Ecology and Sustainable Conservation of the Nase, Chondrostoma nasus: A Literature Review
Abstract
:1. Introduction—Generality
2. Methodology and Worldwide Publication Trend on Nase
3. European Repartition
4. Morphology and Identification
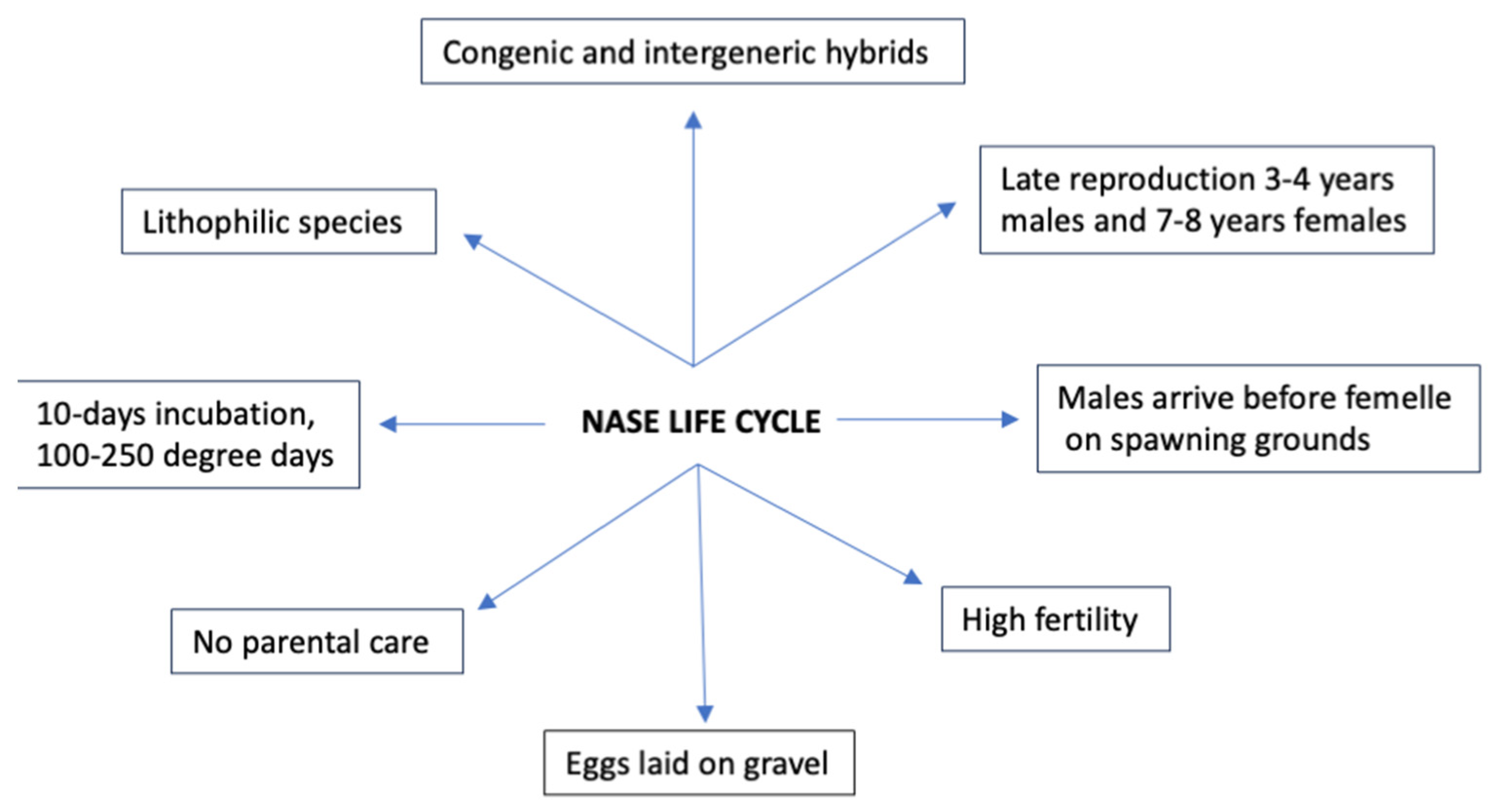
5. Reproduction and Life Cycle
6. Diet
7. Movement Dynamics of Adult and Young Stages
7.1. Adult
7.2. Young Stages
8. Characteristics of Spawning Areas and Habitats of Juvenile and Adult
8.1. Spawning Area
8.2. Juvenile Habitats
8.3. Adult Habitats
9. Impact of Anthropogenic Pressures, Environmental Disruptions, and Restoration Measures
9.1. Water Pollution
9.2. Loss of Habitat and Hydromorphological Perturbations
9.3. Disruption of Hydrological Regimes—Hydropeaking
9.4. Obstruction of Movements
9.5. Mean Threats, Guidelines for Conservation, and Key Research Questions for the Future
10. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philippart, J.C. Problématique de la conservation, de l’exploitation halieutique et de l’aménagement des ressources ichtyologiques dans une grosse rivière de la zone à barbeau: L’Oruthe liégeoise. Cah. Ethol. 1981, 1, 39–80. [Google Scholar]
- Mann, R.H.K.; Penczak, T. Fish production in rivers: A review. Pol. Arch. Hydrobiol. 1986, 33, 233–247. [Google Scholar]
- Keckeis, H.; Bauer-Nemeschkal, E.; Kamler, E. Effects of reduced oxygen level on the mortality and hatching rate of Chondrostoma nasus embryos. J. Fish Biol. 1996, 49, 430–440. [Google Scholar] [CrossRef]
- Ovidio, M.; Philippart, J.C. Movement patterns and spawning activity of individual nase Chondrostoma nasus (L.) in flow-regulated and weir-fragmented rivers. J. Appl. Ichthyol. 2008, 24, 256–262. [Google Scholar] [CrossRef]
- Hudson, A.G.; Vonlanthen, P.; Seehausen, O. Population structure, inbreeding and local adaptation within an endangered riverine specialist: The nase (Chondrostoma nasus). Conserv. Genet. 2014, 15, 933–951. [Google Scholar] [CrossRef]
- Nelva, A. The penetration of the nase, Chondrostoma nasus nasus (Pisces, Cyprinidae), in the French hydrographic network and its consequences. Bull. Fr. Pêche Piscic. 1997, 344–345, 253–269. [Google Scholar] [CrossRef]
- Wetjen, M.; Hübner, D.; Seehausen, O.; Schulz, R. Genetic diversity of endangered Chondrostoma nasus in the River Rhine system: Conservation genetics considerations on stocking and reintroduction. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 25. [Google Scholar] [CrossRef]
- Schiemer, F.; Keckeis, H.; Winkler, G.; Flore, L. Large rivers: The relevance of ectonal structure and hydrological properties for the fish fauna. Arch. Hydrobiol. 2001, 135 (Suppl. S12), 487–508. [Google Scholar] [CrossRef]
