Biology, Ecology, Impacts and Management of the Invasive Weed, Blue Heliotrope (Heliotropium amplexicaule Vahl)—A Review
Abstract
1. Introduction
2. Nomenclature and Taxonomy
- Domain: Eukaryote
- Kingdom: Plantae
- Phylum: Spermatophyta
- Subphylum: Angiospermae
- Class: Dicotyledonae
- Order: Boraginales
- Family: Boraginaceae
- Genus: Heliotropium
- Species: Heliotropium amplexicaule Vahl.
3. Distribution and History
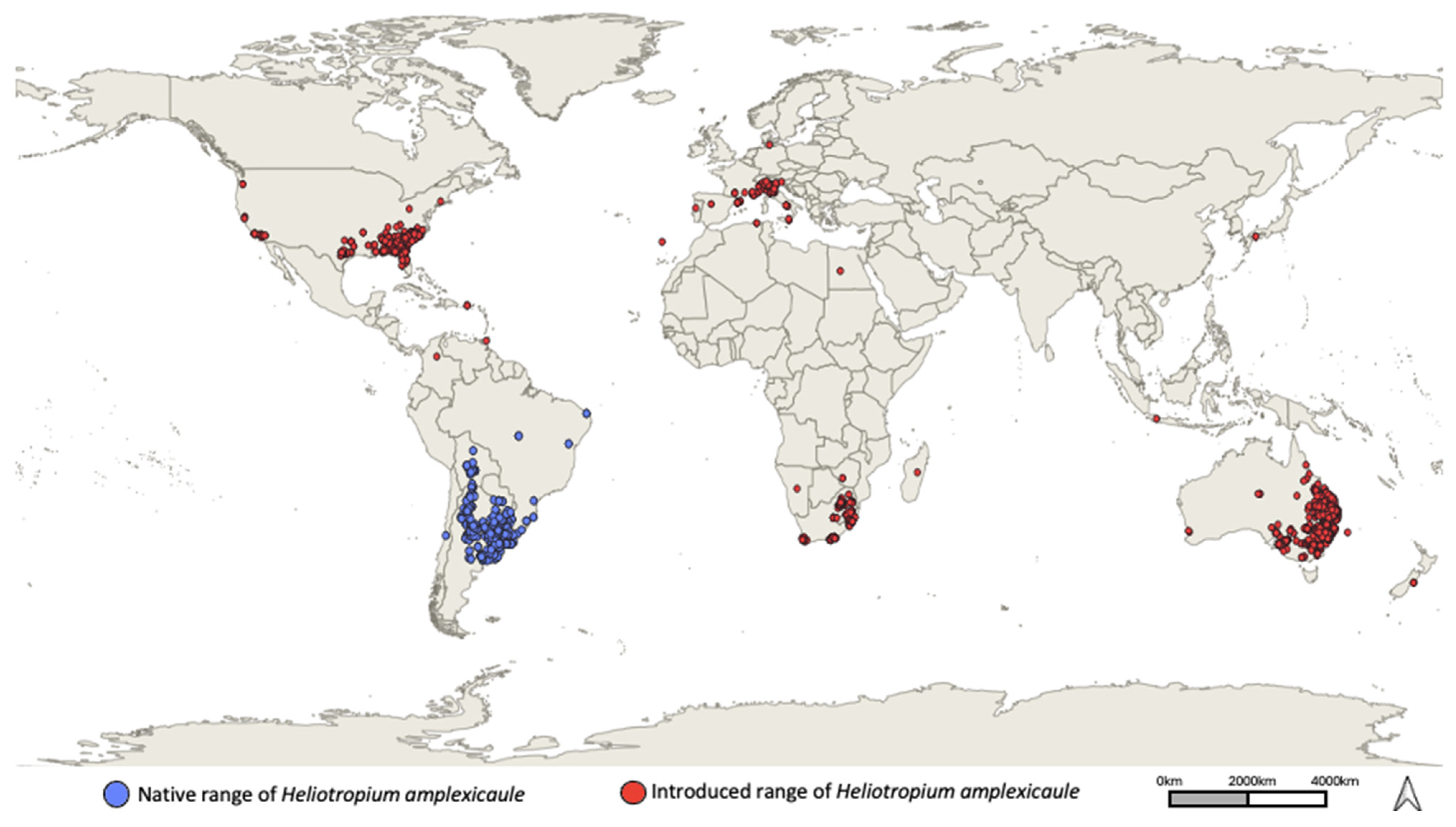
3.1. Native Range
3.2. Introduced Range
3.3. Habitat Suitability
3.4. Future Projections
4. Biology and Ecology
4.1. Botanical Description
4.2. Life Cycle and Phenology
4.3. Seed Biology, Dispersal, and Population Dynamics
4.4. Stress Tolerance
5. Impacts and Interference
5.1. Agricultural Impacts
5.2. Environmental Impacts
5.3. Human Health
5.4. Socioeconomic Impacts
6. Management
6.1. Biosecurity Measures and Prevention
6.2. Chemical Control
6.3. Physical and Cultural Control
6.4. Biological Control
7. Integrated Weed Management—A Case Study
8. Conclusions and Future Research Directions
- (i)
- The use of cultivation or ecological burning to increase seed germination, followed by the application of a registered herbicide to help exhaust the soil seedbank.
