Abstract
Microplastics (MPs) pose a significant and increasing threat globally, with plastics accounting for around 60–80% of marine trash. Plastic particles with a size of 5000 µm or less are referred to as microplastics (MPs). These MPs can enter the marine organisms either through their diet or by trophic transfer. This can potentially expose people to these particles. According to the literature, around 21.9% of fish, 18.4% of seabirds, 11.7% of arthropods, and 7.8% of molluscs in marine systems are at risk from plastic pollution. The LATAM region experiences significant MP contamination that primarily originates from wastewater treatment plants, industrial effluents, maritime sources, and the decomposition of macro–mesoplastics. The majority of research conducted in the LATAM region focuses on MPs in natural habitats, specifically examining the presence of MPs in biota (such as fish, mussels, squids, turtles, and even insects) and sediments. In order to conduct a thorough analysis of the sources and spread of microplastics (MPs) in marine organisms, we conducted a comprehensive assessment of the available literature on microplastic research in Latin American countries. The objective was to evaluate the origin, destinations, and pathways via which MPs are transferred. An assessment of the prevalence of microplastics (MPs) in marine organisms would yield significant insights into the potential health hazards posed by plastic pollution to humans.
1. Introduction
Humans have been using plastics indiscriminately since their discovery in the 1950s, causing a substantial increase in the volume of debris [1]. The term “plastics” encompasses a diverse range of materials that are produced through the polymerization of monomers sourced from petroleum or gas. Plastic pollution is presently the foremost and most prevalent environmental issue in the oceans [2]. Records of plastic debris in the oceans have been recorded since the 1970s. Carpenter [3] initially documented the existence of polystyrene microspheres, with an average diameter of 0.5 mm, in the coastal waters of the North Atlantic (specifically New England, USA). These microspheres were also found in the digestive systems of eight different fish species in the Niantic Bay region of Connecticut. Plastic production in 2021 surpassed 390.7 million tons [4]. Although plastics have greatly enhanced our quality of life, over 50% of them are utilized as single-use products, resulting in a significant amount of daily waste [5]. Improper disposal and inadequate recycling have transformed plastic litter into a worldwide issue [6]. The economic impact of plastic pollution in the oceans is projected to be as high as EUR 21 billion [7]. The inevitable slow breakdown of plastic in the natural environment results in a significant and prolonged presence of plastic in the ecosystem [8,9,10,11]. Furthermore, around 80% of plastic waste in the ocean originates from land, recreational activities on beaches, and maritime transportation. Additionally, around 18% of this debris is specifically linked to the fishing sector [12,13]. Out of all the polymers of plastics, there are six classes that are produced globally in large quantities, namely polyethylene (PE), both high- and low-density, polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), including expanded EPS, polyurethane (PUR), and polyethylene terephthalate (PET) [14]. MPs are classified as primary and secondary plastics. Primary MPs are consciously manufactured in a microscopic size for industrial purposes, such as for use as abrasives or in cosmetics [15]. On the other hand, secondary MPs are formed when larger plastic debris undergoes weathering processes, such as photo-oxidation, in oceanic and coastal environments [13,15]. They can also be generated through anthropogenic activities, such as the production of microfibers from the mechanical degradation of textiles during the laundry process and migration with wastewater discharges [16]. The plastic particles that are neutrally buoyant are the most likely to be ingested [17], both intentionally and accidentally [18,19]. Due to their size, MPs are easily taken up or ingested by all types of aquatic organisms, including microalgae [20], zooplankton [21], teleost fish [22], and megafauna [23,24]. The ingestion of MPs can lead to gastrointestinal obstruction, hunger, and death [25], as well as changes in the gut microbiota in aquatic creatures [26,27]. Prior to photo- and thermo-oxidative processes and subsequent fragmentation [28], plastics can be categorized based on their size. These categories include mesoplastics (5–40 mm), microplastics (1–5000 µm) [29], and nanoplastics (less than 0.1 µm) [30]. Fibers are regarded as the most prevalent form of particulate matter (PM) due to their worldwide presence, ranging from tropical regions to polar ice caps [31,32,33]. These fibers are also found in littoral sediments, subtidal zones, and surface and sub-surface waters and deep waters [33,34,35]. As a result, they are accidentally ingested by various marine organisms, including plankton, shrimps, clams, fishes, and marine mammals [32,36,37,38,39]. According to [40,41], 87% of studies on plastic litter pollution focused on marine habitats, whereas only 13% were conducted in freshwater ecosystems. Microplastics (MPs) have spread throughout all regions of the marine environment, with varying concentrations. Some areas, such as ocean gyres, have particularly high concentrations, known as hotspots [25]. One example is the Pacific gyre, also referred to as the “Great Pacific Garbage Patch” (GPGP), where a significant accumulation of marine litter has occurred within the vortex of the gyre [42].
LATAM comprises 48 countries, the majority of which have coastal access to either the Pacific or Atlantic Ocean. This geographical advantage contributes to the region’s high productivity, as it relies heavily on the natural resources and ecosystem services offered by the ocean. The LATAM region features almost 50,000 km of coastline, encompassing a multitude of coastal environments, namely beaches, marshes, rocky areas, and reefs, all of which are susceptible to plastic pollution. Despite the fact that approximately 80% of MPs are particles in the oceans originated from rivers, the majority of research concentrates on studying their behavior in the oceans, with only limited information available for rivers [43]. MPs have been studied throughout the LATAM region in diverse coastal habitats and reported in more than 39 indexed publications. Despite limited studies in this field compared to North America or Europe, the invasion of MPs in LATAM cannot be neglected. When evaluating the physiological condition of the estuaries in Latin America, it is important to consider the rugged shoreline of the region. Estuaries play a crucial role in maritime ecology by providing essential ecosystem services. They also contribute to the regional economy by supplying food and raw materials and serve as a hub for demographic development, recreational activities, and cultural events [44]. They are of significant ecological importance for the biota, as they provide nurseries and alimentation and serve as breeding grounds [45,46,47,48,49]. Estuarine regions across the globe are usually flanked by large cities, and the development of these cities has a direct effect on the coastal ecosystems in the region [50], and LATAM estuaries are of no exception. Thus, the mismanagement and illegal disposal of vast amounts of plastic trash in marine ecosystems has made plastic pollution a significant environmental issue in recent times [51,52].
