft, M.S.; investigation, K.M., M.W., B.S. and J.S.; formal analysis, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the European Agricultural Fund for Rural Development RDP for 2014–2020/Measure 16 Cooperation within the project entitled “Innovative technology for the production of berries on the example of raspberries with an increased content of bioactive compounds and increased commercial value” project number 00024.DDD6509.00014.2019.07. Publication co-financed from the funds of the Ministry of Education and Science under contract No. KONF/SP/0507/2023/01 dated 13 January 2024 in the amount of 92113.85 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Treder, J.; Nowak, J. Cocopeat as growing medium in bedding plant production. Zesz. Probl. Postep. Nauk. Rol. 2002, 485, 351–358. (In Polish) [Google Scholar]
- Sawicka, B.; Barbaś, P.; Skiba, D.; Krochmal-Marczak, B.; Pszczółkowski, P. Evaluation of the Quality of Raspberries (Rubus idaeus L.) Grown in Balanced Fertilization Conditions. Commodities 2023, 2, 220–245. [Google Scholar] [CrossRef]
- Qiu, C.P.; Xu, Q.H.; Gaudreau, L.; Gosselin, A.; Gauthier, L.; Van Sterthem, A.; Desjardins, Y. Yield improvement of red raspberry by soilless cultivation with two propagation methods under northern Canadian climate. Acta Hortic. 2016, 1133, 195–200. [Google Scholar] [CrossRef]
- Verdonk, O. Reviewing, and evaluation of new materials used as substrates. Acta Hortic. 1984, 150, 467–473. [Google Scholar] [CrossRef]
- Mariotti, B.; Martini, S.; Raddi, S.; Tani, A.; Jacobs, D.F.; Oliet, J.A.; Maltoni, A. Coconut Coir as a Sustainable Nursery Growing Media for Seedling Production of the Ecologically Diverse Quercus Species. Forests 2020, 11, 522. [Google Scholar] [CrossRef]
- Nawrocka-Grześkowiak, U. Influence of coir on the rooting of Calluna vulgaris L. cuttings. Zesz. Probl. Postępów Nauk. Rol. 2005, 504, 479–486. (In Polish) [Google Scholar]
- Dyśko, J.; Kaniszewski, S.; Kowalczyk, W.; Dziedziczak, K.; Kowalski, B.; Moraczewski, A.; Podsiedlik, W.; Wojtysiak, J. Ecological fibrous soilless substrates for greenhouse cultivation. Probl. Eksploat. 2012, 2, 37–56. (In Polish) [Google Scholar]
- Balawejder, M.; Matłok, N.; Piechowiak, T.; Szostek, M.; Kapusta, I.; Niemiec, M.; Komorowska, M.; Wróbel, M.; Mudryk, K.; Szeląg-Sikora, A.; et al. The Modification of Substrate in the Soilless Cultivation of Raspberries (Rubus Idaeus L.) as a Factor Stimulating the Biosynthesis of Selected Bioactive Compounds in Fruits. Molecules 2023, 28, 118. [Google Scholar] [CrossRef]
- Mishra, L.; Basu, G. 8—Coconut fibre: Its structure, properties and applications. In Woodhead Publishing Series in Textiles, Handbook of Natural Fibres, 2nd ed.; Kozłowski, R.M., Mackiewicz-Talarczyk, M., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 231–255. ISBN 9780128183984. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Odutuga, O.L.; Effiong, J.U.; De Jesus Silvera Sarmiento, A.; Mortazavi, S.J.; Hu, J.W. Development of Fibre-Reinforced Cementitious Mortar with Mineral Wool and Coconut Fibre. Materials 2022, 15, 4520. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, A.; Filipek-Mazur, B.; Komorowska, M.; Niemiec, M.; Bar-Michalczyk, D.; Kuboń, M.; Tabor, S.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Sikora, J.; et al. Environmental and production aspects of using fertilizers based on waste elemental sulfur and organic materials. Materials 2022, 15, 3387. [Google Scholar] [CrossRef] [PubMed]
- Mog, L. Smartphone-Operated Smart Farm Watering System Using Long-Range Communication Technology. Agric. Eng. 2023, 27, 59–74. [Google Scholar] [CrossRef]
- Opstad, N.; Sønsteby, A.; Espelien, H.G.; Myrheim, U. Fertigation timing and fertilizer composition affects growth and yield in raspberry long cane and field production. Acta Hortic. 2012, 946, 361–366. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Sadowska, U.; Kuboń, M.; Gliniak, M.; Sikora, J. Sunflower husk biochar as a key agrotechnical factor enhancing sustainable soybean production. Agriculture 2021, 11, 305. [Google Scholar] [CrossRef]
- Wyzińska, M.; Berbeć, A.K.; Grabiński, J. Impact of Biochar Dose and Origin on Winter Wheat Grain Quality and Quantity. Agriculture 2023, 14, 39. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J. Biochar affects macronutrient leaching from soilless substrate. HortScience 2012, 47, 1136–1140. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Lian, Y.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manag. 2022, 317, 115441. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 17828_2016-02E; Solid Biofuels—Determination of Bulk Density. Polish Committee for Standardization: Warszawa, Poland, 2015.
- Matlok, N.; Szostek, M.; Antos, P.; Gajdek, G.; Gorzelany, J.; Bobrecka-Jamro, D.; Balawejder, M. Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants. Appl. Sci. 2020, 10, 2579. [Google Scholar] [CrossRef]
- Xing, X.; Fan, F.; Jiang, W. Characteristic of biochar pellets from corn straw under different pyrolisis temperatures. R. Soc. Open Sci. 2018, 5, 172346. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Chapter 13—Effect of Internal and External Factors on Root Growth and Development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 331–346. ISBN 9780123849052. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Sulman, B.N.; Mayes, M.; Patterson, C.M.; Classen, A.T. Plant root stimulate the decomposition of complex, but not simple, soil carbon. Funct. Ecol. 2019, 34, 899–910. [Google Scholar] [CrossRef]
- Nowak, J.S. Air-Water properties of growing media. Zesz. Probl. Postępów Nauk. Rolniczych 2005, 504, 175–184. (In Polish) [Google Scholar]
- Badr, A.; Bruggemann, W. Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescense measurements and leaf relative water content. Photosynthetica 2020, 58, 638–645. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging tecchnique in horticultural reaserch: A review. Sci. Horticulare 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Górny, A.G. Photosynthetic activity of flag leaves in diallel crosses of spring har ley under varied nutrition and soil moisture. Cercal Res. Commun. 2001, 29, 159–166. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W. Chlorophyll fluorescence measurements in environmental studies. Kosmos 2016, 65, 197–205. [Google Scholar]
- Baker, N.R.; Oxborough, K. Chlorophyll Fluorescence as a Probe of Photosynthetic Productivity. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 65–82. [Google Scholar]
- Borowski, E.; Nurzyński, J. Effect of different growing substrates on the photosynthesis parameters and fruit yield of greenhouse-grown tomato. Acta Sci. Pol. Hortorum Cultus 2012, 11, 95–105. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).