- Lelek, A. The Freshwater Fishes of Europe. Threatened fishes of Europe; Aula Verlag: Wiesbaden, Germany, 1987. [Google Scholar]
- Lusk, S. The status of Chondrostoma nasus in waters of the Czech Republic. Folia Zool. 1995, 44, 1–8. [Google Scholar]
- Luskova, V.S.; Lusk, S.; Halacka, K. Yearly dynamics of enzyme activities and metabolite concentrations in blood plasma of Chondrostoma nasus. Folia Zool. 1995, 44, 75–82. [Google Scholar]
- Freyhof, J. Remarks on the status of Chondrostoma nasus in the river Rhine. Folia Zool. 1997, 46, 61–66. [Google Scholar]
- Hauer, C.; Gunther, U.; Schmutz, S.; Habersack, H. Morphodynamic Effects on the Habitat of Juvenile Cyprinids (Chondrostoma nasus) in a Restored Austrian Lowland River. Environ. Manag. 2008, 42, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Ramler, D.; Keckeis, H. Effects of large-river restoration measures on ecological fish guilds and focal species of conservation in a large European river (Danube, Austria). Sci. Total Environ. 2019, 686, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Scherbaum, S.; Nagel, C.; Fuchs, Y.; Geist, J. Characterizing egg transport of Chondrostoma nasus (L.): A combined laboratory and field experiment. J. Ecoh. 2022, 1–11. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. Comprehensive analysis of >30 years of data on stream fish population trends and conservation status in Bavaria, Germany. Biol. Conserv. 2018, 226, 311–320. [Google Scholar] [CrossRef]
- Zbinden, S.; Maier, K.J. Contribution to the knowledge of the distribution and spawning grounds of Chondrostoma nasus (Pisces, Cyprinidae) in Switzerland. In Conservation of Endangered Freshwater Fish in Europe; Kirchhofer, A., Hefti, D., Eds.; Birkhaån User, Verlag: Basel, Switzerland, 1996; pp. 287–297. [Google Scholar]
- IUCN. IUCN Red List of Threatened Species; Version 2014.1.; IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Philippart, J.C. L’érosion de la Biodiversité: Les Poissons. Dossier Scientifique Réalisé Dans le Cadre de L’élaboration du Rapport Analytique 2006–2007 sur l’Etat de l’Environnement Wallon, Ministère de la Région Wallonne; Direction Générale des Ressources Naturelles et de l’Environnement Namur: Namur, Belgium, 2007; 306p. [Google Scholar]
- Penaz, M. Chondrostoma nasus, its reproduction strategy and possible reasons for a widely observed population decline-a review. In Conservation of Endangered Freshwater Fish in Europe; Kirchhofer, A., Hefti, D., Eds.; Birkhauser Verlag: Basel, Switzerland, 1996; pp. 278–285. [Google Scholar]
- Kottelat, M.; Freyhof, J. Chondrostoma nasus. The IUCN Red List of Threatened Species. Version 2014.3. Available online: www.iucnredlist.org (accessed on 5 December 2023).