2. Materials and Methods
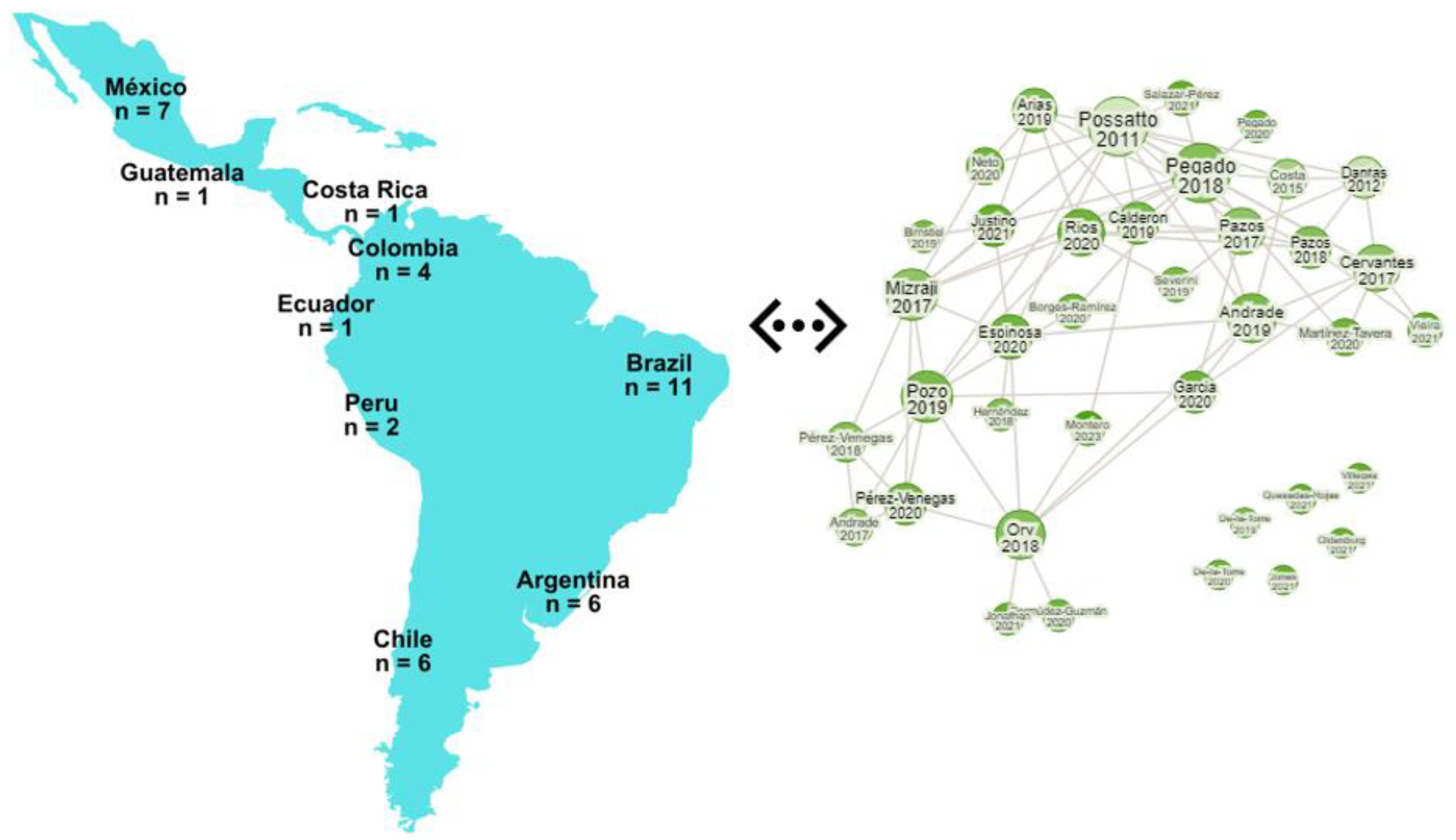
In this review, a comprehensive investigation was carried out to examine all accessible scholarly works with an emphasis on the qualitative and quantitative assessments of microplastics in Latin America. An exhaustive review of the scientific literature on microplastics in marine organisms was conducted using various search engines, including Science Direct, Google Scholar, ISI Web of Science, PLOS ONE, and PubMed. The search was focused on specific keywords such as microplastics in Latin America, fishes, sharks, dolphins, organisms, marine, and seafood to maximize the retrieval of relevant articles. The scope of the review included the following nations in Latin America: Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Guatemala, Mexico, and Peru. Subsequently, the downloaded research articles were thoroughly scrutinized, focusing on the study’s objectives. The research was categorized according to the biota and corresponding habitat studied in each investigation. In total, 40 published articles from various Latin American nations were chosen, and the distribution of publications is as follows: Argentina (n = 6), Brazil (n = 11), Chile (n = 6), Colombia (n = 4), Costa Rica (n = 1), Ecuador (n = 2), Guatemala (n = 1), Mexico (n = 7), and Peru (n = 2) (Figure 1).
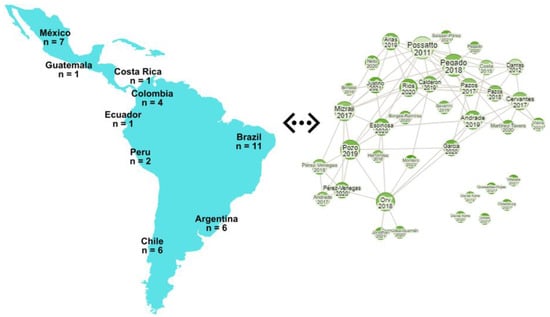
Figure 1.
Geographical representation of the number of studies used for the review and the research rabbit image that represents the linkage between the published articles in the LATAM region.
3. Results and Discussion
3.1. Research on MPs in LATAM
In 2021, the Latin American plastics industry represented around 4% of global production. However, it faced challenges in terms of competitiveness, which were impacted on factors such as political and economic stability, advances in infrastructure, the availability of supplies, workforce training, and the modernization of industrial machinery and processes. Recent data suggest that the total plastic exports from Latin America is worth USD 26.8 billion. LATAM has the third highest per person plastic waste generation rate, 0.99 kg/person/day [53]. In addition, most of the waste management infrastructure is developing, and for this reason, hundreds of tons of plastics debris are accumulating in diverse environments such as terrestrial and marine [54,55].
Additionally, Latin America and the Caribbean are emerging as new destinations for plastic waste from world powers, especially the United States, and there has been a substantial increase in these shipments to Mexico, Ecuador, and some Central American countries in recent years. The outcome of this situation can potentially result in the contamination of the ocean with plastic waste, where about 8 MT of plastic ends up in the world’s oceans every year, and there are about six huge patches of plastic waste and other forms of garbage in the ocean.
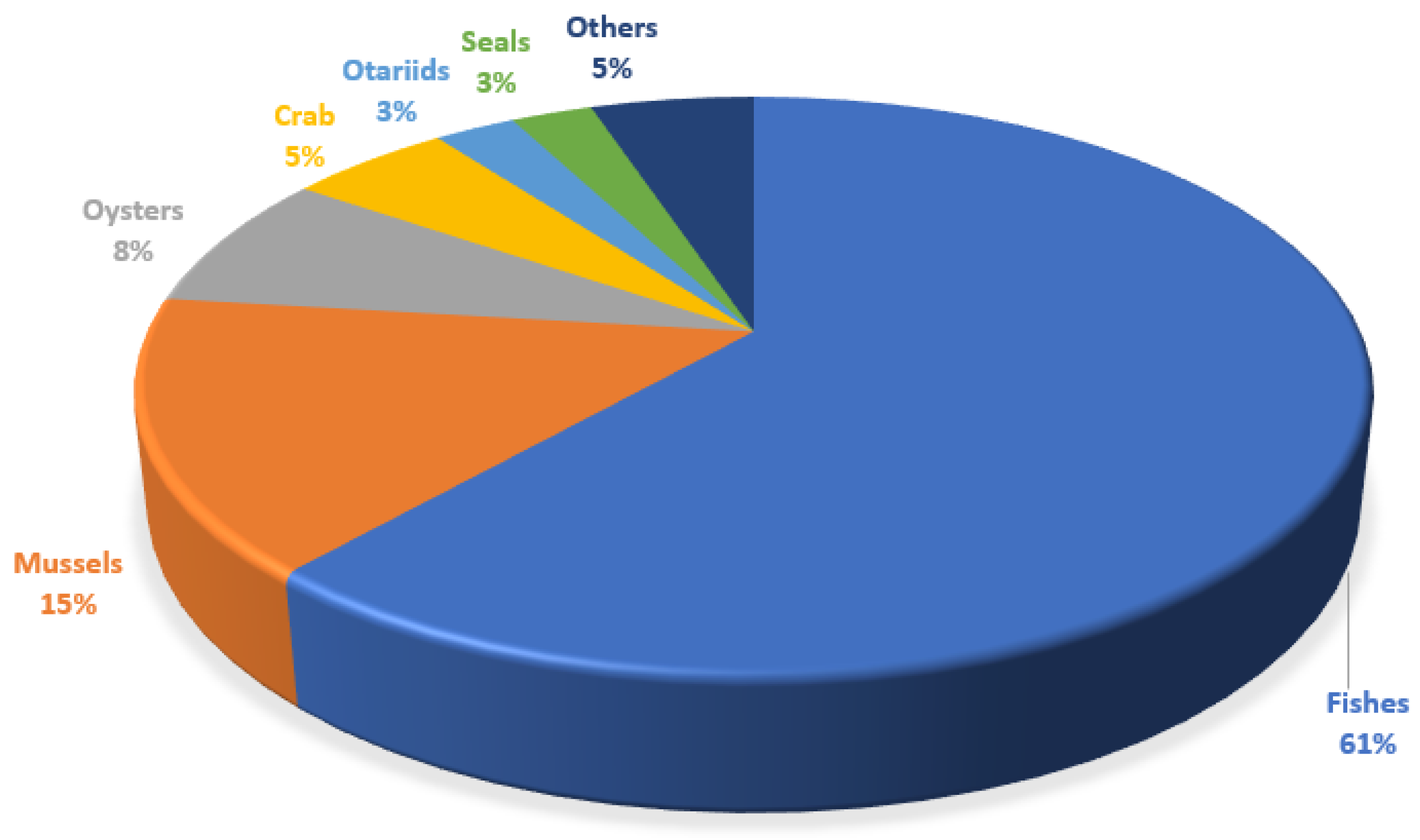
The accumulation of plastic on the seabed can lead to the consumption of microplastics by fish and other marine animals, which can have a negative impact on the safety of human food. The accumulation of microplastics (MPs) in living organisms started in the 1960s, when researchers discovered plastic particles in the digestive system of birds living in the maritime environment [5,15,25,55]. In this study, it was observed that fish were the most analyzed species, followed by molluscs and crabs. The marine biota was procured from the marine environment (45%), rivers (27.5%), estuaries (12.5%), mangroves (2.5%), and markets (7.5%). In the specific case of otariids, scats were taken from the ground (5%). Overall, 208 species were analyzed (Figure 2).
