Effects of Soil Bacterial Taxa under Different Precipitation Gradients on the Multi-Functionality of the Rhizosphere Soils under Caragana intermedia Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Test Design and Collection of Soil Samples
2.3. Test Method
2.3.1. Determination of Soil Properties
2.3.2. Metagenomic Sequencing and Bioinformatics Analysis
2.4. Data Analysis
3. Results and Analysis
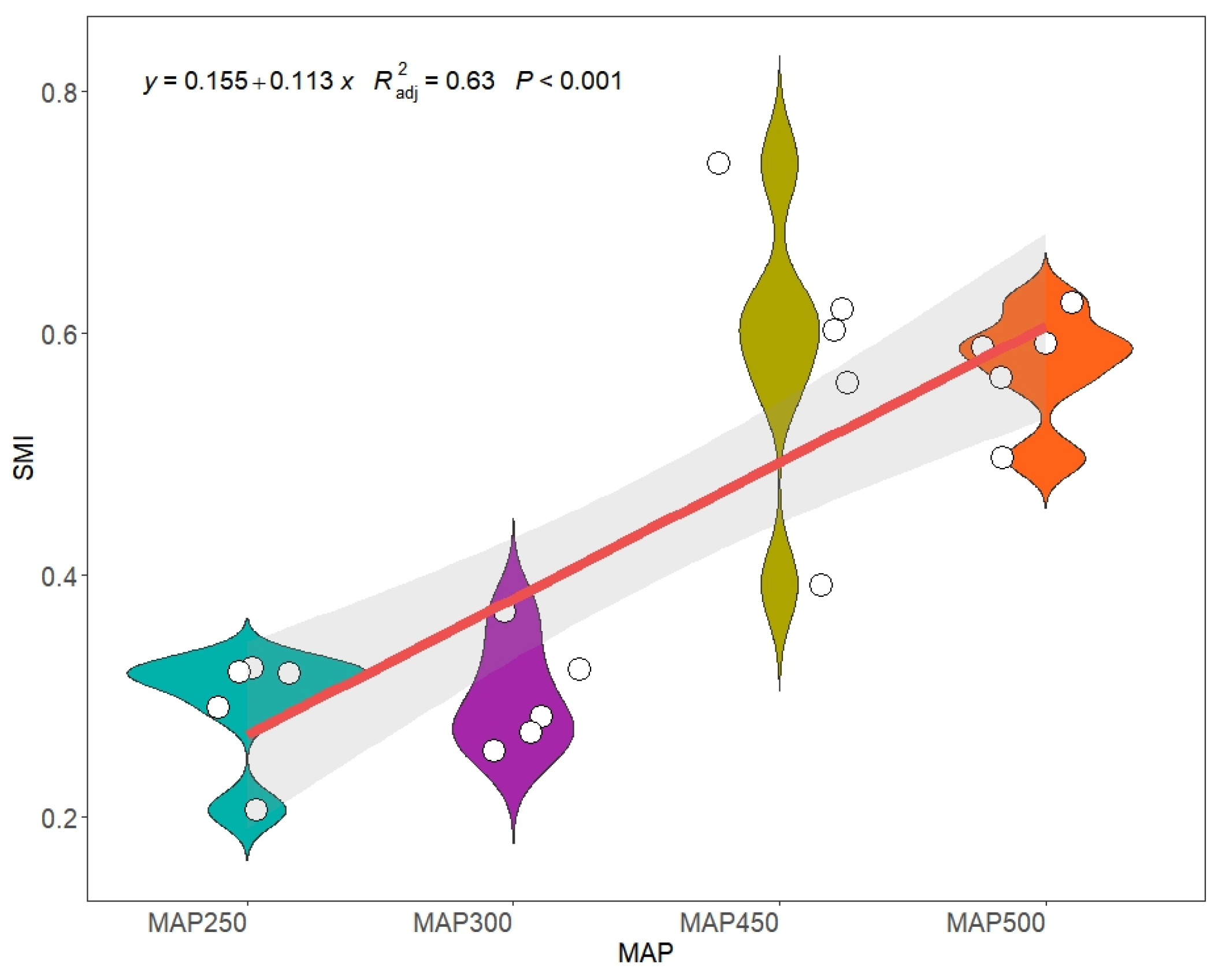
3.1. Effect of the Precipitation Gradient on Soil Multi-Functionality and Soil Factors in the Rhizosphere
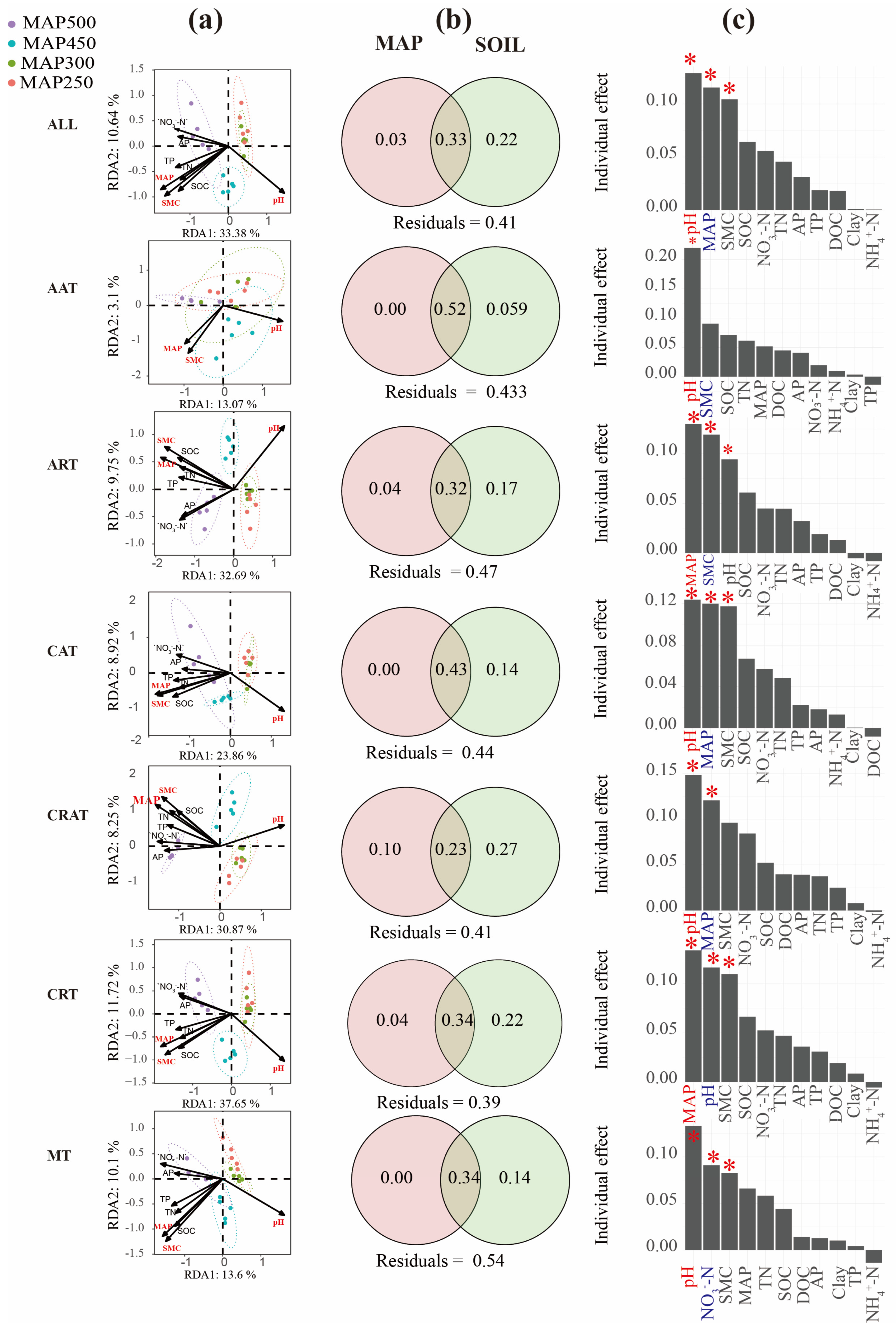
3.2. Influence of Precipitation Gradients and Rhizosphere Soil Attributes on the Soil Bacterial Community Composition
3.3. Relationship between Multi-Functionality and Bacterial Taxa of Rhizosphere Soil
4. Discussions
4.1. Precipitation Gradient Significantly Affected the Rhizosphere Soil Multi-Functionality of the Caragana intermedia Forests
4.2. Precipitation Gradient Significantly Affects Rhizosphere Soil Bacterial Taxa of Caragana intermedia Forest
4.3. Drivers of Rhizosphere Soil Bacterial Taxa and Their Relationships with Soil Multi-Functionality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheffield, J.; Wood, E.F.; Roderick, M.L. Little change in global drought over the past 60 years. Nature 2012, 491, 435–438. [Google Scholar] [CrossRef]
- Stocker, T.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- King, A.D.; Pitman, A.J.; Henley, B.J.; Ukkola, A.M.; Brown, J.R. The role of climate variability in Australian drought. Nat. Clim. Chang. 2020, 10, 177–179. [Google Scholar] [CrossRef]
- WMO. WMO Statement on the State of the Global Climate in 2017; World Meteorological Organisation (WMO): Geneva, Switzerland, 2018.