- Costedoat, C.; Pech, N.; Chappaz, R.; Gilles, A. Novelties in Hybrid Zones: Crossroads between Population Genomic and Ecological Approaches. PLoS ONE 2007, 2, e357. [Google Scholar] [CrossRef] [PubMed]
- Keith, P.; Allardi, J. The introduced freshwater fish of France: Status, impacts and management. In Stocking and Introduction of Fish; Cowx, I.G., Ed.; Fishing News Books; MPG Books Ltd.: Bodmin, Cornwall, 1998; pp. 153–166. [Google Scholar]
- FAO. FAO Database on Introduced Aquatic Species; FAO Database on Introduced Aquatic Species; FAO: Rome, Italy, 1997. [Google Scholar]
- Bianco, P.G. Introductions, chief elements of native freshwater fish degradation and use of indices and coefficients in quantifying the situation in Italy. In Protection of Aquatic Biodiversity: Proceedings of the World Fisheries Congress, Theme 3; Philipp, D.P., Epifanio, J.M., Marsden, J.E., Claussen, J.E., Wolotira, R.J., Jr., Eds.; Oxford and IBH Publishing Co. Ltd.: New Delhi, India, 1995; pp. 175–198. [Google Scholar]
- Bianco, P.G. An update on the status of native and exotic freshwater fishes of Italy. J. Applied Ichthyol. 2014, 30, 62–67. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germany, 1972; 646p. [Google Scholar]
- Bruslé, J.; Quignard, J.P. Biologie Des Poissons D’eau Douce Européens, 2nd ed.; Collection Aquaculture—Pisciculture; Edition Technique & Documentation Lavoisier: Cachan, France, 2013; 740p. [Google Scholar]
- Philippart, J.C. Incidences de la pollution organique et de l’eutrophisation sur la faune ichtyologique de la Semois. Ann. Limnol. 1980, 16, 77–89. [Google Scholar] [CrossRef]
- Herold, J.P.; Kupfer, M.; Corolla, J.P. In DORIS, 30/04/2014: Chondrostoma nasus (Linnaeus, 1758). 2019. Available online: https://doris.ffessm.fr/ref/specie/2164 (accessed on 20 December 2023).
- Libois, R.; Hallet, C. Contribution to the identification of skull remains of freshwater fish from Belgium and Northen France. In Fiches d’Ostéologie Animale Pour L’archéologie; Dresse, J., Desse-Berset, N., Eds.; Centre de Recherche Archéologique CNRS: Juan-les-Pins, France, 1988; 24p. [Google Scholar]
- Demol, T. Guide Identification des Poissons de Wallonie. Service Public de Wallonie, Direction Générale Opérationnelle de l’Agriculture, des Ressources Naturelles et de l’Environnement; Département de l’Etude du Milieu Naturel et agricole, Série “Faune-Flore-Habitats”: Gembloux, Belgium, 2011; 126p. [Google Scholar]
- Kortet, R.; Taskinen, J.; Vainikka, A.; Ylonen, H. Breeding tubercles, papillomatosis and dominance behaviour of male roach (Rutilus rutilus) during the spawning period. Ethology 2004, 110, 591–601. [Google Scholar] [CrossRef]
- Vater, M. Age and Growth of the undermouth Chondrostoma nasus in the Slovak stretch of the Danube River. Biologia 1997, 52, 653–661. [Google Scholar]
- Philippart, J.C. Ecologie des populations de poissons et caractéristiques physiques et chimiques des rivières dans le bassin de la Meuse belge. Bull. Soc. R. Sci. Liège 1989, 25, 75–198. [Google Scholar]
- Poncin, P. Reproduction des Poissons de Nos Rivières; Belge, L.P., Ed.; Fédération Sportive des Pêcheurs Françophones de Belgique: Bruxelles, Belgium, 1996; 50p. [Google Scholar]
- Keckeis, H.; Frankiewicz, P.; Schiemer, F. The importance of inshore areas for spawning nase Chondrostoma nasus (Cyprinidae) in a free-flowing section of a large river (Danube, Austria). Arch. Hydrobiol. Large Rivers 1996, 10 (Suppl. S113), 51–64. [Google Scholar] [CrossRef]