- (ii)
- A combination of physical (chipping/removal and destruction of whole plants) and chemical controls (spot applications) of small or isolated populations.
- (iii)
- A combination of biological control agents that target different aspects of the plant’s biology.
- (iv)
- The strategic use of suppressive pasture and crop species and/or cultivars as well as the grazing management of grasslands to maintain healthy vegetation that can compete with H. amplexicaule.
- (v)
- A combination of each of the above methods, depending on the size and location of the population.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasim, G.; Shabbir, A. Invasive weed species—A threat to sustainable agriculture. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 523–556. [Google Scholar]
- Chauhan, B.S. Grand challenges in weed management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Kumar Rai, P. Environmental degradation by invasive alien plants in the Anthropocene: Challenges and prospects for sustainable restoration. Anthr. Sci 2022, 1, 5–28. [Google Scholar]
- Briese, D.T. Heliotropium amplexicaule Vahl—Blue heliotrope. In Biological Control of Weeds in Australia; Julien, M.H., McFadyen, R.E.C., Cullen, J.M., Eds.; CSIRO Publishing: Collingwood, VC, Australia, 2012; pp. 282–288. [Google Scholar]
- Swamy, R.K.; Sing, A.; Surveswaran, S.; Rao, K.S. Heliotropium amplexicaule (Boraginaceae-Heliotropioideae): A new record for Indian sub-continent. Rheeda 2015, 25, 148–152. [Google Scholar]
- da Silva, E. The Ecology and Control of Blue Heliotrope (Heliotropium amplexicaule Vahl.); Project DAN 35. Final report for the Wool Research and Development Corporation; New South Wales Agriculture: Trangie, NSW, Australia, 1991; p. 33.
- Briese, D.T.; Zapater, M. Biological Control of Blue Heliotrope; RIRDC Publication No. 01/119; Rural Industries Research and Development Corporation: Canberra, Australia, 2001; pp. 1–24.
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Collingwood, VIC, Australia, 2001; p. 698. [Google Scholar]
- Dellow, J.J.; Bourke, C.A.; McCaffery, A.C. AGFACTS—Blue Heliotrope; NSW Agriculture: Orange, NSW, Australia, 2004; pp. 1–10.
- Carpinelli de Jesus, M.; Hungerford, N.L.; Carter, S.J.; Anuj, S.R.; Blanchfield, J.T.; De Voss, J.J.; Fletcher, M.T. Pyrrolizidine alkaloids of blue heliotrope (Heliotropium amplexicaule) and their presence in Australian honey. J. Agric. Food Chem. 2019, 67, 7995–8006. [Google Scholar] [CrossRef]
- Briese, D.; Walker, A.; Zapater, M. Implementation of the Blue heliotrope Biological Control Strategy: Host-Specificity Testing of Longitarsus sp. RIRDC Publication No. 05/003; Rural Industries Research and Development Corporation: Canberra, ACT, Australia, 2005; pp. 1–3.
- Potter, S.; Sheehan, M.R.; Virtue, J.G. A Best Practice Manual for Managing Blue Heliotrope (Heliotropium amplexicaule) in NSW; New South Wales Department of Primary Industries: Orange, NSW, Australia, 2023; pp. 1–128.