- Sun, Y.-F.; Shen, J.-P.; Zhang, C.-J.; Zhang, L.-M.; Bai, W.-M.; Fang, Y.; He, J.Z. Responses of soil microbial community to nitrogen fertilizer and precipitation regimes in a semi-arid steppe. J. Soils Sediments 2018, 18, 762–774. [Google Scholar] [CrossRef]
- Shi, L.; Lin, Z.; Yao, Z.; Peng, C.; Hu, M.-a.; Yin, N.; Lu, X.; Zhou, H.; Liu, K.; Shao, X. Increased precipitation rather than warming increases ecosystem multi-functionality in an alpine meadow. Plant Soil 2024, 498, 357–370. [Google Scholar] [CrossRef]
- Ducklow, H.W. Long-term studies of the marine ecosystem along the west Antarctic Peninsula. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 1945–1948. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Han, X.; Deng, Y. Higher precipitation strengthens the microbial interactions in semi-arid grassland soils. Glob. Ecol. Biogeogr. 2018, 27, 570–580. [Google Scholar] [CrossRef]
- Hu, W.; Ran, J.; Dong, L.; Du, Q.; Ji, M.; Yao, S.; Sun, Y.; Gong, C.; Hou, Q.; Gong, H. Aridity-driven shift in biodiversity–soil multi-functionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef]
- Pedrós-Alió, C. Marine microbial diversity: Can it be determined? Trends Microbiol. 2006, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Galand, P.E.; Casamayor, E.O.; Kirchman, D.L.; Lovejoy, C. Ecology of the rare microbial biosphere of the Arctic Ocean. Proc. Natl. Acad. Sci. USA 2009, 106, 22427–22432. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Yu, L.; Heidelberg, J.F.; Kirchman, D.L. Activity of abundant and rare bacteria in a coastal ocean. Proc. Natl. Acad. Sci. USA 2011, 108, 12776–12781. [Google Scholar] [CrossRef] [PubMed]
- Pedrós-Alió, C. The rare bacterial biosphere. Annu. Rev. Mar. Sci. 2012, 4, 449–466. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Yu, Z.; Wilkinson, D.M. The biogeography of abundant and rare bacterioplankton in the lakes and reservoirs of China. ISME J. 2015, 9, 2068–2077. [Google Scholar] [CrossRef]
- Cheng, J.M.; Du, F.; Wan, H. Solid Collocation of Water Collected Shrub-Grass and Water Regulation in Semi-Arid Region of Loess Plateau. Acta Agrestia Ainica 2000, 8, 210–219. (In Chinese) [Google Scholar]
- An, S.S.; Huang, Y.M. Study On the Ameliorate Benefits of Caragana Korshinskii Shrubwood to Soil Properties in Loess Hilly Area. Sci. Silvae Sin. 2006, 42, 70–74. (In Chinese) [Google Scholar]