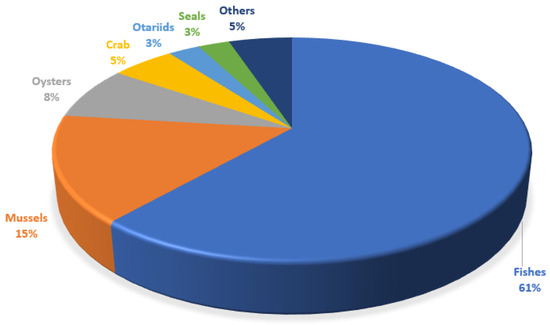
Figure 2.
Quantitative representation of the different groups of marine organisms used for the characterization of MPs in the LATAM region.
According to the literature and the digital resources used in this review, the first research article about the detection of MPs in LATAM in marine biota came out in 2012 in Brazil [56]. Then, there was a span without publications (2013–2017), which may be because most of the studies had been focused on the detection of microplastics on beaches. As of 2017, the number of publications about this topic had slightly increased, reaching only 12 publications in 2020, and throughout this year, 2021, 8 research articles focusing specifically on the detection of MPs in marine biota have been published. There is a concern that, despite the high impact of this problem, MP research on marine biota in LATAM has been scarce and unexplored.
Plastic debris pollution is a well-known and widely reported problem across LATAM. While most research has focused on the detection of MPs in beaches or water (sea, rivers, estuaries, sediments, etc.), only a few countries in LATAM have investigated the consumption of MPs by marine biota. Additionally, the current knowledge about MP sources, transport occurrence, and potential impacts on marine biota and human health has not been well described. This is a significant issue, especially considering that LATAM boasts approximately 199,000 km of coastline [57,58].
3.2. Shapes and Colors of MPs
Practically all species contained MPs, either in large quantities or only in glitter; while their shape and size varied, the color and type of polymers remained relatively similar across countries. This may be an attribute of the fact that microplastics are widespread throughout the world, can be transported by rivers, winds, and wastewater, among others, and reach several ecosystems, regardless of the place where they were produced [59,60].
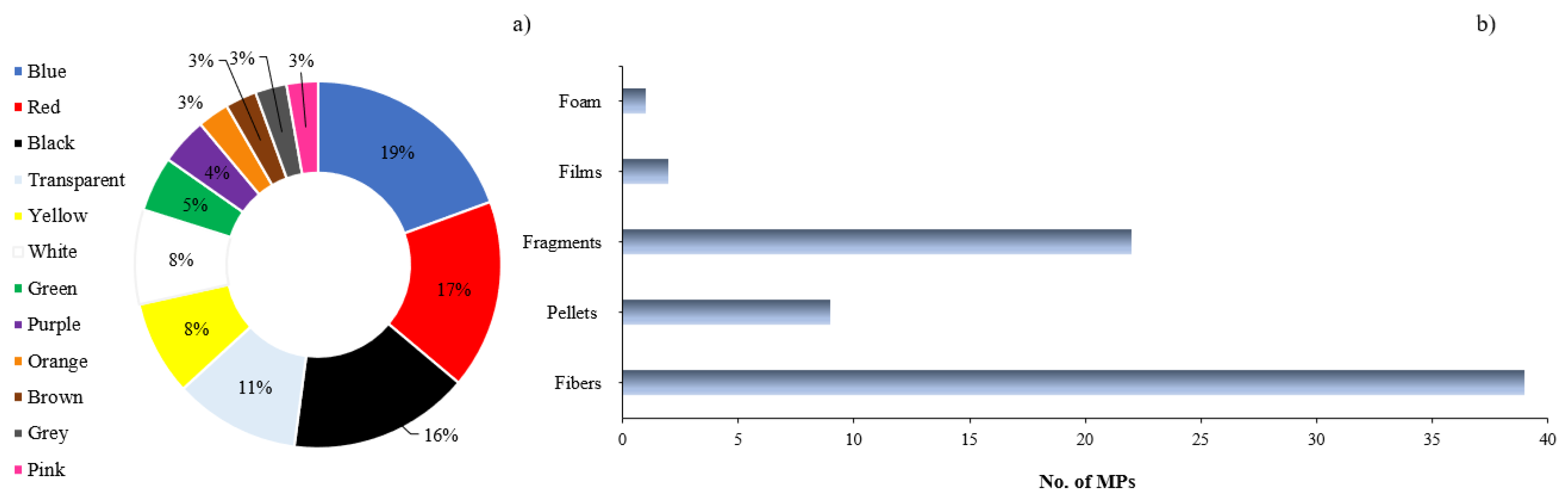
In all cases, the most common shapes found in the organism were fibers, followed by fragments, pellets, and other shapes such as films. According to [33], fibers are the most prevalent types of microplastics (MPs) worldwide, even reaching remote locations such as polar glaciers. Due to their aerodynamic shape and sedimentation velocity, fibers can undergo long-distance atmospheric transport [61]. Plastics exposed to weathering in the ocean are the primary source of secondary MPs [62].
According to Refs. [13,63], one can generate fibers by hand, using washing machines, or through fishing activities. It is important to consider that approximately 97% of the studies were carried out near human settlements; only [63] developed a study in a place with fewer settlements, the Guafo Island in the Chilenean Patagonia, and found fibers in seal scats.
The MPs in marine biota in LATAM had a wide range of colors: blue, white, red, transparent, pink, orange, yellow, green, brown, grey, and purple. Blue, red, and black were the most commonly found colors, with pink, yellow, brown, and green being the least reported (Figure 3). The authors of [64,65] reported that blue and green are related to nets and fishing gear, while a yellow color signifies oxidation or photooxidation and indicates the presence of persistent organic pollutants [66,67].
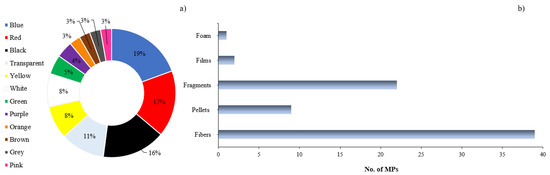
Figure 3.
(a) percentage of color distribution of MPs; (b) number of MPs per form reported in diverse investigations of the LATAM region.