- Teletchea, F.; Fostier, A.; Kamler, E.; Gardeur, J.N.; Le Bail, P.Y.; Jalabert, B.; Fontaine, P. Comparative analysis of reproductive traits in 65 freshwater fish species: Application to the domestication of new fish species. Rev. Fish Biol. Fish. 2009, 19, 403–430. [Google Scholar] [CrossRef]
- Rakowitz, G.; Berger, B.; Kubecka, J.; Keckeis, H. Functional role of environmental stimuli for the spawning migration in Danube nase Chondrostoma nasus (L.). Ecol. Freshw. Fish 2008, 17, 502–514. [Google Scholar] [CrossRef]
- Keckeis, H.; Bauer-Nemeschkal, E.; Menschutkin, V.V.; Nemeschkal, H.L.; Kamler, E. Effects of female attributes and egg properties on offspring viability in a rheophilic cyprinid, Chondrostoma nasus. Can. J. Fish. Aquat. Sci. 2000, 57, 789–796. [Google Scholar] [CrossRef]
- Cattanéo, F.; Carrel, G.; Lamouroux, N.; Breil, P. Relationship between hydrology and cyprinid reproductive success in the Lower Rhône at Montélimar, France. Arch. Hydrobiol. 2001, 151, 427–450. [Google Scholar] [CrossRef]
- Parkinson, D.; François, P.; Perpinien, G.; Philippart, J.C. Habitat de reproduction des poissons et processus géomorphologiques dans les rivières à fond caillouteux: Essai de synthèse et applications à quelques rivières du bassin de la Meuse. Bull. Soc. Géogr. Liège 1999, 36, 31–52. [Google Scholar]
- Maier, K.-J.; Zeh, M.; Ortlepp, J.; Zbinden, S.; Hefti, D. Distribution et Reproduction des Espèces du Genre Chondrostoma en Suisse: Le nase (C. nasus)—La sofie (C. toxostoma)—La savetta (C. soetta). Informations Concernant la Pêche no 53; Office fédéral de L’environnement, des Forêts et du Paysage (OFEEP): Berne, Switzerland, 1995; 64p. [Google Scholar]
- Ostaszewska, T.; Sysa, P. Development of hepatocytes in nase (Chondrostoma nasus (L.) larvae following hatch. Arch. Pol. Fish. 2004, 12, 151–161. [Google Scholar]
- Lechner, A.; Keckeis, H.; Schludermann, E.; Loisl, F.; Humphries, P.; Glas, M.; Tritthart, M.; Habersack, H. Shoreline configurations affect dispersal patterns of fish larvae in a large river. ICES J. Mar. Sci. 2014, 71, 930–942. [Google Scholar] [CrossRef]
- Costedoat, C.; Pech, N.; Chappaz, R.; Salducci, D.; Lim, P.; Gilles, A. Study of introgressive hybridization between Chondrostoma t. toxostoma and Chondrostoma n. nasus (Teleostei, Cyprinidae) using multiple approaches. Cybium 2004, 28, 51–61. [Google Scholar] [CrossRef]
- Hanfling, B.; Bolton, P.; Harley, M.; Carvalho, G.R. A molecular approach to detect hybridisation between crucian carp (Carassius carassius) and non-indigenous carp species (Carassius spp. and Cyprinus carpio). Freshw. Biol. 2005, 50, 403–417. [Google Scholar] [CrossRef]
- Simkova, A.; Navratilova, P.; Davidova, M.; Ondračkova, M.; Sinama, M.; Chappaz, R.; Gilles, A.; Costedoat, C. Does invasive Chondrostoma nasus shift the parasite community structure of endemic Parachondrostoma toxostoma in sympatric zones? Parasit. Vectors 2012, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- VetešnÍk, L.; Halačka, K.; Papoušek, I.; Mendel, J.; ŠImkovÁ, A. The first record of a natural hybrid of the roach Rutilus rutilus and nase Chondrostoma nasus in the Danube River Basin, Czech Republic: Morphological, karyological and molecular characteristics. J. Fish Biol. 2009, 74, 1669–1676. [Google Scholar] [CrossRef]
- Reckendorfer, W.W.; Keckeis, H.; Tiitu, V.; Winkler, G.; Zornig, H.; Schiemer, F. Diet shifts in 0+ nase, Chondrostoma nasus: Size-specific differences and the effect of food availability. Arch. Hydrobiol. 2001, 13512 (Suppl. S4), 425–440. [Google Scholar] [CrossRef]
- Sysa, P.; Ostaszewska, T.; Olejniczak, M. Development of digestive system and swim bladder of larvalnase (Chondrostoma nasus L.). Aquac. Nutr. 2006, 12, 331–339. [Google Scholar] [CrossRef]