- Lakshmanan, V.; Ray, P.; Craven, K.D. Toward a resilient, functional microbiome: Drought tolerance-alleviating microbes for sustainable agriculture. Plant Stress Toler. Methods Protoc. 2017, 1631, 69–84. [Google Scholar]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Methods for Agricultural Chemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 1999; pp. 238–240. (In Chinese) [Google Scholar]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Dai, T.; Zhang, Y.; Tang, Y.; Bai, Y.; Tao, Y.; Huang, B.; Wen, D. Identifying the key taxonomic categories that characterize microbial community diversity using full-scale classification: A case study of microbial communities in the sediments of Hangzhou Bay. FEMS Microbiol. Ecol. 2016, 92, fiw150. [Google Scholar] [CrossRef] [PubMed]
- Peng, S. 1-km monthly mean temperature dataset for China (1901–2021). In A Big Earth Data Platform for Three Poles; Northwest Institute of Eco-Environment and Resources, CAS: Lanzhou, China, 2019. [Google Scholar]
- Smith, M.D. The ecological role of climate extremes: Current understanding and future prospects. J. Ecol. 2011, 99, 651–655. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Zhang, Z.; Li, W.; Liu, W.; Xiao, N.; Liu, H.; Wang, L.; Li, Z.; Ma, J. Decreased soil multi-functionality is associated with altered microbial network properties under precipitation reduction in a semiarid grassland. iMeta 2023, 2, e106. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.W.; Yang, J.C.; Li, H. Analysis on water vapor characteristics of an extreme rainstorm in the arid region of WesternHexi Corridor. Meteorol. Mon. 2021, 47, 412–423. (In Chinese) [Google Scholar]
- Bachar, A.; Al-Ashhab, A.; Soares, M.I.M.; Sklarz, M.Y.; Angel, R.; Ungar, E.D.; Gillor, O. Soil microbial abundance and diversity along a low precipitation gradient. Microb. Ecol. 2010, 60, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Lu, Y. Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Gadd, G.M.; Yang, Y.; Yuan, W.; Gu, J.; Ye, L.; Liu, W. Environmental adaptation is stronger for abundant rather than rare microorganisms in wetland soils from the Qinghai-Tibet Plateau. Mol. Ecol. 2021, 30, 2390–2403. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Y.; Wei, Y.; Huang, Y.; Zhang, H.; He, W.; Sheng, H.; An, L. Effect of aridity and dune type on rhizosphere soil bacterial communities of Caragana microphylla in desert regions of northern China. PLoS ONE 2019, 14, e0224195. [Google Scholar] [CrossRef]
- Cordero, I.; Leizeaga, A.; Hicks, L.C.; Rousk, J.; Bardgett, R.D. High intensity perturbations induce an abrupt shift in soil microbial state. ISME J. 2023, 17, 2190–2199. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Placella, S.A.; Brodie, E.L.; Firestone, M.K. Rainfall-induced carbon dioxide pulses result from sequential resuscitation of phylogenetically clustered microbial groups. Proc. Natl. Acad. Sci. USA 2012, 109, 10931–10936. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- Luo, L.S.; Wang, Y.P.; Du, S. Effects of simulated rainfall reduction on soil nutrients and microbe community in Robinia pseudoacacia plantation. Acta Ecol. Sin. 2023, 43, 5916–5925. [Google Scholar]