3.3. Identification and Characterization of MPs
In the literature reviewed, the identification of MPs involved the removal of organic matter from the samples using various chemicals at different concentrations, temperatures, and digesting durations. These chemicals include H2O2, HNO3, KOH, NaOH, and HCl, with concentrations ranging from 10% to 30%. The duration and temperature of digestion varied between 12 and 72 h and 40 and 100 degrees Celsius, respectively. This indicates a lack of validated digestion and standardization processes in the field of MPs, not only in LATAM but globally [68]. The morphology and size were assessed using several types of microscopes, including an SEM (Scanning Electron Microscope) in certain cases.
Regarding characterization, the predominant analytical technique employed in most investigations to determine the nature of polymers was Fourier transform infrared spectroscopy (FT-IR). This is due to its ability to discern the chemical composition of plastic particles. FT-IR is the predominant analytical technique employed globally for identifying polymer types [69]. Nevertheless, this method has many drawbacks, including its limited sensitivity, restricted range of plastic particle sizes, and susceptibility to resolution issues caused by factors such as the presence of organic materials [70,71].
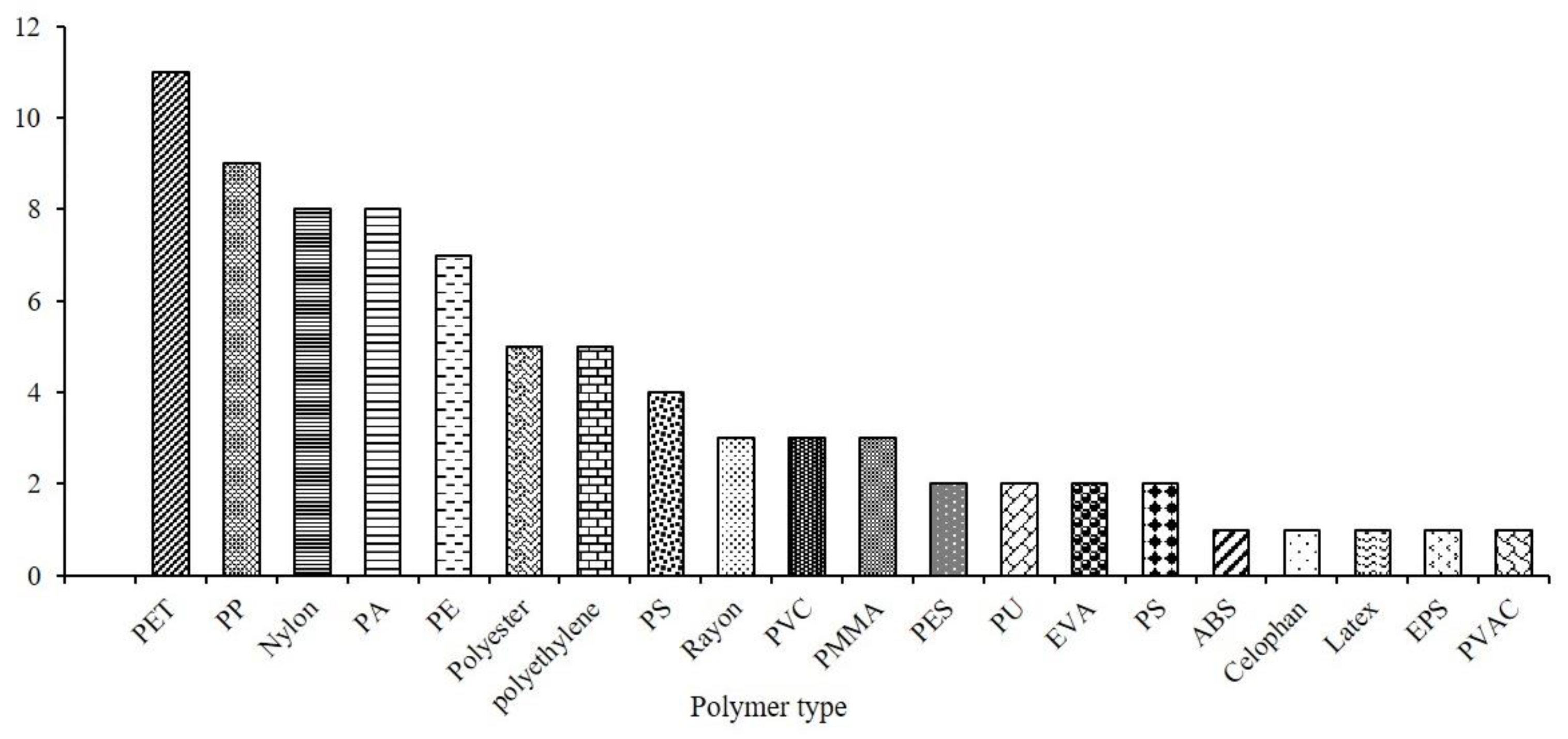
In LATAM, twenty-one types of polymers were identified: polyethylene terephthalate (PET) > polypropylene (PP) > and nylon were the most frequent (Figure 4). Ref. [13] emphasizes that PET stands as one of the most widely produced polymers globally, making it a prime candidate for marine environments. The presence of nylon stems from its widespread use in fishing gear production [72].
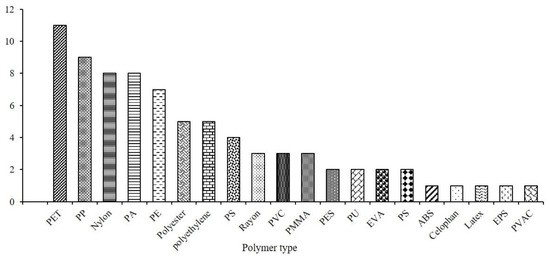
Figure 4.
Polymer characterization of MPs reported in the marine organisms of the LATAM region.
3.4. Fish
3.4.1. Freshwaters
Domestic and industrial activities in urban areas primarily cause MP pollution in freshwater fish [73]. Most of the analyzed fish samples contained MPs, especially fibers, in their gastrointestinal tracts.
In freshwater, [41] report the presence of microplastics in 100% of the studied Prochilodus lineatus (according to [41], MPs were found in the digestive tracts of all 21 species of Prochilodus lineatus that were studied in the Parana River. These MPs were mostly fibers and were in black, yellow, red, and transparent colors. Ref. [74] studied the presence of MPs in adults of Oreochromis niloticus (n = 18) from natural and farmed sources. The study demonstrated that 44% of the farmed fish and 75% of the wild fish were significantly contaminated by microplastics. Nearly 53% of the particles were identified as cellophane/cellulose, while PET, PES and PE were also observed as prevalent MPs. Ref. [75] described the presence of MPs in fish (n = 87), where 96% of the MPs found were fibers, and no correlation between the number of MPs and the size or feeding habit of the fish was present. Ref. [67] discovered that 83% of the digestive tract of Hoplosternum littorale (n = 48) contained fibers, with an average of 3.6 particles per fish. A study conducted on 15 gastrointestinal tracts of Oreochromis niloticus from the Atoyac river revealed the extraction of 139 fibers. The color distribution of these fibers was primarily black (40%), followed by blue (19%), and red and white (14%). It is worth noting that polyamide (PA), PES, and synthetic cellulose were the most prevalent types of fibers found.
3.4.2. Sea and Estuary Waters
In sea and estuary waters, Ref. [76] analyzed 292 planktivorous fish from four families (Atherinopsidae, Clupeidae, Engraulidae, and Scombridae) and reported the presence of MPs in 6 fish gut samples. Ref. [77] studied 1095 fish, representing 15 species, and found 1384 particles of plastic debris that were recovered from 552 gastrointestinal tracts. The polymer composition of MPs mainly included PA (51.2%), PE (36.6%), PP (7.3%), and polyacrylic (4.9%). Likewise, in another study, 965 specimens of eight commercially exploited fish species from different habitats off the southeast-south coast of Brazil were analyzed, and all the studied species contained MPs [78].