- Panchan, R.; Pinter, K.; Schmutz, S.; Unfer, G. Seasonal migration and habitat use of adult barbel (Barbus barbus) and nase (Chondrostoma nasus) along a river stretch of the Austrian Danube River. Environ. Biol. Fishes 2022, 105, 1601–1616. [Google Scholar] [CrossRef]
- Ovidio, M.; Hanzen, C.; Gennotte, V.; Michaux, J.; Benitez, J.P.; Dierckx, A. Is adult translocation a credible way to accelerate the recolonization process of Chondrostoma nasus in a rehabilitated river? Cybium 2016, 40, 43–49. [Google Scholar] [CrossRef]
- De Leeuw, J.J.; Winter, H.V. Migration of rheophilic fish in the large lowland rivers Meuse and Rhine, the Netherlands. Fish. Manag. Ecol. 2008, 15, 409–415. [Google Scholar] [CrossRef]
- Cuchet, M. Fish Protection and Downstream Migration at Hydropower Intakes. Investigation of Fish Behavior under Laboratory Conditions. Ph.D Thesis, Technische Universitat Munchen, Munchen, Germany, 2013; 164p. [Google Scholar]
- Fielenbach, J. Zeitliche und Räumliche Verteilungsmuster der Nase Chondrostoma nasus (L.) in der Sieg; Diplomarbeit Friedrich Wilhelms Universität Bonn: Bonn, Germany, 1996; 112p. [Google Scholar]
- Huber, M.; Kirchhofer, A. Radio telemetry as a tool to study habitat use of nase (Chondrostoma nasus L.) in medium-sized rivers. Hydrobiologia 1998, 371–372, 309–319. [Google Scholar] [CrossRef]
- Ovidio, M.; Baras, E.; Goffaux, D.; Birtles, C.; Philippart, J.C. Environmental unpredictability rules the autumn migrations of trout (Salmo trutta) in the Belgian Ardennes. Hydrobiologia 1998, 372, 262–273. [Google Scholar]
- Benitez, J.P.; Dierckx, A.; Nzau Matondo, B.; Rollin, X.; Ovidio, M. Movement behaviours of potamodromous fish within a large anthropised river after the reestablishment of the longitudinal connectivity. Fish. Res. 2008, 207, 140–149. [Google Scholar] [CrossRef]
- Ovidio, M.; Dierckx, A.; Benitez, J.P. Movement behaviour and fishway performance for endemic and exotic species in a large anthropized river. Limnologica 2023, 99, 126061. [Google Scholar] [CrossRef]
- Benitez, J.-P.; Dierckx, A.; Rimbaud, G.; Nzau Matondo, B.; Renardy, S.; Rollin, X.; Gillet, A.; Dumonceau, F.; Poncin, P.; Philippart, J.-C.; et al. Assessment. of Fish Abundance, Biodiversity and Movement Periodicity Changes in a Large River over a 20-Year Period. Environments 2022, 9, 22. [Google Scholar] [CrossRef]
- Prokes, M.; Penaz, M. The course of spawning, early development and longitudinal growth of the nase carp, Chondrostoma nasus, in the Rokytna and Jihlava rivers. Folia Zool. 1978, 27, 269–278. [Google Scholar]
- Baras, E.; Nindaba, J. Diel dynamics of habitat use by riverine young–of–the–year Barbus barbus and Chondrostoma nasus (Cyprinidae). Arch. Hydrobiol. 1999, 146, 431–448. [Google Scholar] [CrossRef]
- Nagel, C.; Mueller, M.; Pander, J.; Stoeckle, B.C.; Kuehn, R.; Geist, J. Going with the flow: Spatio-temporal drift patterns of larval fish in a large alpine river. Freshw. Biol. 2021, 66, 1765–1781. [Google Scholar] [CrossRef]
- Wolter, C.; Sukhodolov, A. Random displacement versus habitat choice of fish larvae in rivers. River Res. Appl. 2008, 24, 661–672. [Google Scholar] [CrossRef]
- Schludermann, E.; Tritthart, M.; Humphries, P.; Keckeis, H. Dispersal and retention of larval fish in a potential nursery habitat of a large temperate river: An experimental study? Can. J. Fish. Aquat. Sci. 2012, 69, 1302–1315. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Glas, M.; Tritthart, M.; Habersack, H.; Andorfer, L.; Humphries, P. The influence of discharge, current speed, and development on the downstream dispersal of larval nase (Chondrostoma nasus) in the River Danube. Can. J. Fish. Aquat. Sci. 2018, 75, 247–259. [Google Scholar] [CrossRef]