- Zhang, X.; Qi, L.; Li, W.; Hu, B.X.; Dai, Z. Bacterial community variations with salinity in the saltwater-intruded estuarine aquifer. Sci. Total Environ. 2021, 755, 142423. [Google Scholar] [CrossRef]
- Ma, X.; Chao, L.; Li, J.; Ding, Z.; Wang, S.; Li, F.; Bao, Y. The distribution and turnover of bacterial communities in the root zone of seven Stipa species across an arid and semi-arid steppe. Front. Microbiol. 2021, 12, 782621. [Google Scholar] [CrossRef]
- Zeng, Q.; An, S. Identifying the Biogeographic Patterns of Rare and Abundant Bacterial Communities Using Different Primer Sets on the Loess Plateau. Microorganisms 2021, 9, 139. [Google Scholar] [CrossRef]
- Pillai, P.; Gouhier, T.C.; Vollmer, S.V. Ecological rescue of host-microbial systems under environmental change. Theor. Ecol. 2017, 10, 51–63. [Google Scholar] [CrossRef]
- Tecon, R.; Or, D. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol. Rev. 2017, 41, 599–623. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, X.; Nuccio, E.E.; Yuan, M.; Zhang, N.; Xue, K.; Cohan, F.M.; Zhou, J.; Sun, B. Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 2020, 22, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhao, Z.; Xia, L.; Wang, S.; Yu, G.; Miao, A. Responses of abundant and rare prokaryotic taxa in a controlled organic contaminated site subjected to vertical pollution-induced disturbances. Sci. Total Environ. 2022, 853, 158625. [Google Scholar] [CrossRef] [PubMed]
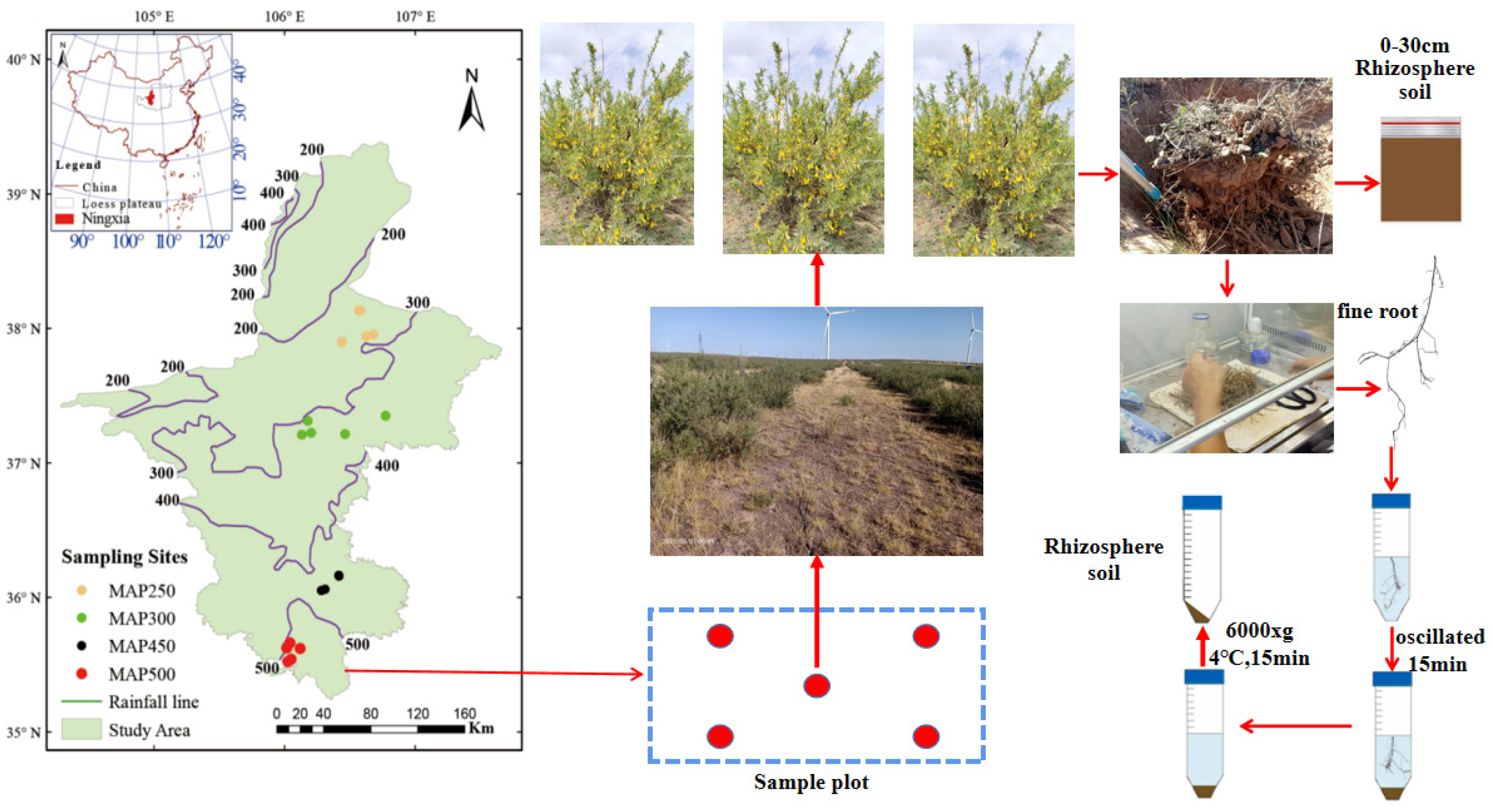






| Precipitation | Latitude and Longitude (°) | Precipitation (mm) | Altitude (m) | Forest Age (Years) | Stand Density (Bush/hm2) | Ground Diameter (cm) | Height (m) | Crown Breadth (cm) | |
|---|---|---|---|---|---|---|---|---|---|
| MAP250 | 37.9–38.1 | 106.4–106.7 | 228.9–256.4 | 1236–1441 | 20 | 2687.1 ± 1873 | 10.1 ± 3.6 | 107.1 ± 39.3 | 99.9 ± 43.0 |
| MAP300 | 37.2–37.34 | 106.1–106.8 | 284.2–319.6 | 1417–1671 | 20 | 2067.7 ± 689.3 | 13.3 ± 3.8 | 125.1 ± 28.3 | 125.1 ± 36.7 |
| MAP450 | 36.1–36.2 | 106.3–106.4 | 443.4–451.6 | 1716–1929 | 20 | 2067.7 ± 734.3 | 14.8 ± 3.6 | 174.0 ± 19.9 | 154.6 ± 39.1 |
| MAP500 | 35.5–35.6 | 106.0–106.1 | 484.6–531.4 | 2019–2158 | 20 | 3121.6 ± 996.5 | 12.0 ± 4.2 | 177.0 ± 36.9 | 196.4 ± 78.1 |
| Precipitation Gradient | pH | SMC (%) | Sand (%) | Silt (%) | Clay (%) | SMI |
|---|---|---|---|---|---|---|