The pelagic species exhibit greater quantities, occurrence rates, variety, and sizes of ingested microplastics (MPs) compared to demersalpelagic or demersal organisms. The pelagic skipjack tuna Katsuwonus pelamis (Scombridae) had the highest frequency of MP ingestion of 25.8%. On the other hand, the demersal bluewing searobin Prionotus punctatus (Triglidae) had the lowest frequency of MP ingestion of 5%. The MPs were predominantly fibers and fragments, which likely originated from fishing materials. Ref. [79] examined a total of 189 fish specimens collected from the estuary of the Amazon River, encompassing 22 different families and 46 distinct species. A total of 228 particles were extracted from the gastrointestinal tracts of 26 fish specimens, which belonged to 14 different species. These particles were classified into four categories: pellets (97.4%), sheets (1.3%), pieces (0.4%), and threads (0.9%).Ref. [80] reported an average of 1.31 ± 2.59 microplastic particles (MPs) per fish, resulting in a total of 316 MPs found in 240 sampled individuals. The results suggest variations in the abundance of microplastics (MPs) in demersal fish. Haemulon plumierii had the highest count of MPs, totaling 115 (73 fibers and 42 fragments). Ref. [81] examined the occurrence of microplastic (MP) ingestion by fish in the mangrove ecosystems of Cispata, Caribbean Colombia. Out of the 302 fish specimens belonging to 22 different species, a total of 69 MPs were found in their digestive tracts, accounting for 7% of the fish analyzed. The MPs were categorized as 55% filaments, 23% fragments, 19% films, and 3% foam. Ref. [82] conducted a study on 30 gastrointestinal tracts of the herring Opisthonema complex (Clupeiformes: Clupeidae) from the Central Pacific of Costa Rica and reported MPs in all the samples, with an average of 36.7 pieces per fish. Among these, 79.5% were fibers and 20.5% were particles. Most of the fibers were categorized as polypropylene (PP). Ref. [83] examined the gastrointestinal tracts of four fish species from the estuary Cienega Grande de Santa Marta in northern Colombia. Out of the total samples purchased from a local market, 17 (12.1%) were discovered to contain MPs. The mullet species, scientifically known as Mugil incilis, exhibited the highest quantity of microplastics (MPs), followed by Caranx hippos, Caquetaia kraussii, and Eugerres plumieri. Out of the 19 MPs that were discovered, 17 of them (89.5%) were fibers, while the remaining 2 (10.5%) were fragments. Polyethylene terephthalate (PET) and polyethylene (PE) were the predominant types of fibers, with nylon, acrylic, and additional PE also being used. This coastal area is the second most contaminated by particulate matter in its sediments, with only the region including Cartagena, Colombia, having higher contamination levels [84] Possatto et al. [85] examined a total of 182 organisms in a study focused on three significant catfish species: Cathorops spixii (n = 60), Cathorops agassizii (n = 60), and Sciades herzbergii (n = 62). The study was conducted in the estuaries of the Southwestern Atlantic region in the northeastern part of Brazil. In the case of C. spixii and C. agassizii, 18% and 33% of the individuals, respectively, exhibited a presence of plastic litter in their stomachs. S. herzbergii had a contamination rate of 18% among its individuals. Every stage of development (juveniles, sub-adults, and adults) possessed MPs. The contamination was primarily caused by nylon pieces derived from cables utilized in fisheries operations. The extremely small size, as well as the appealing color and ability to float, make it effortless for the filtered fish to consume and swallow the microplastics. Animals may inadvertently or intentionally consume microplastics (MPs) by mistaking these particles for their usual food sources, such as plankton or zooplankton, especially if the MPs are already present within or connected to their prey [80,86].
3.5. Otariid
Currently, there is a lack of research on marine mammals in comparison to fish. However, two studies conducted in Chile, [87,88], focused on investigating the pollution of microplastics in the feces of otariids and seals. As stated by [88], the act of searching for microplastics in animal feces is a non-invasive technique that serves as an effective means of monitoring plastic exposure. In Northern Chilean Patagonia [87], scat samples from 51 female South American fur seals (Arctocephalus australis) in Guafo Island were collected. In the case of [88], researchers collected 205 scat samples from four species of otariids, Arctocephalus philippi, Otaria byronia, Arctocephalus australis, and Arctocephalus australis un-named, in six different sites along the coastline of Peru and Chile: Punta San Juan, San Juan Fernández Archipielago, Puyuya Islet, Chiloé Island, Punta Chaiguaco, and Guafo Island.
Both studies yielded consistent findings, indicating the presence of microplastics in all the tested scat samples. The predominant form of microplastics observed was fibers, followed by fragments. The primary colors found in [89] were blue, followed by white and red. Similarly, in [56], the dominating colors were blue, white, black, and then red. On Guafo Island, a study found that 66% of the samples collected had microplastics (MPs) in them. Another study detected MPs in 67% of the samples. In both cases, the most common color of the microplastics was blue. This finding aligns with the observation that blue fibers are the most prevalent shape of microplastics in the lower trophic levels of the Pacific Ocean [77].
Through FTIR analysis, it was determined that 85% of the fibers/fragments found in the feces originated from materials such as cotton, PET, and nylon. Another crucial factor to consider is the feeding behavior of otariids, as they consume mesopelagic fishes, which are species that come into contact with microplastics [89,90,91].
3.6. Crabs
Studies of MPs in crabs were carried out only in two countries, in Chile by [92] and in Ecuador by [93]. The species that were examined in the study were Lithodes santolla, often known as southern king crab, with a sample size of 30 individuals in Chile. In addition, Leptuca festae and Minuca ecuadoriensis, each with a sample size of 30 individuals, were evaluated in Ecuador. The samples were collected from L. santolla in the Nassau Bay in Cape Horn, Chile, and from L. festae and M. Ecuadoriensis in the estuary of the Guayas River.
Fibers are also the most prevalent MP shape in crabs, and these have been found in stomachs and other tissues. Ref. [91] exclusively examined the stomachs, whereas [94] investigated the gills, hepatopancreas, and digestive tract, which includes the esophagus, stomach, and intestines. Both studies [92,93] showed that the studied crabs had similar quantities (n = 30) in their stomachs and were blue in color. The size of MPs in L. santolla ranged from 3 to 20 mm, but the size was not quantified for L. festae and M. ecuadoriensis. None of the authors identified the type of polymer.
In Chile, the primary causes of plastic pollution in Cape Horn are related to fishing and transportation activities carried out by ocean currents [94]. As stated by [95], crabs can consume microplastics (MPs) either directly or indirectly through the food chain. However, [96] emphasizes that both oral ingestion and uptake through the gills are significant routes of plastic intake in areas with substantial plastic pollution. The study conducted by [97] found a higher presence of MPs in gills compared to stomachs, suggesting that gills may act as the primary route of entry.
3.7. Mollusc
Research on MPs in shellfish species has been carried out in LATAM countries like Argentina, Brazil, Ecuador, and Peru. The analyzed species mainly included Aulacomya atra, Limnoperma fortunei, Mytilus chilensis, Perna perna, Sacostrea palmula, Sacostrea palmula, Lepas anafiteria, Semimytilua algosus, Megabalanus peninsularis, Nerita scabricosa, Chiton sulcatos, Eudicaris galapagensis, Holothuria kefersteini, Chiton granosus, Tegula atra, Crassoteos gigas, Crassostea gasar, Crassotrea rhizophorae, Bathymodiolus platifrons, and Egeria congica.
Microplastics were detected in all species and nations. The primary shape seen in most cases was fibers, followed by fragments and pellets. However, in the Paranagua estuary system and Amazon estuary in Brazil, the most abundant shape was pellets, followed by fibers, films, and fragments [78,97]. Ref. [98] linked the variations in shapes to the proximity of the samples to the ports and fishing areas, while [99] mentioned that the Amazon estuary is not only home to one of the greatest petrochemical industries in Latin America but also a significant source of plastic pollution.