- Zens, B.; Glas, M.; Tritthart, M.; Habersack, H.; Keckeis, H. Movement patterns and rheoreaction of larvae of a fluvial specialist (nase, Chondrostoma nasus): The role of active versus passive components of behaviour in dispersal. Can. J. Fish. Aquat. Sci. 2018, 75, 193–200. [Google Scholar] [CrossRef]
- Maier, K.-J. On the nase Chondrostoma nasus spawning area situation in Switzerland. Folia Zool. 1997, 46, 79–87. [Google Scholar]
- Melcher, A.; Schmutz, S. The importance of structural features for spawning habitat of nase Chondrostoma nasus (L.) and barbel Barbus barbus (L.) in a pre-Alpine River. River Syst. 2010, 19, 33–42. [Google Scholar] [CrossRef]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early-life-stage development of the European nase (Chondrostoma nasus L.). Ecol Freshw Fish. 2018, 27, 920–932. [Google Scholar] [CrossRef]
- Nagel, C.; Mueller, M.; Pander, J.; Geist, J. Making up the bed: Gravel cleaning as a contribution to nase (Chondrostoma nasus L.) spawning and recruitment success. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2269–2283. [Google Scholar] [CrossRef]
- Winkler, G.; Keckeis, H.; Reckendorfer, W.; Schiemers, F. Temporal and spatial dynamic of 0+ Chondrostoma nasus, at the inshore zone of a large river. Folia Zool. 1996, 46, 151–168. [Google Scholar]
- Keckeis, H.; Winkler, G.; Flore, l.; Reckendorfer, W.; Schiemer, F. Spatial and seasonal characteristics of o+ fish nursery habitats of nase, chondrostoma nasus in the river danube, austria. Folia Zool. 1997, 46, 133–150. [Google Scholar]
- Copp, G. Comparative microhabitat use of cyprinid larvae and juveniles in a lotic floodplain channel. Environ. Biol. Fishes 1990, 33, 181–193. [Google Scholar] [CrossRef]
- Tissot, L.; Souchon, Y. Synthèse des tolérances thermiques des principales espèces de poissons des rivières et fleuves de plaine de l’ouest européen. Hydroecologie Appl. 2010, 17, 17–76. [Google Scholar] [CrossRef]
- Dedual, M. Démographie du Hotu (Chondrostoma nasus nasus) en relation avec la gestion d’une usine hydro-électrique. Hydrology in Mountainous Regions. Artificial Reservoirs; Water and SIope. In Proceedings of Two Lausanne Symposia, August 1990; IAHS Publisher: Wallingford, UK, 1990. [Google Scholar]
- Gerke, M.; Hübnerb, D.; Schneider, J.; Winkelmanna, C. Can top-down effects of cypriniform fish be used to mitigate eutrophication effects in medium-sized European rivers? Sci. Total Environ. 2021, 755, 142547. [Google Scholar] [CrossRef]
- Hübner, D.; Gerke, M.; Fricke, R.; Schneider, J.; Winkelmann, C. Cypriniform fish in running waters reduce hyporheic oxygen depletion in a eutrophic river. Freshw. Biol. 2020, 755, 1518–1528. [Google Scholar] [CrossRef]
- Juradja, P. Effect of channelization and regulation on fish recruitment in a flood plain river. Regulated Rivers. Res. Manag. 1995, 10, 207–215. [Google Scholar]
- Chovanec, A.; Schiemer, F.; Waidbacher, H.; Spolwind, R. Rehabilitation of a heavily modified river section of the Danube in Vienna (Austria): Biological assessment of landscape linkages on different scales. International. Int. Rev. Hydrobiol. 2002, 2–3, 183–195. [Google Scholar] [CrossRef]
- Pander, J.; Nagel, C.; Ingermann, H.; Geist, J. Water level induced changes of habitat quality determine fish community composition in restored and modified riverbanks of a large alpine river. Int. Rev. Hydrobiol. 2022, 107, 46–59. [Google Scholar] [CrossRef]