- Schulze, K.J.; Unwin, J. Long term control of blue heliotrope (Heliotropium amplexicaule) using Graslan® and tropical grasses. In Proceedings of the 21st Annual Conference of the Grassland Society of NSW, Wagga Wagga, NSW, Australia, 25–27 July 2006; The Grassland Society of NSW: Orange, NSW, Australia, 2006; pp. 150–151. [Google Scholar]
- Mortensen, D.A.; Bastiaans, L.; Sattin, M. The role of ecology in the development of weed management systems: An outlook. Weed Res. 2000, 40, 49–62. [Google Scholar] [CrossRef]
- Riemens, M.; Sønderskov, M.; Moonen, A.-C.; Storkey, J.; Kudsk, P. An Integrated Weed Management framework: A pan-European perspective. Eur. J. Agron. 2022, 133, 126443. [Google Scholar] [CrossRef]
- Briese, D.T.; Walker, A. A new perspective on the selection of test plants for evaluating the host-specificity of weed biological control agents: The case of Deuterocampta quadrijuga, a potential insect control agent of Heliotropium amplexicaule. Biol. Control 2002, 25, 273–287. [Google Scholar] [CrossRef]
- Ibáñez, S.; Luebert, F.; Gómez, M. First record of Heliotropium amplexicaule (Heliotropiaceae) in Chile. Gayana Bot. 2011, 68, 93–96. [Google Scholar] [CrossRef]
- Craven, L. A taxonomic revision of Heliotropium (Boraginaceae) in Australia. Aust. Syst Bot. 1996, 9, 521–657. [Google Scholar] [CrossRef]
- Hilger, H.H.; Diane, N. A systematic analysis of Heliotropiaceae (Boraginales) based on trnL and ITS1 sequence data. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. 2003, 125, 19–51. [Google Scholar] [CrossRef]
- Briese, D.T.; McLaren, D.A.; Pettit, W.; Zapater, M.; Anderson, F.; Delhey, R.; Distel, R. New biological control projects against weeds of South American origin in Australia: Blue heliotrope and serrated tussock. In Proceedings of the 10th Symposium on the Biological Control of Weeds, Bozeman, MT, USA, 4–14 July 1999; Bozeman, Montana State University: Bozeman, MT, USA, 2000; pp. 215–223. [Google Scholar]
- Briese, D.T.; Walker, A. Choosing the right plants to test: The host-specificity of Longitarsus sp. (Coleoptera: Chrysomelidae) a potential biological control agent of Heliotropium amplexicaule. Biol. Control 2008, 44, 271–285. [Google Scholar] [CrossRef]
- Kambhar, S.V.; Kotresha, K. A record on distribution of Heliotropium amplexicaule Vahl (boraginaceae) in Dharwad, Karnataka, India. J. Econ. Taxon. Bot. 2015, 39, 1–4. [Google Scholar]
- GBIF. Heliotropium amplexicaule, Global Biodiversity Information Facility 2024. Viewed Online May 2024. Available online: https://www.gbif.org/occurrence/download/0005146-240506114902167 (accessed on 1 May 2024).
- Cunningham, G.M.; Mulham, W.E.; Milthorpe, P.L.; Leigh, J.H. Plants of Western New South Wales; Inkata Press: Melbourne, VIC, Australia, 1992; p. 766. [Google Scholar]
- Milne, B.R. Agnote DPI 160: Blue Heliotrope—Management and Control; NSW Agriculture: Orange, NSW, Australia, 1996; pp. 1–3.
- Everest, S.L. Poisonous Plants of Australia; Angus and Robertson: Sydney, NSW, Australia, 1981; pp. 119–120. [Google Scholar]
- Zapater, M.; Bartoloni, N.; Perez-Camargo, G.; Briese, D.T. Natural impact of the flea-beetle, Longitarsus sp., on Heliotropium amplexicaule in Argentina and its potential for use as a biological control agent in Australia. In Proceedings of the 11th International Symposium on Biological Control of Weeds, Canberra, Australia, 28 April–2 May 2003; Cullen, J.M., Briese, D.T., Kriticos, D.J., Lonsdale, W.M., Morin, L., Scott, J.K., Eds.; CSIRO Entomology: Canberra, ACT, Australia, 2004; pp. 208–214. [Google Scholar]
- Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 1st ed.; Inkata Press: Melbourne, Australia, 1992; p. 692. [Google Scholar]
- Steel, J.; Kohout, M.; Newell, G. Climate Change and Potential Distribution of Weeds. Whither the Weeds under Climate Change? Department of Primary Industries, Biosciences Research Division: Frankston, VIC, Australia, 2008; pp. 1–3. [Google Scholar]
- Weed Futures. Heliotropium amplexicaule—Weed Futures Determining Current and Future Weed Threats in Australia; viewed online May 2024; Macquarie University Australia: Sydney, NSW, Australia, 2024; Available online: https://weedfutures.net/species.php?id=1097 (accessed on 1 May 2024).