In Argentina, in the Bahía Blanca Estuary, the presence of MPs in Crassostrea gigas is attributed to two PVC and PP plants located near the estuary. Additionally, the water waste treatment plant contributes a high number of fibers due to inadequate primary treatment of domestic washings, which fails to remove the fibers [62].
Various types of polymers were documented in Ecuador and Peru, including PET, PP, PE, PS, rayon, PVC, PES, PE, Polyester, and PS. In the other experiments, either only one type of polymer was identified, or the researchers did not determine their composition [98,100].
It is widely recognized that molluscs can consume microplastics, either deliberately or unintentionally [101,102]. The likelihood of this happening relies on the characteristics of the plastic debris, such as its size, shape, and density [102]. The MPs discovered in mollusk species ranged from 0.03 to 12 mm. Given this size, it is unlikely that MPs would cross the gastrointestinal barrier. Instead, nanoplastics have the potential to travel from the gut into the circulatory system and tissues, leading to cytotoxicity. Further studies on this are required to support this statement [102,103] and delve deeper into the modes and mechanisms of damage.
Some studies that have investigated microplastics in different species of mussels have demonstrated that exposure to polymeric particles, such as polystyrene, leads to deformities, genetic damage, neurotoxicity, and oxidative stress. These findings emphasize that smaller particles tend to create more severe issues [104,105].
The consumption of an average portion of mussels (250 g wet weight) might result in the ingestion of 90 plastic particles. Similarly, a portion of six oysters (100 g) may include 50 plastic particles [106]. However, it should be noted that the quantities of plastic particles can vary significantly throughout different regions of the world. Consuming shellfish could be a relevant pathway for humans to ingest microplastics. Regrettably, there is currently a lack of studies assessing the potential risks posed to the human body by these plastic particles when consumed.
Research on microplastics in molluscs and crabs is being conducted globally, including in Latin America. Countries such as Argentina, Brazil, Ecuador, and Peru have undertaken studies on this topic. Chile and Guatemala are the only countries in Central and South America where researchers have discovered rare and understudied animals, specifically otariids (eared seals) and corals. Some of the studies performed in Latin America countries are summarized in Table 1. The predominant form of MPs were fibers, followed by fragments and pellets, with a smaller presence of foams and films. It is noteworthy that there was no consistent pattern of color or shape distribution across North, Central, and South America. However, all the articles consistently documented the presence of blue fragments.

Table 1.
Comparative studies of MPs’ presence, types in different species, and polymers from Central and South American countries.
The most studied species are been fishes, and different studies in Brazil report about 51 different species of fish, followed by Mexico and Colombia with 38 and 27 species, respectively, and it is important to say that Brazil has been the only country in Latin America where microplastics in stingrays have been studied. In countries such as Peru and Guatemala, the research has focused on molluscs and corals.
In countries like Argentina, Colombia, Costa Rica, Guatemala, and Peru, the size of MPs was not reported, either because the authors did not determine it, or they reported it as the number of microplastics/cm2. The size of the MPs showed variations both between different countries and within different studies performed within the same country. The predominant types of polymers were PET, PP, nylon, PA, and PE. The size of the discovered microplastics was only documented in a limited number of studies carried out in Brazil, Chile, Ecuador, and Mexico. The method employed in all investigations for polymer identification was Fourier transform infrared (FTIR) spectroscopy using attenuated total reflectance (ATR) and micro-FTIR.
4. Concluding Views
Even though LATAM encompasses 48 countries, there is a dearth of knowledge and research regarding microplastic pollution in this region. Several of these studies have confirmed the presence of microplastics in a diverse range of marine animals, spanning from Mexico in the north to Argentina in the south. However, the aim of this research has been limited to identifying the color and morphologic characteristics of MPs, and rarely has the polymer type been identified, with PET, PP, and nylon being found more frequently, and fibers being the predominant shape.
From all marine species, fishes, followed by mussels, are the main object of study, and this is understood to be related to their human consumption relevance. Out of all the countries in LATAM, so far, only researchers in Chile have conducted a study on the pollution of MPs in marine mammals. Research about the MP threat should be considered from a broader view, including other species and trophic levels. Further studies are necessary to know if these polymers could be both bioaccumulated and biomagnified through the food webs, generating impacts in marine species themselves or on human health.
Finally, it should be emphasized that Latin America is a region in which there are several and critical hotspots for waste management and which have a large coastline area, and these conditions have a strong relationship with the invasion of plastics in oceans. However, research on this problem is still deficient, and studies regarding the source, environmental trace, and pathways of their incorporation into trophic webs are indispensable. Therefore, this review focusses on the knowledge gap regarding the presence of microplastics in marine organisms.
Author Contributions
Conceptualizations, L.A.-M. and J.M.P.; methodology, R.T.-L. and P.G.-G.; validation and formal analysis, S.B.S.; data curation, P.G.-G.; writing-review and editing, S.B.S. and L.A.-M.; supervision, L.A.-M. and Jonathan Muthuswamy Ponniah; and founding acquisition, L.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This article was founded by Instituto Politécnico Nacional, SIP20242931 and APC was founded by the Instituto Politécnico Nacional.
Institutional Review Board Statement
Not applicable due to studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this review. Data sharing is not applicable to this article.
Acknowledgments
R.T.L. and P.G.P. wish to thank CONAHCYT (Mexico) for the research fellowship. S.B.S., L.A.M. and M.P.J. wish to express their gratitude to Sistema Nacional de Investigadores (SNI) and CONAHCYT, E.D.I. & C.O.F.A.A., IPN, Mexico. This article is a contribution (partial) from Earth System Science Group (ESSG), Mexico and India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; GESAMP: London, UK, 2015. [Google Scholar]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Marine debris occurrence and treatment: A review. Renew. Sustain. Energy Rev. 2016, 64, 394–402. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal water. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Europe, P. Plastics—The Facts 2022—An Analysis of European Plastics Production; Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Nerland, I.L.; Halsband, C.; Allan, I.; Thomas, K.V. Microplastics in Marine Environments: Occurrence, Distribution and Effects; Kristians and Norwegian Institute for Water Research: Oslo, Norway, 2014; pp. 12–15. [Google Scholar]