- Pander, J.; Geist, J. The Contribution of Different Restored Habitats to Fish Diversity and Population Development in a Highly Modified River: A Case Study from the River Günz. Water 2008, 10, 1202. [Google Scholar] [CrossRef]
- Hauer, C.; Gunther, U.; Schmutz, S.; Habersack, H. The importance of morphodynamic processes at riffles used as spawning grounds during the incubation time of nase (Chondrostoma nasus). Hydrobiologia 2007, 579, 15–27. [Google Scholar] [CrossRef]
- Wild, R.; Nagel, C.; Geist, J. Climate change effects on hatching success and embryonic development of fish: Assessing multiple stressor responses in a large-scale mesocosm study. Sci. Total Environ. 2023, 893, 164834. [Google Scholar] [CrossRef] [PubMed]
- Zingraff-Hamed, A.; Noack, M.; Greulich, S.; Schwarzwälder, K.; Pauleit, S.; Wantzen, K.M. Model-Based Evaluation of the Effects of River Discharge Modulations on Physical Fish Habitat Quality. Water 2018, 10, 374. [Google Scholar] [CrossRef]
- Führer, S.; Hayes, D.S.; Hasler, T.; Graf, D.; Fauchery, E.; Mameri, D.; Schmutz, S.; Auer, S. Stranding of larval nase (Chondrostoma nasus L.) depending on bank slope, down-ramping rate and daytime. Front. Environ. Sci. 2022, 10, 966418. [Google Scholar] [CrossRef]
- Hayes, D.S.; Auer, S.; Fauchery, E.; Graf, D.; Hasler, T.; Mameri, D.; Schmutz, S.; Führer, S. The interactive effect of river bank morphology and daytime on downstream displacement and stranding of cyprinid larvae in hydropeaking conditions. Ecohydrol. Hydrobiol. 2023, 23, 152–161. [Google Scholar] [CrossRef]
- Mameri, D.; Hayes, D.S.; Führer, S.; Fauchery, E.; Schmutz, S.; Monserat, A.; Hasler, T.; Graf, D.R.M.; Santos, J.M.; Ferreira, M.T.; et al. Cold thermopeaking-induced drift of nase Chondrostoma nasus larvae. Aquat. Sci. 2023, 85, 56. [Google Scholar] [CrossRef]
- Lusk, S. Influence of Valley dams on the change of fish communities inhabiting streams in the Dyke river drainage area. Folia Zool. 1994, 44, 45–56. [Google Scholar]
- Kappus, B.M.; Jansen, W.; Bohmer, J.; Rahmann, H. Historical and present distribution and recent habitat use of nase, chondrostoma nasus, in the lower jagst river (badenwurttemberg, Germany). Folia Zool. 1997, 46, 51–60. [Google Scholar]
- Penczak, T.; Głowacki, L.; Galicka, W.; Koszalinski, H. A long-term study (1985–1995) of fish populations in the impounded Warta River, Poland. Hydrobiologia 1998, 368, 157–173. [Google Scholar] [CrossRef]
- Le Pichon, C.; Tales, E.; Gorges, G.; Baudry, J.; Boët, P. Using a continuous riverscape survey to examine the effects of the spatial structure of functional habitats on fish distribution. J. Freshw. Ecol. 2015, 31, 1–19. [Google Scholar] [CrossRef]
- Meulenbroek, P.; Drexler, S.; Nagel, C.; Geistler, M.; Waidbacher, H. The importance of a constructed near-nature-like Danube fish by-pass as a lifecycle fish habitat for spawning, nurseries, growing and feeding: A long-term view with remarks on management. Mar. Freshw. Res. 2018, 69, 1857–1869. [Google Scholar] [CrossRef]
- Zitek, A.; Pacher, K.; Erlenburg, W.F.; Schmutz, S. Attraction and Passage Efficiency of a Fish Pass Within a Chain of Impoundments at the River Drau, Villach, Austria. In Proceedings of the International Conference of Ecohydraulics, Vienna, Austria, 17–21 September 2012. [Google Scholar]
- Epple, T.; Friedmann, A.; Wetzel, K.F.; Born, O. The life cycle of nase (Chondrostoma nasus) before and after the construction of hydropower plants in the river Iller (Bavaria, Germany) and its migration behavior through fish-bypass channels. Danub. News 2020, 22, 41. Available online: https://www.danube-iad.eu (accessed on 10 December 2023).