- Jeanes, J.A. Boraginaceae. In Flora of Victoria; Walsh, N.G., Entwisle, T.J., Eds.; Inkata Press: Melbourne, Australia, 1999; pp. 387–411. [Google Scholar]
- Wilson, B.J.; Hawton, D.; Duff, A.A. Crop Weeds of Northern Australia: Identification at Seedling and Mature Stages; Queensland Department of Primary Industries: Brisbane, QLD, Australia, 1995; p. 160.
- Cope, R.; Ossedryver, S. Poisonous Plants of Australia and New Zealand. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Academic Press: Cambridge, MA, USA, 2018; p. 911. [Google Scholar]
- Newell, D. Beating blue heliotrope. In Proceedings of the 9th Biennial Noxious Weeds Conference Australia; Michelmore, M., Ed.; NSW Agriculture: Orange, NSW, Australia, 1997; p. 1. [Google Scholar]
- Hunt, J.R. The Ecology of Common Heliotrope (Heliotropium europaeum L.) in a Mediterranean Dry-Land Cropping System; Faculty of Land and Food Resources, The University of Melbourne: Melbourne, VC, Australia, 2005; pp. 1–166. [Google Scholar]
- Ketterer, P.J.; Glower, P.E.; Smith, L.W. Blue heliotrope (Heliotropium amplexicaule) poisoning in cattle. Aust. Vet. J. 1987, 64, 115–117. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.A. Australia’s Poisonous Plants, Fungi and Cyanobacteria: A Guide to Species of Medicinal and Veterinary Importance; Commonwealth Scientific and Industrial Research Organisation (CSIRO) Publishing: Collingwood, UK, 2012; pp. 272–273. [Google Scholar]
- Glover, P.E.; Ketterer, P.J. Blue heliotrope kills cattle. Qld Agric. 1987, 113, 109–110. [Google Scholar]
- Burrows, G.E.; Tyrl, R.J. Toxic Plants of North America; Iowa State University Press: Ames, IA, USA, 2001; p. 1342. [Google Scholar]
- Crews, C. Natural Toxicants: Alkaloids. Ref. Modul. Food Sci. 2014, 2, 251–260. [Google Scholar]
- Esposito, M.; Crimaldi, M.; Cirillo, V.; Sarghini, F.; Maggio, A. Drone and sensor technology for sustainable weed management: A review. Chem. Biol. Technol. Agric. 2021, 8, 18. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S. Advancements and developments in the detection and control of invasive weeds: A global review of the current challenges and future opportunities. Weed Sci. 2024, 72, 205–215. [Google Scholar] [CrossRef]
- Liang, W.C.; Yang, Y.J.; Chao, C.M. Low-Cost Weed Identification System Using Drones. In Proceedings of the Seventh International Symposium on Computing and Networking Workshops (CANDARW), Nagasaki, Japan, 26–29 November 2019; pp. 260–263. [Google Scholar]
- Thompson, C. Blue Heliotrope: A Weed to Watch Out For, NSW Government. 2024. Available online: https://www.lls.nsw.gov.au/help-and-advice/weeds-and-plant-diseases/weed-identification-and-management/blue-heliotrope-weed-management-guide-a-plan-of-attack (accessed on 1 May 2024).
- NSWDPI Blue Heliotrope (Heliotropium amplexicaule), New South Wales Department of Primary Industries. 2024. Available online: https://weeds.dpi.nsw.gov.au/Weeds/BlueHeliotrope#:~:text=To%20improve%20the%20effectiveness%20of,Avoid%20spraying%20stressed%20plants (accessed on 1 May 2024).