- Arias, A.H.; Ronda, A.C.; Oliva, A.L.; Marcovecchio, J.E. Evidence of microplastic ingestion by fish from the Bahía Blanca estuary in Argentina, South America. Bull. Environ. Contam. Toxicol. 2019, 102, 750–756. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Aanesen, M.; Austen, M.C.; Borger, T.; Clark, J.R.; Cole, M.; Hooper, T.; Lindeque, P.K.; Pascoe, C.; Wyles, K.J. Global ecological, social, and economic impacts of marine plastic. Mar. Pollut. Bull. 2019, 142, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Hakkarainen, M.; Varma, I.K.; Albertsson, A. Degradable polyethylene: Fantasy or reality. Environ. Sci. Technol. 2011, 45, 4217–4227. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Rios, L.M.; Jones, P.R.; Moore, C.; Narayan, U.V. Quantitation of persistent organic pollutants adsorbed on plastic debris from the northern Pacific Gyre’s eastern garbage patch. J. Environ. Monit. 2010, 12, 2226–2236. [Google Scholar] [CrossRef]
- Sheavly, S.B.; Register, K. M Marine debris & plastics: Environmental concerns, sources, impacts and solutions. J. Polym. Environ. 2007, 15, 301–305. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- McKeen, L.W. Film Properties of Plastics and Elastomers, 4th ed.; William Andrew: Oxford, UK, 2017; pp. 1–23. [Google Scholar]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding Type Affects Microplastic Ingestion in a Coastal Invertebrate Community. Mar. Pollut. Bull. 2015, 102, 95–101. [Google Scholar] [CrossRef]
- Cliff, G.; Dudley, S.F.J.; Ryan, P.G.; Singleton, N. Large sharks and plastic debris in KwaZulu-Natal, South Africa. Mar. Freshw. Res. 2002, 53, 575–581. [Google Scholar] [CrossRef]
- Laist, D.W. Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In Marine Debris; Coe, J.M., Rogers, D.B., Eds.; Springer: New York, NY, USA, 1997; pp. 99–139. [Google Scholar]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220 Pt B, 1282–1288. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Baini, M.; Panti, C.; Galli, M.; Jiménez, B.; Muñoz, A.J.; Marsili, L.; Finoia, M.G.; Ramírez, M.D. Are whale sharks exposed to persistent organic pollutants and plastic pollution in the Gulf of California (Mexico)? First ecotoxicological investigation using skin biopsies. Comp. Biochem. Physiol. C 2017, 199, 48–58. [Google Scholar] [CrossRef]
- Germanov, E.S.; Marshall, A.D.; Bejder, L.; Fossi, M.C.; Loneragan, N.R. Microplastics: No Small Problem for Filter-Feeding Megafauna. Trends Ecol. Evol. 2018, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir inensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jasen, M.A.K. Oxidation and fragmentation of plastic in a changing environment; from UV-radiation to biological degradation. Sci. Total Environ. 2020, 851, 158022. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russel, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Obbard, R.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Artic Sea ice. Earth’s Future 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Di Mauro, R.; Kupchik, M.J.; Benfield, M.C. Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ. Pollut. 2017, 230, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Baruque, R.J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.; Windsor, F.M.; Munday, M.; Durance, I. Natural or synthetic—How global trends in textile usage threaten freshwater environments. Sci. Total Environ. 2020, 718, 134689. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Mancuso, M.; Panarello, G.; Falco, F.; Di Paola, D.; Serena, S.; Capillo, G.; Romeo, T.; Presti, G.; Gullotta, E.; Spanò, N.; et al. Investigating the effects of microplastic ingestion in Scyliorhinus canicula from the South of Sicily. Sci. Total Environ. 2022, 850, 157875. [Google Scholar]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic microfiber emissions to land rival those to waterbodies and are growing. PLoS ONE 2020, 15, e0237839. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Bottari, T.; Mancuso, M.; Pedà, C.; De Domenico, F.; Laface, F.; Schirinzi, G.F.; Battaglia, P.; Consoli, P.; Spanò, N.; Greco, S.; et al. Microplastics in the bogue, Boops boops: A snapshot of the past from the southern Tyrrhenian Sea. J. Hazard. Mater. 2022, 424, 127669. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.M.; Elie, A.; Farhan, R.K.; Nuket, S.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Bottari, T.; Houssa, R.; Brundo, M.V.; Mghili, B.; Maaghloud, H.; Mancuso, M. Plastic litter colonization in a brackish water environment. Sci. Total Environ. 2024, 912, 169177. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte, R.B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastics. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt, H.P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [CrossRef]
- Atkins, J.P.; Burdon, D.; Elliott, M.; Gregory, A.J. Management of the marine environment: Integrating ecosystem services and societal benefits with the DPSIR framework in a systems approach. Mar. Pollut. Bull. 2011, 62, 215–226. [Google Scholar] [CrossRef]
- Ferreira, G.V.B.; Barletta, M.; Lima, A.R.A.; Morley, S.A.; Costa, M.F. Dynamics of Marine Debris Ingestion by Profitable Fishes Along the Estuarine Ecocline. Sci. Rep. 2019, 9, 13514. [Google Scholar] [CrossRef]
- Krumme, U.; Brenner, M.; Saint, P.U. Spring-neap cycle as a major driver of temporal variations in feeding of intertidal fishes: Evidence from the sea catfish Sciades herzbergii (Ariidae) of equatorial west Atlantic mangrove creeks. J. Experimen. Mar. Biol. Ecol. 2008, 367, 91–99. [Google Scholar] [CrossRef]
- Lima, A.R.A.; Barletta, M. Lunar influence on prey availability, diet shifts and niche overlap between Engraulidae larvae in tropical mangrove creeks. J. Fish Biol. 2016, 89, 2133–2152. [Google Scholar] [CrossRef] [PubMed]
- Potter, I.C.; Tweedley, J.R.; Elliott, M.; Whitfield, A.K. The ways in which fish use estuaries: A refinement and expansion of the guild approach. Fish Fish. 2013, 16, 230–239. [Google Scholar] [CrossRef]
- Ramos, J.A.A.; Barletta, M.; Dantas, D.V.; Costa, M.F. Seasonal and spatial ontogenetic movements of gerreidae in a Brazilian tropical estuarine ecocline and its application for nursery habitat conservation. J. Fish Biol. 2016, 89, 696–712. [Google Scholar] [CrossRef]
- Freeman, L.A.; Corbett, D.R.; Fitzgerald, A.M.; Lemley, D.A.; Quigg, A.; Steppe, C.N. Impacts of urbanization and Development on Estuarine Ecosystems and Water Quality. Estuaries Coasts 2019, 42, 1821–1883. [Google Scholar] [CrossRef]
- Dauvergne, P. Why is the global governance of plastic failing the oceans? Glob. Environ. Change 2018, 51, 22–31. [Google Scholar] [CrossRef]
- Ostle, C.; Thompson, R.C.; Broughton, D.; Gregory, L.; Wootton, M.; Johns, D.G. The rise in ocean plastics evidenced from a 60-year time series. Nat. Commun. 2019, 10, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Margallo, M.; Ziegler, R.K.; Vázquez, R.I.; Aldaco, R.; Irabien, Á.; Kahhat, R. Enhancing waste management strategies in Latin America under a holistic environmental assessment perspective: A review for policy support. Sci. Total Environ. 2019, 689, 1255–1275. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development; World Bank: Washington, DC, USA, 2018; Available online: http://hdl.handle.net/10986/30317 (accessed on 2 February 2020).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Dantas, D.V.; Barletta, M.; da Costa, M.F. The seasonal and spatial patterns of ingestion of polyfilament nylon fragments by estuarine drums (Sciaenidae). Environ. Sci. Pollut. Res. 2012, 19, 600–606. [Google Scholar] [CrossRef]
- Barragán, J.M. The coasts of Latin America at the end of the century. J. Coast. Res. 2001, 17, 885–899. [Google Scholar]
- Kutralam, M.G.; Pérez, G.F.; Elizalde, M.I.; Shruti, V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; pp. 8–16. [Google Scholar]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. Wiley Interdiscip. Rev. Wate Water 2018, 5, 1268. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibers influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Severini, M.D.F.; Villagran, D.M.; Buzzi, N.S.; Sartor, G.C. Microplastics in oysters (Crassostrea gigas) and water at the Bahía Blanca Estuary (Southwestern Atlantic): An emerging issue of global concern. Reg. Stud. Mar. Sci. 2019, 32, 100829. [Google Scholar]
- Mac Namara, C.; Gabriele, A.; Amador, C.; Bakalis, S. Dynamics of textile motion in front-loading domestic washing machine. Chem. Eng. Sci. 2012, 75, 14–27. [Google Scholar] [CrossRef]