| Adult Movements | Young Stage Movements |
|---|---|
| Home ranges up to tens of Km. | Seasonal active movements of young stages between shallow habitats in summer and deeper habitats in winter. |
| Frequent high mobility within the home range. | Downstream drift is from mid-April to late June, with a peak in late April and early May. |
| Spawning migrations are stimulated by decreasing flow and increasing water temperature within 7.5–12 °C. | Drift in every period of the diel cycle. |
| Spawning migration occurred between late March and early May. | Drift occurs in free embryos, young larvae, intermediate larvae, older larvae, and young juveniles. |
| Spawning movements usually occur upstream unless obstacles are present that may provoke downstream migration. | Drift has a strong active component. |
| Migration occurs during the four phases of the diel period (day, night, dusk, and dawn). | Juveniles realise upstream movement through fish passes from late June to early November, with peaks in late June and early July. |
| Frequent movements in shoals. | |
| Frequent important post-spawning downstream movements. |
| Spawning Habitats | Juvenile Habitats | Adult Habitats |
|---|---|---|
| Shallow habitats (15–50 cm). | Narrow, shallow, and slow-flowing reaches. | Medium-sized watercourses with gravelly and stony bottom substrates. |
| 2.7–10 cm diameter substrate. | Large juveniles (>30 mm) are associated with gravel banks with coarse substrate and higher velocities. | Barbel zone and grayling zone preferred, but presence in bream zone. |
| 0.7–1.1 m/s water velocities. | Larvae in water have velocities ranging from 0.01 to 0.1 ms−1. | 0.5 to >1 m/s water velocities. |
| Overhanging riparian vegetation. | Overlap of habitats with other cyprinid species. | Deep water (up to 9 m). |
| Permeable and well-oxygenated hyporheic zones. | Optimal temperature around 15 °C, tolerance 4–24 °C. | |
| Preference for slope 3.2–0.8‰, pH 6.6–7.9, dissolved oxygen 10.2–12.2, and N-NH4 < 450 mg/L. |
| Mean Threats to Nase Populations | |
|---|---|
| Thermal Pollution | Lack of oxygen |
| Channelling | Eutrophication |
| River flow perturbations | Chemical pollutants and micropollutants |
| Hydropeaking during the spawning season | Poor diversity in microhabitats |
| Insufficient minimum flow conditions | Sedimentation and the presence of fin particles |
| Obstacles to upstream movement | Poor availability of spawning substrate |
| Obstacles to downstream movement | Poor availability of stony-gravelly bottom substrate |
| Guidelines for Nase Conservation |
|---|
| Restore the good quality of the aquatic environment by eliminating all forms of inorganic and organic chemical pollution. |
| Ensure sufficient availability and quality of habitats for reproduction, nurseries, and residence. |
| Guarantee the availability and stability of spawning substrates, at least for the duration of incubation. |
| Prohibit all types of hydraulic exploitation works that lead to significant variations in flow, water level, temperature, and turbidity and that degrade spawning grounds (stony bottoms), nurseries, and residences. |
| Determining a suitable catch size for recreational fishing. |
| Avoid the reduction in large areas of potential habitat by canalization of watercourses (incompatible with the microhabitats of juveniles), dredging and cleaning (destruction of gravelly and stony beds essential for reproduction), catchment water leading to a permanent reduction in water height, water intakes from hydroelectric power stations causing a sudden variation in flow (hydropeaking), and direct discharge for cooling water. |
| Guarantee free movement throughout the life cycle (river annexes, tributaries) by equipping with effective fish-passes (or other devices) to ensure the rise of nase and the continued recolonization of upstream sectors. |
| Limit development work (cleaning, reprofiling, channelling, and backfilling of banks) as much as possible. |
| Choose suitable times of the year (outside the breeding season) to carry out certain essential work. |
| Develop new spawning grounds and nurseries in the most altered waterways according to ecological principles. |
| When the construction of a fish-pass is not possible, carry out intra- and inter-river translocations of non-introgressed wild spawners to initiate or support the reconstitution of stable self-reproducing populations. |
| Restore and protect all potential spawning grounds and nurseries habitats. |
| Subject Area | Research Questions |
|---|---|
| European repartition |
|
| Morphology and identification |
|
| Reproduction and life cycle |
|
| Diet |
|
| Movement dynamics in adult and young stages |
|
| Characteristics of the spawning area and habitats of juveniles and adults |
|
| Impact of anthropogenic pressures and restoration measures |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovidio, M.; Nzau Matondo, B. Ecology and Sustainable Conservation of the Nase, Chondrostoma nasus: A Literature Review. Sustainability 2024, 16, 6007. https://doi.org/10.3390/su16146007
Ovidio M, Nzau Matondo B. Ecology and Sustainable Conservation of the Nase, Chondrostoma nasus: A Literature Review. Sustainability. 2024; 16(14):6007. https://doi.org/10.3390/su16146007
Chicago/Turabian StyleOvidio, Michaël, and Billy Nzau Matondo. 2024. "Ecology and Sustainable Conservation of the Nase, Chondrostoma nasus: A Literature Review" Sustainability 16, no. 14: 6007. https://doi.org/10.3390/su16146007