- Grundy, A.C.; Mead, A.; Bond, W.; Clark, G.; Burston, S. The impact of herbicide management on long-term changes in the diversity and species composition of weed populations. Weed Res. 2011, 51, 187–200. [Google Scholar] [CrossRef]
- Boutin, C.; Strandberg, B.; Carpenter, D.; Mathiassen, S.K. Herbicide impact on non-target plant reproduction: What are the toxicological and ecological implications? Environ. Pollut. 2014, 185, 295–305. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Fernando, W.G.D.; Tennakoon, K.U. Achievements, developments and future challenges in the field of bioherbicides for weed control: A global review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef]
- Briese, D.T.; Zapater, M.; Andorno, A.; Perez-Camargo, G. A two-phase open-field test to evaluate the host-specificity of candidate biological control agents for Heliotropium amplexicaule. Biol. Control 2002, 25, 259–272. [Google Scholar] [CrossRef]


| Plant Life Cycle Event | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seed germination | ||||||||||||
| Active growth period | ||||||||||||
| Flowering | ||||||||||||
| Growth from rootstocks | ||||||||||||
| Dormant/less active growth | ||||||||||||
| Type of Socioeconomic Impact | Reason | References |
|---|---|---|
| Animal health consequences | Juvenile or inexperienced livestock may feed on H. amplexicaule which can cause poisoning or reduce livestock fertility rates, resulting in loss of livestock or increased cost of care. | [12,33,36,37] |
| Increased cost of land management | Control works required for large infestations of H. amplexicaule can be costly and time consuming. | [10,12,36,37] |
| Land devaluation | Large infestations of H. amplexicaule require ongoing work to control which can be costly and may reduce or restrict land activity (e.g., number of livestock). | [12,36,37] |
| Neighbour disputes | Disputes may arise if neighbours do not work together to control H. amplexicaule infestations. Alternatively, control works can be undone if surrounding properties do not work together. | [12,36] |
| Reduced recreational access | Infested landscapes may have reduced recreational access and limited access to waterways or important structures such as feeding areas or water points for livestock. | [12] |
| Reduced stocking rates | Invasion by H. amplexicaule can result in reduced pasture production by outcompeting and displacing desirable species. This results in the carrying capacity of the land being reduced. Additional food sources may be required on the infested landscape which can increase the cost of farm production. | [12,26,37] |
| Seed contamination | Livestock or visitors to the infested area may unintentionally spread H. amplexicaule seeds into new areas. | [12] |
| Herbicide Active Ingredient | Herbicide Group Classification and Mode of Action | References |
|---|---|---|
| 2,4-D | Group 4: Disruptors of plant cell growth (Auxin mimics) | [12,45] |
| Aminopyralid | Group 4: Disruptors of plant cell growth (Auxin mimics) | [12] |
| Amitrole | Group 32: Inhibition of solanesyl diphosphate synthase | [12,45] |
| Ammonium thiocyanate | Group 34: Inhibition of lycopene cyclase | [12] |
| Dicamba | Group 4: Disruptors of plant cell growth (Auxin mimics) | [12,45] |
| Fluroxypyr | Group 4: Disruptors of plant cell growth (Auxin mimics) | [12,45] |
| Glyphosate | Group 9: Inhibition of 5-enolpyruvyl shikimate-3 phosphate synthase (EPSP inhibition) | [12,35,44] |
| Metsulfuron-methyl | Group 9: Acetolactate synthase (ALS) or acetohydroxyacid synthase (AHAS) inhibitors | [12,35,45] |
| Picloram | Group 4: Disruptors of plant cell growth (Auxin mimics) | [12,45] |
| Sulfonylurea | Group 9: ALS or AHAS inhibitors | [12] |
| Tebuthiuron | Group 5: Inhibition of photosynthesis at photosystem II | [12,45] |
| Triazine | Group 5: Inhibition of photosynthesis at photosystem II | [12,35] |
| Triclopyr | Group 4: Disruptors of plant cell growth (Auxin mimics) | [12,45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, J.; Peerzada, A.M.; Bajwa, A.A. Biology, Ecology, Impacts and Management of the Invasive Weed, Blue Heliotrope (Heliotropium amplexicaule Vahl)—A Review. Sustainability 2024, 16, 5923. https://doi.org/10.3390/su16145923
Roberts J, Peerzada AM, Bajwa AA. Biology, Ecology, Impacts and Management of the Invasive Weed, Blue Heliotrope (Heliotropium amplexicaule Vahl)—A Review. Sustainability. 2024; 16(14):5923. https://doi.org/10.3390/su16145923
Chicago/Turabian StyleRoberts, Jason, Arslan Masood Peerzada, and Ali Ahsan Bajwa. 2024. "Biology, Ecology, Impacts and Management of the Invasive Weed, Blue Heliotrope (Heliotropium amplexicaule Vahl)—A Review" Sustainability 16, no. 14: 5923. https://doi.org/10.3390/su16145923
APA StyleRoberts, J., Peerzada, A. M., & Bajwa, A. A. (2024). Biology, Ecology, Impacts and Management of the Invasive Weed, Blue Heliotrope (Heliotropium amplexicaule Vahl)—A Review. Sustainability, 16(14), 5923. https://doi.org/10.3390/su16145923