- Bessa, F.; Barria, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, M.C.; Cózar, A. The colors of the ocean plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]
- Silva, C.J.S.; Silva, J.D.B.; de França, E.J.; de Araújo, M.C.B.; Gusmão, F. Microplastics ingestion by a common tropical freshwater fishing resource. Environ. Pollut. 2017, 221, 218–226. [Google Scholar] [CrossRef]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Barbosa, F.; Adeyemi, J.A.; Bocato, M.Z.; Comas, A.; Campiglia, A. A critical viewpoint on current issues, limitations, and future research needs on micro- and nanoplastic studies: From the detection to the toxicological assessment. Environ. Res. 2020, 182, 109089. [Google Scholar] [CrossRef]
- Renner, G.; Schmidt, T.C.; Schram, J. Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Curr. Opin. Environ. Sci. Health 2018, 1, 55–61. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.L.L.; da Costa, J.P.; Duarte, A.C.; Rocha, S.T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef] [PubMed]
- Timmers, M.A.; Kistner, C.A.; Donohue, M.J. Marine Debris of the Northwestern Hawaiian Islands: Ghost Net Identification; UNIHI-SEAGRANT-AR-05-01; U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Sea Grant College Program: Washington, DC, USA, 2005; 31p. [Google Scholar]
- Martinez, T.E.; Duarte, M.A.M.; Sujitha, S.B.; Rodriguez, E.P.F.; Rosano, O.G.; Expósito, N. Microplastics and metal burdens in freshwater Tilapia (Oreochromis niloticus) of a metropolitan reservoir in Central Mexico: Potential threats for human health. Chemosphere 2021, 266, 128968. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.G.; Suárez, D.C.; Li, J.; Rotchell, J.M. A comparison of microplastic contamination in freshwater fish from natural and farmed sources. Environ. Sci. Poll. Res. 2021, 28, 14488–14497. [Google Scholar] [CrossRef] [PubMed]
- Pazos, R.S.; Maiztegui, T.; Colautti, D.C.; Paracampo, A.H.; Gómez, N. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017, 122, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.C.; Amezcua, F.; Rosales, V.A.; Green, L.; Pollorena, M.J.E.; Sarmiento, M.M.A.; Tomita, R.I.; Gil, M.B.D.; Hernandez, L.M.Y.; Muro, T.V.M.; et al. First insight into plastics ingestion by fish in the Gulf of California, Mexico. Mar. Pollut. Bull. 2021, 171, 112705. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.J.G.; Rodrigues, L.F.; Ortega, I.; Rodrigues, L.d.S.; Lacerda, L.d.F.A.; Coletto, J.L.; Kessler, F.; Cardoso, G.L.; Madureira, L.; Proietti, C.M. Ingestion of plastic debris by commercially important marine fish in southeast-south Brazil. Environ. Pollut. 2020, 267, 115508. [Google Scholar] [CrossRef]
- Pegado, T.d.S.e.S.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- Borges, R.M.M.; Mendoza, F.E.F.; Escalona, S.G.; Rendón-von, O.J. Plastic density as a key factor in the presence of microplastic in the gastrointestinal tract of commercial fishes from Campeche Bay, Mexico. Environ. Pollut. 2020, 267, 115659. [Google Scholar] [CrossRef]
- Garcés, O.O.; Mejía, E.K.A.; Sierra, L.T.; Patiño, A.; Blandón, L.M.; Espinosa, D.L.F. Prevalence of microplastic contamination in the digestive tract of fishes from mangrove ecosystem in Cispata, Colombian Caribbean. Mar. Pollut. Bull. 2020, 154, 111085. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, G.L.; Alpízar, V.C.; Gatgens, G.J.; Jiménez, H.G.; Rodríguez, A.M.; Molina, H.; Villalobos, J.; Paniagua, S.A.; Vega, B.J.R.; Rojas, J.K. Microplastic ingestion by a herring Opisthonema sp. in the Pacific coast of Costa Rica. Reg. Stud. Mar. Sci. 2020, 38, 101367. [Google Scholar]
- Calderon, E.A.; Hansen, P.; Rodríguez, A.; Blettler, M.C.; Syberg, K.; Khan, F.R. Microplastics in the digestive tracts of four fish species from the Ciénaga Grande de Santa Marta Estuary in Colombia. Wat. Air Soil Pollut. 2019, 230, 257. [Google Scholar] [CrossRef]
- Montero, A.A.G.; Costa-Redondo, L.C.; Vasco-Echeverri, O.; Rengifo, V.A.A. Microplastic pollution in coastal areas of Colombia. Mar. Environ. Res. 2023, 190, 106027. [Google Scholar] [CrossRef]
- Possatto, F.E.; Barletta, M.; Costa, M.F.; Ivar do Sul, J.A.; Dantas, D.V. Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Mar. Pollut. Bull. 2011, 62, 1098–1102. [Google Scholar] [CrossRef]
- Turner, J.T. The importance of small planktonic copepods and their roles in pelagic marine food webs. J. Zool. Res. 2004, 43, 255–266. [Google Scholar]
- Pérez, V.D.J.; Seguel, M.; Pavés, H.; Pulgar, J.; Urbina, M.; Ahrendt, C.; Galbán, M.C. First detection of plastic microfibers in a wild population of South American fur seals (Arctocephalus australis) in the Chilean Northern Patagonia. Mar. Pollut. Bull. 2018, 136, 50–54. [Google Scholar] [CrossRef]
- Perez, V.D.J.; Toro, V.C.; Ayala, F.; Brito, B.; Iturra, L.; Arriagada, M.; Seguel, M.; Barrios, C.; Sepúlveda, M.; Oliva, D.; et al. Monitoring the occurrence of microplastic ingestion in Otariids along the Peruvian and Chilean coasts. Mar. Pollut. Bull. 2020, 153, 110966. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, R.; Ahrendt, C.; Perez, V.D.; Vargas, J.; Pulgar, J.; Aldana, M.; Galbán, M.C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Narmatha, M.S.; Jeyasanta, I.; Patterson, J. Occurrence of microplastics in epipelagic and mesopelagic fishes from Tuticorin, Southeast coast of India. Sci. Total Environ. 2020, 720, 137614. [Google Scholar]
- Andrade, C.; Ovando, F. First record of microplastics in stomach content of the southern king crab Lithodes santolla (Anomura: Lithodidadae), Nassau bay, Cape Horn, Chile. An. Del Inst. De La Patagon. 2017, 45, 59–65. [Google Scholar] [CrossRef]
- Villegas, L.; Cabrera, M.; Capparelli, M.V. Assessment of microplastic and organophosphate pesticides contamination in fiddler crabs from a Ramsar site in the estuary of Guayas River, Ecuador. Bull. Environ. Contam. Toxicol. 2021, 107, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, I.A.; Thiel, M. Floating marine debris in fjords, gulfs and channels of southern Chile. Mar. Pollut. Bull. 2009, 5, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.G.; Grose, J.; Pahl, S.; Thompson, R.C.; Wyles, K.J. Microplastics in personal care products: Exploring perceptions of environmentalists, beauticians, and students. Mar. Pollut. Bull. 2016, 113, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and Retention of Microplastics by the Shore Crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.S.; Baptista, N.J.A.; Crapez, M.A.C.; Gaylarde, C.; Pierri, B.d.S.; Saldaña, S.M.; Bainy, A.C.D.; Nogueira, D.J.; Fonseca, E.M. Occurrence of microplastics and heavy metals accumulation in native oysters Crassostrea Gasar in the Paranaguá estuarine system, Brazil. Mar. Pollut. Bull. 2021, 166, 112225. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.S.; Porter, A.; Muñoz, P.J.P.; Alarcón, R.D.; Galloway, T.S.; Godley, B.J.; Santillo, D.; Vagg, J.; Lewis, C. Plastic contamination of a Galapagos Island (Ecuador) and the relative risks to native marine species. Sci. Total Environ. 2021, 789, 147704. [Google Scholar] [CrossRef]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hiraia, H.; Iwasaa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef]
- De la Torre, G.; Mendoza, C.L.; Pilar, R. Microplastic contamination in market bivalve Argopecten purpuratus from Lima, Peru. Manglar 2019, 16, 85–89. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the edible tissues of shellfishes sold for human consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49. [Google Scholar] [CrossRef]
- Cole, M.; Liddle, C.; Consolandi, G.; Drago, C.; Hird, C.; Lindeque, P.K.; Galloway, T.S. Microplastics, microfibres and nanoplastics cause variable sub-lethal responses in mussels (Mytilus spp.). Mar. Pollut. Bull. 2020, 160, 111552. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco, M.L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Baun, A.; Almeda, R.; Hartmann, N.B. Ingestion and effects of micro- and nanoplastics in blue mussel (Mytilus edulis) larvae. Mar. Pollut. Bull. 2019, 140, 423–430. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Colin, R.J. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).