| MAP 250 | 8.52 ± 0.05a | 1.27 ± 0.58c | 44.98 ± 25.9a | 48.72 ± 22.82b | 6.29 ± 3.79b | 0.29 ± 0.05b |
| MAP 300 | 8.65 ± 0.07a | 2.62 ± 0.83b | 41.53 ± 10.07a | 50.88 ± 9.04b | 7.59 ± 1.39ab | 0.3 ± 0.05b |
| MAP 450 | 8.63 ± 0.06a | 8 ± 0.59a | 20.15 ± 1.96b | 72.58 ± 1.25a | 7.27 ± 0.87ab | 0.58 ± 0.13a |
| MAP 500 | 8.32 ± 0.21b | 8.58 ± 0.62a | 14.16 ± 2.82b | 76.74 ± 2.25a | 9.11 ± 0.75a | 0.57 ± 0.05a |
| Precipitation | ALL | AAT | ART | MT | CAT | CRT | CRAT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | R2 | p | R2 | p | R2 | p | R2 | p | |
| ALL | 0.676 | 0.001 | 0.540 | 0.002 | 0.669 | 0.001 | 0.583 | 0.001 | 0.664 | 0.001 | 0.706 | 0.001 | 0.724 | 0.001 |
| MAP 500/MAP 450 | 0.626 | 0.013 | 0.702 | 0.010 | 0.581 | 0.008 | 0.585 | 0.011 | 0.556 | 0.004 | 0.654 | 0.011 | 0.697 | 0.010 |
| MAP 500/MAP 300 | 0.660 | 0.018 | 0.516 | 0.020 | 0.643 | 0.006 | 0.572 | 0.011 | 0.628 | 0.011 | 0.700 | 0.010 | 0.743 | 0.008 |
| MAP 500/MAP 250 | 0.667 | 0.003 | 0.534 | 0.020 | 0.666 | 0.019 | 0.509 | 0.009 | 0.659 | 0.008 | 0.705 | 0.010 | 0.703 | 0.014 |
| MAP 450/MAP 300 | 0.497 | 0.008 | 0.268 | 0.101 | 0.518 | 0.005 | 0.432 | 0.006 | 0.531 | 0.010 | 0.543 | 0.008 | 0.475 | 0.015 |
| MAP 450/MAP 250 | 0.568 | 0.008 | 0.319 | 0.053 | 0.588 | 0.007 | 0.507 | 0.008 | 0.637 | 0.006 | 0.589 | 0.004 | 0.501 | 0.010 |
| MAP 300/MAP 250 | 0.143 | 0.267 | 0.004 | 0.970 | 0.179 | 0.111 | 0.219 | 0.052 | 0.118 | 0.340 | 0.157 | 0.192 | 0.194 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Bai, X.; Hu, S.; Zhang, M.; Wang, Y.; Yu, X. Effects of Soil Bacterial Taxa under Different Precipitation Gradients on the Multi-Functionality of the Rhizosphere Soils under Caragana intermedia Forests. Sustainability 2024, 16, 6032. https://doi.org/10.3390/su16146032
Dong L, Bai X, Hu S, Zhang M, Wang Y, Yu X. Effects of Soil Bacterial Taxa under Different Precipitation Gradients on the Multi-Functionality of the Rhizosphere Soils under Caragana intermedia Forests. Sustainability. 2024; 16(14):6032. https://doi.org/10.3390/su16146032
Chicago/Turabian StyleDong, Liguo, Xiaoxiong Bai, Sile Hu, Min Zhang, Ying Wang, and Xuan Yu. 2024. "Effects of Soil Bacterial Taxa under Different Precipitation Gradients on the Multi-Functionality of the Rhizosphere Soils under Caragana intermedia Forests" Sustainability 16, no. 14: 6032. https://doi.org/10.3390/su16146032
APA StyleDong, L., Bai, X., Hu, S., Zhang, M., Wang, Y., & Yu, X. (2024). Effects of Soil Bacterial Taxa under Different Precipitation Gradients on the Multi-Functionality of the Rhizosphere Soils under Caragana intermedia Forests. Sustainability, 16(14), 6032. https://doi.org/10.3390/su16146032






